Abstract
Considering the high prevalence and the complex pharmacological management of immune-mediated inflammatory diseases (IMIDs), the search for new therapeutic approaches for their treatment is vital. Although the immunomodulatory and anti-inflammatory effects of mesenchymal stromal cells (MSCs) have been extensively studied as a potential therapy in this field, direct MSC implantation presents some limitations that could slow down the clinical translation. Since the beneficial effects of MSCs have been mainly attributed to their ability to secrete a plethora of bioactive factors, their secretome has been proposed as a new and promising pathway for the treatment of IMIDs. Formed from soluble factors and extracellular vesicles (EVs), the MSC-derived secretome has been proven to elicit immunomodulatory effects that control the inflammatory processes that occur in IMIDs. This article aims to review the available knowledge on the MSC secretome, evaluating the advances in this field in terms of its composition, production and application, as well as analyzing the pending challenges in the field. Moreover, the latest research involving secretome administration in IMIDs is discussed to provide an updated state-of-the-art for this field. Finally, novel secretome delivery alternatives are reviewed, paying special attention to hydrogel encapsulation as one of the most convenient and promising strategies.
Keywords:
MSCs; secretome; hydrogel; extracellular vesicles; exosomes; immunomodulation; IMIDs; IBD; GvHD; OA 1. Introduction
Immune-mediated inflammatory diseases (IMIDs) are pathological conditions characterized by defective immune responses that lead to chronic tissue damage [1]. This group of chronic and highly disabling conditions that share inflammatory pathways includes inflammatory bowel disease (IBD), osteoarthritis (OA) or graft-versus-host-disease (GvHD), among others [1,2,3]. The prevalence of some of these pathologies is reaching epidemic proportions, causing a huge economic impact on health-care systems worldwide [4].
The pharmacological management of IMIDs is complex, since current treatments are mainly symptomatic and generally based on corticosteroids, biological therapies or immunosuppressants in the most severe cases [5]. The frequent resistance to these drugs together with their important side effects importantly limit their clinical use [6]. Thus, intense research is taking place to develop new therapeutic strategies for the treatment of IMIDs. Among them, therapies based on mesenchymal stromal cells (MSCs) represent a novel and promising alternative [7]. Over the last decades, intense debate has taken place regarding the most adequate terminology and phenotypic characterization for MSCs, which is discussed in Box 1 [8,9,10,11,12,13,14,15,16,17].
Initially, the interest in MSCs was based on their ability to differentiate into stromal tissue cells and their engraftment capacity. Indeed, their regenerative effects have been explored in many studies from the very beginnings of this research field. However, it soon became apparent that, once implanted in the body, cells did not survive long enough to engraft and differentiate into tissue cells, whereas, they were able to produce and release bioactive factors to their microenvironment exerting important effects [18,19]. Indeed, the set of bioactive factors produced by MSCs—also known as the MSC-derived secretome—has been demonstrated to modulate the exacerbated or erratic immune responses observed in IMIDs by regulating cells from both innate and adaptive immunity [20]. Thus, MSC therapies have evolved into a new picture frame. Their grafting capacity has taken a back seat, and, instead, attention has now shifted to the secretory properties. It is not the cells for tissue construction that are required, but the beneficial effects of the released factors that are pursued [21]. Since reaching that inflection point, the secretory properties of MSCs have been thoroughly studied [19,22].
It is important to mention that MSCs present a high immunoplasticity and hence, the content of their secretome varies depending on the cell microenvironment [23]. By means of toll-like receptors (TLRs) present in their membrane, MSCs detect the cytokines present in their surroundings. It has been widely reported that TLR4 activation with factors such as LPS activates the MSC1 phenotype, in which they mostly elaborate proinflammatory mediators. In contrast, factors such as interferon γ (IFN-γ), tumor necrosis factor α (TNF-α) or poly-I:C result in TLR3 activation and MSC polarization towards an MSC2 phenotype leading to the secretion of mediators that have been proven to regulate and control the function of immune effector cells and lead to tissue repair and homeostasis [4,24,25,26]. Therefore, this MSC2 phenotype represents a promising avenue for the treatment of IMIDs, in which there is an exacerbated/erratic immune response.
However, despite the intense research in the field and the important advances, there are still some limitations in the therapeutic use of MSCs (Figure 1). One of these issues is the lack of protocol standardization. Discrepancies among published results are frequently attributed to variances in cell source, cell isolation and culture techniques or administration routes [22]. Another important concern is the poor retention and survival of the implanted cells. It has been reported that less than 1% of MSCs survive more than one week after systemic administration [27]. This therapeutic window may be insufficient in the majority of the applications [27,28]. Furthermore, to obtain an adequate secretion profile that serves as a treatment of IMIDs, MSCs have to present an immunomodulatory MSC2 phenotype. However, in many IMIDs, the chronic inflammation levels reached are not sufficient to polarize the cells towards such a phenotype and their production of immunomodulatory factors is very limited, compromising the efficacy of the therapy [3].
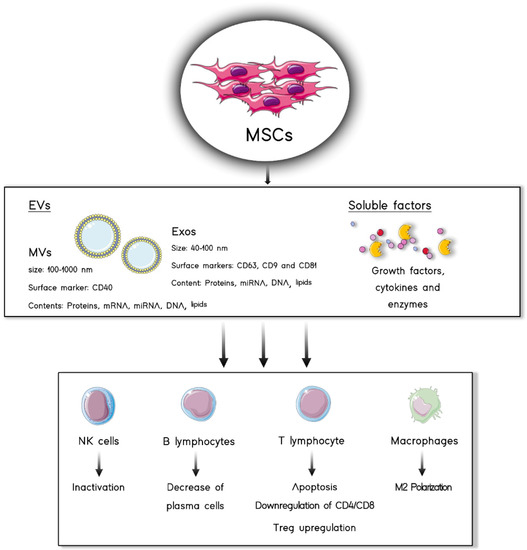
Figure 1.
MSC secretome is composed of two different fractions: EVs and soluble factors. Both of them are capable of eliciting immunomodulatory effects on immune cells such as lymphocytes, macrophages and NK cells.
Regarding safety, although intravenous administration of human MSCs is generally accepted as safe, only showing postinfusion febrile and site reactions, the likelihood of more severe side effects such as thrombosis or adverse inflammatory effects is still present [18,22,27,29,30]. Another obstacle for the clinical translation of cell-based therapies is that their legal regulation is complex and not well defined in some of the cases.
Box 1. MSC designation and identification.
In a series of studies performed between the 1960s and 1980s, Friedenstein and co-workers isolated adherent cells from mouse bone marrow (BM) which formed fibroblast-like colonies and were capable of generating bone tissue when transplanted [8,9,10,11]. Posterior studies demonstrated that these cells were also able to generate cartilage and adipose tissue, and that they were present in embryonic and adult tissues such as umbilical cord, adipose tissue, skin or dental pulp [11,13]. In the late 1990s the term “mesenchymal stem cells” was first used by Arnold Caplan. MSC definition has been controversial over the years because of a lack of unified criteria for their terminology and characteristics. Thus, terms like “mesenchymal stem cells”, “marrow stromal cells”, “multipotent stromal cell” or “mesenchymal stromal cells” have been used arbitrarily. To tackle this problem, in 2005, by means of a position statement, the International Society for Cellular Therapy (ISCT) established the term “mesenchymal stromal cells” as the most suitable for the MSCs acronym [15]. A year later, the ISCT proposed a list of phenotypic characteristics that identify MSCs [15]. In this context, the ISCT stated that MSCs are plastic-adherent cells that grow forming fibroblast-like colonies and present the ability to differentiate into three different cell lineages: osteoblasts, chondroblasts and adipocytes. Moreover, according to the ISCT, MSCs are characterized by the presence of surface markers including CD73, CD90 and CD105; and the absence of CD45, CD34, CD14 or CD11b, CD79a or CD19 and class II histocompatibility complex antigen (HLA-DR) [14]. Nevertheless, these criteria have been widely criticized for not being accurate enough and the correct MSC defining criteria and designation is still polemic. In general terms, the ISCT guidelines have been taken as an updateable basic knowledge about MSCs, useful for defining them but not enough to describe them completely [8].
Because of these issues—and taking into account that the secretory effects of MSCs are considered the main factor responsible for their therapeutic properties [27,28]—the attention has now shifted to the MSC-derived secretome, which will be deeply reviewed in Section 2. The use of the secretome derived from MSCs instead of the cells themselves can overcome some of the important limitations above mentioned. One of the key advantages of secretome-based therapies is that they resolve multiple safety concerns. The secretome is not a living proliferative cell population and this avoids the potential adverse effects that could occur with the implantation of cells. Furthermore, related to safety, the danger of tumorigenicity related to cryopreservants used for cell storage has vanished [31]. On the other hand, while MSC implantation involves the unknown production of bioactive factors, the MSC-derived secretome can be evaluated for content prior to its administration [29]. This contributes to protocol standardization since it enables measurement of the dosage and consequently, the obtained results are more scalable and reproducible [27]. Moreover, this overcomes the issue of the MSC-phenotype, since cells can be in vitro preconditioned to present an immunomodulatory-MSC2 phenotype and collect the resulting enriched, conditioned media. Considering all this, it is possible to say that cell-free therapies based on the MSC-derived secretome open a new path to safer therapies that are scalable, standardizable and easier to regulate [3].
2. MSC-Derived Immunomodulatory Secretome
MSCs can mediate immunomodulation through cell−cell interactions by means of membrane ligands, such as programmed death-ligand 1 (PD-L1) and FAS ligand (FAS-L), which bind to inhibitory receptors on immune cells [32,33]. However, as previously mentioned, the bioactive factors that MSCs secrete have been recognized as the main factors responsible for their effects [26]. The set of bioactive factors released by these cells to the extracellular space is known as MSC-derived secretome [34]. In a descriptive approach, this secretome presents two differentiated parts: a first one formed from soluble factors and a second one composed of extracellular vesicles (EVs) [35] (Figure 2). In this section, we will revise the composition of the secretome derived from the MSC2 phenotype.
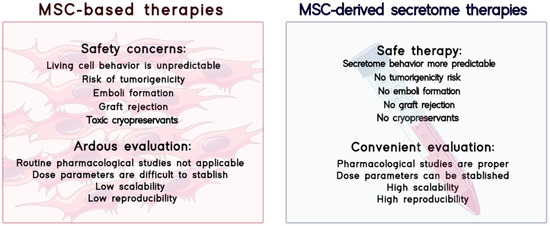
Figure 2.
Comparison between MSC-based therapies and MSC-derived secretome therapies in terms of safety and evaluation of therapies.
2.1. Soluble Factors
Among the soluble factors secreted by the anti-inflammatory MSC2 phenotype, it is possible to find a plethora of molecules including growth factors, cytokines and enzymes. In the following lines, some of the most relevant factors will be discussed, highlighting the effects that make them potential candidates for the treatment of IMIDs [36].
Prostaglandin-E2 (PGE2) has been described as one of the soluble factors of the MSC secretome [3]. The release of this anti-inflammatory factor has been related to natural killer (NK) cell inhibition, macrophage polarization towards an M2 phenotype, suppression of T cell proliferation and the promotion of regulatory T cell (Treg) differentiation [37,38,39].
Another important group of factors in the soluble MSC-derived secretome are galectins, which are involved in cell–matrix adhesion, cell–cell interactions and transmembrane signaling. Furthermore, some galectins produced by MSCs have also been demonstrated to present immunomodulatory properties, as is the case of galectin 1 (Gal-1) and galectin 9 (Gal-9). The former has been reported to inhibit T cell proliferation and to regulate cytokine release, whereas the latter is involved in the inhibition of T and B cells [40,41].
Indoleamine 2, 3-dioxygenase (IDO) is a cytosolic metabolic enzyme that can also be secreted [7]. IDO catabolizes tryptophan and the depletion of this amino acid causes T cell anergy. Moreover, in this metabolic pathway, toxic kynurenine metabolites are produced, which exert a cytotoxic action on effector T cells and inhibit NK and B cells [40]. Furthermore, IDO stimulates macrophage polarization to the M2 phenotype, characterized by IL-10 production which inhibits T cell proliferation and promotes Treg differentiation [1,2,3,4,5,6,8,10,11,12,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,42,43,44,45].
Another interesting factor released by MSCs is interleukin 1 (IL-1) receptor antagonist (IL-1Ra), which when bound to IL-1 receptors impedes the proinflammatory effects of this cytokine. Thus, among others, IL-1Ra limits CD4+ T cell activation, helper T cell (Th) differentiation, B cell proliferation and macrophage IL-1β secretion [46,47,48].
TNF-α-induced gene/protein 6 (TSG-6) is another secretory factor responsible for the anti-inflammatory activity exerted by MSCs [49]. Indeed, it has been demonstrated to mediate immunosuppressive effects on multiple immune cells. Among them, TSG-6 is able to inhibit neutrophil migration [50,51], to suppress Th1 immune responses [52] and to promote Treg production [51]. Regarding macrophages, TSG-6 limits TNF-α secretion by activated macrophages [53,54] and promotes macrophage polarization towards an anti-inflammatory M2 phenotype [55,56]. As another example, leukemia inhibitory factor (LIF) produced by MSCs has also been related to the immunomodulatory effects of the secretome [57].
Among the multiple growth factors released by MSCs there are some with described immunomodulatory effects. One of the most remarkable is transforming growth factor β (TGF-β) because of its ability to inhibit Th activation, NK proliferation and cytotoxic activity and dendritic cell (DC) maturation [58,59,60]. Moreover, TGF-β also promotes the differentiation of T cells into Tregs [60]. Similarly, hepatocyte growth factor (HGF), also present in secretome, is a pleiotropic factor especially relevant for its ability to inhibit T cell proliferation and DC activity and stimulate Treg differentiation [61,62].
2.2. EVs
The second fraction of the MSC-derived secretome is composed of EVs. EVs are membrane-packed vesicles that act as a system of intercellular communication. They consist of a lipid bilayer that envelops bioactive molecules including lipids, proteins or nucleic acid species [35]. The lipid bilayer is not only an envelope since it is enriched with proteins like traspanins, integrins and other ligands that allow the traffic, adhesion and endocrine effects of EVs [34,35,36]. MSC-derived EVs are classified into three groups attending mainly to their biogenesis mechanism, size and surface markers.
Apoptotic bodies are the biggest ones with a size of ≥1000 nm, they are formed by cell apoptosis processes and usually express cell death markers such as Caspase 3 or Annexin V [34].
The second group are microvesicles (MVs)—also known as shedding vesicles—which are formed by the budding of the plasma membrane of the source cells. The formation process requires the reorganization of the cytoskeleton and the liberation is intracellular calcium dependent. Their diameter varies from 100 to 1000 nm and their density goes from 1.04 to 1.07 g/mL [63]. Surface markers in MVs include CD40 and their membrane is usually enriched in lipids such as cholesterol, sphingomyelin and ceramide [63]. The MV cargo is formed mainly by mRNAs and miRNAs and their interaction with target cells occurs by specific receptor−ligand interactions between MVs and target cells [36].
The third group are exosomes, which have an endosomal origin [64]. They are formed via the endocytosis−exocytosis pathway [36]. Briefly, exosome formation starts when a small amount of intracellular fluid is included in an early endosome [65]. This early endosome matures and expands into a late endosome, which can merge with other endosomal membranes to form intraluminal bodies—also known as multivesicular bodies (MVBs) [66]. The exosomes are finally formed when MVBs are released to the extracellular environment by exocytosis [67]. The release process is regulated by proteins including p53 and TSG101 and controlled by the cytoskeleton activation pathway but it is not affected by calcium signaling [29,36]. As previously mentioned, they have a size of 40–100 nm and its density goes from 1.13 to 1.19 g/mL [66]. As the exosomes present bilayered lipid membranes they can also include small amounts of phosphatidylserine and high amounts of cholesterol, sphingolipids and ceramides [36]. They normally express CD9, CD63, CD81 and CD79 markers as well as clathrins, anexines and other biomarkers [68]. When the exosomes are released to the extracellular environment, their main objective is to establish communication with other cells [36]. The interaction between exosomes and target cells can occur by three different mechanisms, such as direct fusion of the exosomes with cell membrane or endocytosis resulting in the transmission of the content to the target cell intracellular milieu. This transmission can also occur by the interaction between the lipid ligand receptors present on cells and the lipids on the exososomal membrane [36,64,69]. Their cargo includes mostly genetic material such as DNA or miRNA noncoding fragments as well as proteins [64,70,71].
Interestingly, the cargo of the last two groups—MVs and exosomes—has been proven to elicit major cellular effects. Their mRNA content has been suggested to elicit direct influence on cell differentiation and proliferation, while the miRNA cargo has been reported to modulate their phenotype. Indeed, several miRNA contained in EVs have been reported to present immunoregulatory and regenerative properties [29,72,73]. In their protein fraction, EVs can contain multiple the soluble components of the secretome aforementioned, presenting important immunomodulatory effects [29,74,75]. Considering all the above mentioned, the EV fraction of the secretome itself is a potential candidate for the treatment of IMIDs [34].
3. Secretome Isolation
Once centrifuged to remove cell debris, the expended medium where the MSCs are cultured, usually known as “conditioned medium” (CM) contains both fractions of the MSC-derived secretome [63]. According to the latest research, the EV fraction could mediate an important effect in the management of IMIDs and therefore, more and more attention is being paid to its isolation, since it implies the concentration of the bioactive components [35,76].
The gold standard technique for EV isolation is ultracentrifugation (Figure 3) [35,77]. The described isolation protocols recommend a series of centrifugations of the CM to remove cell debris, followed by ultracentrifugation to pellet the EVs [35,78,79]. Although ultracentrifugation has been demonstrated to isolate EVs, it is a time-consuming technique and it is limited by the risk of disruption of EV integrity [80]. Furthermore, problems may appear during the process as it is a laborious protocol and the aggregation of the EVs could easily occur due to gravitational forces [35,81]. Moreover, during the centrifugation of the CM, the soluble factors of the secretome may also become deposited [82]. This event could open a discussion as to whether the therapeutic effects of the EVs are solely related to the effect of the MVs and exosomes [35].
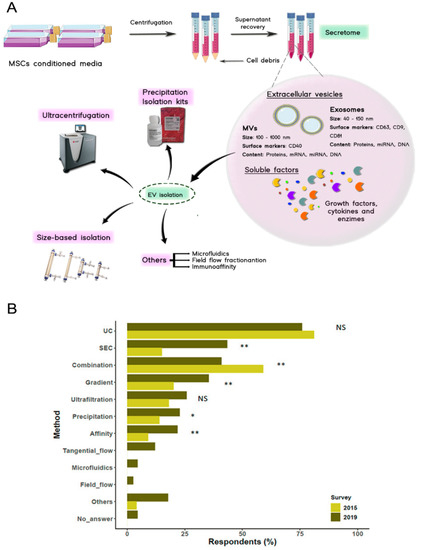
Figure 3.
(A) Secretome isolation starts with a centrifugation stage where cell debris are pelleted and discarded, after that, secretome fractions can be isolated by employing different techniques such as centrifugation, size-based isolation techniques or commercial kits among others. (B) Results of the survey carried out by the ISCT regarding the techniques used for the isolation of EVs in 2019. Ultracentrifugation and a combination of size-based isolation techniques are the most commonly used protocols for EV isolation. (NS, non-significant, * p < 0.05, ** p < 0.01. Missing bars: not queried in the 2015 survey) (Adapted with permission from [82]).
Size-based isolation techniques have also been very useful. Ultrafiltration devices, tangential flow filtration (TFF) systems or size exclusion chromatography (SEC) have turned out to be interesting strategies [82,83]. Blind-elution chromatography has returned good results in the purification of the EVs in combination with a prefiltration with TFF devices [80].
Commercialized rapid isolation kits are also widely employed for EV isolation [80]. All of them are based on the isolation by precipitation methods. Some of the available kits are ExosQuick (System Biosciences), Exos-spin (Cell Guidance Systems) or Total Exosome Isolation Reagent (Thermo Fisher, Waltham, MA, USA). Unfortunately, although commercial kits are useful for separating EVs from the soluble fraction of the secretome, the achieved purity is often rather poor [79]. Less employed techniques include affinity-based capture [77]. For example, immunoaffinity methods have been used for the identification and isolation of specifically marked EVs. This method could be useful if specific EVs are required for the intended therapy [77].
Nowadays, in laboratory practice, ultracentrifugation and size exclusion techniques are often combined since ultracentrifugation enables vesicle isolation and size exclusion techniques to allow their purification. However, many efforts are being made to optimize and gain knowledge on these procedures, since it is well known that the isolation method affects the amount, type and purity of EVs as well as their effect [35]. Furthermore, isolation and purification techniques could be useful to concentrate the bioactive compounds, which may be beneficial for lowering therapeutic dose volume and therefore, to obtain more manageable treatments. Despite the advances made so far, there is still a need for isolation techniques that present a high yield, maintain the characteristics of the obtained products and are not cost prohibitive but scalable [63].
4. Factors That Introduce Variability in the Secretome Composition
4.1. Donor Source
In many studies where MSC-based therapy is explored, autologous cells are harvested from donor patients to avoid cell rejection. Nevertheless, factors such as the age or health condition of the donor have been shown to pose a great influence on the growth, development and immunomodulatory capacity of the primary MSCs [84]. Indeed, the secretome has been shown to vary its composition depending on these factors. For example, the secretome obtained from donors with multiple sclerosis had less neuroprotective effect in comparison to that of healthy patients [85]. Likewise, obesity of donors has shown to have a great influence on the composition of the cargo of the EVs derived from adipose tissue MSCs (AT-MSCs), reducing the concentration of macrophage inflammatory protein (MIP) and miRNA-126 [86]. This effect could be limited by using allogeneic sources. However, primary MSCs—both autologous or allogeneic—are less long-lived and their characteristics and behavior change throughout the cell passages. Similarly, donor age has an impact on the MSC secretory profile. Aging has been associated with MSC senescence which can lead to an important modification of their secretome, especially in terms of miRNA. Indeed, senescent cells adopt a different secretory profile known as senescent-associated secretory phenotype (SASP). As well as differences in content, aging cells have been reported to present a higher EV production as well as EV size reduction [87,88]. This means that the composition of the MSC-derived secretome can be changeable and not reproducible.
4.2. Cell Source
Another factor that presents a direct effect on the composition of the MSC-based secretome is the cell tissue source. The differences in the secretome originated from alternative cell sources has been evidenced thanks to proteomic studies. One of them showed that there is a significant difference in the protein profile released from bone marrow (BM-MSCs), AT-MSCs and dental pulp-derived MSCs. However, the secretion of proteins that have been related to the therapeutic potential of the secretome is constitutive in all the cellular sources analyzed so far [89]. In this way, the differences in secretome from these different tissues are mainly quantitative with a greater or lesser variability on the concentration of the secreted bioactive compounds depending on the cell source. Backing up these results, studies comparing the secretome protein expression of human MSCs derived from BM, Wharton’s jelly (WJ) and exfoliated deciduous teeth (SHED) demonstrated quantitative differences in a variety of growth factors and cytokines. In particular, IL-10 expression in the secretome was higher in WJ-MSCs and SHED-MSCs while the latter was also enriched in HGF [90]. In another comparative study, the secretome derived from WJ-MSCs, AT-MSCs and BM-MSCs was demonstrated to have different concentrations of the proteins of interest. In particular, the WJ-MSC-derived secretome was enriched in factors like RANTES, IL1-Ra TGF-β, HGF and IL-6 [91,92].
It is important to mention that variability introduced by the cell donor or origin makes the MSCs unpredictable and as a result, there is an increasing interest in their replacement by immortalized cell lines [93]. According to the latest research, immortalized MSC-derived secretome does not vary depending on tissue source or cell passage [74,94]. Furthermore, since an important quantity of cells are required for an optimal yield of secretome production, the use of immortalized MSCs with lower cost and better longevity results is appealing. All of these considerations show the way to new prospects for the use of immortalized MSCs lines in secretome-based therapies.
To date, the effect of cell source on EV composition has not been directly explored. Instead, a few studies have addressed it indirectly by comparing the global therapeutic effects obtained by EVs originated from alternative tissue sources. In a comparative study, exosomes obtained from menstrual MSCs (mMSCs) presented greater therapeutic effects on neurodegenerative pathologies like Parkinson’s disease when compared to BM, umbilical cord and chorion-derived MSCs [95]. Considering that tissue source definitely introduces variability in the whole secretome composition, it is highly likely that it also affects the EV fraction. Therefore, in EV-based therapies, exploring this avenue would shed light on which could be the source presenting EVs with a better therapeutic profile for the treatment of certain diseases.
4.3. MSCs Preconditioning Strategies: Towards an Msc2 Anti-Inflammatory Secretome
The possibility of manipulating the MSCs’ microenvironment to polarize them towards an MSC2 phenotype that renders a secretome loaded with anti-inflammatory factors is currently in vogue. In the following sections, we review some of the most explored strategies that could be implemented in secretome-derived therapies for the treatment of IMIDs.
4.3.1. Hypoxia
The influence of controlled oxygen exposure when culturing MSCs in vitro has been largely studied [96,97,98]. Normally, in vitro cell culture is carried out under an 18–21% oxygen concentration. However, it has been reported that hypoxic culture conditions, with an oxygen tension varying between 0–10% created using a cell-culture chamber or bioreactors, could enhance the secretion of the immunomodulatory factors by MSCs. Indeed, hypoxic preconditioning has been demonstrated to increase MSC production of pleiotropic growth factors such as HGF, IGF-1 or brain-derived neurotrophic factor (BDNF) and immunomodulatory factors such as IDO or PGE2, among others [99,100,101,102,103]. In a study conducted by Yu et al., the secretome derived from hypoxia preconditioned MSCs exerted an immunomodulatory effect on microglia cells modulating their proinflammatory cytokine secretion and promoting their polarization towards an anti-inflammatory phenotype [104]. These effects were attributed to a secretome enriched in factors such as IL-10, HGF or PGE2 [105].
According to the latest research, EV concentration and cargo could also vary when hypoxic preconditioning is employed. Hypoxic conditions have been reported to not alter the average size, morphological appearance and surface markers of MSC-derived EVs [106,107]. Not so well established is whether hypoxia could promote or demote EV production by MSCs. Despite some studies reporting that EVs release could be triggered by culturing MSCs in hypoxic conditions [104], others revile this hypothesis, advocating the opposite [25,107]. This controversy might be based on the use of different hypoxic conditions, since slight changes in oxygen concentration and exposure time may have an important impact [104]. Regarding the therapeutic effect of hypoxia preconditioned EVs, it has been observed that they are capable of exerting immunomodulatory effects by enhancing macrophage polarization through M2 anti-inflammatory phenotypes [25,108]. It has been proposed that EVs could enrich their cargo when preconditioned, this being the principle role of their miRNA cargo [109,110,111]. Among others, miRNA-21 has been suggested as a key mediator on preconditioned EVs’ immunomodulatory effects [108].
The mechanisms involved in these effects have not been accurately described yet. However, it has been reported that hypoxia induces the secretion of hypoxia-inducible factor (HIF-1), which influences cell metabolism, differentiation, proliferation, migration and survival [97]. In addition, there are indications that the induction of HIF-1 is directly related to the mechanisms of membrane fusion and budding and therefore in the release process of EVs [112,113,114,115,116]. Moreover, it is believed that since in vivo damaged tissues are frequently hypoxic (1–7%), the culture of MSCs in this environment increases their viability after being implanted [3,117]. Nevertheless, it is important to note that whereas some studies have observed increased cell survival after exposure to hypoxic conditions [118], others have reported cytotoxic effects [119]. These disparate outcomes could be due, again, to differences in the severity and exposure time of the hypoxic conditions [3,120].
4.3.2. Three-Dimensional (3D) Culture
Even though MSCs are normally cultured in monolayers, 3D cultures have been shown to enhance immunomodulatory factor production by MSCs [27]. There are several strategies for MSC 3D culture, one of the most used is spheroid formation [53,121]. In this scenario, less oxygen reaches the inner layer cells, providing a hypoxic microenvironment, which together with the promotion of cell−cell contacts, has been reported to enhance immunomodulatory factor secretion [53]. Recent studies report a higher secretion of granulocyte—colony stimulating factor (G-CSF) and IL-1Ra by MSCs when cultured in spheroids. In addition, the secretome obtained from 3D cultured MSCs (3D-MSC-secretome) has been proven to stimulate macrophage polarization preventing the release of proinflammatory factors such as TNF-α and stimulating their IL-10 secretion [53,122,123]. Furthermore, an increase in anti-inflammatory factors such as PGE2 has also been observed [53,124]. However, this approach could limit the viability of grafted cells since their inner core could present oxygen and nutrient diffusion limitations [121]. Efforts now focus on 3D-MSC-secretome characterization and, although not intense, research has been performed in this regard, first approaches have already been carried out. Specifically, the 3D-MSC-secretome has been characterized and compared to the secretome obtained from 2D-cultured MSCs (2D-MSC-secretome) demonstrating that the spheroid culture of MSCs could lead to an enrichment of the obtained secretome profile on anti-inflammatory factors such as IL-10. In contrast, the 2D-MSC-secretome presented higher levels of pro-inflammatory cytokines such as IL-6 or IL-2 [125]. In terms of the EV fraction of the secretome, it has been described that 3D-cultured MSCs have a higher efficiency on EV production compared to 2D-cultured MSCs. Moreover, EVs obtained from 3D-cultured MSCs (3D-MSC-EVs) have been shown to display superior functions in some studies [126,127].
Other strategies such as cell culture on scaffolds have also been employed for 3D-MSC-secretome obtainment. This method has been demonstrated to have a direct impact on secretome composition and function increasing its HGF levels [128]. However, one of the most interesting approaches for obtaining a 3D-MSC secretome is the encapsulation of MSCs in hydrogels [129,130]. Hydrogels enable the immediate cell microenvironment to be tuned in terms of mechanical properties—elasticity, stiffness—and also, to decorate it with motifs present in the natural extracellular matrix, all of which contribute to secretome enrichment [131]. This new approach has recently been combined with the biochemical stimulation of cells prolonging the immunomodulatory MSCs phenotype [132,133].
4.3.3. Biochemical Stimuli
In recent years, the exposure of MSCs to biochemical stimuli has been widely explored. This process is also known as licensing. As previously mentioned, it has been described that TLR-3 activation with inflammatory cytokines such as IFN-γ, TNF-α or IL-1 polarizes MSCs towards an MSC2 anti-inflammatory phenotype. As a result, exposing MSCs to these factors has become a widely used strategy on therapies based on the MSC-derived secretome for the treatment of IMIDs [134,135].
For instance, cell exposure to IFN-γ has been proven to unfetter the production of factors such as HGF, Gal-9, IDO or PGE2, thus eliciting immunomodulatory and regenerative effects [7,27,63,136,137]. Similarly, MSC licensing with TNF-α has been related to secretome enrichment with tissue repairing and immunomodulatory growth factors [138,139]. Significantly, this enrichment is greater when proinflammatory stimuli with TNF-α is combined with hypoxic conditions during preconditioning [107,140]. Interestingly, the combination of both cytokines, IFN-γ and TNF-α, has been demonstrated to have a synergistic effect to polarize MSCs towards the MSC2 phenotype [44].
In this vein, recent studies have shown that exposure to IL-1β and TNF-α enriches the secretome in proteins such as granulocyte-macrophage colony stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1) and matrix metalloproteinases 8 and 9 [107]. Interestingly, other studies have demonstrated that IL-1β not only enriches the factors above mentioned but also HGF and IL1-Ra, which exert important immunomodulatory effects [141].
Variations in the EV-secretion profile have also been studied after MSC exposure to biochemical stimuli. It has been shown that IFN-γ is capable of inducing minor changes on EV shape and size when being used in high concentrations [142]. However, these changes do not always occur [106,107] and there is still the need to confirm if minor modifications on EV size would affect their therapeutic potential. The concentration of EVs released by licensed MSCs has also been shown to be higher. In particular, AT-MSCs were demonstrated to release a higher quantity of EVs when subjected to proinflammatory stimuli. However, although this increase in the secretion of EVs points to a greater therapeutic effect, it is vital to characterize the effect of licensing on the EV cargo. In this regard, recent research has demonstrated that the proinflammatory licensing of MSCs also enhances the immunomodulatory effects of EVs. In a study performed by Serejo et al., EVs derived from AT-MSCs licensed with IFN-γ inhibited T-cell proliferation to a higher extent than unlicensed EVs. These effects were attributed to an increase of IDO and Gal-1 within the EV cargo [143]. Similar effects have been described when licensing MSCs with TNF-α and IL-1β [143,144,145].
Although IFN-γ, TNF-α and IL-1 are the most studied factors, some others have also been demonstrated to boost the EV immunomodulatory effects. For instance, the use of thapsigargin has also been tested. The resultant WJ-MSC-derived EVs showed an increased yield and expression of TGFβ, cyclooxygenase 2 (COX2) and IDO compared to control EVs. Moreover, licensed EVs exerted direct immunomodulatory effects by suppressing T-cell proliferation and differentiation while increasing Treg proliferation and macrophage M2 polarization. Remarkably, these immunomodulatory effects of the EVs obtained by licensed MSCs were related to therapeutic outcomes observed in dextran sodium sulfate (DSS)-induced colitis mice models [146].
5. Application of the MSC Secretome for the Treatment of IMIDs
Considering the therapeutic potential of the MSC-derived secretome for the treatment of IMIDs, its use in animal models is being extensively studied.
5.1. IBD
IBD is a chronic IMID, which comprises ulcerative colitis and Crohn’s disease. Characteristic of IBD is the inflammation of the gastrointestinal tract, which causes several symptoms such as diarrhea, bloody stool and pain spikes. The pathology of IBD has been related to immune disbalances such as excessive T cell activation, reduction of Treg differentiation and overproduction of proinflammatory mediators in the gastrointestinal tract [147]. Consequently, the immunomodulatory properties of the MSC-derived secretome have become an interesting new avenue for its treatment.
Both local or systemic administration of MSC-derived EVs in dextran sodium sulphate (DSS)-induced mice models have been demonstrated to improve the clinical severity of colitis, improving survival rates and showing less gut inflammation on histopathological studies [148,149,150]. These beneficial properties of the EVs have been related to the polarization of macrophages towards an M2 anti-inflammatory phenotype, as well as to oxidative stress reduction in the affected tissues when being administered by intraperitoneal injection or locally into injured tissue through laparotomy [150,151]. Preconditioning the MSCs with IFN-γ and TNF-α has also been demonstrated to enrich the EVs in immunomodulatory proteins such as HGF, PGE2 or TGF-β eliciting superior effects when administered by intraperitoneal injection [148]. Similarly, MSC-derived CM has been proven to downregulate the expression of proinflammatory cytokines such as TNF-α or IL-1 and upregulate the anti-inflammatory factors such as IL-10 in DSS-induced colitis mice models [152].
A different strategy that has also been explored is the in vitro exposure of macrophages to the MSC-derived secretome. Once implanted in a 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis mice model, preconditioned macrophages were demonstrated to improve animal survival rate by decreasing weight loss and bloody stool, and thus alleviating colitis progression and avoiding disease recurrence [153].
5.2. OA
OA is a disease that causes the progressive degeneration of joints [154,155,156]. It is characterized by cartilage degradation, calcification and inflammation processes that lead to symptoms such as reduced movement capacity, joint stiffness and diffuse pain [154]. Remarkably, OA has been related to poor cartilage formation−degradation balance with low rates of chondrocyte proliferation and a notable cell apoptosis [154]. Moreover, immune disbalances such as macrophage overactivation have also been described in OA. Interestingly, the MSC-derived secretome has been studied as a new therapeutic approach for the treatment of OA showing promising results in preclinical animal models [154,157].
In a series of studies performed in OA mice models, an exosome intra-articular injection was shown to have both protective and therapeutic effects in the damaged joints [155,156]. MSC-derived exosomes have also been demonstrated to prevent cartilage and bone from degradation improving disease severity [154]. Moreover, exosomes have been proven to elicit regulatory effects in chondrocytes, increasing their proliferation and migration rates as well as enhancing their type II collagen synthesis, which improves cartilage mechanic resistance [155,158]. It is remarkable that these beneficial effects have been attributed to the immunomodulatory activity of exosomes including the upregulation of anti-inflammatory mediators such as IL-10, IL-13 or IL-4, the downregulation of proinflammatory mediators such as TNF-α or IL-1 and the polarization of macrophages towards their M2 anti-inflammatory phenotype, among others [154,159,160].
Indeed, it has been observed that MSC-derived CM, as well as isolated EVs are able to ameliorate OA symptoms by preventing joint deterioration and improving tissue remodeling in mice joint disease models [161]. Finally, it is also worth mentioning that MSC preconditioning strategies, such as the 3D culture of MSCs, increases the effectiveness of secretome-based treatments in OA, accomplishing higher synthesis rates of glycosaminoglycan (GAG) in damaged cartilage as well as recovering chondrocyte proliferation and migration [125].
5.3. GvHD
Hematopoietic stem cell transplantation (HSTC) is a clinical curative approach for the treatment of hematological malignancies. When HSCT is performed, GvHD can become a risky limitation. GvHD occurs when donor T cells respond to the human leucocyte antigens (HLA) of the host cells. This incompatibility between donor and host cells results in immune system disorders with varied clinical manifestations of disease on the skin, liver or gastrointestinal tract, among others [162].
The immunomodulatory properties of MSCs were first reported to be useful for treating GvHD caused by HSCT in 2004 [163]. Since such promising findings, several studies, including both preclinical and human clinical trials have extensively evaluated the use of MSCs for the treatment of GvHD. However, results are varying and controversial [164,165]. This has mainly been attributed to heterogeneity of protocols that include disparities in cell source and dosage, and could also be caused by the ability of the specific microenvironment in each specific patient to polarize the anti-inflammatory MSC2 phenotype. In this scenario, the use of cell-free therapies could represent an interesting alternative [3].
Indeed, EVs have shown promising outcomes in the treatment and prevention of GvHD [162]. Clinical manifestations of both acute and chronic GvHD have been proven to be milder when employing MSC-EVs as a treatment. Among them, skin, liver and gastrointestinal disease symptoms have been reverted and controlled [166]. Recently, Dal collo et al. tested the effects of intraperitoneally administered BM-MSC-derived EVs on an in vivo mice model of acute GvHD [139]. The results demonstrated that EVs were not only capable of improving disease severity but also of preventing its development, increasing survival of the animals [139].
Furthermore, in acute GvHD mice models, MSC-derived EVs administered intravenously have been proven to modulate cytokine production, enhancing IL-10 production and controlling TNF-α, IL-17 and IL-2 expression, which are typically related to the inflammatory stages in GvHD [167].
Isolated exosomes themselves have also been tested on GvHD models. As Lai and coworkers demonstrated in their research with GvHD mouse models, MSC-derived exosomes intravenously injected decreased proinflammatory cytokine secretion as well as being demonstrated to elicit T cell activation in host bone marrow and spleen [168,169].
Preclinical studies have shown promising results in pathologies such as IBD, OA or GvHD (Table 1).

Table 1.
Preclinical studies involving secretome application on inflammatory bowel disease (IBD), osteoarthritis (OA) and graft-versus-host disease (GvHD) animal models.
5.4. Coronavirus Disease 2019 (COVID-19) and Acute Respiratory Distress Syndrome (ASDR)
COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a contagious respiratory disease declared a pandemic on 11 March, 2020. Symptoms of COVID-19 include fever, cough, fatigue, dyspnea and smell sense loss. The viral infection produces a torrent release of proinflammatory cytokines leading, in many cases, to fatal outcomes and the death of patients. One of the common complications in cases of coronavirus infection is the acute respiratory failure known as acute respiratory distress syndrome (ARDS). Physiopathologically, ARDS occurs when there is continuous damage to the pulmonary epithelium. This triggers an excessive inflammatory response in the tissue, weakening it and causing edema and dysfunction. This excessive immune response has been related to the presence of proinflammatory cytokines and the activation of macrophages and neutrophils in the pulmonary alveoli [170]. Due to this altered immunological component in ARDS, the immunomodulatory effect of MSCs could be especially beneficial for the treatment of COVID-19 cases with this complication. Indeed, a clinical trial on allogenic MSCs on severe COVID-19 patients demonstrated the therapy to be beneficial for COVID-19-induced ARDS. In the study, five patients were injected with the treatment and the clinical symptoms of ARDS improved dramatically [171]. In fact, the results in this trial were so encouraging that the compassionate use of MSCs of severe COVID-19 cases has been recently approved by the FDA. However, additional research is required in larger population samples.
Regarding cell-free therapy based on the MSC-derived secretome, recent studies have revealed that it could also have therapeutic potential in COVID-19/ARDS treatment. Indeed, in preclinical studies of ARDS, MVs have been reported to be a valuable strategy. Worthy of mention, an endotoxin-induced mouse model of acute lung inflammation (ALI) showed a reduction in lung-edema when MVs were instilled intra-tracheally and similar results were shown in lipopolysaccharide ALI-induced mice. The mechanism behind these effects has not been elucidated yet, but it is believed that part of the mRNA cargo of MVs, such as the Ang-1 mRNA or KFG mRNA, is related to the therapeutic effects.
The effect of MSC-derived exosomes has already been studied in a phase I clinical trial of COVID-19 induced ARDS. In the trial, 24 severe COVID-19 patients presenting ARDS were intravenously injected 15 mL of BM-MSC-derived exosomes with a non-specified dose. The treatment was demonstrated to improve the survival of patients as well as to control neutrophil and macrophage lung infiltration and lung edema appearance, in comparison to the status prior to exosome treatment. Moreover, no adverse effects were detected during the trial and patient survival was determined to be above the 80% [172].
6. Secretome Delivery
The immunomodulatory effects of the MSC-derived secretome have been widely demonstrated. However, the immediate challenge in this field is still to find a safe, effective and controlled manner for its delivery [173]. In the treatment of IMIDs, as in many other pathologies, it is especially important to obtain a controlled and sustained release of bioactive factors [174]. Nude secretome administration has not been successful in many cases [175]. When administered systemically, a significant fraction is clarified and removed by macrophages, that is to say, permanence is very limited [175,176,177,178,179]. Similarly, topically administered nude secretome also showed a low bioavailability [173,175]. These facts do not only impair the effectiveness of the treatment but also increase their cost [180,181]. Secretome obtainment, especially if EV isolation is required, is arduous and large-scale production has not yet been achieved with reproducibility [182,183]. Thus, secretome delivery needs to have a higher yield and dose wastage should be avoided.
Encapsulation of the secretome into administration delivery matrices is a possible solution to these problems [184,185]. One of the most interesting strategies may be hydrogel encapsulation, since it could enable a sustained and controlled release extending the half-life of the secretome and decreasing its administration frequency [186]. As a result, lower secretome production would be needed and dosage availability would be higher, enhancing also patient comfort and compliance [173,175].
Hydrogels are three-dimensional, hydrophilic, porous and soft matrices that present high water content [187]. They are formed by the crosslinking of natural—e.g., chitosan, hyaluronic acid (HA) or alginate—or synthetic—e.g., poly-ethylene glycol (PEG) or poly-lactic-glycolic acid (PLGA)—polymers with crosslinking agents [175]. Natural polymers can be also distinguished by their source since they can be extracted from human or non-human sources such as other animals, plants or bacteria. Unlike other strategies, hydrogels are easily produced [188] and they can be formulated under mild conditions—room temperature and moisture conditions, gentle stirring, no need for cell-toxic chemicals. This is of paramount importance for the encapsulation of thermosensitive, brittle or breakable factors [189]. Furthermore, it is possible to elaborate biocompatible hydrogels by using the adequate polymers [173,190]. Moreover, the final products are manageable and easily modifiable for comfortable administration [187,191]. In this regard, the formulation of injectable hydrogels enables the in situ crosslinking, which once administered, adapt to and fill the space of the local milieu. Therefore, the encapsulation of the different fractions of the MSC-derived secretome in hydrogels is a promising alternative for their delivery [192]. In that sense, special interest has been paid to the hydrogel encapsulation of the EV-fraction of the secretome.
Different hydrogel encapsulation strategies have been used for EV delivery, which vary in polymer composition—including both natural and synthetic polymers—and the encapsulation technique. According to the encapsulation techniques, EVs can be included in the hydrogel before, after or at the same time as the crosslinkers [173] (Figure 4). The order of encapsulation will depend mostly on the selected polymers and the porosity of the produced matrices. An example illustrating the first technique where polymers are mixed up with EVs before the crosslinker addition is the system designed by Quin et al. They developed an EV-loaded hydrogel made of thiolated hyaluronic acid, thiolated heparin and thiolated gelatin with polyethylene glycol diacrylate (PEGDA) as a crosslinker. This process of mixing EVs and polymers before adding the crosslinkers is the most employed as it is simple and suitable for the majority of the polymers employed [193].
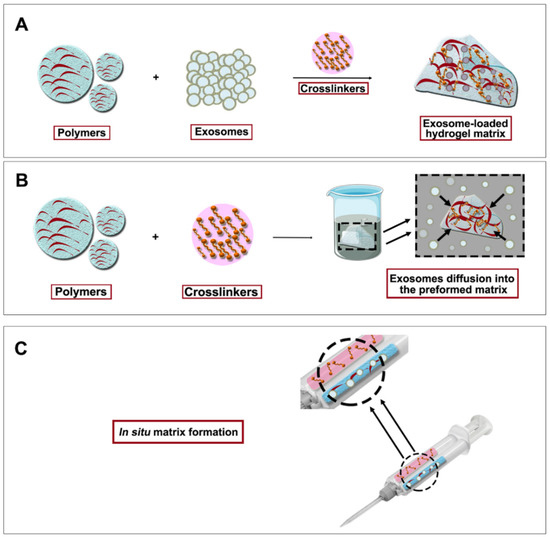
Figure 4.
Exosome-loaded hydrogel formation strategies. (A) Exosomes are first mixed with polymers and then crosslinkation is performed. (B) Breathing method where hydrogel matrix is first built up and exosomes are allowed to diffuse into the matrix. (C) In situ matrix formation where hydrogel components and exosomes are simultaneously mixed with the use of a double syringe system.
The “breathing” encapsulation method has also been employed for EV encapsulation in hydrogels [173]. Briefly, polymers and crosslinkers are mixed and afterwards, the formed matrix is submerged into an EV solution, resulting in their entrance into the hydrogel matrix. This breathing is suitable for porous systems since EVs would have to cross their matrix for the encapsulation [194].
Following the third approach, a polypeptide hydrogel made of Pluronic F127, oxidative hyaluronic acid, and poly-ε-L-lysine was developed by Wang and co-workers [195]. In this case, polymers, crosslinkers and EVs were mixed at the same time. This mixture order allows the in situ gelation of the hydrogel and its immediate administration in vivo. Simultaneous mixing can be used when polymer gelation is quick and a rapid encapsulation of EVs is needed [195].
The release of EVs from hydrogels can occur through different mechanisms, including diffusion if the matrix porosity allows it, polymer-EV interactions and matrix degradation. Therefore, changes in factors such as hydrogel composition, the chemical nature of its components, the crosslinking method used for its gelation or even the environment where the hydrogel is administered will directly affect the EV release and thus, the performance of the system. Furthermore, the mechanical properties of hydrogels can be a determining factor in the administration of the formulations, making it more or less difficult. Commonly, a shear-thinning behavior of the hydrogels is desirable—being less viscous when administered but recovering elasticity and shape when remaining in steady state—which enables manageable administration of treatments and increased permanence rates on injured areas [196].
Apart from the hydrogels containing the EV-fraction, whole secretome hydrogel encapsulation has also been explored so far. In this vein, one of the most promising approaches is the combination of the MSC preconditioning culture strategies for secretome enrichment and the subsequent secretome hydrogel encapsulation for its delivery into injured areas [176]. An example of this strategy is the one employed by Waters et al., which employs AT-MSC-3D culture to enrich their secretome in regenerative factors. Hydrogel encapsulation of the obtained secretome was employed as a successful treatment in cardiac infarcted mouse models [197]. Interestingly, the most explored strategies for secretome encapsulation are both the breathing and the in situ crosslinking methods because of their simplicity. However, and as it happens, in EV-encapsulation, the followed strategy depends mostly on the polymers used for hydrogel formation [176,198].
However, even if secretome hydrogel encapsulation has been demonstrated to be promising, this field is in its infancy [199]. Despite hydrogels being widely employed in cell therapy, their use in secretome delivery has not yet been fully exploited [200,201,202]. Indeed, as shown in Table 2, most of the studies in which EVs or secretome are encapsulated in hydrogels are focused on regenerative medicine applications such as osteoporosis, endometrial tissue repair, hind limb ischemia, wound healing or cardiac tissue repair [155,195,203,204,205,206,207,208,209,210,211,212,213,214,215,216]. Therefore, future research is guaranteed to explore the potential of this approach for the delivery of immunomodulatory secretome for the treatment of IMIDs.

Table 2.
Studies involving hydrogel encapsulation of exosomes for increased treatment permanence around injured areas. MSC-EVs (mesenchymal stromal cell-derived extracellular vesicles) u-MSCs (umbilical-derived MSCs); p-MSCs (placenta-derived MSCs); a-MSC (adipose tissue-derived MSCs) e-MSC (embryonic stromal cells) 3D-a-MSC-secretome (3D cultured adipose-derived MSC-derived secretome).
7. Concluding Remarks
Despite numerous studies showing that the immunomodulatory effects of MSCs are promising in inflammation management, the cell-based therapies present important limitations. Concerns regarding cell administration—including implant rejection, risk of thrombosis or adverse inflammatory reactions, as well as the difficult reproducibility and bioactive factor evaluation—have put MSC-based therapies at stake. Several studies show that the immunomodulatory effects of MSCs can be mainly attributed to their secretome, which has led to a turning point where cellular therapies could be replaced by cell-free therapies. In this sense, the MSC-derived immunomodulatory/anti-inflammatory secretome is the new promising vein of gold, overcoming the drawbacks of cell-based therapies. This is encouraging the scientific community to study its therapeutic effects and the interest in the field is increasing rapidly.
Regarding its application, using secretome as a treatment on IMIDs such as IBD, osteoarthritis or GvHD has been demonstrated to elicit therapeutic effects. However, there is still a great research pathway ahead since there are still limitations for this therapeutic potential to overcome. First, an exhaustive characterization of secretome is necessary to ensure comprehension and reproducibility of the treatments in which it is used. There are signs of a variable composition of the secretome depending on conditioning factors both external and inherent to the source cells, but the magnitude of this variability has not been accurately determined in the research carried out so far. Similarly, there is no unified consensus on the best methods to optimize and customize the secretome content, which is mandatory to achieve adaptable therapies with a broad spectrum of applications.
Another milestone that has not yet been reached is the development of finely tuned methods for secretome obtention. A great variety of methods for the isolation and purification of the whole secretome or EVs have been proposed, however, advances still need to take place to optimize the yield and scalability.
Moreover, as mentioned above, one of the pillars still under research is the development of secretome delivery strategies. The controlled and sustained release of secretome factors being the main goal, hydrogel encapsulation could be one of the most appropriate delivery strategies because of the simplicity and efficiency of the approach. Although EV encapsulation in hydrogels has lately been explored for tissue regeneration purposes, no studies have been published regarding MSC-secretome delivery devices for IMID treatment. Therefore, the study of this strategy is warranted in the near future. All these facts open a new promising avenue for the development of secretome-based therapies for the treatment of IMIDs.
Funding
This research was funded by the projects SAF2017-82292-R (MINECO), AEI/FEDER, UE, ICTS “NANBIOSIS” Drug Formulation Unit (U10) and the Basque Country Government (Grupos Consolidados, No ref: IT907-16). Elena Munoz-Perez thanks the Basque Government for the predoctoral grant (PRE_2020_1_0279). A. Gonzalez-Pujana thanks the University of the Basque Country (UPV/EHU) for the postdoctoral grant (ESPDOC20/119).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Figures were created using Servier Medical Art templates, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com, last accessed 13 September 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory Mechanisms of Mesenchymal Stem and Stromal Cells in Inflammatory Diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Mesenchymal Stromal Cell Based Therapies for the Treatment of Immune Disorders: Recent Milestones and Future Challenges. Expert Opin. Drug Deliv. 2020, 17, 189–200. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L. Stem Cell Therapies for Inflammatory Bowel Disease. Curr. Gastroenterol. Rep. 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Bonovas, S.; Cicerone, C.; Allocca, M.; Furfaro, F.; Correale, C.; Danese, S. The Safety of Biological Pharmacotherapy for the Treatment of Ulcerative Colitis. Expert Opin. Drug Saf. 2017, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.; Tse, H.; Fu, Q.; Lian, Q. Mesenchymal Stem Cells and Immunomodulation: Current Status and Future Prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Le Blanc, K.; Davies, L.C. Mesenchymal Stromal Cells and the Innate Immune Response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone Marrow Osteogenic Stem Cells: In Vitro Cultivation and Transplantation in Diffusion Chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal Cells Responsible for Transferring the Microenvironment of the Hemopoietic Tissues. Cloning in Vitro and Retransplantation in Vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells International 2018, 2018, 8031718. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells*. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. the International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Miroslaw, J. MSCs from Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Hoogduijn, M.J.; Lombardo, E. Mesenchymal Stromal Cells Anno 2019: Dawn of the Therapeutic Era? Concise Review. Stem Cells Transl. Med. 2019, 8, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Sigmarsdottir, T.; McGarrity, S.; Rolfsson, O.; Yurkovich, J.T.; Sigurjonsson, O.E. Current Status and Future Prospects of Genome-Scale Metabolic Modeling to Optimize the use of Mesenchymal Stem Cells in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 239. [Google Scholar] [CrossRef]
- Regmi, S.; Pathak, S.; Kim, J.O.; Yong, C.S.; Jeong, J.H. Mesenchymal Stem Cell Therapy for the Treatment of Inflammatory Diseases: Challenges, Opportunities, and Future Perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological Functions of Mesenchymal Stem Cells and Clinical Implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 Or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Naftali-Shani, N.; Levin-Kotler, L.P.; Palevski, D.; Amit, U.; Kain, D.; Landa, N.; Hochhauser, E.; Leor, J. Left Ventricular Dysfunction Switches Mesenchymal Stromal Cells Toward an Inflammatory Phenotype and Impairs their Reparative Properties Via Toll-Like Receptor-4. Circulation 2017, 135, 2271–2287. [Google Scholar] [CrossRef] [PubMed]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in Vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef]
- FDA Warns about Stem Cell Therapies. 2019. Available online: https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies (accessed on 13 September 2021).
- Noel, P.R.; Barnett, K.C.; Davies, R.E.; Jolly, D.W.; Leahy, J.S.; Mawdesley-Thomas, L.E.; Shillam, K.W.; Squires, P.F.; Street, A.E.; Tucker, W.C.; et al. The Toxicity of Dimethyl Sulphoxide (DMSO) for the Dog, Pig, Rat and Rabbit. Toxicology 1975, 3, 143–169. [Google Scholar] [CrossRef]
- de Mare-Bredemeijer, E.L.; Mancham, S.; Verstegen, M.M.; de Ruiter, P.E.; van Gent, R.; O’Neill, D.; Tilanus, H.W.; Metselaar, H.J.; de Jonge, J.; Kwekkeboom, J.; et al. Human Graft-Derived Mesenchymal Stromal Cells Potently Suppress Alloreactive T-Cell Responses. Stem Cells Dev. 2015, 24, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liao, L.; Shao, B.; Su, X.; Shuai, Y.; Wang, H.; Shang, F.; Zhou, Z.; Yang, D.; Jin, Y. Knockdown of MicroRNA Let-7a Improves the Functionality of Bone Marrow-Derived Mesenchymal Stem Cells in Immunotherapy. Mol. Ther. 2017, 25, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.A.; Lotvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Novel Frontiers in Regenerative Medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; O’Connor, R.A.; Crittenden, S.; Forster, T.; Yu, C.; Zheng, X.; Smyth, D.; Robb, C.T.; Rossi, F.; Skouras, C.; et al. Prostaglandin E(2) Constrains Systemic Inflammation through an Innate Lymphoid Cell-IL-22 Axis. Science 2016, 351, 1333–1338. [Google Scholar] [CrossRef]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung Resident Mesenchymal Stem Cells Isolated from Human Lung Allografts Inhibit T Cell Proliferation Via a Soluble Mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal Stem Cells Program Macrophage Plasticity by Altering their Metabolic Status Via a PGE2-Dependent Mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef]
- Gieseke, F.; Bohringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Muller, I. Human Multipotent Mesenchymal Stromal Cells use Galectin-1 to Inhibit Immune Effector Cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schuttrumpf, J. Galectin-9 is a Suppressor of T and B Cells and Predicts the Immune Modulatory Potential of Mesenchymal Stromal Cell Preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Li Volti, G.; Tibullo, D.; Vanella, L.; Giallongo, C.; Di Raimondo, F.; Forte, S.; Di Rosa, M.; Signorelli, S.S.; Barbagallo, I. The Heme Oxygenase System in Hematological Malignancies. Antioxid. Redox Signal. 2017, 27, 363–377. [Google Scholar] [CrossRef]
- Abram, D.M.; Fernandes, L.G.R.; Ramos Filho, A.C.S.; Simioni, P.U. The Modulation of Enzyme Indoleamine 2,3-Dioxygenase from Dendritic Cells for the Treatment of Type 1 Diabetes Mellitus. Drug Des. Devel. Ther. 2017, 11, 2171–2178. [Google Scholar] [CrossRef][Green Version]
- Jin, P.; Zhao, Y.; Liu, H.; Chen, J.; Ren, J.; Jin, J.; Bedognetti, D.; Liu, S.; Wang, E.; Marincola, F.; et al. Interferon-Gamma and Tumor Necrosis Factor-Alpha Polarize Bone Marrow Stromal Cells Uniformly to a Th1 Phenotype. Sci. Rep. 2016, 6, 26345. [Google Scholar] [CrossRef]
- Francois, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC Suppression Correlates with Cytokine Induction of Indoleamine 2,3-Dioxygenase and Bystander M2 Macrophage Differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 Acts Directly on CD4 T Cells to Enhance their Antigen-Driven Expansion and Differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, N.; Djonov, V.; Volarevic, V. The Role of Interleukin 1 Receptor Antagonist in Mesenchymal Stem Cell-Based Tissue Repair and Regeneration. Biofactors 2020, 46, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A Multifunctional Protein with Anti-Inflammatory and Tissue-Protective Properties. Matrix Biol. 2019, 78-79, 60–83. [Google Scholar] [CrossRef] [PubMed]
- Hertsenberg, A.J.; Shojaati, G.; Funderburgh, M.L.; Mann, M.M.; Du, Y.; Funderburgh, J.L. Corneal Stromal Stem Cells Reduce Corneal Scarring by Mediating Neutrophil Infiltration After Wounding. PLoS ONE 2017, 12, e0171712. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice Via Release of TSG6, Independently of their Localization to the Intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef] [PubMed]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. TSG-6 Produced by hMSCs Delays the Onset of Autoimmune Diabetes by Suppressing Th1 Development and Enhancing Tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of Human Mesenchymal Stromal Cells (MSCs) into 3D Spheroids Enhances their Antiinflammatory Properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 Released from Intradermally Injected Mesenchymal Stem Cells Accelerates Wound Healing and Reduces Tissue Fibrosis in Murine Full-Thickness Skin Wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNFalpha-Stimulated Gene-6 (TSG6) Activates Macrophage Phenotype Transition to Prevent Inflammatory Lung Injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Ha Bhang, D.; Chan Jung, Y.; Youn, H.Y. TSG-6 Secreted by Human Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorates DSS-Induced Colitis by Inducing M2 Macrophage Polarization in Mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.; Mazurier, C.; Bouchet, S.; Francois, S.; Chapel, A.; Thierry, D.; Gorin, N.C.; Fouillard, L. Leukemia Inhibitory Factor: Role in Human Mesenchymal Stem Cells Mediated Immunosuppression. Cell. Immunol. 2008, 253, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Strobl, H.; Knapp, W. TGF-Beta1 Regulation of Dendritic Cells. Microbes Infect. 1999, 1, 1283–1290. [Google Scholar] [CrossRef]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.M.; Jack, R.S.; Wunderlich, F.T.; Bruning, J.C.; Muller, W.; et al. Interleukin-10 Signaling in Regulatory T Cells is Required for Suppression of Th17 Cell-Mediated Inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef]
- Oh, S.A.; Li, M.O. TGF-Β: Guardian of T Cell Function. J. Immunol. 2013, 191, 3973–3979. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular Or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte Growth Factor Inhibits CNS Autoimmunity by Inducing Tolerogenic Dendritic Cells and CD25+Foxp3+ Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The Current Landscape of the Mesenchymal Stromal Cell Secretome: A New Paradigm for Cell-Free Regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mardpour, S.; Hamidieh, A.A.; Taleahmad, S.; Sharifzad, F.; Taghikhani, A.; Baharvand, H. Interaction between Mesenchymal Stromal Cell-Derived Extracellular Vesicles and Immune Cells by Distinct Protein Content. J. Cell. Physiol. 2019, 234, 8249–8258. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New Molecular Targets of Diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC Exosome Works through a Protein-Based Mechanism of Action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.J.; Bae, E.H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M.; et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Lawson, C.; Kovacs, D.; Finding, E.; Ulfelder, E.; Luis-Fuentes, V. Extracellular Vesicles: Evolutionarily Conserved Mediators of Intercellular Communication. Yale J. Biol. Med. 2017, 90, 481–491. [Google Scholar] [PubMed]
- Park, K.S.; Bandeira, E.; Shelke, G.V.; Lasser, C.; Lotvall, J. Enhancement of Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef]
- Adamo, A.; Brandi, J.; Caligola, S.; Delfino, P.; Bazzoni, R.; Carusone, R.; Cecconi, D.; Giugno, R.; Manfredi, M.; Robotti, E.; et al. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front. Immunol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Wu, H.; Fan, H.; Shou, Z.; Xu, M.; Chen, Q.; Ai, C.; Dong, Y.; Liu, Y.; Nan, Z.; Wang, Y.; et al. Extracellular Vesicles Containing miR-146a Attenuate Experimental Colitis by Targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019, 68, 204–212. [Google Scholar] [CrossRef]
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.B.; Padmanabhan, J.; Lee, C.N.; de Kleijn, D.P.; Lim, S.K. Enabling a Robust Scalable Manufacturing Process for Therapeutic Exosomes through Oncogenic Immortalization of Human ESC-Derived MSCs. J. Transl. Med. 2011, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Patel, T. The Mesenchymal Stem Cell Secretome as an Acellular Regenerative Therapy for Liver Disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Bostancioglu, R.B.; Welsh, J.A.; Zickler, A.M.; Murke, F.; Corso, G.; Felldin, U.; Hagey, D.W.; Evertsson, B.; Liang, X.M.; et al. Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures. Front. Immunol. 2018, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Corso, G.; Mager, I.; Lee, Y.; Gorgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and Scalable Purification of Extracellular Vesicles using Combined Bind-Elute and Size Exclusion Chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Thery, C.; Falcon-Perez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Monguio-Tortajada, M.; Galvez-Monton, C.; Bayes-Genis, A.; Roura, S.; Borras, F.E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-Versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Sarkar, P.; Redondo, J.; Kemp, K.; Ginty, M.; Wilkins, A.; Scolding, N.J.; Rice, C.M. Reduced Neuroprotective Potential of the Mesenchymal Stromal Cell Secretome with Ex Vivo Expansion, Age and Progressive Multiple Sclerosis. Cytotherapy 2018, 20, 21–28. [Google Scholar] [CrossRef]
- Togliatto, G.; Dentelli, P.; Gili, M.; Gallo, S.; Deregibus, C.; Biglieri, E.; Iavello, A.; Santini, E.; Rossi, C.; Solini, A.; et al. Obesity Reduces the Pro-Angiogenic Potential of Adipose Tissue Stem Cell-Derived Extracellular Vesicles (EVs) by Impairing miR-126 Content: Impact on Clinical Applications. Int. J. Obes. (Lond.) 2016, 40, 102–111. [Google Scholar] [CrossRef]
- Neri, S.; Borzi, R.M. Molecular Mechanisms Contributing to Mesenchymal Stromal Cell Aging. Biomolecules 2020, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential Effects of Extracellular Vesicles from Aging and Young Mesenchymal Stem Cells in Acute Lung Injury. Aging 2019, 11, 7996–8014. [Google Scholar] [CrossRef] [PubMed]
- Tachida, Y.; Sakurai, H.; Okutsu, J. Proteomic Comparison of the Secreted Factors of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue and Dental Pulp. J. Proteom. Bioinform. 2015, 8, 266. [Google Scholar] [CrossRef]
- Konala, V.B.R.; Bhonde, R.; Pal, R. Secretome Studies of Mesenchymal Stromal Cells (MSCs) Isolated from Three Tissue Sources Reveal Subtle Differences in Potency. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 689–700. [Google Scholar] [CrossRef]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Protein Synthesis and Secretion in Human Mesenchymal Cells Derived from Bone Marrow, Adipose Tissue and Wharton’s Jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Gimona, M.; Brizzi, M.F.; Choo, A.B.H.; Dominici, M.; Davidson, S.M.; Grillari, J.; Hermann, D.M.; Hill, A.F.; de Kleijn, D.; Lai, R.C.; et al. Critical Considerations for the Development of Potency Tests for Therapeutic Applications of Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles. Cytotherapy 2021, 23, 373–380. [Google Scholar] [CrossRef]
- Yeo, R. Efficiency of Exosome Production Correlates Inversely with the Developmental Maturity of MSC Donor. J. Stem Cell Res. Ther. 2013, 3. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal Stem Cell-Derived Exosomes from Different Sources Selectively Promote Neuritic Outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jin, H.J.; Heo, J.; Ju, H.; Lee, H.Y.; Kim, S.; Lee, S.; Lim, J.; Jeong, S.Y.; Kwon, J.; et al. Small Hypoxia-Primed Mesenchymal Stem Cells Attenuate Graft-Versus-Host Disease. Leukemia 2018, 32, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef]
- Ejtehadifar, M.; Shamsasenjan, K.; Akbarzadehlaleh, P.; Zahedi, S.; Kazemi, N. The Effects of Hypoxia on U937 Cell Line in Mesenchymal Stem Cells Co-Culture System. Adv. Pharm. Bull. 2016, 6, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Paquet, J.; Deschepper, M.; Moya, A.; Logeart-Avramoglou, D.; Boisson-Vidal, C.; Petite, H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl. Med. 2015, 4, 809–821. [Google Scholar] [CrossRef]
- Kong, P.; Xie, X.; Li, F.; Liu, Y.; Lu, Y. Placenta Mesenchymal Stem Cell Accelerates Wound Healing by Enhancing Angiogenesis in Diabetic Goto-Kakizaki (GK) Rats. Biochem. Biophys. Res. Commun. 2013, 438, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, Y.; Zhou, L.; Zhu, H.Y.; Song, Y.C.; Hu, L.; Wang, Y.; Li, Q.P. Pretreatment of Mesenchymal Stem Cells with Angiotensin II Enhances Paracrine Effects, Angiogenesis, Gap Junction Formation and Therapeutic Efficacy for Myocardial Infarction. Int. J. Cardiol. 2015, 188, 22–32. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Huo, Y.; Yang, Y.; Wang, Y. Hypoxia-Pretreated Human MSCs Attenuate Acute Kidney Injury through Enhanced Angiogenic and Antioxidative Capacities. Biomed. Res. Int. 2014, 2014, 462472. [Google Scholar] [CrossRef]
- Lan, Y.W.; Choo, K.B.; Chen, C.M.; Hung, T.H.; Chen, Y.B.; Hsieh, C.H.; Kuo, H.P.; Chong, K.Y. Hypoxia-Preconditioned Mesenchymal Stem Cells Attenuate Bleomycin-Induced Pulmonary Fibrosis. Stem Cell Res. Ther. 2015, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Z.; Qu, G.; Wang, H.; Lin, L.; Li, X.; Xie, X.; Lei, Y.; He, X.; Chen, Y.; et al. Hypoxic Preconditioning Enhances the Efficacy of Mesenchymal Stem Cells-Derived Conditioned Medium in Switching Microglia Toward Anti-Inflammatory Polarization in Ischemia/Reperfusion. Cell. Mol. Neurobiol. 2021, 41, 505–524. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Hyland, M.; Mennan, C.; Wilson, E.; Clayton, A.; Kehoe, O. Pro-Inflammatory Priming of Umbilical Cord Mesenchymal Stromal Cells Alters the Protein Cargo of their Extracellular Vesicles. Cells 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the Effects of Preconditioning with Inflammatory Cytokines and Hypoxia on the Angiogenic Potential of Mesenchymal Stromal Cell (MSC)-Derived Soluble Proteins and Extracellular Vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular Vesicles Secreted by Hypoxia Pre-Challenged Mesenchymal Stem Cells Promote Non-Small Cell Lung Cancer Cell Growth and Mobility as Well as Macrophage M2 Polarization Via miR-21-5p Delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018, 3290372. [Google Scholar] [CrossRef]
- Zimta, A.A.; Baru, O.; Badea, M.; Buduru, S.D.; Berindan-Neagoe, I. The Role of Angiogenesis and Pro-Angiogenic Exosomes in Regenerative Dentistry. Int. J. Mol. Sci. 2019, 20, 406. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal Stem Cells Release Exosomes that Transfer miRNAs to Endothelial Cells and Promote Angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and Extracellular Vesicles: A Review on Methods, Vesicular Cargo and Functions. J. Extracell Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-Inducible Factors and RAB22A Mediate Formation of Microvesicles that Stimulate Breast Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, 3234. [Google Scholar] [CrossRef] [PubMed]
- Kastner, N.; Mester-Tonczar, J.; Winkler, J.; Traxler, D.; Spannbauer, A.; Ruger, B.M.; Goliasch, G.; Pavo, N.; Gyongyosi, M.; Zlabinger, K. Comparative Effect of MSC Secretome to MSC Co-Culture on Cardiomyocyte Gene Expression Under Hypoxic Conditions in Vitro. Front. Bioeng. Biotechnol. 2020, 8, 502213. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, R.; Li, Z.; Wang, Y.; Hu, R. Hypoxia Changes Chemotaxis Behaviour of Mesenchymal Stem Cells Via HIF-1alpha Signalling. J. Cell. Mol. Med. 2019, 23, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis Via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Beegle, J.; Lakatos, K.; Kalomoiris, S.; Stewart, H.; Isseroff, R.R.; Nolta, J.A.; Fierro, F.A. Hypoxic Preconditioning of Mesenchymal Stromal Cells Induces Metabolic Changes, Enhances Survival, and Promotes Cell Retention in Vivo. Stem Cells 2015, 33, 1818–1828. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, M.; Dangelmajer, S.; Lee, Y.M.; Wijesekera, O.; Castellanos, C.X.; Denduluri, A.; Chaichana, K.L.; Li, Q.; Zhang, H.; et al. Hypoxia-Cultured Human Adipose-Derived Mesenchymal Stem Cells are Non-Oncogenic and have Enhanced Viability, Motility, and Tropism to Brain Cancer. Cell Death Dis. 2015, 6, e1797. [Google Scholar] [CrossRef] [PubMed]
- Özer, S.; Vatansever, H.S.; Özdal-Kurt, F. Cytotoxic Effect of Hypoxic Environment in Mesenchymal Stem Cell. Proceedings 2018, 2, 1592. [Google Scholar] [CrossRef]
- Antebi, B.; Rodriguez, L.A.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-Term Physiological Hypoxia Potentiates the Therapeutic Function of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Burand, A.J.; Di, L.; Boland, L.K.; Boyt, D.T.; Schrodt, M.V.; Santillan, D.A.; Ankrum, J.A. Aggregation of Human Mesenchymal Stromal Cells Eliminates their Ability to Suppress Human T Cells. Front. Immunol. 2020, 11, 143. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Zhou, L.; Zhang, L.; Jiang, B. Signal Factors Secreted by 2D and Spheroid Mesenchymal Stem Cells and by Cocultures of Mesenchymal Stem Cells Derived Microvesicles and Retinal Photoreceptor Neurons. Stem Cells Int. 2017, 2017, 2730472. [Google Scholar] [CrossRef] [PubMed]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Ylostalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-Activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-Inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.P.; Camoes, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Barcia, R.N.; Cruz, P.; Cruz, H.; Simoes, S.; Santos, J.M. The Secretome Derived from 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yun, H.W.; Park, D.Y.; Choi, B.H.; Min, B.H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-Dimensional Culture of MSCs Produces Exosomes with Improved Yield and Enhanced Therapeutic Efficacy for Cisplatin-Induced Acute Kidney Injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the Impact of 2D and 3D Culture Conditions on the Therapeutic Effects of Human Mesenchymal Stem Cell Secretome on Corneal Wound Healing in Vitro and Ex Vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef]
- Mao, A.S.; Ozkale, B.; Shah, N.J.; Vining, K.H.; Descombes, T.; Zhang, L.; Tringides, C.M.; Wong, S.W.; Shin, J.W.; Scadden, D.T.; et al. Programmable Microencapsulation for Enhanced Mesenchymal Stem Cell Persistence and Immunomodulation. Proc. Natl. Acad. Sci. USA 2019, 116, 15392–15397. [Google Scholar] [CrossRef]
- Attia, N.; Santos, E.; Abdelmouty, H.; Arafa, S.; Zohdy, N.; Hernandez, R.M.; Orive, G.; Pedraz, J.L. Behaviour and Ultrastructure of Human Bone Marrow-Derived Mesenchymal Stem Cells Immobilised in Alginate-Poly-L-Lysine-Alginate Microcapsules. J. Microencapsul. 2014, 31, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Stafford, A.; Mooney, D.J. Sequential Modes of Crosslinking Tune Viscoelasticity of Cell-Instructive Hydrogels. Biomaterials 2019, 188, 187–197. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Vining, K.H.; Zhang, D.K.Y.; Santos-Vizcaino, E.; Igartua, M.; Hernandez, R.M.; Mooney, D.J. Multifunctional Biomimetic Hydrogel Systems to Boost the Immunomodulatory Potential of Mesenchymal Stromal Cells. Biomaterials 2020, 257, 120266. [Google Scholar] [CrossRef]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal Stem Cells: A Friend Or Foe in Immune-Mediated Diseases. Stem Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Boland, L.; Burand, A.J.; Brown, A.J.; Boyt, D.; Lira, V.A.; Ankrum, J.A. IFN-Gamma and TNF-Alpha Pre-Licensing Protects Mesenchymal Stromal Cells from the Pro-Inflammatory Effects of Palmitate. Mol. Ther. 2018, 26, 860–873. [Google Scholar] [CrossRef]
- Carvalho, A.E.S.; Sousa, M.R.R.; Alencar-Silva, T.; Carvalho, J.L.; Saldanha-Araujo, F. Mesenchymal Stem Cells Immunomodulation: The Road to IFN-Gamma Licensing and the Path Ahead. Cytokine Growth Factor Rev. 2019, 47, 32–42. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for Interferon-Gamma in the Immunomodulatory Activity of Human Bone Marrow Mesenchymal Stem Cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Dal Collo, G.; Adamo, A.; Gatti, A.; Tamellini, E.; Bazzoni, R.; Takam Kamga, P.; Tecchio, C.; Quaglia, F.M.; Krampera, M. Functional Dosing of Mesenchymal Stromal Cell-Derived Extracellular Vesicles for the Prevention of Acute Graft-Versus-Host-Disease. Stem Cells 2020, 38, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Selvasandran, K.; Makhoul, G.; Jaiswal, P.K.; Jurakhan, R.; Li, L.; Ridwan, K.; Cecere, R. A Tumor Necrosis Factor-Alpha and Hypoxia-Induced Secretome Therapy for Myocardial Repair. Ann. Thorac. Surg. 2018, 105, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.J.; Trouillas, M. IL-1beta-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing Via Matrix Metalloproteinases and Transforming Growth Factor-Beta1. J. Investig. Dermatol. 2020, 140, 688–698.e21. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-Gamma-Primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia Coli-Induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Serejo, T.R.T.; Silva-Carvalho, A.E.; Braga, L.D.C.F.; Neves, F.A.R.; Pereira, R.W.; Carvalho, J.L.; Saldanha-Araujo, F. Assessment of the Immunosuppressive Potential of INF-Gamma Licensed Adipose Mesenchymal Stem Cells, their Secretome and Extracellular Vesicles. Cells 2019, 8, 22. [Google Scholar] [CrossRef]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human Multipotent Mesenchymal Stromal Cells Cytokine Priming Promotes RAB27B-Regulated Secretion of Small Extracellular Vesicles with Immunomodulatory Cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, H.; Cui, S.; Zhou, Y.; Zhang, T.; Zhou, Y. IFN-Gamma Promoted Exosomes from Mesenchymal Stem Cells to Attenuate Colitis Via miR-125a and miR-125b. Cell Death Dis. 2020, 11, 603. [Google Scholar] [CrossRef]
- Joo, H.; Oh, M.K.; Kang, J.Y.; Park, H.S.; Chae, D.H.; Kim, J.; Lee, J.H.; Yoo, H.M.; Choi, U.; Kim, D.K.; et al. Extracellular Vesicles from Thapsigargin-Treated Mesenchymal Stem Cells Ameliorated Experimental Colitis Via Enhanced Immunomodulatory Properties. Biomedicines 2021, 9, 209. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and Adaptive Immunity in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, Q.; Bhang, D.; Song, W.; Youn, H. TNF-A and INF-Γ Primed Canine Stem Cell-Derived Extracellular Vesicles Alleviate Experimental Murine Colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from Mesenchymal Stromal Cells Reduce Murine Colonic Inflammation Via a Macrophage-Dependent Mechanism. JCI Insight 2019, 4, e131273. [Google Scholar] [CrossRef]
- Duan, L.; Huang, H.; Zhao, X.; Zhou, M.; Chen, S.; Wang, C.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Extracellular Vesicles Derived from Human Placental Mesenchymal Stem Cells Alleviate Experimental Colitis in Mice by Inhibiting Inflammation and Oxidative Stress. Int. J. Mol. Med. 2020, 46, 1551–1561. [Google Scholar] [CrossRef]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Attenuate Dextran Sodium Sulfate-Induced Ulcerative Colitis by Promoting M2 Macrophage Polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef]
- Legaki, E.; Roubelakis, M.; Theodoropoulos, G.; Lazaris, A.; Kollia, A.; Karamanolis, G.; Marinos, E.; Gazouli, M. Therapeutic Potential of Secreted Molecules Derived from Human Amniotic Fluid Mesenchymal Stem/Stroma Cells in a Mice Model of Colitis. Stem Cell Rev. Rep. 2016, 12, 604–612. [Google Scholar] [CrossRef]
- Anderson, P.; Souza-Moreira, L.; Morell, M.; Caro, M.; O’Valle, F.; Gonzalez-Rey, E.; Delgado, M. Adipose-Derived Mesenchymal Stromal Cells Induce Immunomodulatory Macrophages which Protect from Experimental Colitis and Sepsis. Gut 2013, 62, 1131–1141. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cells Derived Exosomes and Microparticles Protect Cartilage and Bone from Degradation in Osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from Embryonic Mesenchymal Stem Cells Alleviate Osteoarthritis through Balancing Synthesis and Degradation of Cartilage Extracellular Matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Ma, D.; Xu, K.; Zhang, G.; Liu, Y.; Gao, J.; Tian, M.; Wei, C.; Li, J.; Zhang, L. Immunomodulatory Effect of Human Umbilical Cord Mesenchymal Stem Cells on T Lymphocytes in Rheumatoid Arthritis. Int. Immunopharmacol. 2019, 74, 105687. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs Promoted Cartilage Repair and Chondrocyte Proliferation in Osteoarthritis. Biochem. J. 2018, 475, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Castejon, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Colombini, A.; Vigano, M.; de Girolamo, L. Secreted Factors and EV-miRNAs Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1582. [Google Scholar] [CrossRef]
- Woo, C.H.; Kim, H.K.; Jung, G.Y.; Jung, Y.J.; Lee, K.S.; Yun, Y.E.; Han, J.; Lee, J.; Kim, W.S.; Choi, J.S.; et al. Small Extracellular Vesicles from Human Adipose-Derived Stem Cells Attenuate Cartilage Degeneration. J. Extracell Vesicles 2020, 9, 1735249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Tieu, A.; Slobodian, M.; Shorr, R.; Burger, D.; Lalu, M.M.; Allan, D.S. Preclinical Studies of MSC-Derived Extracellular Vesicles to Treat Or Prevent Graft Versus Host Disease: A Systematic Review of the Literature. Stem Cell Rev. Rep. 2020, 17, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of Severe Acute Graft-Versus-Host Disease with Third Party Haploidentical Mesenchymal Stem Cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, J.; Yu, J.; Wei, W. Cellular and Molecular Mechanisms in Graft-Versus-Host Disease. J. Leukoc. Biol. 2016, 99, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.M.; Chaudhry, M.S. GVHD: Biology Matters. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, Z.; Zhao, X.; Yang, N.; Wang, F.; Deng, A.; Zhao, S.; Luo, L.; Wei, H.; Guan, L.; et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016, 25, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Miura, Y.; Fujishiro, A.; Shindo, T.; Shimazu, Y.; Hirai, H.; Tahara, H.; Takaori-Kondo, A.; Ichinohe, T.; Maekawa, T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells 2018, 36, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Chen, X.; Guo, L.; Wang, Y.; Liu, X.; Liu, Y.; Zhou, T.; Huang, T.; Geng, S.; Luo, C.; et al. A Potent Immunomodulatory Role of Exosomes Derived from Mesenchymal Stromal Cells in Preventing cGVHD. J. Hematol. Oncol. 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal Stromal Cell Exosome-Enhanced Regulatory T-Cell Production through an Antigen-Presenting Cell-Mediated Pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care. Med. 2019, 40, 31–39. [Google Scholar]
- Iglesias, M.; Butron, P.; Torre-Villalvazo, I.; Torre-Anaya, E.A.; Sierra-Madero, J.; Rodriguez-Andoney, J.J.; Tovar-Palacio, A.R.; Zentella-Dehesa, A.; Dominguez-Cherit, G.; Rodriguez-Reyna, T.S.; et al. Mesenchymal Stem Cells for the Compassionate Treatment of Severe Acute Respiratory Distress Syndrome due to COVID 19. Aging Dis. 2021, 12, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Weiss, D.J.; Witwer, K.W.; Rohde, E. Re: “Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19” by Sengupta et al. Stem Cells Dev. 2020, 29, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of Chronic Inflammation in Disease Development and Prevention: Challenges and Opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and Hydrogel Encapsulated Exosome-Based Therapies in Regenerative Medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef]
- Waters, R.; Pacelli, S.; Maloney, R.; Medhi, I.; Ahmed, R.P.; Paul, A. Stem Cell Secretome-Rich Nanoclay Hydrogel: A Dual Action Therapy for Cardiovascular Regeneration. Nanoscale 2016, 8, 7371–7376. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice After Intravenous Injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell Type-Specific and Common Characteristics of Exosomes Derived from Mouse Cell Lines: Yield, Physicochemical Properties, and Pharmacokinetics. Eur. J. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef]
- Fiorino, G.; Sturniolo, G.C.; Bossa, F.; Cassinotti, A.; di Sabatino, A.; Giuffrida, P.; Danese, S. A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis. Cells 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of Isolating Extracellular Vesicles Impact Down-Stream Analyses of their Cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Liu, B.; Lee, B.W.; Nakanishi, K.; Villasante, A.; Williamson, R.; Metz, J.; Kim, J.; Kanai, M.; Bi, L.; Brown, K.; et al. Cardiac Recovery Via Extended Cell-Free Delivery of Extracellular Vesicles Secreted by Cardiomyocytes Derived from Induced Pluripotent Stem Cells. Nat. Biomed. Eng. 2018, 2, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Shah, S.A.; Kousar, M. Novel Biodegradable pH-Sensitive Hydrogels: An Efficient Controlled Release System to Manage Ulcerative Colitis. Int. J. Biol. Macromol. 2019, 136, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin Iii, R.; In Het Panhuis, M. An Overview of the Suitability of Hydrogel-Forming Polymers for Extrusion-Based 3D-Printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–123. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Lenzini, S.; Bargi, R.; Chung, G.; Shin, J.W. Matrix Mechanics and Water Permeation Regulate Extracellular Vesicle Transport. Nat. Nanotechnol. 2020, 15, 217–223. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation in Vitro and Promote Bone Regeneration in Vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound Healing Effects of a Curcuma Zedoaria Polysaccharide with Platelet-Rich Plasma Exosomes Assembled on Chitosan/Silk Hydrogel Sponge in a Diabetic Rat Model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained Release of Endothelial Progenitor Cell-Derived Extracellular Vesicles from Shear-Thinning Hydrogels Improves Angiogenesis and Promotes Function After Myocardial Infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.H.; Paul, A. Stem Cell-Inspired Secretome-Rich Injectable Hydrogel to Repair Injured Cardiac Tissue. Acta Biomater. 2018, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Bakota, E.L.; Wang, Y.; Danesh, F.R.; Hartgerink, J.D. Injectable Multidomain Peptide Nanofiber Hydrogel as a Delivery Agent for Stem Cell Secretome. Biomacromolecules 2011, 12, 1651–1657. [Google Scholar] [CrossRef]
- Shoma Suresh, K.; Bhat, S.; Guru, B.R.; Muttigi, M.S.; Seetharam, R.N. A Nanocomposite Hydrogel Delivery System for Mesenchymal Stromal Cell Secretome. Stem Cell Res. Ther. 2020, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Moreira, J.; Ribeiro-Samy, S.; Gomes, E.D.; Tam, R.Y.; Shoichet, M.S.; Reis, R.L.; Sousa, N.; Salgado, A.J. Modulation of Bone Marrow Mesenchymal Stem Cell Secretome by ECM-Like Hydrogels. Biochimie 2013, 95, 2314–2319. [Google Scholar] [CrossRef]
- Chierchia, A.; Chirico, N.; Boeri, L.; Raimondi, I.; Riva, G.A.; Raimondi, M.T.; Tunesi, M.; Giordano, C.; Forloni, G.; Albani, D. Secretome Released from Hydrogel-Embedded Adipose Mesenchymal Stem Cells Protects Against the Parkinson’s Disease Related Toxin 6-Hydroxydopamine. Eur. J. Pharm. Biopharm. 2017, 121, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Dewi, R.E.; Goldstone, A.B.; Cohen, J.E.; Steele, A.N.; Woo, Y.J.; Heilshorn, S.C. Regulating Stem Cell Secretome using Injectable Hydrogels with in Situ Network Formation. Adv. Healthc. Mater. 2016, 5, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, W.; Peng, Z.; Lv, S.; Guan, Y.; An, G.; Zhang, Y.; Huang, T.; Wang, Y. The Therapeutic Effect of Secretome from Human Umbilical Cord-Derived Mesenchymal Stem Cells in Age-Related Osteoporosis. Artif. Cells Nanomed Biotechnol. 2019, 47, 1357–1366. [Google Scholar]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, 1900411. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of Small Extracellular Vesicles in Sodium Alginate Hydrogel as a Novel Therapeutic Strategy for Myocardial Infarction. Theranostics 2019, 9, 7403–7416. [Google Scholar] [CrossRef]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Pan, D.; Li, H.; Shen, J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int. J. Nanomed. 2020, 15, 5911–5926. [Google Scholar] [CrossRef]
- Zhang, S.; Thiebes, A.L.; Kreimendahl, F.; Ruetten, S.; Buhl, E.M.; Wolf, M.; Jockenhoevel, S.; Apel, C. Extracellular Vesicles-Loaded Fibrin Gel Supports Rapid Neovascularization for Dental Pulp Regeneration. Int. J. Mol. Sci. 2020, 21, 4226. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020, 20, 4298–4305. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, J.; Zhou, X.; Sun, J.; Zhu, B.; Duan, C.; Chen, P.; Guo, X.; Zhang, T.; Guo, H. A New Self-Healing Hydrogel Containing hucMSC-Derived Exosomes Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 564731. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomes with Hydroxyapatite-Embedded Hyaluronic Acid-Alginate Hydrogel for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhou, J.; Liu, B.; Liang, C.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Y.; Shao, L.; Zhu, B.; et al. Delivery of miR-675 by Stem Cell-Derived Exosomes Encapsulated in Silk Fibroin Hydrogel Prevents Aging-Induced Vascular Dysfunction in Mouse Hindlimb. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 322–332. [Google Scholar] [CrossRef]
- Han, C.; Zhou, J.; Liang, C.; Liu, B.; Pan, X.; Zhang, Y.; Wang, Y.; Yan, B.; Xie, W.; Liu, F.; et al. Human Umbilical Cord Mesenchymal Stem Cell Derived Exosomes Encapsulated in Functional Peptide Hydrogels Promote Cardiac Repair. Biomater. Sci. 2019, 7, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).