Immune Response after Skin Delivery of a Recombinant Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia coli in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of a Recombinant Plasmid Encoding LTB Subunit Gene
2.2. Expression of rLTB Recombinant Protein
2.2.1. Proteomic Analysis
2.2.2. Purification of rLTB Recombinant Protein
2.2.3. Analysis of rLTB Recombinant Protein Conformation
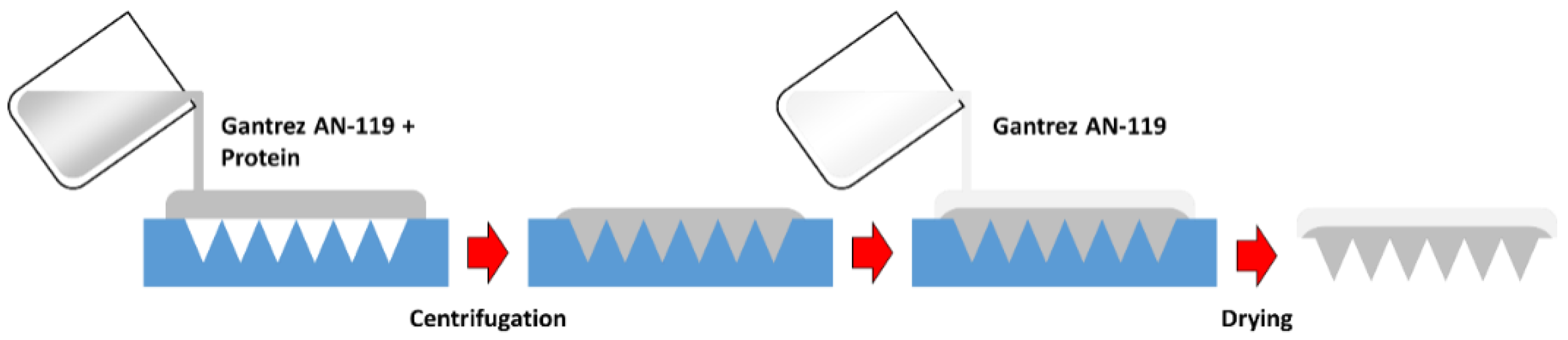
2.3. Polymeric MN Arrays Formulation
2.4. Mechanical Characterization of MNs
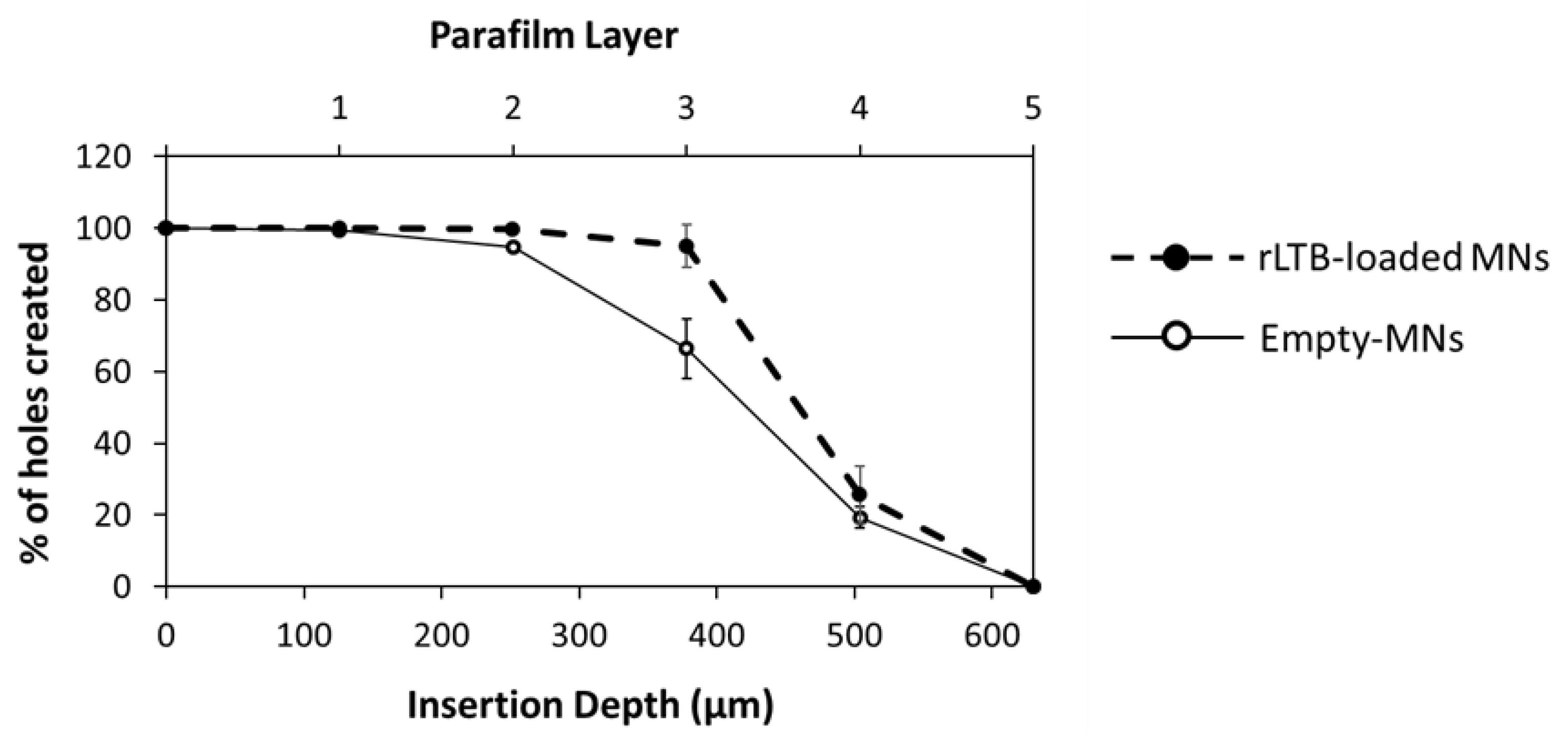
2.4.1. Insertion Test
2.4.2. Skin Insertion Studies
2.5. Immunization Studies
2.6. Detection of Fecal Antibodies by ELISA
2.7. Determination of IL-17A, TNFα IFN-γ, and IL-4 Production
2.8. Statistical Analysis
3. Results
3.1. rLTB Construction and Expression
3.2. Polymeric MN Arrays Formulation Containing rLTB
3.3. Skin Insertion and Dissolution Studies
3.4. Insertion in Neonatal Porcine Skin
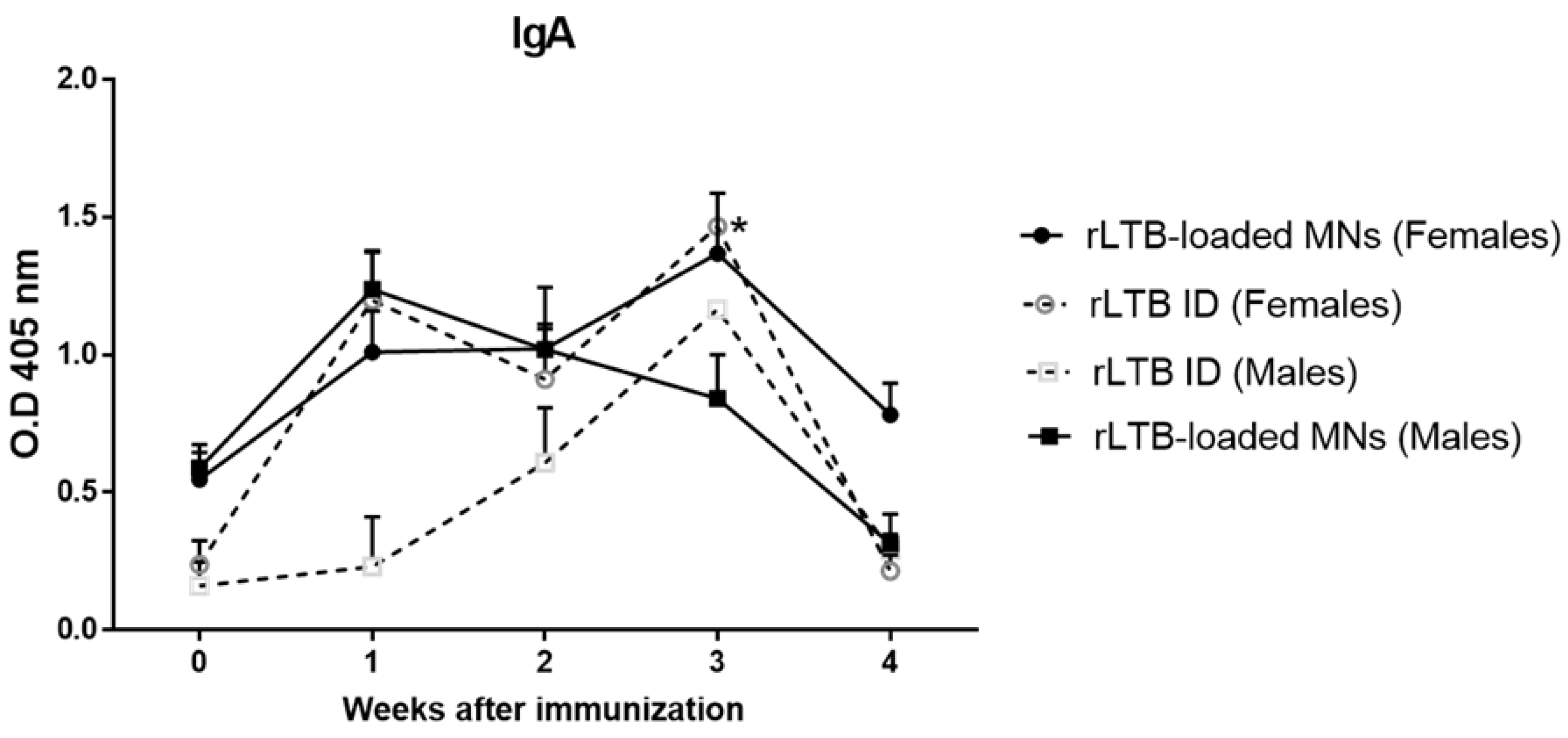
3.5. Evaluation of Mucosal Antibody Responses
3.6. Analysis of Cell-Mediated Immune Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.D.; Bagamian, K.H.; Muhib, F.; Baral, R.; Laytner, L.A.; Amaya, M.; Wierzba, T.; Rheingans, R. Potential impact and cost-effectiveness of future ETEC and Shigella vaccines in 79 low- and lower middle-income countries. Vaccine X 2019, 2, 100024. [Google Scholar] [CrossRef]
- Olson, S.; Hall, A.; Riddle, M.S.; Porter, C.K. Travelers’ diarrhea: Update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop. Dis. Travel Med. Vaccines 2019, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Walker, R.; Porter, C.K.; Muhib, F.; Chilengi, R.; Cravioto, A.; Guerrant, R.; Svennerholm, A.-M.; Qadri, F.; Baqar, S.; et al. Enterotoxigenic Escherichia coli (ETEC) vaccines: Priority activities to enable product development, licensure, and global access. Vaccine 2021, 39, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Isidean, S.D.; Riddle, M.S.; Savarino, S.J.; Porter, C.K. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 2011, 29, 6167–6178. [Google Scholar] [CrossRef]
- Duan, Q.; Xia, P.; Nandre, R.; Zhang, W.; Zhu, G. Review of Newly Identified Functions Associated with the Heat-Labile Toxin of Enterotoxigenic Escherichia coli. Front. Cell. Infect. Microbiol. 2019, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Li, C.; Li, C.; Li, P.; Fu, E.; Xie, Y.; Jin, F. Heat-labile enterotoxin-induced PERK-CHOP pathway activation causes intestinal epithelial cell apoptosis. Front. Cell. Infect. Microbiol. 2017, 7, 244. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Harro, C.; DeNearing, B.; Ram, M.; Feller, A.; Cage, A.; Bauers, N.; Bourgeois, A.L.; Walker, R.; Sack, D.A. Characterization of mucosal immune responses to enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: Response profiles after primary infection and homologous rechallenge with strain H10407. Clin. Vaccine Immunol. 2015, 23, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhong, Z.; Luo, Y.; Cox, E.; Devriendt, B. Heat-stable enterotoxins of enterotoxigenic Escherichia coli and their impact on host immunity. Toxins 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frech, S.A.; DuPont, H.L.; Bourgeois, A.L.; McKenzie, R.; Belkind-Gerson, J.; Figueroa, J.F.; Okhuysen, P.C.; Guerrero, N.H.; Martinez-Sandoval, F.G.; Meléndez-Romero, J.H. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: A phase II, randomised, double-blind, placebo-controlled field trial. Lancet 2008, 371, 2019–2025. [Google Scholar] [CrossRef]
- Diaz, Y.; Govasli, M.L.; Zegeye, E.D.; Sommerfelt, H.; Steinsland, H.; Puntervoll, P. Immunizations with enterotoxigenic Escherichia coli heat-stable toxin conjugates engender toxin-neutralizing antibodies in mice that also cross-react with guanylin and uroguanylin. Infect. Immun. 2019, 87, e00099-19. [Google Scholar] [CrossRef] [Green Version]
- Romani, N.; Flacher, V.; Tripp, C.H.; Sparber, F.; Ebner, S.; Stoitzner, P. Targeting Skin Dendritic Cells to Improve Intradermal Vaccination. Curr. Top. Microbiol. Immunol. 2011, 351, 113–138. [Google Scholar] [CrossRef] [Green Version]
- Pastor, Y.; Larrañeta, E.; Erhard, Á.; Quincoces, G.; Peñuelas, I.; Irache, J.M.; Donnelly, R.; Gamazo, C. Dissolving microneedles for intradermal vaccination against shigellosis. Vaccines 2019, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Huarte, J.; Espuelas, S.; Martínez-Oharriz, C.; Irache, J.M. Nanoparticles from Gantrez-based conjugates for the oral delivery of camptothecin. Int. J. Pharm. X 2021, 3, 100104. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W. Bemerkungen zur Eignung der Schweinehaut als biologisches Modell für die Haut des Menschen. Der Hautarzt 1996, 47, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Valenti, D.; Cencetti, C.; Diez-Sales, O.; Nacher, A.; Mir-Palomo, S.; Terencio, M.C.; Demurtas, D.; et al. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Moffatt, K.; Alkilani, A.Z.; Vicente-Pérez, E.M.; Barry, J.; McCrudden, M.T.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays Can Be Effectively Inserted in Skin by Self-Application: A Pilot Study Centred on Pharmacist Intervention and a Patient Information Leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef]
- Mani, S.; Toapanta, F.R.; McArthur, M.A.; Qadri, F.; Svennerholm, A.-M.; Devriendt, B.; Phalipon, A.; Cohen, D.; Sztein, M.B. Role of antigen specific T and B cells in systemic and mucosal immune responses in ETEC and Shigella infections, and their potential to serve as correlates of protection in vaccine development. Vaccine 2019, 37, 4787–4793. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Aktar, A.; Afrin, S.; Rahman, M.A.; Aktar, S.; Uddin, T.; Rahman, M.A.; Mahbuba, D.A.; Chowdhury, F.; Khan, A.I. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl. Trop. Dis. 2014, 8, e2822. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, A.; Jertborn, M.; Svennerholm, A.-M. Induction of long term mucosal immunological memory in humans by an oral inactivated multivalent enterotoxigenic Escherichia coli vaccine. Vaccine 2016, 34, 3132–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárdeno, A.; Magnusson, M.K.; Quiding-Järbrink, M.; Lundgren, A. Activated T follicular helper-like cells are released into blood after oral vaccination and correlate with vaccine specific mucosal B-cell memory. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Virdi, V.; Palaci, J.; Laukens, B.; Ryckaert, S.; Cox, E.; Vanderbeke, E.; Depicker, A.; Callewaert, N. Yeast-secreted, dried and food-admixed monomeric IgA prevents gastrointestinal infection in a piglet model. Nat. Biotechnol. 2019, 37, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Amcheslavsky, A.; Wallace, A.L.; Ejemel, M.; Li, Q.; McMahon, C.T.; Stoppato, M.; Giuntini, S.; Schiller, Z.A.; Pondish, J.R.; Toomey, J.R.; et al. Anti-CfaE nanobodies provide broad cross-protection against major pathogenic enterotoxigenic Escherichia coli strains, with implications for vaccine design. Sci. Rep. 2021, 11, 2751. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.; Balmert, S.C.; Carey, C.D.; Erdos, G.; Falo, L.D. Emerging skin-targeted drug delivery strategies to engineer immunity: A focus on infectious diseases. Expert Opin. Drug. Deliv. 2021, 18, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Prausnitz, M.R. Enabling skin vaccination using new delivery technologies. Drug Deliv. Transl. 2011, 1, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, A.I.; Martins, R.d.; Tamayo, I.; de Souza, J.; Lasarte, J.J.; Mansilla, C.; Esparza, I.; Irache, J.M.; Gamazo, C. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine 2011, 29, 7130–7135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamayo, I.; Irache, J.M.; Mansilla, C.; Ochoa-Repáraz, J.; Lasarte, J.J.; Gamazo, C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Hutton, A.R.J.; Quinn, H.L.; McCague, P.J.; Jarrahian, C.; Rein-Weston, A.; Coffey, P.S.; Gerth-Guyette, E.; Zehrung, D.; Larrañeta, E.; Donnelly, R.F. Transdermal delivery of vitamin K using dissolving microneedles for the prevention of vitamin K deficiency bleeding. Int. J. Pharm. 2018, 541, 56–63. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Anti-Wrinkle Efficacy of Cross-Linked Hyaluronic Acid-Based Microneedle Patch with Acetyl Hexapeptide-8 and Epidermal Growth Factor on Korean Skin. Ann. Dermatol. 2019, 31, 263. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Elderman, M.; van Beek, A.; Brandsma, E.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex impacts Th1 cells, Tregs, and DCs in both intestinal and systemic immunity in a mouse strain and location-dependent manner. Biol. Sex Differ. 2016, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Kahlenberg, J.M.; Gudjonsson, J.E. A vestigial pathway for sex differences in immune regulation. Cell. Mol. Immunol. 2017, 14, 578–580. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Tsoi, L.C.; Xing, X.; Beamer, M.A.; Swindell, W.R.; Sarkar, M.K.; Berthier, C.C.; Stuart, P.E.; Harms, P.W.; Nair, R.P.; et al. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat. Immunol. 2017, 18, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y.-I.; Nagao, S.; Ohashi, K.; Takahashi, H.; Marunouchi, T. Sex Differences in the Densities of Epidermal Langerhans Cells of the Mouse. J. Investig. Dermatol. 1987, 88, 541–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells as Immune Regulators within the Skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.; Lu, C.-Y.; Sue, S.-C.; Chen, T.-H.; Jan, J.-T.; Huang, M.-H.; Huang, C.-H.; Chen, C.-C.; Chiang, B.-L.; Huang, L.-M.; et al. Type IIb Heat Labile Enterotoxin B Subunit as a Mucosal Adjuvant to Enhance Protective Immunity against H5N1 Avian Influenza Viruses. Vaccines 2020, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Aliahmadi, E.; Gramlich, R.; Grützkau, A.; Hitzler, M.; Krüger, M.; Baumgrass, R.; Schreiner, M.; Wittig, B.; Wanner, R.; Peiser, M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur. J. Immunol. 2009, 39, 1221–1230. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Marshall, A.S.; Silva, J.R.; Bannerman, C.A.; Gilron, I.; Ghasemlou, N. Skin-Resident γδ T Cells Exhibit Site-Specific Morphology and Activation States. J. Immunol. Res. 2019, 2019, 9020234. [Google Scholar] [CrossRef] [Green Version]
- Born, W.K.; Kemal Aydintug, M.; O’Brien, R.L. Diversity of γδ T-cell antigens. Cell. Mol. Immunol. 2013, 10, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frossard, C.P.; Asigbetse, K.E.; Burger, D.; Eigenmann, P.A. Gut T cell receptor-γδ(+) intraepithelial lymphocytes are activated selectively by cholera toxin to break oral tolerance in mice. Clin. Exp. Immunol. 2015, 180, 118–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosine, N.; Miceli-Richard, C. Innate Cells: The Alternative Source of IL-17 in Axial and Peripheral Spondyloarthritis? Front. Immunol. 2021, 11, 3206. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Turner, J.E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013, 14, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215–e00218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brereton, C.F.; Sutton, C.E.; Ross, P.J.; Iwakura, Y.; Pizza, M.; Rappuoli, R.; Lavelle, E.C.; Mills, K.H. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. J. Immunol. 2011, 186, 5896–5906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Tapping, R.I.; Martin, M.H.; Nawar, H.; Lyle, E.A.; Russell, M.W.; Connell, T.D. Toll-Like Receptor 2 Mediates Cellular Activation by the B Subunits of Type II Heat-Labile Enterotoxins. Infect. Immun. 2005, 73, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pharmather. Press Releases. 2021. Available online: https://www.pharmather.com/news/pharmather-enters-into-process-development-agreeement-with-lts-lohmann-for-ketamine-microneedle-patch (accessed on 12 January 2022).
- World Health Organization. WHO Preferred Product Characteristics for Vaccines Against Enterotoxigenic Escherichia coli. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/341507/9789240021839-eng.pdf?sequence=1 (accessed on 15 December 2021).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzosa, M.; Nemeskalova, A.; Zúñiga-Ripa, A.; Salvador-Bescós, M.; Larrañeta, E.; Donnelly, R.F.; Gamazo, C.; Irache, J.M. Immune Response after Skin Delivery of a Recombinant Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia coli in Mice. Pharmaceutics 2022, 14, 239. https://doi.org/10.3390/pharmaceutics14020239
Berzosa M, Nemeskalova A, Zúñiga-Ripa A, Salvador-Bescós M, Larrañeta E, Donnelly RF, Gamazo C, Irache JM. Immune Response after Skin Delivery of a Recombinant Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia coli in Mice. Pharmaceutics. 2022; 14(2):239. https://doi.org/10.3390/pharmaceutics14020239
Chicago/Turabian StyleBerzosa, Melibea, Alzbeta Nemeskalova, Amaia Zúñiga-Ripa, Miriam Salvador-Bescós, Eneko Larrañeta, Ryan F. Donnelly, Carlos Gamazo, and Juan M. Irache. 2022. "Immune Response after Skin Delivery of a Recombinant Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia coli in Mice" Pharmaceutics 14, no. 2: 239. https://doi.org/10.3390/pharmaceutics14020239
APA StyleBerzosa, M., Nemeskalova, A., Zúñiga-Ripa, A., Salvador-Bescós, M., Larrañeta, E., Donnelly, R. F., Gamazo, C., & Irache, J. M. (2022). Immune Response after Skin Delivery of a Recombinant Heat-Labile Enterotoxin B Subunit of Enterotoxigenic Escherichia coli in Mice. Pharmaceutics, 14(2), 239. https://doi.org/10.3390/pharmaceutics14020239








