Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma—A Comprehensive Update
Abstract
1. Introduction
2. Neuroblastoma
2.1. Aetiology, Prevalence and Characteristics
2.2. Risk Factors, Tumour Staging and Treatment
3. ALK in Health and Disease
3.1. Structure and Function of ALK
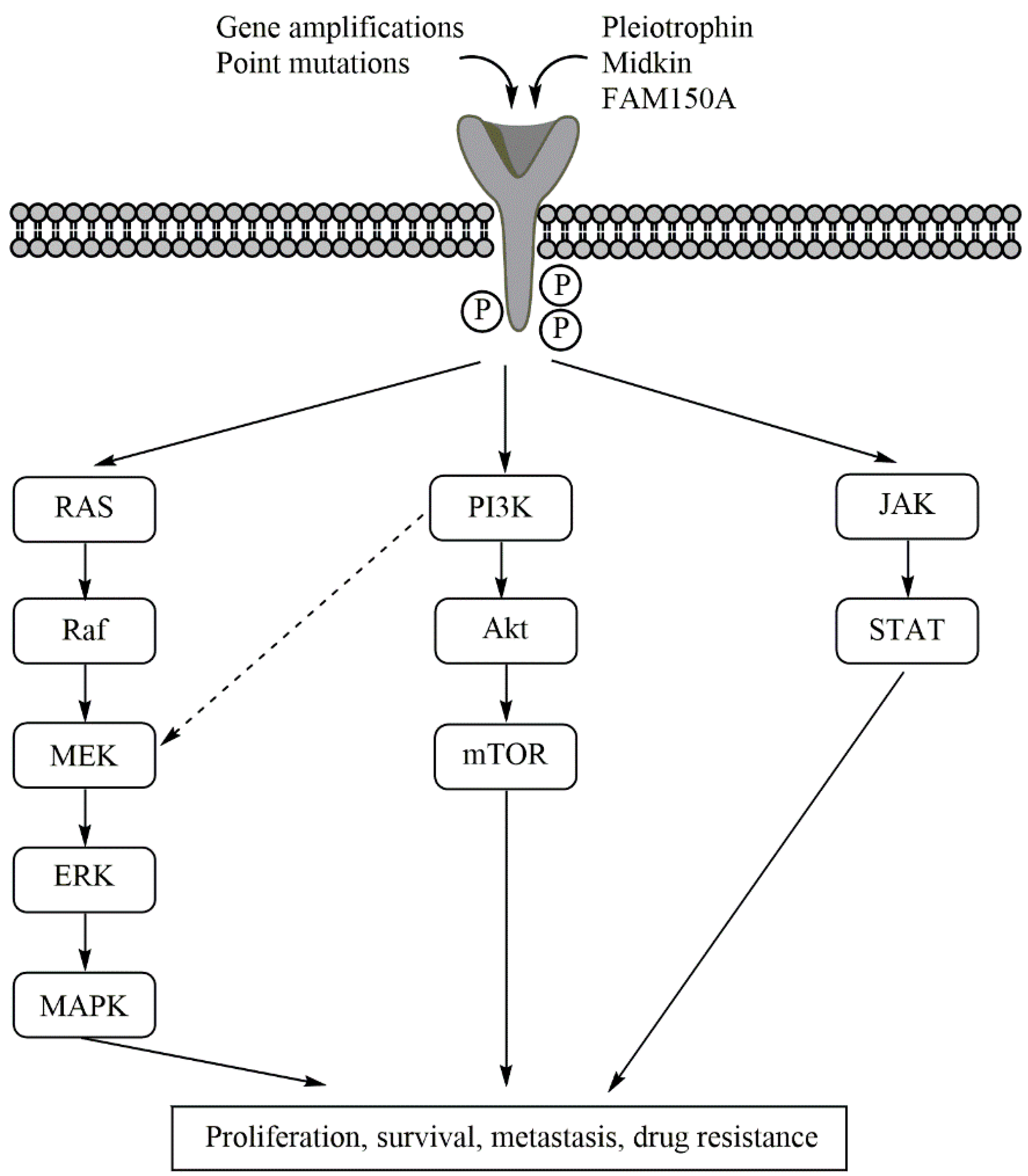
3.2. The Role of ALK in Tumorigenesis
4. ALK Inhibitors in Monotherapy
4.1. The ALK Inhibitor Pioneer Crizotinib
- In a phase I clinical study of eleven refractory NBL patients, one patient obtained complete remission, and an additional three patients obtained stable disease. Of these patients, two had an R1275Q germline mutation, while the two somatic point mutations were R1275L and F1174L. The remaining seven patients, among them one harbouring the ALK R1275Q mutation, showed disease progression [32];
- In a follow-up phase II clinical study, twenty relapsed/refractory NBL cases (no germline mutations, no point mutation at F1245) were included, and only three patients showed complete or partial response. All of these patients, in addition to two with stable disease, harboured the ALK R1275Q mutation [44];
- A case report included two patients with ALK overexpression. It is not stated whether this overexpression resulted from ALK amplifications or increased mRNA expression. Both patients showed complete response, but one patient relapsed after 32 weeks of crizotinib monotherapy. The other patient remained in remission without further treatment during the two months of follow-up [52].
4.2. A Wide Selection of Second-Generation of ALK Inhibitors
4.2.1. TAE-684
4.2.2. Ceritinib/LDK-378
4.2.3. ASP3026
4.2.4. Brigatinib/AP26133
4.2.5. Ensartinib/X-396
4.2.6. AZD3463
4.2.7. CEP-37440
4.2.8. Alectinib/CH5424802
4.2.9. Belizatinib/TSR-011
4.2.10. Entrectinib/RDX-101
4.3. Third-Generation of ALK Inhibitors
4.3.1. Lorlatinib/PF-06463992
4.3.2. Repotrectinib/TPX-0005
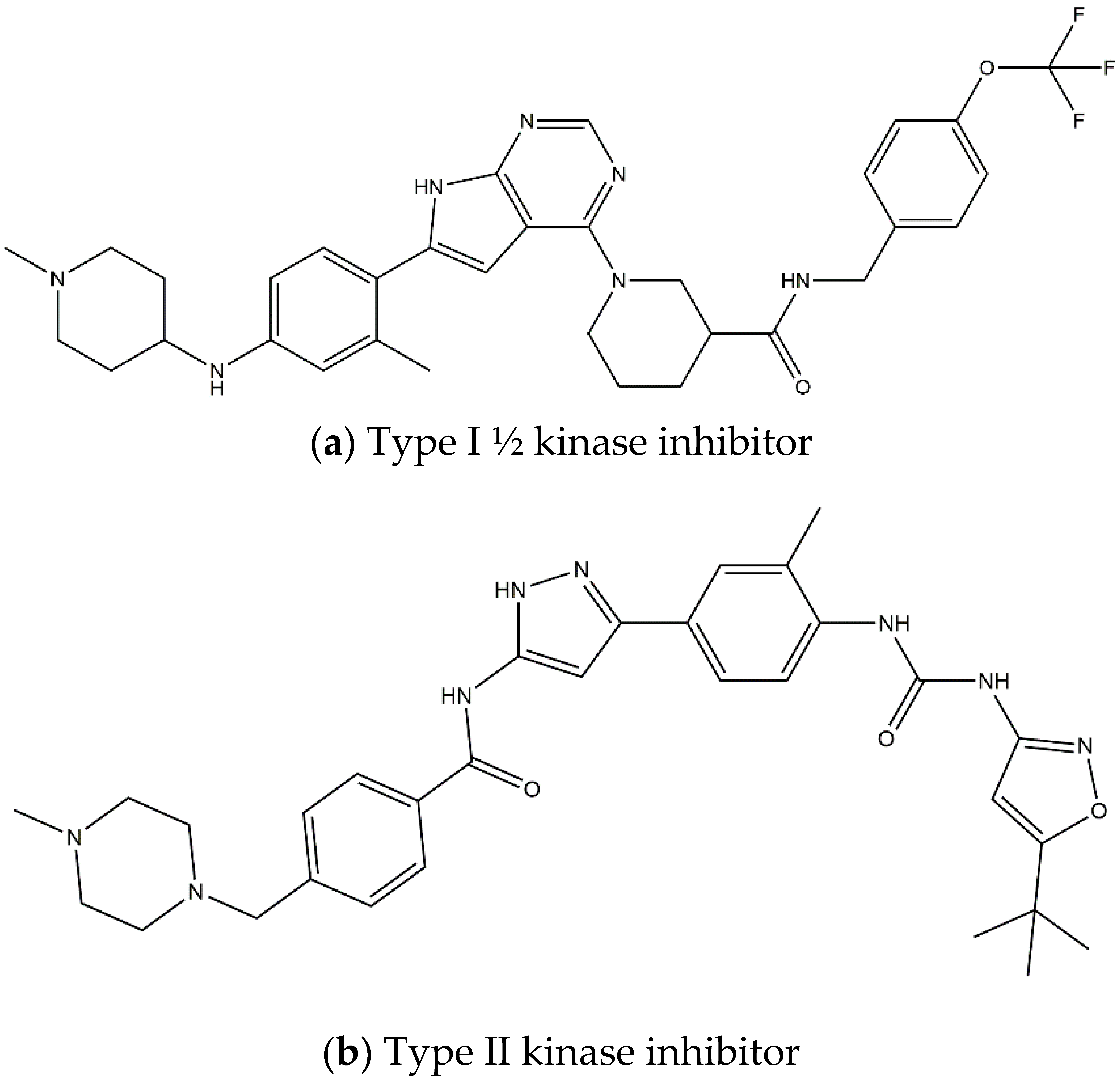
4.4. Improved Targeting Effects: Types I ½ and II Kinase Inhibitors
4.5. Other Inhibitors
4.5.1. HSP90 Inhibition
4.5.2. Cell Differentiation by Retinoic Acids
5. Combination Therapy in NBL Treatment
5.1. Combinational Therapy with Pathway Inhibitors
5.1.1. Inhibitors of the PI3K-Akt-mTOR Pathway
5.1.2. Inhibitors of the RAS/MAPK Pathway
5.2. Combinatory Therapy with Other Agents
5.2.1. Inhibition of the Ubiquitin Kinase MDM2
5.2.2. Inhibition of Histone Deacytylases
5.2.3. Inhibition of Cyclin Dependant Kinases
5.2.4. Inhibition of the Axl Kinase
5.2.5. Inhibition of the Wnt/β-Catenin Pathway
5.3. On the Verge—The Combination of ALK Inhibitors with TRAIL
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- An, X.; Tiwari, A.K.; Sun, Y.; Ding, P.R.; Ashby, C.R., Jr.; Chen, Z.S. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk. Res. 2010, 34, 1255–1268. [Google Scholar] [CrossRef]
- Dogra, R.; Bhatia, R.; Shankar, R.; Bansal, P.; Rawal, R.K. Enasidenib: First Mutant IDH2 Inhibitor for the Treatment of Refractory and Relapsed Acute Myeloid Leukemia. Anti-Cancer Agents Med. Chem. 2018, 18, 1936–1951. [Google Scholar] [CrossRef]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Fasano, M.; Martinelli, E.; Troiani, T.; Ciardiello, F.; Morgillo, F. Role and targeting of anaplastic lymphoma kinase in cancer. Mol. Cancer 2018, 17, 30. [Google Scholar] [CrossRef]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27 (Suppl. 3), iii4–iii15. [Google Scholar] [CrossRef]
- Holla, V.R.; Elamin, Y.Y.; Bailey, A.M.; Johnson, A.M.; Litzenburger, B.C.; Khotskaya, Y.B.; Sanchez, N.S.; Zeng, J.; Shufean, M.A.; Shaw, K.R.; et al. ALK: A tyrosine kinase target for cancer therapy. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001115. [Google Scholar] [CrossRef]
- Kong, X.; Pan, P.; Sun, H.; Xia, H.; Wang, X.; Li, Y.; Hou, T. Drug Discovery Targeting Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2019, 62, 10927–10954. [Google Scholar] [CrossRef]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular pathogenesis and therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef]
- Nakagawara, A.; Li, Y.; Izumi, H.; Muramori, K.; Inada, H.; Nishi, M. Neuroblastoma. Jpn. J. Clin. Oncol. 2018, 48, 214–241. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, S.; Kadomatsu, K. Origin and initiation mechanisms of neuroblastoma. Cell Tissue Res. 2018, 372, 211–221. [Google Scholar] [CrossRef]
- Strobl-Mazzulla, P.H.; Bronner, M.E. Epithelial to mesenchymal transition: New and old insights from the classical neural crest model. Semin. Cancer Biol. 2012, 22, 411–416. [Google Scholar] [CrossRef][Green Version]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef]
- Yi, B.; Yang, J.; Wang, L. The growth inhibitory effect of 17-DMAG on ALK and MYCN double-positive neuroblastoma cell line. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 3229–3235. [Google Scholar] [CrossRef]
- Cervantes-Madrid, D.; Szydzik, J.; Lind, D.E.; Borenäs, M.; Bemark, M.; Cui, J.; Palmer, R.H.; Hallberg, B. Repotrectinib (TPX-0005), effectively reduces growth of ALK driven neuroblastoma cells. Sci. Rep. 2019, 9, 19353. [Google Scholar] [CrossRef]
- Aygun, N. Biological and genetic features of neuroblastoma and their clinical importance. Curr. Pediatr. Rev. 2018, 14, 73–90. [Google Scholar] [CrossRef]
- Pacenta, H.L.; Macy, M.E. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug Des. Dev. Ther. 2018, 12, 3549–3561. [Google Scholar] [CrossRef]
- Ritenour, L.E.; Randall, M.P.; Bosse, K.R.; Diskin, S.J. Genetic susceptibility to neuroblastoma: Current knowledge and future directions. Cell Tissue Res. 2018, 372, 287–307. [Google Scholar] [CrossRef]
- London, W.B.; Castel, V.; Monclair, T.; Ambros, P.F.; Pearson, A.D.; Cohn, S.L.; Berthold, F.; Nakagawara, A.; Ladenstein, R.L.; Iehara, T.; et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3286–3292. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Schleiermacher, G.; Mosseri, V.; London, W.B.; Maris, J.M.; Brodeur, G.M.; Attiyeh, E.; Haber, M.; Khan, J.; Nakagawara, A.; Speleman, F.; et al. Segmental chromosomal alterations have prognostic impact in neuroblastoma: A report from the INRG project. Br. J. Cancer 2012, 107, 1418–1422. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef]
- Brisse, H.J.; McCarville, M.B.; Granata, C.; Krug, K.B.; Wootton-Gorges, S.L.; Kanegawa, K.; Giammarile, F.; Schmidt, M.; Shulkin, B.L.; Matthay, K.K.; et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011, 261, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.; Desai, A.V. The Evolution of Risk Classification for Neuroblastoma. Children 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef]
- Esposito, M.R.; Aveic, S.; Seydel, A.; Tonini, G.P. Neuroblastoma treatment in the post-genomic era. J. Biomed. Sci. 2017, 24, 14. [Google Scholar] [CrossRef]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef]
- Zaizen, Y.; Taniguchi, S.; Noguchi, S.; Suita, S. The effect of N-myc amplification and expression on invasiveness of neuroblastoma cells. J. Pediatr. Surg. 1993, 28, 766–769. [Google Scholar] [CrossRef]
- Hasan, M.K.; Nafady, A.; Takatori, A.; Kishida, S.; Ohira, M.; Suenaga, Y.; Hossain, S.; Akter, J.; Ogura, A.; Nakamura, Y.; et al. ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma. Sci. Rep. 2013, 3, 3450. [Google Scholar] [CrossRef]
- Bresler, S.C.; Weiser, D.A.; Huwe, P.J.; Park, J.H.; Krytska, K.; Ryles, H.; Laudenslager, M.; Rappaport, E.F.; Wood, A.C.; McGrady, P.W.; et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell 2014, 26, 682–694. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef]
- Siaw, J.T.; Wan, H.; Pfeifer, K.; Rivera, V.M.; Guan, J.; Palmer, R.H.; Hallberg, B. Brigatinib, an anaplastic lymphoma kinase inhibitor, abrogates activity and growth in ALK-positive neuroblastoma cells, Drosophila and mice. Oncotarget 2016, 7, 29011–29022. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Mossé, Y.P. Anaplastic Lymphoma Kinase as a Cancer Target in Pediatric Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 546–552. [Google Scholar] [CrossRef]
- Kelleher, F. Close Neighbours on Chromosome 2 the ALK and MYCN Genes. Implications for Targeted Therapeutics in Neuroblastoma; IntechOpen: London, UK, 2013. [Google Scholar]
- Azarova, A.M.; Gautam, G.; George, R.E. Emerging importance of ALK in neuroblastoma. Semin. Cancer Biol. 2011, 21, 267–275. [Google Scholar] [CrossRef]
- De Brouwer, S.; De Preter, K.; Kumps, C.; Zabrocki, P.; Porcu, M.; Westerhout, E.M.; Lakeman, A.; Vandesompele, J.; Hoebeeck, J.; Van Maerken, T.; et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4353–4362. [Google Scholar] [CrossRef]
- Schönherr, C.; Ruuth, K.; Kamaraj, S.; Wang, C.L.; Yang, H.L.; Combaret, V.; Djos, A.; Martinsson, T.; Christensen, J.G.; Palmer, R.H.; et al. Anaplastic lymphoma kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012, 31, 5193–5200. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Foster, J.H.; Voss, S.D.; Hall, D.C.; Minard, C.G.; Balis, F.M.; Wilner, K.; Berg, S.L.; Fox, E.; Adamson, P.C.; Blaney, S.; et al. Activity of crizotinib in patients with ALK-aberrant relapsed/refractory neuroblastoma: A Children’s Oncology Group Study (ADVL0912). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lee, J.S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell 2012, 21, 362–373. [Google Scholar] [CrossRef]
- Epstein, L.F.; Chen, H.; Emkey, R.; Whittington, D.A. The R1275Q neuroblastoma mutant and certain ATP-competitive inhibitors stabilize alternative activation loop conformations of anaplastic lymphoma kinase. J. Biol. Chem. 2012, 287, 37447–37457. [Google Scholar] [CrossRef]
- Lambertz, I.; Kumps, C.; Claeys, S.; Lindner, S.; Beckers, A.; Janssens, E.; Carter, D.R.; Cazes, A.; Cheung, B.B.; De Mariano, M.; et al. Upregulation of MAPK negative feedback regulators and RET in mutant ALK neuroblastoma: Implications for targeted treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3327–3339. [Google Scholar] [CrossRef]
- George, R.E.; Sanda, T.; Hanna, M.; Fröhling, S.; Luther, W., 2nd; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef]
- Wang, H.Q.; Halilovic, E.; Li, X.; Liang, J.; Cao, Y.; Rakiec, D.P.; Ruddy, D.A.; Jeay, S.; Wuerthner, J.U.; Timple, N.; et al. Combined ALK and MDM2 inhibition increases antitumor activity and overcomes resistance in human ALK mutant neuroblastoma cell lines and xenograft models. eLife 2017, 6, e17137. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef]
- Bresler, S.C.; Wood, A.C.; Haglund, E.A.; Courtright, J.; Belcastro, L.T.; Plegaria, J.S.; Cole, K.; Toporovskaya, Y.; Zhao, H.; Carpenter, E.L.; et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci. Transl. Med. 2011, 3, 108ra114. [Google Scholar] [CrossRef]
- Verma, P.; Jain, S.; Kapoor, G. Complete response with crizotinib in two children with chemotherapy resistant neuroblastoma. South Asian J. Cancer 2017, 6, 89–90. [Google Scholar] [PubMed]
- Geoerger, B.; Schulte, J.; Zwaan, C.M.; Casanova, M.; Fischer, M.; Moreno, L.; Trahair, T.; Jimenez, I.; Kang, H.J.; Pappo, A.S.; et al. Phase I study of ceritinib in pediatric patients (Pts) with malignancies harboring a genetic alteration in ALK (ALK+): Safety, pharmacokinetic (PK), and efficacy results. J. Clin. Oncol. 2015, 33, 10005. [Google Scholar] [CrossRef]
- Li, T.; LoRusso, P.; Maitland, M.L.; Ou, S.H.; Bahceci, E.; Ball, H.A.; Park, J.W.; Yuen, G.; Tolcher, A. First-in-human, open-label dose-escalation and dose-expansion study of the safety, pharmacokinetics, and antitumor effects of an oral ALK inhibitor ASP3026 in patients with advanced solid tumors. J. Hematol. Oncol. 2016, 9, 23. [Google Scholar] [CrossRef]
- Debruyne, D.N.; Bhatnagar, N.; Sharma, B.; Luther, W.; Moore, N.F.; Cheung, N.K.; Gray, N.S.; George, R.E. ALK inhibitor resistance in ALK(F1174L)-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene 2016, 35, 3681–3691. [Google Scholar] [CrossRef]
- Cameron, L.; Solomon, B. Treatment of ALK-Rearranged Non-Small Cell Lung Cancer: Recent Progress and Future Directions. Drugs 2015, 75, 1059–1070. [Google Scholar] [CrossRef]
- Friboulet, L.; Li, N.; Katayama, R.; Lee, C.C.; Gainor, J.F.; Crystal, A.S.; Michellys, P.Y.; Awad, M.M.; Yanagitani, N.; Kim, S.; et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014, 4, 662–673. [Google Scholar] [CrossRef]
- Pan, P.; Yu, H.; Liu, Q.; Kong, X.; Chen, H.; Chen, J.; Liu, Q.; Li, D.; Kang, Y.; Sun, H.; et al. Combating drug-resistant mutants of anaplastic lymphoma kinase with potent and selective type-I(1/2) inhibitors by stabilizing unique DFG-shifted loop conformation. ACS Cent. Sci. 2017, 3, 1208–1220. [Google Scholar] [CrossRef]
- Guan, J.; Fransson, S.; Siaw, J.T.; Treis, D.; Van den Eynden, J.; Chand, D.; Umapathy, G.; Ruuth, K.; Svenberg, P.; Wessman, S.; et al. Clinical response of the novel activating ALK-I1171T mutation in neuroblastoma to the ALK inhibitor ceritinib. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002550. [Google Scholar] [CrossRef]
- Mori, M.; Ueno, Y.; Konagai, S.; Fushiki, H.; Shimada, I.; Kondoh, Y.; Saito, R.; Mori, K.; Shindou, N.; Soga, T.; et al. The selective anaplastic lymphoma receptor tyrosine kinase inhibitor ASP3026 induces tumor regression and prolongs survival in non-small cell lung cancer model mice. Mol. Cancer Ther. 2014, 13, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Iragavarapu, C.; Mustafa, M.; Akinleye, A.; Furqan, M.; Mittal, V.; Cang, S.; Liu, D. Novel ALK inhibitors in clinical use and development. J. Hematol. Oncol. 2015, 8, 17. [Google Scholar] [CrossRef]
- Di Paolo, D.; Yang, D.; Pastorino, F.; Emionite, L.; Cilli, M.; Daga, A.; Destafanis, E.; Di Fiore, A.; Piaggio, F.; Brignole, C.; et al. New therapeutic strategies in neuroblastoma: Combined targeting of a novel tyrosine kinase inhibitor and liposomal siRNAs against ALK. Oncotarget 2015, 6, 28774–28789. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Guan, S.; Cao, W.; Wang, H.; Chen, Z.; Zhao, Y.; Yu, Y.; Zhang, H.; Pang, J.C.; et al. Novel ALK inhibitor AZD3463 inhibits neuroblastoma growth by overcoming crizotinib resistance and inducing apoptosis. Sci. Rep. 2016, 6, 19423. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.R.; Cheng, M.; Learn, K.S.; Wagner, J.; Gingrich, D.E.; Lisko, J.G.; Curry, M.; Mesaros, E.F.; Ghose, A.K.; Quail, M.R.; et al. Discovery of Clinical Candidate CEP-37440, a Selective Inhibitor of Focal Adhesion Kinase (FAK) and Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2016, 59, 7478–7496. [Google Scholar] [CrossRef]
- Alam, M.W.; Borenäs, M.; Lind, D.E.; Cervantes-Madrid, D.; Umapathy, G.; Palmer, R.H.; Hallberg, B. Alectinib, an anaplastic lymphoma kinase inhibitor, abolishes alk activity and growth in ALK-positive neuroblastoma cells. Front. Oncol. 2019, 9, 579. [Google Scholar] [CrossRef]
- Kodama, T.; Hasegawa, M.; Takanashi, K.; Sakurai, Y.; Kondoh, O.; Sakamoto, H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother. Pharmacol. 2014, 74, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Tsukaguchi, T.; Hiroshima, S.; Kodama, T.; Kobayashi, T.; Fukami, T.A.; Oikawa, N.; Tsukuda, T.; Ishii, N.; Aoki, Y. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011, 19, 679–690. [Google Scholar] [CrossRef]
- Heath, J.A.; Campbell, M.A.; Thomas, A.; Solomon, B. Good clinical response to alectinib, a second generation ALK inhibitor, in refractory neuroblastoma. Pediatr. Blood Cancer 2018, 65, e27055. [Google Scholar] [CrossRef]
- Lin, C.C.; Arkenau, H.T.; Lu, S.; Sachdev, J.; de Castro Carpeño, J.; Mita, M.; Dziadziuszko, R.; Su, W.C.; Bobilev, D.; Hughes, L.; et al. A phase 1, open-label, dose-escalation trial of oral TSR-011 in patients with advanced solid tumours and lymphomas. Br. J. Cancer 2019, 121, 131–138. [Google Scholar] [CrossRef]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef]
- Aveic, S.; Pantile, M.; Seydel, A.; Esposito, M.R.; Zanon, C.; Li, G.; Tonini, G.P. Combating autophagy is a strategy to increase cytotoxic effects of novel ALK inhibitor entrectinib in neuroblastoma cells. Oncotarget 2016, 7, 5646–5663. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- MacFarland, S.P.; Naraparaju, K.; Iyer, R.; Guan, P.; Kolla, V.; Hu, Y.; Tan, K.; Brodeur, G.M. Mechanisms of Entrectinib Resistance in a Neuroblastoma Xenograft Model. Mol. Cancer Ther. 2020, 19, 920–926. [Google Scholar] [CrossRef]
- Redaelli, S.; Ceccon, M.; Zappa, M.; Sharma, G.G.; Mastini, C.; Mauri, M.; Nigoghossian, M.; Massimino, L.; Cordani, N.; Farina, F.; et al. Lorlatinib treatment elicits multiple on- and off-target mechanisms of resistance in ALK-driven cancer. Cancer Res. 2018, 78, 6866–6880. [Google Scholar] [CrossRef]
- Infarinato, N.R.; Park, J.H.; Krytska, K.; Ryles, H.T.; Sano, R.; Szigety, K.M.; Li, Y.; Zou, H.Y.; Lee, N.V.; Smeal, T.; et al. The ALK/ROS1 inhibitor PF-06463922 overcomes primary resistance to crizotinib in ALK-driven neuroblastoma. Cancer Discov. 2016, 6, 96–107. [Google Scholar] [CrossRef]
- Guan, J.; Tucker, E.R.; Wan, H.; Chand, D.; Danielson, L.S.; Ruuth, K.; El Wakil, A.; Witek, B.; Jamin, Y.; Umapathy, G.; et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Dis. Models Mech. 2016, 9, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, K.C.; Kayser, K.; Groshen, S.G.; Chioda, M.; Thurm, H.C.; Chen, J.; Peltz, G.; Granger, M.; Maris, J.; Matthay, K.K.; et al. Phase I trial of lorlatinib in patients with ALK-driven refractory or relapsed neuroblastoma: A New Approaches to Neuroblastoma Consortium study. J. Clin. Oncol. 2020, 38, 10504. [Google Scholar] [CrossRef]
- Dar, A.C.; Shokat, K.M. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu. Rev. Biochem. 2011, 80, 769–795. [Google Scholar] [CrossRef]
- Zuccotto, F.; Ardini, E.; Casale, E.; Angiolini, M. Through the “Gatekeeper Door”: Exploiting the active kinase conformation. J. Med. Chem. 2010, 53, 2681–2694. [Google Scholar] [CrossRef]
- Bryan, M.C.; Whittington, D.A.; Doherty, E.M.; Falsey, J.R.; Cheng, A.C.; Emkey, R.; Brake, R.L.; Lewis, R.T. Rapid development of piperidine carboxamides as potent and selective anaplastic lymphoma kinase inhibitors. J. Med. Chem. 2012, 55, 1698–1705. [Google Scholar] [CrossRef]
- Tu, C.H.; Lin, W.H.; Peng, Y.H.; Hsu, T.; Wu, J.S.; Chang, C.Y.; Lu, C.T.; Lyu, P.C.; Shih, C.; Jiaang, W.T.; et al. Pyrazolylamine derivatives reveal the conformational switching between type I and type II binding modes of anaplastic lymphoma kinase (ALK). J. Med. Chem. 2016, 59, 3906–3919. [Google Scholar] [CrossRef]
- Futami, H.; Sakai, R. All-trans retinoic acid downregulates ALK in neuroblastoma cell lines and induces apoptosis in neuroblastoma cell lines with activated ALK. Cancer Lett. 2010, 297, 220–225. [Google Scholar] [CrossRef]
- Opel, D.; Poremba, C.; Simon, T.; Debatin, K.M.; Fulda, S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007, 67, 735–745. [Google Scholar] [CrossRef]
- Jaboin, J.; Kim, C.J.; Kaplan, D.R.; Thiele, C.J. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 2002, 62, 6756–6763. [Google Scholar]
- Wood, A.C.; Krytska, K.; Ryles, H.T.; Infarinato, N.R.; Sano, R.; Hansel, T.D.; Hart, L.S.; King, F.J.; Smith, T.R.; Ainscow, E.; et al. Dual ALK and CDK4/6 Inhibition Demonstrates Synergy against Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2856–2868. [Google Scholar] [CrossRef]
- Moore, N.F.; Azarova, A.M.; Bhatnagar, N.; Ross, K.N.; Drake, L.E.; Frumm, S.; Liu, Q.S.; Christie, A.L.; Sanda, T.; Chesler, L.; et al. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget 2014, 5, 8737–8749. [Google Scholar] [CrossRef]
- Waetzig, R.; Matthes, M.; Leister, J.; Penkivech, G.; Heise, T.; Corbacioglu, S.; Sommer, G. Comparing mTOR inhibitor Rapamycin with Torin-2 within the RIST molecular-targeted regimen in neuroblastoma cells. Int. J. Med. Sci. 2021, 18, 137–149. [Google Scholar] [CrossRef]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Colmet Daage, L.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Sun, D.; Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef]
- Stockhausen, M.T.; Sjölund, J.; Manetopoulos, C.; Axelson, H. Effects of the histone deacetylase inhibitor valproic acid on Notch signalling in human neuroblastoma cells. Br. J. Cancer 2005, 92, 751–759. [Google Scholar] [CrossRef]
- Hagiwara, K.; Tokunaga, T.; Iida, H.; Nagai, H. Combined inhibition of ALK and HDAC induces synergistic cytotoxicity in neuroblastoma cell lines. Anticancer Res. 2019, 39, 3579–3584. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Sun, Y. Inhibition of Axl improves the targeted therapy against ALK-mutated neuroblastoma. Biochem. Biophys. Res. Commun. 2014, 454, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- Unno, K.; Chalmers, Z.R.; Pamarthy, S.; Vatapalli, R.; Rodriguez, Y.; Lysy, B.; Mok, H.; Sagar, V.; Han, H.; Yoo, Y.A.; et al. Activated ALK cooperates with N-Myc via Wnt/β-catenin signaling to induce neuroendocrine prostate cancer. Cancer Res. 2021, 81, 2157–2170. [Google Scholar] [CrossRef]
- van Groningen, T.; Akogul, N.; Westerhout, E.M.; Chan, A.; Hasselt, N.E.; Zwijnenburg, D.A.; Broekmans, M.; Stroeken, P.; Haneveld, F.; Hooijer, G.K.J.; et al. A NOTCH feed-forward loop drives reprogramming from adrenergic to mesenchymal state in neuroblastoma. Nat. Commun. 2019, 10, 1530. [Google Scholar] [CrossRef]
- Van Nes, J.; Hamdi, E.M.W.M.; Van Groningen, T.; Persson, C.U.; Chan, A.; Stroeken, P.; Akogul, N.; Von Stedingk, K.; Valentijn, L.J.; Mohlin, S.; et al. Drug resistance and phenotype of MES- and ADRN-type neuroblastoma cells faithfully reflect consecutive stages of normal adrenergic lineage development. In Proceedings of the ANR2021, Virtual Conference, 25 January 2021. [Google Scholar]
- Westerhout, E.M.S.P.; Van Arkel, J.; Lakeman, A.; Hasselt, N.E.; Akogul, N.; Zwijnenburg, D.A.; Volckmann, R.; Koster, J.; Van Nes, J.; Valentijn, L.J.; et al. Adrenergic and mesenchymal neuroblastoma cells have opposite resistance to ALK inhibitors and TRAIL, allowing dual therapy to impede resistance and relapse development. In Proceedings of the ANR2021, Virtual Conference, 27 January 2021. [Google Scholar]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027. [Google Scholar] [CrossRef]
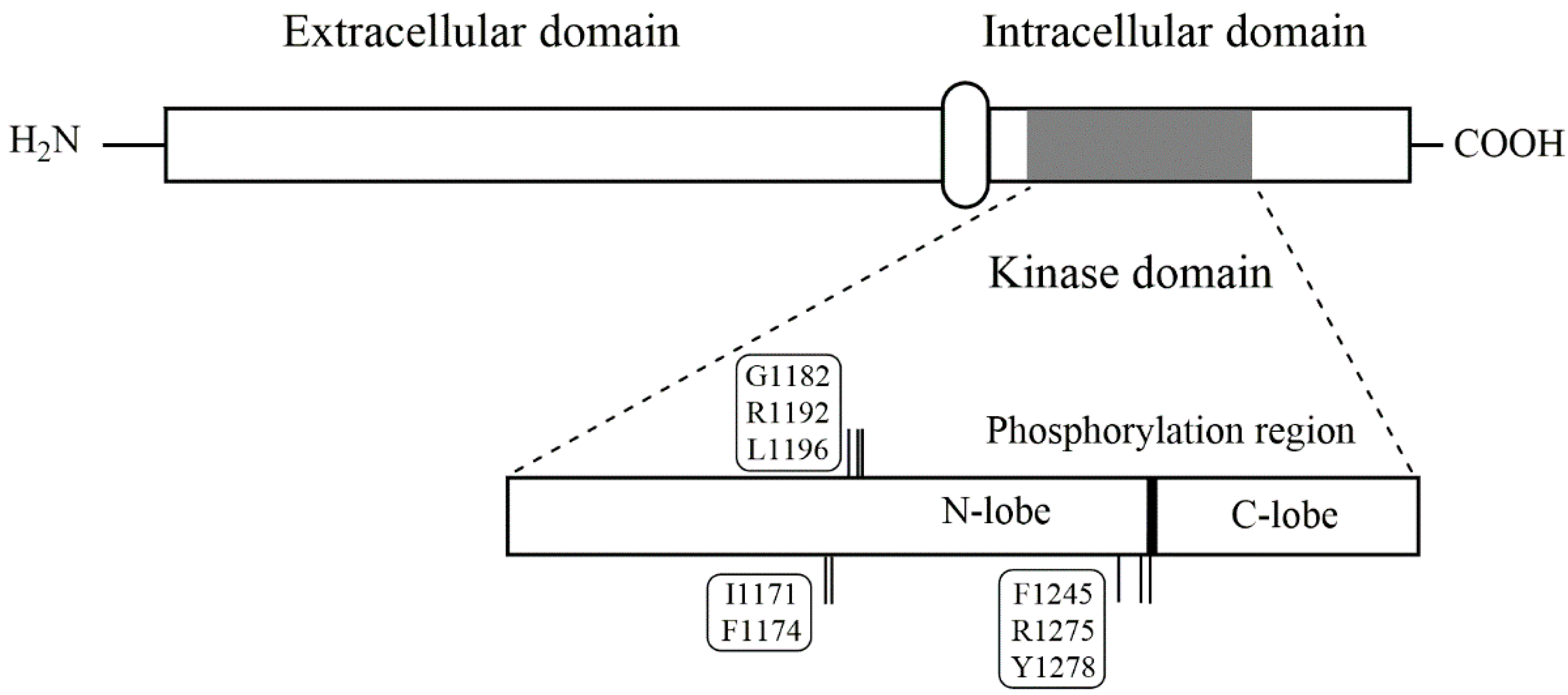
| Specific Positions | Residues |
|---|---|
| Auto-phosphorylation site | Y1278, Y1282, Y1283 |
| NBL germline mutations | G1182, R1192, R1275 |
| NBL hotspot mutations | F1174, F1245, R1275 |
| Less common NBL mutations | I1170, I1171, Y1278 |
| ALK gatekeeper mutation | L1196 |
| Mutations involved in drug resistance | G1202, G1269 |
| First-Generation Inhibitor | |||
| Crizotinib Off-targets: ROS1, c-MET No CNS penetration Sensitive: R1275Q Resistant: F1174L/V | 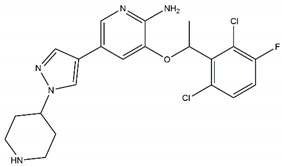 | ||
| Second-Generation Inhibitors | |||
| TAE-684 Sensitive: R1275Q, F1174L Discontinued | 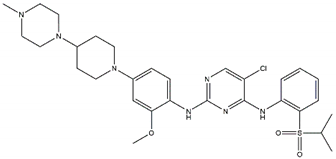 | Ceritinib Off-targets: IGFR1, ROS1 CNS penetration Resistant: F1174L/C | 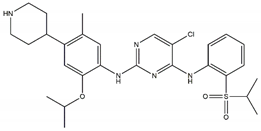 |
| ASP3026 Off-targets: ROS1, ACK Resistant: L1196M | 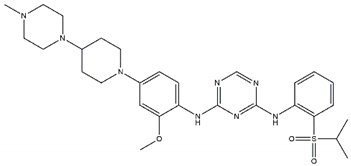 | Brigatinib Off-targets: ROS1, EGFR CNS penetration Sensitive: all NBL-associated point mutations |  |
| Ensartinib Sensitive: R1275Q, F1174L |  | AZD3463 Off-target: IGFR1 Sensitive: NBL hotspot mutations | 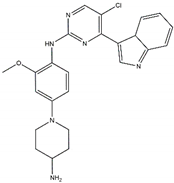 |
| CEP-37440 Off-target: FAK CNS penetration Sensitive: NBL hotspot mutations | 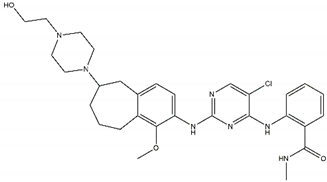 | Alectinib Sensitive: all NBL-associated point mutations, plus amplifications | 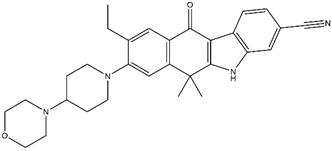 |
| Belizatinib Off-targets: IGFR1, JAK2, TrkA/B/C, c-Src CNS penetration Sensitive: R1275Q, L1196M | 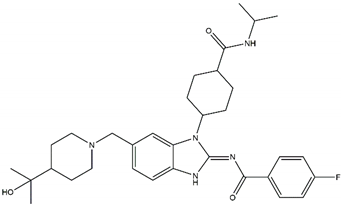 | Entrectinib Off-targets: ROS1, TrkA/B/C CNS penetration Sensitive: ALK amplifications |  |
| Third-Generation Inhibitors | |||
| Lorlatinib Off-target: ROS1 CNS penetration Sensitive: NBL hotspot mutations | 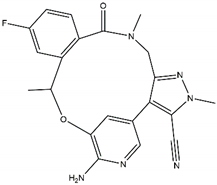 | Repotrectinib Off-targets: ROS1, TrkA/B/C CNS penetration Sensitive: all NBL-associated point mutations | 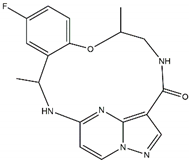 |
| Drug | Phase | Inclusion Criteria | Study Identifier | State | Published Data |
|---|---|---|---|---|---|
| Crizotinib | I/II | Refractory NBL | NCT00949770 | Completed | [32,44] |
| I | Malignant neoplasms | NCT01121588 | Active | ||
| III | High-risk NBL | NCT03126916 | Recruiting | ||
| Ceritinib | I | ALK-activated tumours | NTC01742286 | Completed | [53] |
| I | Relapsed/refractory NBL | NTC02780128 | Recruiting | ||
| II | De novo NBL | NTC02559778 | Recruiting | ||
| ASP3026 | I | Crizotinib-resistant tumours | NCT01284192 | Completed | [54] |
| Ensartinib | II | Relapsed NBL | NTC03213652 | Recruiting | |
| II | Relapsed/refractory NBL | NTC03155620 | Recruiting | ||
| Entrectinib | I/II | ALK-activated tumours | NTC02650401 | Active | |
| Lorlatinib | I | Relapsed/refractory NBL | NCT03107988 | Recruiting | |
| I | De novo NBL | NCT04753658 | Recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brenner, A.K.; Gunnes, M.W. Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma—A Comprehensive Update. Pharmaceutics 2021, 13, 1427. https://doi.org/10.3390/pharmaceutics13091427
Brenner AK, Gunnes MW. Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma—A Comprehensive Update. Pharmaceutics. 2021; 13(9):1427. https://doi.org/10.3390/pharmaceutics13091427
Chicago/Turabian StyleBrenner, Annette K., and Maria W. Gunnes. 2021. "Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma—A Comprehensive Update" Pharmaceutics 13, no. 9: 1427. https://doi.org/10.3390/pharmaceutics13091427
APA StyleBrenner, A. K., & Gunnes, M. W. (2021). Therapeutic Targeting of the Anaplastic Lymphoma Kinase (ALK) in Neuroblastoma—A Comprehensive Update. Pharmaceutics, 13(9), 1427. https://doi.org/10.3390/pharmaceutics13091427





