3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery
Abstract
1. Introduction
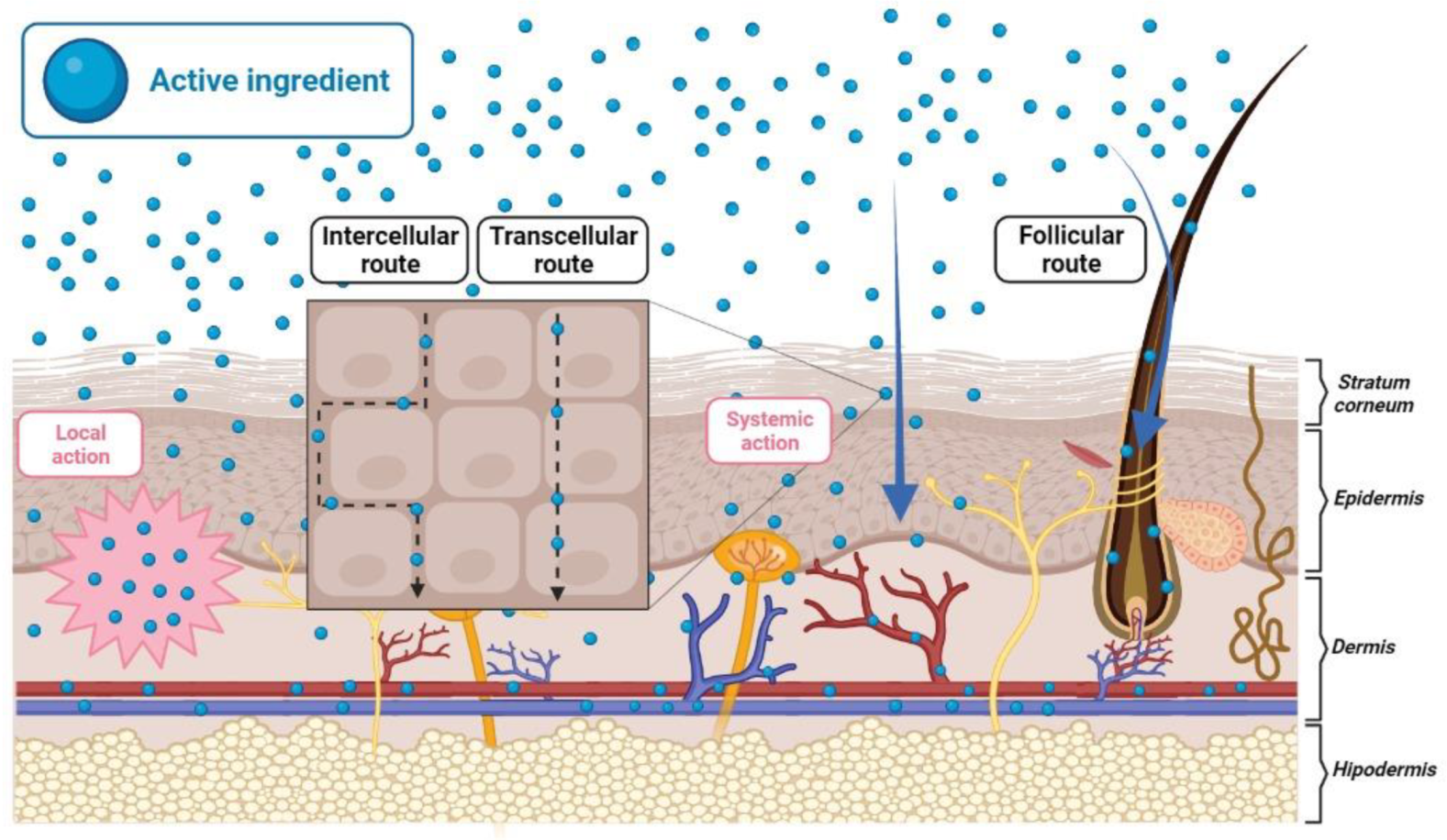
2. Topical Delivery
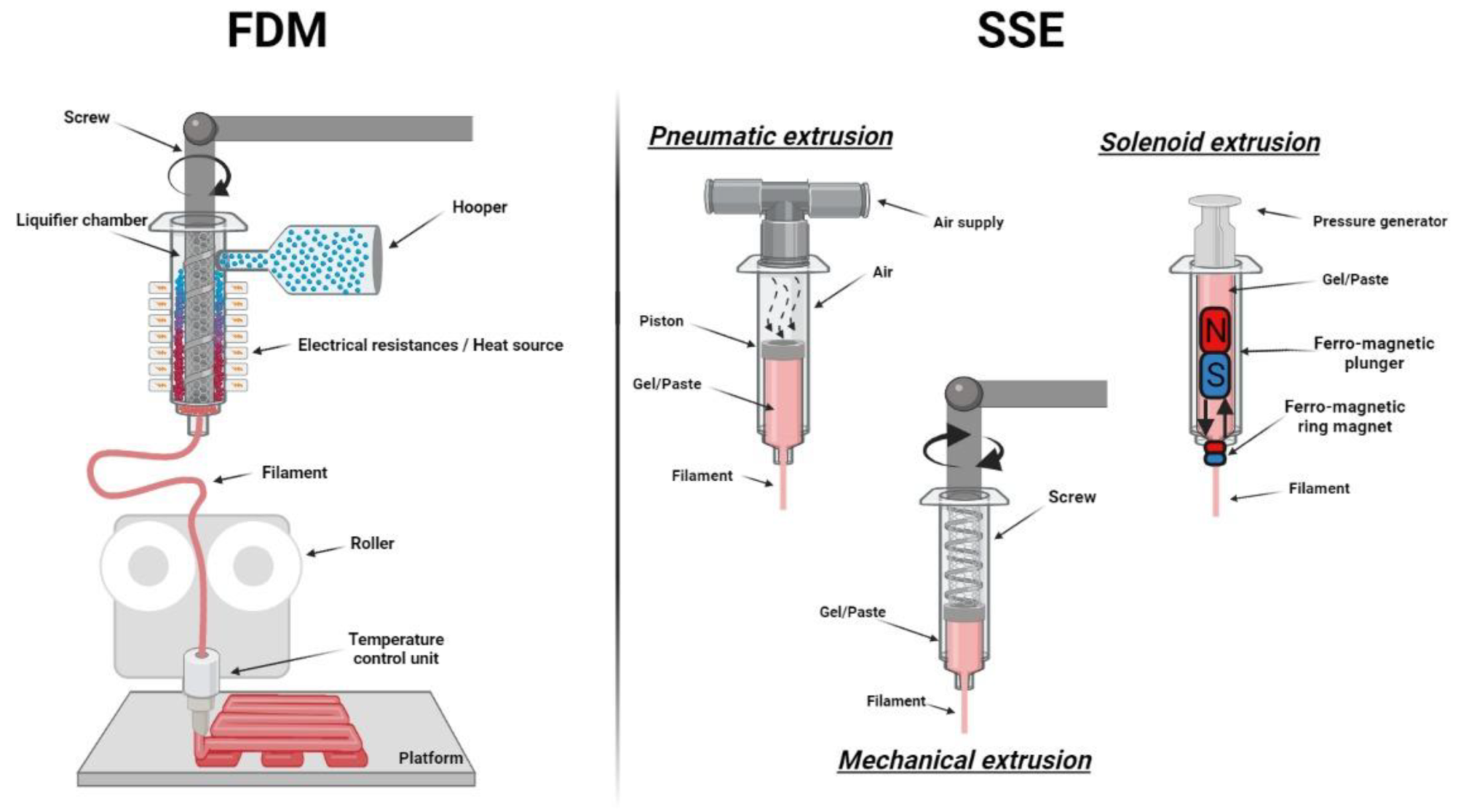
3. 3D Printing Techniques
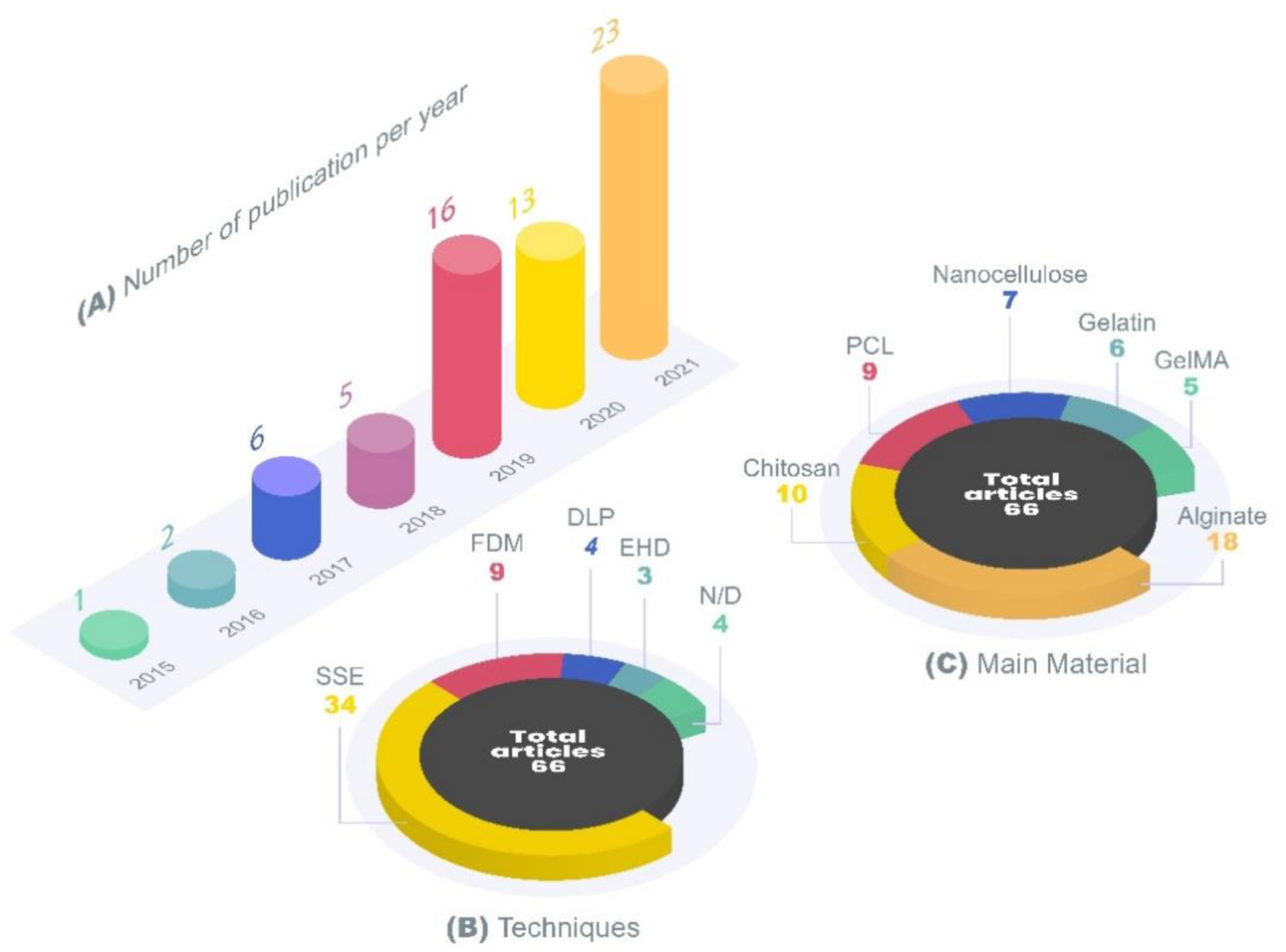
4. Review Methodology
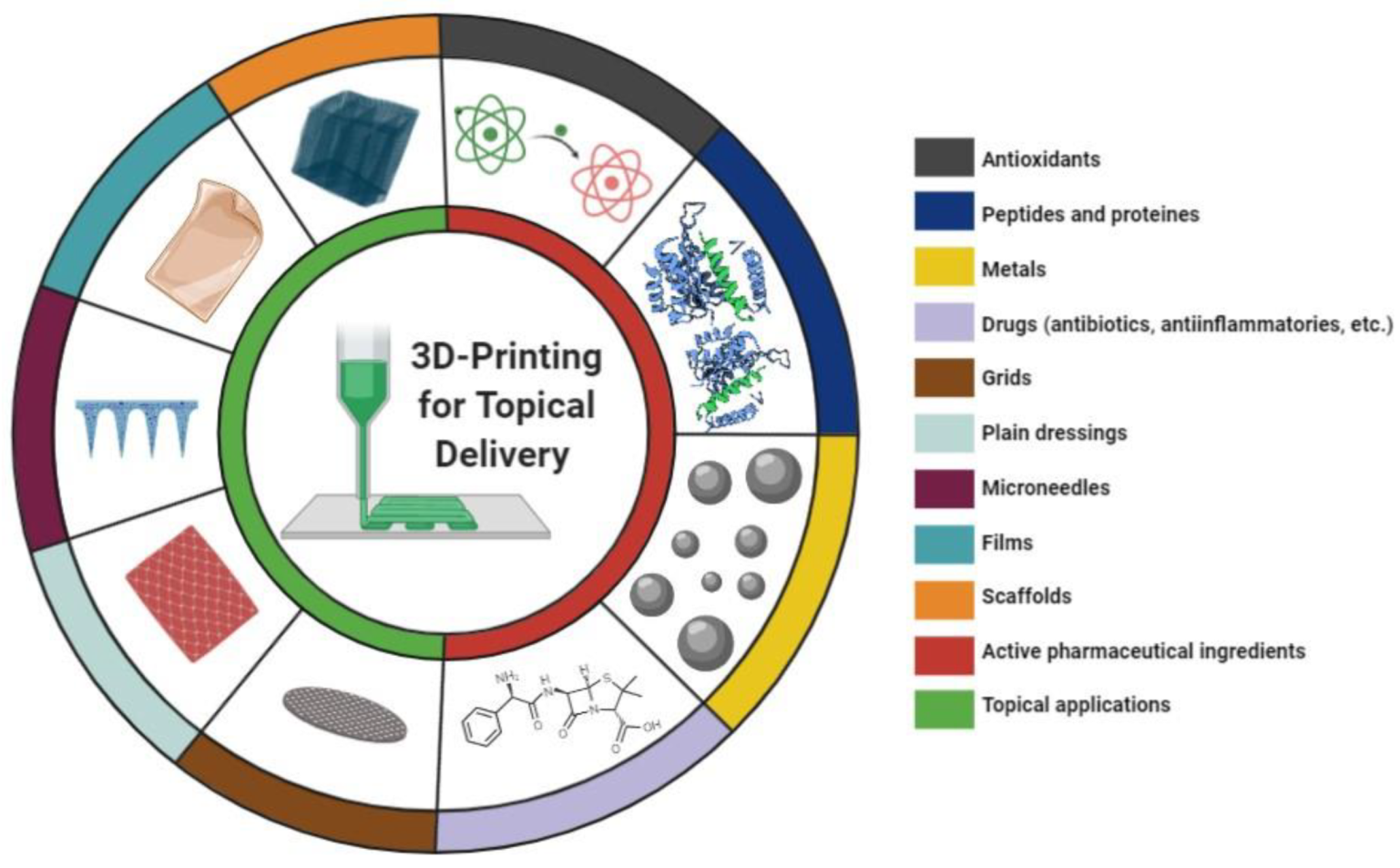
5. 3D printing for Topical Skin Applications
5.1. Plain Dressings
5.1.1. Algae-Derived Biomaterials
5.1.2. Nanocellulose
5.1.3. Chitosan
5.1.4. Synthetic Polymers
5.1.5. Other Polymers
5.2. Active Ingredients Delivery
5.2.1. Drugs
5.2.2. Peptides and Proteins
5.2.3. Metals
5.2.4. Natural Compounds
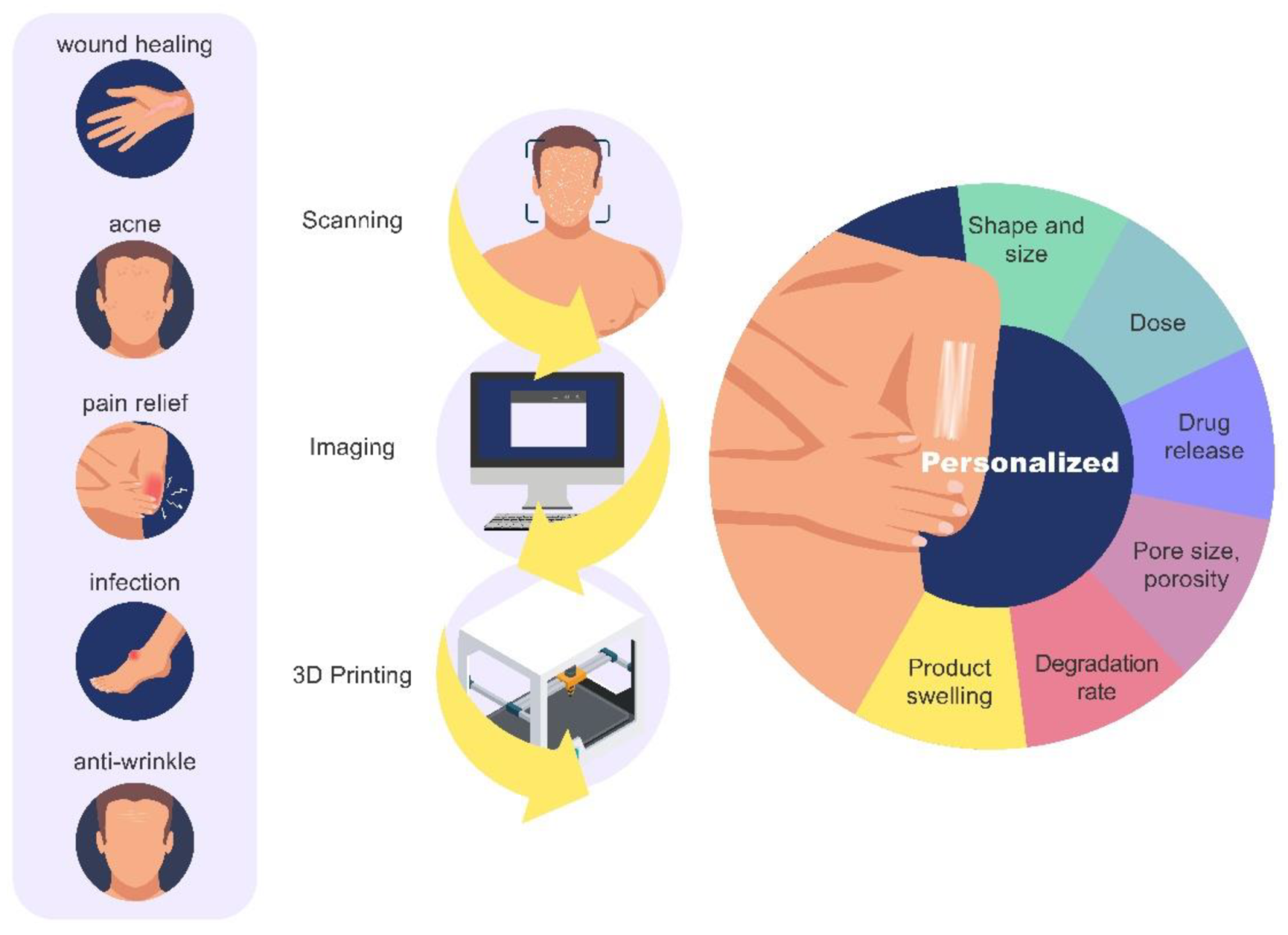
6. Regulation, Safety Considerations and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Machado, A.C.H.R.; Lopes, P.S.; Raffier, C.P.; Haridass, I.N.; Roberts, M.; Grice, J.; Leite-Silva, V.R. Skin Penetration. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, H., Maibach, H., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 741–755. ISBN 9780128020548. [Google Scholar]
- Seth, D.; Cheldize, K.; Brown, D.; Freeman, E.E. Global Burden of Skin Disease: Inequities and Innovations. Curr. Dermatol. Rep. 2017, 6, 204–210. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef] [PubMed]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.; Oliveira, R.S.; Oliveira, T.V.; Velho, M.C.; Konrad, M.V.; da Silva, G.S.; Deon, M.; Beck, R.C.R. 3D Printing and Nanotechnology: A Multiscale Alliance in Personalized Medicine. Adv. Funct. Mater. 2021, 31, 2009691. [Google Scholar] [CrossRef]
- Maver, T.; Smrke, D.M.; Kurečič, M.; Gradišnik, L.; Maver, U.; Kleinschek, K.S. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J. Sol-Gel Sci. Technol. 2018, 88, 33–48. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Approval Letter-Spritam. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf (accessed on 28 July 2021).
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications–Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Y.; Chi Lip Kwok, P.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef]
- Tan, S.H.; Ngo, Z.H.; Leavesley, D.; Liang, K. Recent Advances in the Design of Three-Dimensional and Bioprinted Scaffolds for Full-Thickness Wound Healing. Tissue Eng. Part B Rev. 2021. Ahead of Print. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef]
- Manita, P.G.; Garcia-Orue, I.; Santos-Vizcaino, E.; Hernandez, R.M.; Igartua, M. 3D Bioprinting of Functional Skin Substitutes: From Current Achievements to Future Goals. Pharmaceuticals 2021, 14, 362. [Google Scholar] [CrossRef]
- van Kogelenberg, S.; Yue, Z.; Dinoro, J.N.; Baker, C.S.; Wallace, G.G. Three-Dimensional Printing and Cell Therapy for Wound Repair. Adv. Wound Care 2018, 7, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, F.; Leu, M.C. A Brief Review on 3D Bioprinted Skin Substitutes. Procedia Manuf. 2020, 48, 790–796. [Google Scholar] [CrossRef]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X.; Meng, S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin. Drug Deliv. 2019, 16, 847–867. [Google Scholar] [CrossRef]
- Leite-Silva, V.R.; De Almeida, M.M.; Fradin, A.; Grice, J.E.; Roberts, M.S. Delivery of drugs applied topically to the skin. Expert Rev. Dermatol. 2012, 7, 383–397. [Google Scholar] [CrossRef]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Bahari, L.A. Therapeutic Nanostructures for Dermal and Transdermal Drug Delivery. In Nano- and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 131–146. ISBN 9780323527279. [Google Scholar]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and bioequivalence of topical glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Pandey, V.; Maheshwari, R.; Ghode, P.; Tekade, R.K. Cutaneous and Transdermal Drug Delivery: Techniques and Delivery Systems Dinesh. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 595–650. ISBN 9780128179093. [Google Scholar]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef]
- Mayba, J.N.; Gooderham, M.J. A Guide to Topical Vehicle Formulations. J. Cutan. Med. Surg. 2018, 22, 207–212. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Principles and approaches for optimizing therapy with unique topical vehicles. J. Drugs Dermatol. 2014, 13, 1431–1435. [Google Scholar]
- Walters, K.A. Dermatological and Transdermal Formulations; Walters, K.A., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; ISBN 0824798899. [Google Scholar]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Østergaard Knudsen, N.; Pommergaard Pedersen, G. pH and Drug Delivery. In pH of the Skin: Issues and Challenges; Surber, C., Abels, C., Maibach, H., Eds.; Karger Publishers: Basel, Switzerland, 2018; Volume 54, pp. 143–151. [Google Scholar]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Pavel, T.I.; Chircov, C.; Rădulescu, M.; Grumezescu, A.M. Regenerative Wound Dressings for Skin Cancer. Cancers 2020, 12, 2954. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Douroumis, D.; Boateng, J. 3D Printed Scaffolds for Wound Healing and Tissue Regeneration. In Therapeutic Dressings and Wound Healing Applications; Boateng, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 385–398. ISBN 9781119433316. [Google Scholar]
- Dalisson, B.; Barralet, J. Bioinorganics and Wound Healing. Adv. Healthc. Mater. 2019, 8, 1900764. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. The Shape of Things to Come: Emerging Applications of 3D Printing in Healthcare. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Madla, C.M.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Technologies, Implementation and Regulation: An Overview. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–40. [Google Scholar]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Harrington, T.; Rodriguez Rivero, M.C.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D inkjet printing of biopharmaceuticals–A review of trends and future perspectives in research and manufacturing. Int. J. Pharm. 2021, 599, 120443. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Vadodaria, S.; Mills, T. Jetting-based 3D printing of edible materials. Food Hydrocoll. 2020, 106, 105857. [Google Scholar] [CrossRef]
- Wu, Y. Electrohydrodynamic jet 3D printing in biomedical applications. Acta Biomater. 2021, 128, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Tekko, I.A.; Jomaa, M.H.; Vora, L.; McAlister, E.; Volpe-Zanutto, F.; Nethery, M.; Baine, P.T.; Mitchell, N.; McNeill, D.W.; et al. Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems. Pharm. Res. 2020, 37, 174. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Bhatnagar, D.; Murthy, N.S. Biomedical Polymers Synhtesis and Processing; Damodaran, V.B., Bhatnagar, D., Murthy, N.S., Eds.; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-32051-9. [Google Scholar]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater. Today Proc. 2020, 29, 228–232. [Google Scholar] [CrossRef]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- dos Santos, J.; Deon, M.; da Silva, G.S.; Beck, R.C.R. Multiple variable effects in the customisation of fused deposition modelling 3D-printed medicines: A design of experiments (DoE) approach. Int. J. Pharm. 2021, 597, 120331. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Orlu, M.; Woerdenbag, H.J.; Scarpa, M.; Kiefer, O.; Kottke, D.; Sjöholm, E.; Öblom, H.; Sandler, N.; Hinrichs, W.L.J.; et al. Oromucosal films: From patient centricity to production by printing techniques. Expert Opin. Drug Deliv. 2019, 16, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability analysis of drug loaded pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 554, 292–301. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Alginate-based hydrogel systems for drug releasing in wound healing. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 323–358. ISBN 9780128176405. [Google Scholar]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing Applications. Biomed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Liu, J.; Chi, J.; Wang, K.; Liu, X.; Liu, J.; Gu, F. Full-thickness wound healing using 3D bioprinted gelatin-alginate scaffolds in mice: A histopathological study. Int. J. Clin. Exp. Pathol. 2016, 9, 11197–11205. [Google Scholar]
- O’Neill, J.J.; Johnson, R.A.; Dockter, R.L.; Kowalewski, T.M. 3D bioprinting directly onto moving human anatomy. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 934–940. [Google Scholar] [CrossRef]
- Yu, I.; Kaonis, S.; Chen, R. A Study on Degradation Behavior of 3D Printed Gellan Gum Scaffolds. Procedia CIRP 2017, 65, 78–83. [Google Scholar] [CrossRef]
- Chuan, Y.L.; Pandya, S.A.K. Fabrication of Non-Implant 3D Printed Skin. MATEC Web Conf. 2018, 152, 02016. [Google Scholar] [CrossRef][Green Version]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Streifel, B.C.; Lundin, J.G.; Sanders, A.M.; Gold, K.A.; Wilems, T.S.; Williams, S.J.; Cosgriff-Hernandez, E.; Wynne, J.H. Hemostatic and Absorbent PolyHIPE–Kaolin Composites for 3D Printable Wound Dressing Materials. Macromol. Biosci. 2018, 18, 1700414. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang Molino, B.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Ehman, N.V.; Filgueira, D.; Johansson, J.; Vallejos, M.E.; Felissia, F.E.; Håkansson, J.; Area, M.C. Bagasse—A major agro-industrial residue as potential resource for nanocellulose inks for 3D printing of wound dressing devices. Addit. Manuf. 2019, 28, 267–274. [Google Scholar] [CrossRef]
- Chen, X.; Yue, Z.; Winberg, P.C.; Dinoro, J.N.; Hayes, P.; Beirne, S.; Wallace, G.G. Development of rhamnose-rich hydrogels based on sulfated xylorhamno-uronic acid toward wound healing applications. Biomater. Sci. 2019, 7, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Filgueira, D.; Rodríguez, A.; Chinga-Carrasco, G. Nanocellulose-Based Inks—Effect of Alginate Content on the Water Absorption of 3D Printed Constructs. Bioengineering 2019, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications. Pharmaceutics 2019, 11, 678. [Google Scholar] [CrossRef]
- Shi, L.; Hu, Y.; Ullah, M.W.; Ullah, I.; Ou, H.; Zhang, W.; Xiong, L.; Zhang, X. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019, 11, 035023. [Google Scholar] [CrossRef]
- Xu, W.; Molino, B.Z.; Cheng, F.; Molino, P.J.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On Low-Concentration Inks Formulated by Nanocellulose Assisted with Gelatin Methacrylate (GelMA) for 3D Printing toward Wound Healing Application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three Dimensional Printing Bilayer Membrane Scaffold Promotes Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Chekini, M.; Krivoshapkina, E.; Shkodenko, L.; Koshel, E.; Shestovskaya, M.; Dukhinova, M.; Kheiri, S.; Khuu, N.; Kumacheva, E. Nanocolloidal Hydrogel with Sensing and Antibacterial Activities Governed by Iron Ion Sequestration. Chem. Mater. 2020, 32, 10066–10075. [Google Scholar] [CrossRef]
- Nun, N.; Cruz, M.; Jain, T.; Tseng, Y.-M.; Menefee, J.; Jatana, S.; Patil, P.S.; Leipzig, N.D.; McDonald, C.; Maytin, E.; et al. Thread Size and Polymer Composition of 3D Printed and Electrospun Wound Dressings Affect Wound Healing Outcomes in an Excisional Wound Rat Model. Biomacromolecules 2020, 21, 4030–4042. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, R.I.R.; do Amaral, R.J.F.C.; Reis, R.L.; Marques, A.P.; Murphy, C.M.; O’Brien, F.J. 3D-Printed Gelatin Methacrylate Scaffolds with Controlled Architecture and Stiffness Modulate the Fibroblast Phenotype towards Dermal Regeneration. Polymers 2021, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Nadhif, M.H.; Irsyad, M.; Satrio, M.; Suhaeri, M.; Whulanza, Y. Computational Analysis of Soft Polymer Lattices for 3D Wound Dressing Materials. J. Mech. Eng. 2021, 18, 1–11. [Google Scholar]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, Z.; Li, Y.; Zhou, K.; Cao, C.; Zhang, P.; Li, W. Cryogenically printed flexible chitosan/bioglass scaffolds with stable and hierarchical porous structures for wound healing. Biomed. Mater. 2021, 16, 015004. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose nanofibers and other biopolymers for biomedical applications. A review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Powell, K.A.; Kishnani, H.; Johnsen, P.O.; Pukstad, B.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohydr. Polym. 2017, 157, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Tanaka, M.; Huang, Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Tan, X.; Feldman, S.R.; Chang, J.; Balkrishnan, R. Topical drug delivery systems in dermatology: A review of patient adherence issues. Expert Opin. Drug Deliv. 2012, 9, 1263–1271. [Google Scholar] [CrossRef]
- Bom, S.; Santos, C.; Barros, R.; Martins, A.M.; Paradiso, P.; Cláudio, R.; Pinto, P.C.; Ribeiro, H.M.; Marto, J. Effects of Starch Incorporation on the Physicochemical Properties and Release Kinetics of Alginate-Based 3D Hydrogel Patches for Topical Delivery. Pharmaceutics 2020, 12, 719. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Shaqour, B.; Samaro, A.; Verleije, B.; Beyers, K.; Vervaet, C.; Cos, P. Production of Drug Delivery Systems Using Fused Filament Fabrication: A Systematic Review. Pharmaceutics 2020, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Shaqour, B.; Reigada, I.; Górecka, Ż.; Choińska, E.; Verleije, B.; Beyers, K.; Święszkowski, W.; Fallarero, A.; Cos, P. 3D-Printed Drug Delivery Systems: The Effects of Drug Incorporation Methods on Their Release and Antibacterial Efficiency. Materials 2020, 13, 3364. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Zheng, H.; Chang, M.W.; Ahmad, Z.; Li, J.S. Preparation of active 3D film patches via aligned fiber electrohydrodynamic (EHD) printing. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Sun, H.; Lv, H.; Sun, D.; Chen, N.; Deng, K.; Yang, Y.; Engineer, D.; Zhang, H.; Ren, D.; Engineer, D.; et al. Clinical application of a 3D-printed scaffold in chronic wound treatment: A case series. J. Wound Care 2018, 27, 262–271. [Google Scholar] [CrossRef]
- Cereceres, S.; Lan, Z.; Bryan, L.; Whitely, M.; Wilems, T.; Greer, H.; Alexander, E.R.; Taylor, R.J.; Bernstein, L.; Cohen, N.; et al. Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng. 2019, 3, 026102. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Martin, N.; Fong, M.; Stewart, S.; Irwin, N.; Rial-Hermida, M.; Donnelly, R.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Hewitt, E.; Mros, S.; McConnell, M.; Cabral, J.; Ali, A. Melt-electrowriting with novel milk protein/PCL biomaterials for skin regeneration. Biomed. Mater. 2019, 14, 055013. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, Y.Y.; Derakhshanfar, S.; Xu, K.; Zhong, W.; Luo, G.; Liu, T.; Wang, Y.Y.; Wu, J.; Xing, M. Biomimicry of oil infused layer on 3D printed poly(dimethylsiloxane): Non-fouling, antibacterial and promoting infected wound healing. Mater. Sci. Eng. C 2019, 100, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Cai, F.; Huang, J.; Chen, S.; Liao, Q. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J. Mater. Chem. B 2019, 7, 2954–2961. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, Y. Combination of the Silver–Ethylene Interaction and 3D Printing To Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef]
- Afghah, F.; Ullah, M.; Seyyed Monfared Zanjani, J.; Akkuş Süt, P.; Sen, O.; Emanet, M.; Saner Okan, B.; Culha, M.; Menceloglu, Y.; Yildiz, M.; et al. 3D printing of silver-doped polycaprolactone-poly propylene succinate composite scaffolds for skin tissue engineering. Biomed. Mater. 2020, 15, 035015. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Eleftheriadis, G.K.; Karavasili, C.; Fatouros, D.G. Development of Bio-Active Patches Based on Pectin for the Treatment of Ulcers and Wounds Using 3D-Bioprinting Technology. Pharmaceutics 2020, 12, 56. [Google Scholar] [CrossRef]
- Aranci, K.; Uzun, M.; Su, S.; Cesur, S.; Ulag, S.; Amin, A.; Guncu, M.M.; Aksu, B.; Kolayli, S.; Ustundag, C.B.; et al. 3D Propolis-Sodium Alginate Scaffolds: Influence on Structural Parameters, Release Mechanisms, Cell Cytotoxicity and Antibacterial Activity. Molecules 2020, 25, 5082. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier-Bower, C.; Moosavi Basri, S.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef]
- Ilhan, E.; Cesur, S.; Guler, E.; Topal, F.; Albayrak, D.; Guncu, M.M.; Cam, M.E.; Taskin, T.; Sasmazel, H.T.; Aksu, B.; et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1040–1054. [Google Scholar] [CrossRef]
- Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physico-mechanical and finite element analysis evaluation of 3D printable alginate-methylcellulose inks for wound healing applications. Carbohydr. Polym. 2020, 247, 116666. [Google Scholar] [CrossRef]
- Lim, S.H.; Tiew, W.J.; Zhang, J.; Ho, P.C.-L.; Kachouie, N.N.; Kang, L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef] [PubMed]
- Naseri, E.; Cartmell, C.; Saab, M.; Kerr, R.G.; Ahmadi, A. Development of 3D Printed Drug-Eluting Scaffolds for Preventing Piercing Infection. Pharmaceutics 2020, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Clohessy, R.M.; Holder, R.C.; Gabard, A.R.; Herendeen, G.J.; Christy, R.J.; Burnett, L.R.; Fisher, J.P. In Vivo Evaluation of Three-Dimensional Printed, Keratin-Based Hydrogels in a Porcine Thermal Burn Model. Tissue Eng. Part A 2020, 26, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Xiang, S.; Jiang, C.; Wu, W.; Ruan, S.; Du, Q.; Chen, T.; Xue, Y.; Chen, H.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. AAPS PharmSciTech 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef]
- Azadmanesh, F.; Pourmadadi, M.; Zavar Reza, J.; Yazdian, F.; Omidi, M.; Haghirosadat, B.F. Synthesis of a novel nanocomposite containing chitosan as a three-dimensional printed wound dressing technique: Emphasis on gene expression. Biotechnol. Prog. 2021, 37, e3132. [Google Scholar] [CrossRef]
- Bandiera, A.; Catanzano, O.; Bertoncin, P.; Bergonzi, C.; Bettini, R.; Elviri, L. 3D-printed scaffold composites for the stimuli-induced local delivery of bioactive adjuncts. Biotechnol. Appl. Biochem. 2021, 1–12. [Google Scholar] [CrossRef]
- Barnum, L.; Quint, J.; Derakhshandeh, H.; Samandari, M.; Aghabaglou, F.; Farzin, A.; Abbasi, L.; Bencherif, S.; Memic, A.; Mostafalu, P.; et al. 3D-Printed Hydrogel-Filled Microneedle Arrays. Adv. Healthc. Mater. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3D Printed Chitosan-Based Scaffolds Containing α-Tocopherol Showing Antioxidant and Antimicrobial Activity. Appl. Sci. 2021, 11, 7253. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, B.; Xiong, Y.; Tao, R.; Panayi, A.C.; Chen, L.; Tian, W.; Xue, H.; Shi, L.; Zhang, X.; et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem. Eng. J. 2021, 426, 130634. [Google Scholar] [CrossRef]
- Joseph, B.; Ninan, N.; Visalakshan, R.M.; Denoual, C.; Bright, R.; Kalarikkal, N.; Grohens, Y.; Vasilev, K.; Thomas, S. Insights into the biomechanical properties of plasma treated 3D printed PCL scaffolds decorated with gold nanoparticles. Compos. Sci. Technol. 2021, 202, 108544. [Google Scholar] [CrossRef]
- Lim, S.H.; Kathuria, H.; Amir, M.H.B.; Zhang, X.; Duong, H.T.T.; Ho, P.C.L.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Xu, Z.; Li, S.; Jiang, Y.; Qu, G.W.; Li, Z.; Zhao, Y.; Wu, X.; Ren, J. Fabrication of gelatin-based printable inks with improved stiffness as well as antibacterial and UV-shielding properties. Int. J. Biol. Macromol. 2021, 186, 396–404. [Google Scholar] [CrossRef]
- Naseri, E.; Cartmell, C.; Saab, M.; Kerr, R.G.; Ahmadi, A. Development of N,O-Carboxymethyl Chitosan-Starch Biomaterial Inks for 3D Printed Wound Dressing Applications. Macromol. Biosci. 2021, 2100368. [Google Scholar] [CrossRef]
- Nizioł, M.; Paleczny, J.; Junka, A.; Shavandi, A.; Dawiec-Liśniewska, A.; Podstawczyk, D. 3D Printing of Thermoresponsive Hydrogel Laden with an Antimicrobial Agent towards Wound Healing Applications. Bioengineering 2021, 8, 79. [Google Scholar] [CrossRef]
- Samandari, M.; Aghabaglou, F.; Nuutila, K.; Derakhshandeh, H.; Zhang, Y.; Endo, Y.; Harris, S.; Barnum, L.; Kreikemeier-Bower, C.; Arab-Tehrany, E.; et al. Miniaturized Needle Array-Mediated Drug Delivery Accelerates Wound Healing. Adv. Healthc. Mater. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Shen, H.; Liu, Z.; Hong, J.; Wu, M.; Shiue, S.; Lin, H. Controlled-release of free bacteriophage nanoparticles from 3D-plotted hydrogel fibrous structure as potential antibacterial wound dressing. J. Control. Release 2021, 331, 154–163. [Google Scholar] [CrossRef]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.E.; Oh, J.; Jang, J.H.; Rosas-Gómez, D.A.; Pérez-Gómez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-Controlled Growth Factors Release on Tetrapodal ZnO-Incorporated 3D-Printed Hydrogels for Developing Smart Wound Scaffold. Adv. Funct. Mater. 2021, 31, 1–20. [Google Scholar] [CrossRef]
- Singh, M.; Jonnalagadda, S. Design and characterization of 3D printed, neomycin-eluting poly-L-lactide mats for wound-healing applications. J. Mater. Sci. Mater. Med. 2021, 32, 44. [Google Scholar] [CrossRef]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 2105932. [Google Scholar] [CrossRef]
- Wu, T.; Cui, C.; Fan, C.; Xu, Z.; Liu, Y.; Liu, W. Tea eggs-inspired high-strength natural polymer hydrogels. Bioact. Mater. 2021, 6, 2820–2828. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Kumar Sonker, A.; Gidwani, B. Carrier-Based Drug Delivery System for Treatment of Acne. Sci. World J. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, S.L.; Krishna, K.L.; Ameena Shirin, V.K.; Sankar, R.; Pramod, K.; Gangadharappa, H.V. Drug delivery systems for the treatment of psoriasis: Current status and prospects. J. Drug Deliv. Sci. Technol. 2021, 62, 102364. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, R.; Balraj, S.; Ganesh, J.; John Milton, M.C. Therapeutic agents loaded chitosan-based nanofibrous mats as potential wound dressings: A review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]
- Choi, S.M.; Chaudhry, P.; Zo, S.M.; Han, S.S. Advances in Protein-Based Materials: From Origin to Novel Biomaterials. In Cutting-Edge Enabling Technologies for Regenerative Medicine. Advances in Experimental Medicine and Biology; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; Volume 1078, pp. 161–210. ISBN 9789811309502. [Google Scholar]
- Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Debele, T.A.; Su, W.-P. Polysaccharide and protein-based functional wound dressing materials and applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–22. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wiraja, C.; Chew, S.W.T.; Xu, C. Nanodelivery Systems for Topical Management of Skin Disorders. Mol. Pharm. 2021, 18, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Obst, K.; Friess, W.; Hedtrich, S. Recent advances in topical delivery of proteins and peptides mediated by soft matter nanocarriers. Biotechnol. Adv. 2015, 33, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, N.; Feng, X. The role of internal and external stimuli in the rational design of skin-specific drug delivery systems. Int. J. Pharm. 2021, 592, 120081. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Hussain, M.; Goldberg, D.J. Topical manganese peptide in the treatment of photodamaged skin. J. Cosmet. Laser Ther. 2007, 9, 232–236. [Google Scholar] [CrossRef]
- Maarouf, M.; Vaughn, A.R.; Shi, V.Y. Topical micronutrients in atopic dermatitis-An evidence-based review. Dermatol. Ther. 2018, 31, e12659. [Google Scholar] [CrossRef]
- Pavithran, K. Psoriasis: Topical treatment. Indian J. Dermatol. Venereol. Leprol. 2001, 67, 85. [Google Scholar] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Angioi, R.; Morrin, A.; White, B. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Appl. Sci. 2021, 11, 5192. [Google Scholar] [CrossRef]
- Artem Ataide, J.; Caramori Cefali, L.; Machado Croisfelt, F.; Arruda Martins Shimojo, A.; Oliveira-Nascimento, L.; Gava Mazzola, P. Natural actives for wound healing: A review. Phyther. Res. 2018, 32, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phyther. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-Dimensional Printing of Medicinal Products and the Challenge of Personalized Therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Technical Considerations for Additive Manufactured Medical Devices. Guidance for Industry and Food and Drug Administration Staff. Available online: https://www.fda.gov/media/97633/download (accessed on 31 July 2021).
- Souto, E.B.; Campos, J.C.; Filho, S.C.; Teixeira, M.C.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A.M. 3D printing in the design of pharmaceutical dosage forms. Pharm. Dev. Technol. 2019, 24, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, I.A.; Martynovich, I.V.; Torubarov, I.S. Automatic Print Job Scheduling and Management Over Multiple 3D Printers. In Proceedings of the 5th International Conference on Industrial Engineering (ICIE 2019); Radionov, A.A., Kravchenko, O.A., Guzeev, V.I., Rozhdestvenskiy, Y.V., Eds.; Springer: Cham, Switzerland, 2020; pp. 477–487. [Google Scholar]
- Beer, N.; Hegger, I.; Kaae, S.; De Bruin, M.L.; Genina, N.; Alves, T.L.; Hoebert, J.; Sporrong, S.K. Scenarios for 3D printing of personalized medicines—A case study. Explor. Res. Clin. Soc. Pharm. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Lepowsky, E.; Tasoglu, S. 3D printing for drug manufacturing: A perspective on the future of pharmaceuticals. Int. J. Bioprint. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.J.; de Vasconcellos, F.L.L.; Raffier, C.P.; Roberts, M.S.; Grice, J.E.; Benson, H.A.E.; Leite-Silva, V.R. Alternative Methods to Animal Studies for the Evaluation of Topical/Transdermal Drug Delivery Systems. Curr. Top. Med. Chem. 2018, 18, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare Facilities. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection (accessed on 2 August 2021).
- Food and Drug Administration (FDA). Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. Guidance for Industry. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070342.pdf (accessed on 13 August 2021).
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of Using Sterilization by Gamma Radiation: The Other Side of the Coin. Int. J. Med. Sci. 2018, 15, 274–279. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Tetsuka, H.; Shin, S.R. Materials and technical innovations in 3D printing in biomedical applications. J. Mater. Chem. B 2020, 8, 2930–2950. [Google Scholar] [CrossRef] [PubMed]
| 3D Printing Technique | Main Material | Application | Printed Form | Reference |
|---|---|---|---|---|
| SSE | Nanocellulose | Wound dressing | Grid | [79] |
| SSE | Alginate and gelatin | Recover full-thickness skin wounds | Scaffold | [80] |
| Jet dispensing (micro-jetting system) | Alginate | Robotically deposited bioprinting hydrogel directly onto moving human hand | Hydrogel | [81] |
| SSE | Gellan gum | Wound dressing or cartilage replacement | Scaffold | [82] |
| n/d | ABS | Burn wound healing | Artificial skin | [83] |
| Extrusion based | Chitosan | Skin tissue regeneration and diabetic wound healing | Scaffold | [84] |
| SSE | PEGMA-sodium acrylate-PEGDA and kaolin | Absorbent and hemostatic wound dressing | Dressing | [85] |
| SSE | Nanocellulose | Wound healing, regeneration and tissue repair | Hydrogel scaffold | [86] |
| SSE | Alginate and nanocellulose | Wound dressing devices | Grid wound dressing | [87] |
| SSE | Xylorhamno uronic acid and gelatin | Skin repair and wound healing | Hydrogel scaffold | [88] |
| SSE | Alginate and cellulose nanofibrils | Wound dressing | Hydrogel dressing | [89] |
| SSE | Chitosan and sodium alginate | Skin regeneration | Skin construct/membrane | [90] |
| SSE | PVA and sodium alginate | Bilayered polymeric carriers for wound care | Mats | [91] |
| Cryogenic free-form extrusion | Decellularized small intestinal submucosa | Skin tissue engineering | Scaffold | [92] |
| SSE | Nanocellulose and GelMA | Wound healing | Scaffold | [93] |
| EHD e SSE | PLGA and sodium alginate | Deep wound healing | Membrane scaffold | [94] |
| SSE | Gelatin and cellulose nanocrystals decorated with nitrogen-doped carbon dots | Skin wounds | Hydrogel dressing | [95] |
| Extrusion-based direct-write | PCL and functionalized polyesters | Wound healing | Scaffold dressings | [96] |
| Hybrid extrusion | Alginate, CMC and PCL | Tissue engineering and wound dressing | Scaffold | [97] |
| SSE | GelMA | Wound healing and skin repair | Scaffold | [98] |
| FDM | PVA, PU and PCL | Wound dressing | 3D lattices | [99] |
| n/d | CMC and ε-polylysine | Skin wound repair | Hydrogel dressing | [100] |
| Cryogenic extrusion-based | Chitosan and bioactive glass | Wound healing | Scaffold | [101] |
| 3D Printing Technique | Main Materials | Active Ingredient | Active Incorporation Method | Application | Printed Form | Reference |
|---|---|---|---|---|---|---|
| FDM | NF, FLPA and PCL. | Salicylic acid | Preprint: solvent casting prior filament fabrication by hot melt extrusion | Anti-acne personalized patch | Patch/mask | [113] |
| SLA | PEGDA and PEG | Preprint: dispersion in the polymer | ||||
| DLP | 3DM-Castable resin | Diclofenac | * | Trigger finger treatment | Microneedle | [116] |
| FDM | PCL | Zinc, copper and silver | Preprint: solvent casting prior filament fabrication by hot melt extrusion | Antimicrobial personalized wound dressings | Dressing | [112] |
| EHD | PCL and PVP | Tetracycline | Preprint: blended in the ink | Tetracycline loaded patches for personalized drug delivery | Patch | [117] |
| SSE | Alginate and CMC | Sodium diclofenac and lidocaine | Preprint: blended in the ink | Pain-relieving scaffolds for wound healing applications | Scaffold | [9] |
| n/d | PLLA and gelatin | Platelet rich fibrinogen | Postprint: blended in the 3D printing powder | 3D printed scaffolds for chronic wounds | Scaffold | [118] |
| SSE | PEGDA, mineral oil and Kolliphor® P188 | Gallium maltolate | Postprint: soaking method | Hydrogel wound dressing with antimicrobial agent | Hydrogel dressing | [119] |
| FDM | Lignin, PLA and castor oil | Tetracycline | Preprint: blended with polymers prior hot melt extrusion for filament fabrication | Drug loaded meshes with antioxidant activity for wound dressing application | Mesh | [120] |
| Curcumin | * | |||||
| Jet dispensing | Chitosan | Fluorescein sodium | Preprint: blended in the ink | Films for wound healing applications | Film | [121] |
| MEW | PCL | Milk proteins (whey protein and lactoferrin) | Preprint: blended in the ink | Milk proteins loaded scaffold for deep skin tissue regeneration | Scaffold | [122] |
| SSE | Chitosan and pectin | Lidocaine hydrochloride | Preprint: blended in the ink | Hydrogel dressing loaded with local anesthetic for wound dressing | Hydrogel scaffold | [123] |
| SSE | PDMS | Silver nanoparticles | Preprint: blended in the ink | Wound dressing with silver nanoparticles and oil infusion for wound healing activity | Dressing membrane | [124] |
| SSE | GeLMA | PDGF-BB | Preprint: blended in the ink | Skin-inspired bilayer scaffold for diabetic wound healing | Scaffold | [125] |
| Silver nanoparticles | Postprint: impregnation | |||||
| FDM | PLA | Silver nanoparticles | * | Antibacterial Superporous Hydrogels Wound Dressing | Porous dressing templates | [126] |
| Melt extrusion | PCL-PPSu block copolymers | Silver | Preprint: polymer impregnation prior printing | Scaffold with antimicrobial properties for skin tissue engineering | Scaffold | [127] |
| SSE | Pectin and manuka honey | Chitosan and β-cyclodextrin/propolis extract inclusion complexes | Preprint: blended in the ink | Bio-active dressing patch for ulcers and wound healing applications | Patch | [128] |
| SSE | Sodium alginate | Propolis | Preprint: blended in the ink | Propolis-sodium alginate scaffolds for wound healing applications | Scaffold | [129] |
| SSE | Alginate and starch | Rhodamine B | Preprint: blended in the ink | Topical hydrogel patch for drug delivery | Hydrogel patch | [111] |
| FDM | Resin VeroClear, and Tango black | BSA, VEGF, cefazolin | * | Wound bandage with miniaturized needle array for wireless actively delivery of drugs | Miniaturized needle array | [130] |
| SSE | Sodium alginate and PEG | Satureja cuneifolia extract | Preprint: blended in the ink | 3D printed loaded scaffold for diabetic wound treatment | Scaffold | [131] |
| SSE | Alginate and methylcellulose | Manuka honey, aloe vera gel and eucalyptus essential oil | Preprint: blended in the ink | Hydrogel loaded with bioactive components for wound healing applications | Hydrogel film | [132] |
| DLP | 3DM-Castable resin | Acetyl-hexapeptide-3 | * | Microneedle patch with different geometries and curvature for anti-wrinkle peptide delivery | Microneedle patch | [133] |
| SSE | PLGA | Mupirocin | Preprint: blended in the ink | 3D printed scaffold to cover piercing studs for preventing piercing infections | Scaffold | [134] |
| DLP | Keratin | Halofuginone | Postprint: impregnation | Keratin loaded scaffold for burn wounds healing | Scaffold | [135] |
| SSE | Sodium polyacrylate | Cryptotanshinone niosome | Preprint: blended in the ink | Cryptotanshinone loaded niosome for topical delivery in acne treatment | Hydrogel | [136] |
| SSE | Cellulose nanocrystals and chitosan methacrylamide | VEGF, BSA, silver nanoparticles and gentamicin | Preprint: blended in the ink | 3D-printed multifunctional wound dressing | Hydrogel | [137] |
| EHD | Bacterial cellulose and PCL | amoxicillin, ampicillin, and kanamycin | Preprint: blended in the ink | Antibiotic patches for local transdermal delivery in wound healing applications | Patch | [138] |
| FDM | PLA | Cu-CDs | Postprint: soaking method | Nanocomposite containing PLA/HA/chitosan/Cu-CDs/rosmarinic acid for wound healing applications | Scaffold | [139] |
| N/D | Chitosan and alginate | Epidermal Growth Factor | Postprint: solution was embedded or directly dropped in the scaffolds | Multifunctional dressings for local release of therapeutic adjuncts | Scaffold | [140] |
| Material jetting | VeroClear RGD810 and TangoBlack FLX973 resins | Fetal bovine serum, VEGF and rhodamine B | Postprint: by filling the microneedles | Microneedle arrays for drug delivery applications | Microneedles patch | [141] |
| Freeze-deposition | Chitosan | α-tocopherol | Preprint: blended in the ink | Active dressings for chronically infected wounds | Scaffold | [142] |
| Cryogenic extrusion based | Mesoporous bioglass, sodium alginate and decellularized small intestinal submucosa | Exosomes | Postprint: exosome solution was wrapped in the scaffold | Hydrogel scaffold for diabetic wound healing | Hydrogel scaffold | [143] |
| FDM | PCL | Gold nanoparticles | Postprint: soaking method | Scaffold loaded with gold nanoparticles for skin regeneration | Scaffold | [144] |
| DLP | Vinyl pyrrolidone and PEGDA | Acetyl-hexapeptide-3 | Preprint: blended in the polymers resin | Personalized microneedle patch for anti-wrinkle peptide delivery | Microneedle patch | [145] |
| SSE | Polyacrylamine and gelatin | Silver nanoparticles | Preprint: blended in the ink | Printable inks with antibacterial and anti-UV properties | Scaffolds | [146] |
| SSE | Starch and N, O-carboxymethyl chitosan | Mupirocin | Preprint: blended in the ink | Hybrid biomaterial ink for 3D printed wound dressings | Scaffold | [147] |
| SSE | Methylcellulose, alginate, PNIPAAm | Octenisept® (octenidine dihydrochloride and 2-phenoxyethanol) | Postprint: soaking method | Thermoresponsive 3D printed hydrogel loaded with antimicrobial agent for wound healing applications | Hydrogel dressing | [148] |
| FDM | VeroClear resin | VEGF | * | Miniaturized needle array for VEGF intradermal delivery for wound healing application. | Miniaturized needle array | [149] |
| SSE | Alginate | Bacteriophage nanoparticles | Preprint: blended in the ink | Bacteriophage-based antibacterial wound dressing | Hydrogel dressing | [150] |
| SSE | GelMA and gelatin | VEGF and ZnO | Preprint: blended in the ink | Smart wound scaffold with antibacterial active | Hydrogel patch | [151] |
| Hot melt extrusion-based | PLLA | Neomycin | Postprint: soaking method | Neomycin loaded mats for wound healing applications | Mats | [152] |
| SSE | Chitosan methacrylate | Lidocaine hydrochloride and levofloxacin | Preprint: blended in the ink | Wound dressing for thermal burns | Hydrogel dressing | [153] |
| SSE | Sodium alginate and GelMA | Tea polyphenols | Postprint: soaking method | Hydrogel with antibacterial and antioxidant activities for wound healing and treating | Hydrogel scaffolds | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. https://doi.org/10.3390/pharmaceutics13111946
de Oliveira RS, Fantaus SS, Guillot AJ, Melero A, Beck RCR. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics. 2021; 13(11):1946. https://doi.org/10.3390/pharmaceutics13111946
Chicago/Turabian Stylede Oliveira, Rafaela Santos, Stephani Silva Fantaus, Antonio José Guillot, Ana Melero, and Ruy Carlos Ruver Beck. 2021. "3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery" Pharmaceutics 13, no. 11: 1946. https://doi.org/10.3390/pharmaceutics13111946
APA Stylede Oliveira, R. S., Fantaus, S. S., Guillot, A. J., Melero, A., & Beck, R. C. R. (2021). 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics, 13(11), 1946. https://doi.org/10.3390/pharmaceutics13111946









