Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of SD and co-SD Particles by Organic Solution Advanced Spray Drying in Closed Mode
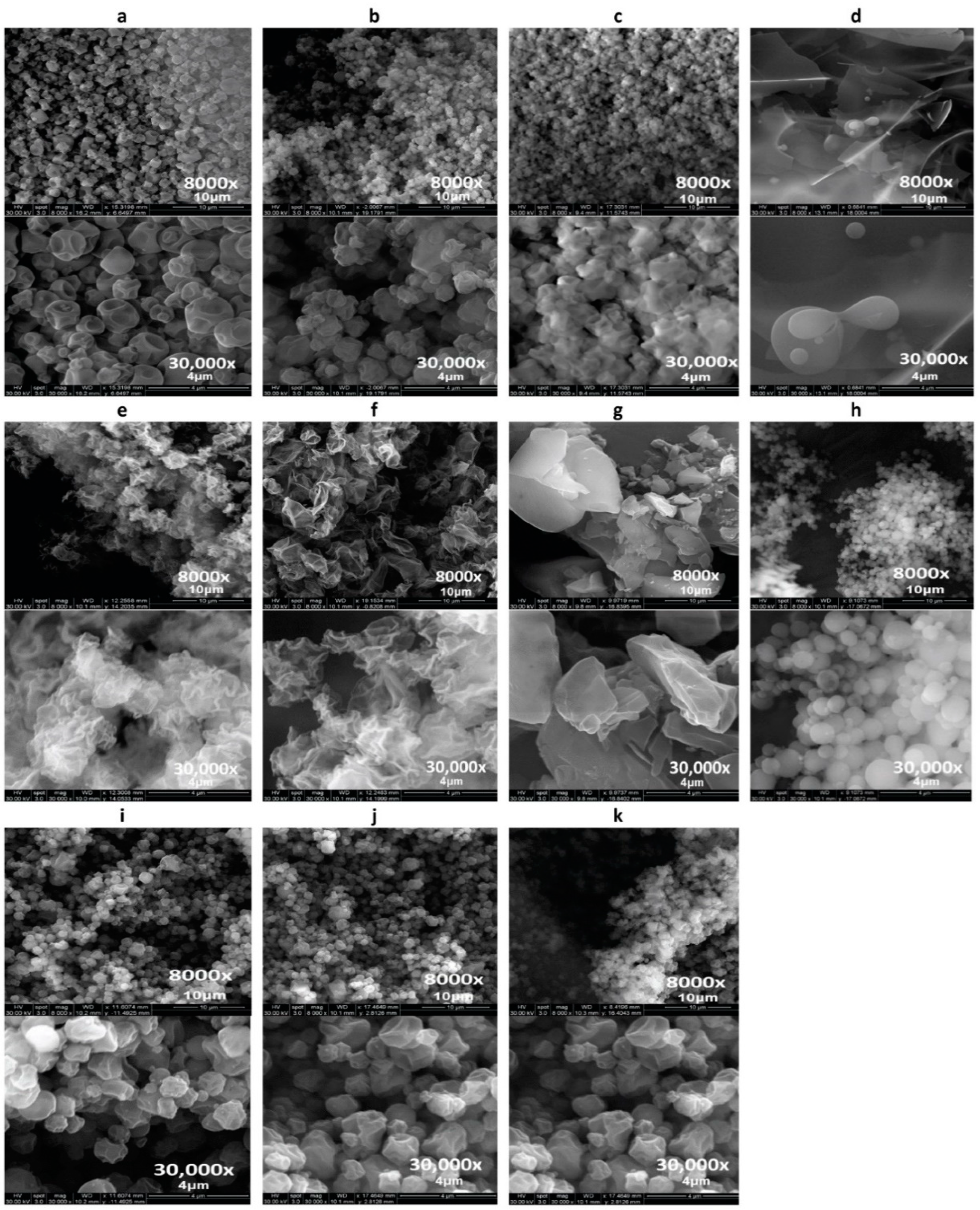
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Particle Sizing and Size Distribution
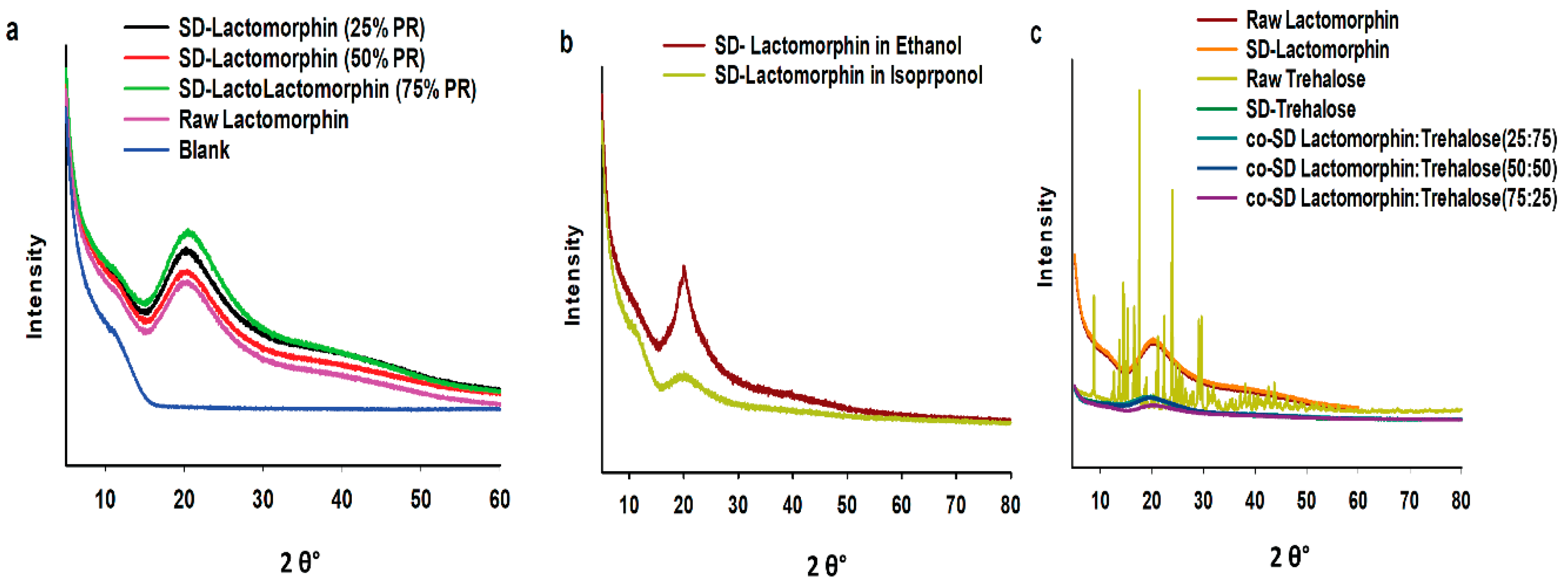
2.2.4. X-ray Powder Diffraction (XRPD)
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Hot Stage Microscopy (HSM) under Cross-Polarizers
2.2.7. Karl Fisher Titration (KFT)
2.2.8. Confocal Raman Spectroscopy (CRM)
2.2.9. Attenuated Total Reflectance-Fourier-Transform Infrared (ATR-FTIR)
2.2.10. In Vitro Aerosol Dispersion Performance
2.2.11. Solubility Studies
2.2.12. Partitioning Studies
2.2.13. HPLC Analysis for Solubility and LogP Experiments
2.2.14. Thermal Degradation Study of Lactomorphin
2.2.15. In Vitro Cell Culture Assays and Cell Viability Experiments after Treated with Lactomorphin
2.2.16. Computational Predictions of Physicochemical Parameters (PCP) of Lactomorphin
3. Results
3.1. Scanning Electron Microscopy (SEM)
3.2. Particle Sizing and Size Distribution by Image Analysis of SEM Micrographs
3.3. X-ray Powder Diffraction (XRPD)
3.4. Differential Scanning Calorimetry (DSC)
3.5. HSM under Cross-Polarizer Lens
3.6. Karl Fisher Titration (KFT)
3.7. ATR-FTIR Spectroscopy
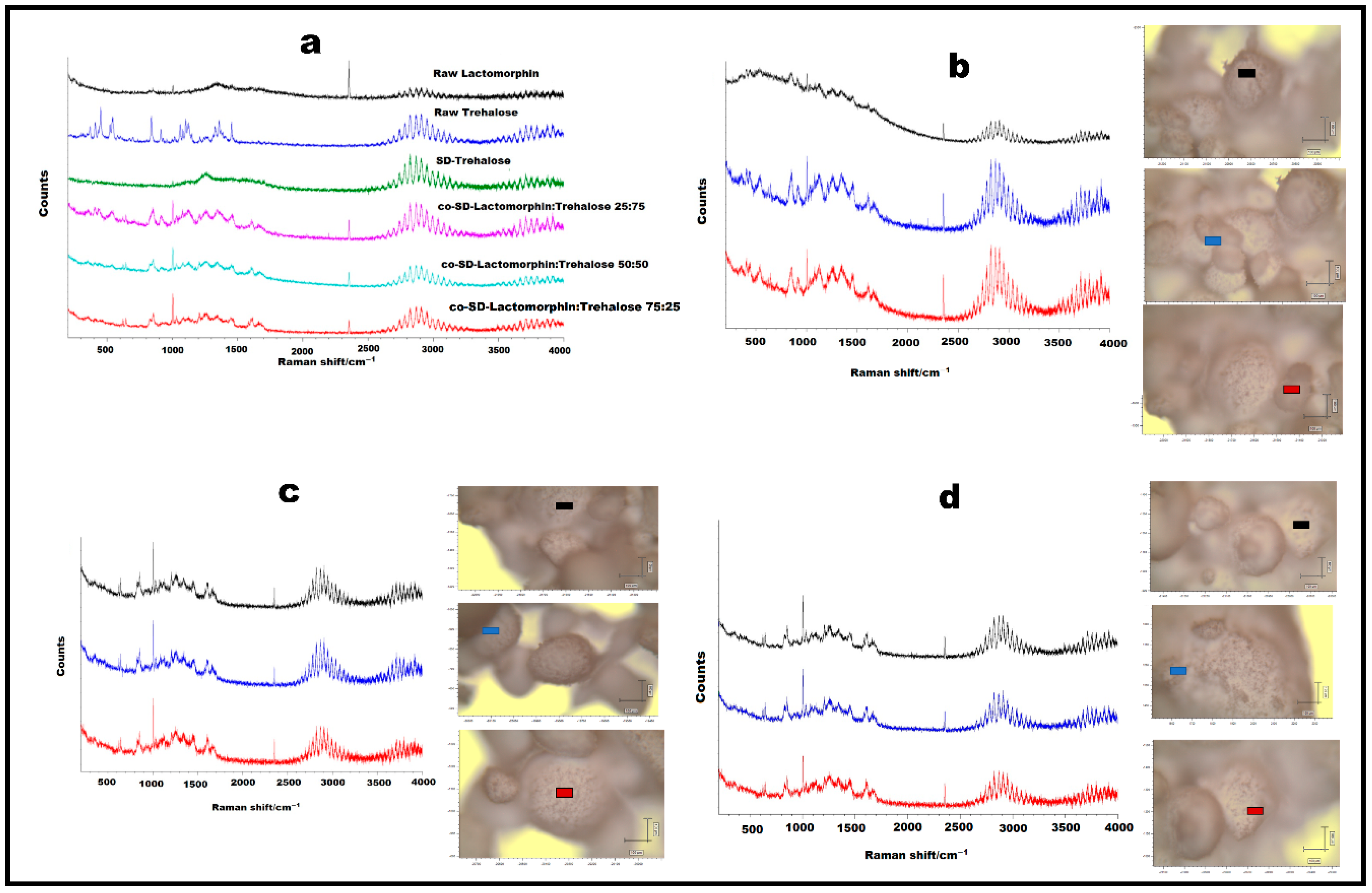
3.8. Confocal Raman Microspectroscopy (CRM)
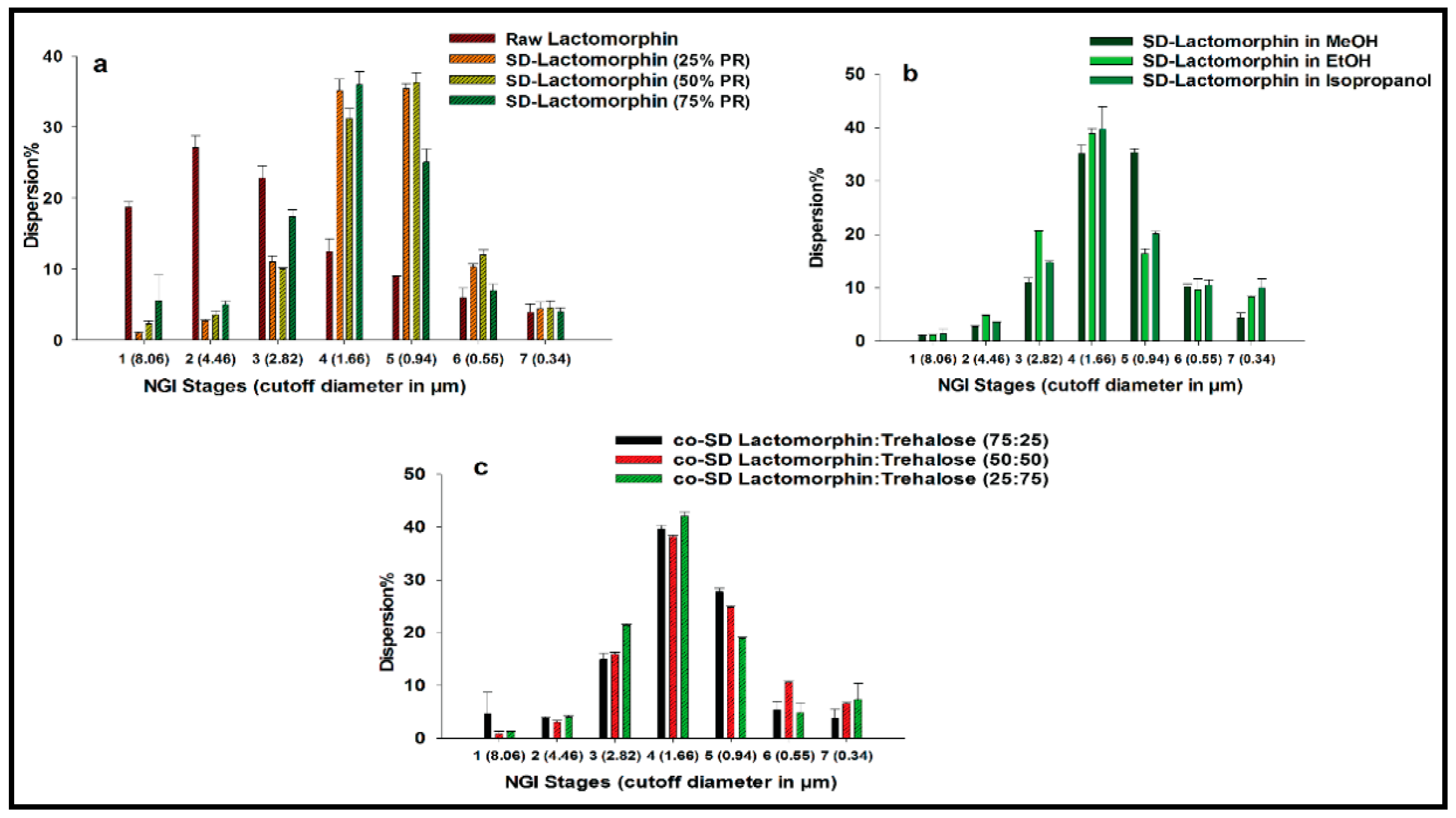
3.9. In Vitro Aerosol Dispersion Performance
3.10. Chromatography
3.11. Solubility
3.12. Partitioning Study (LogP)
3.13. Thermal Degradation Study
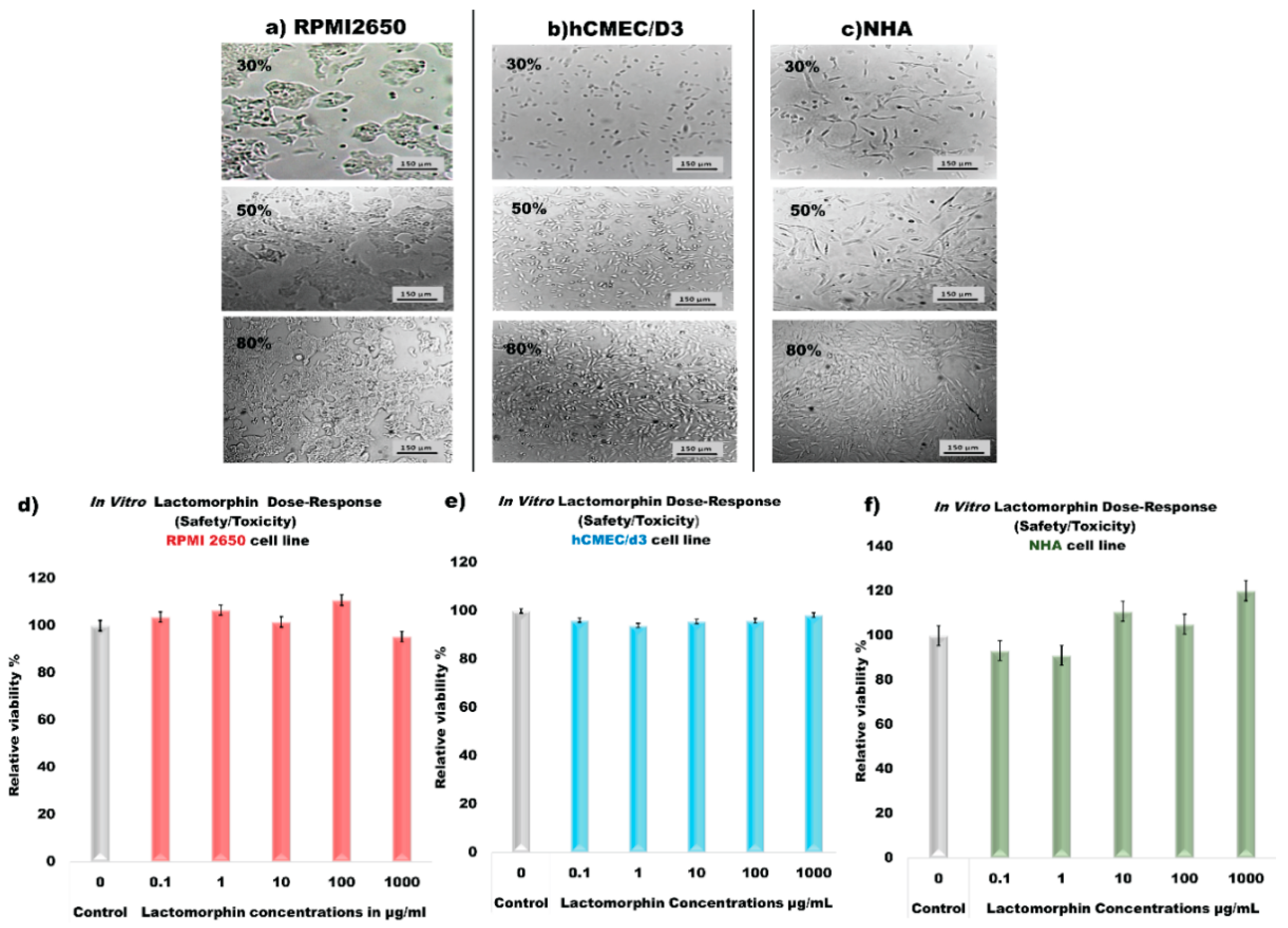
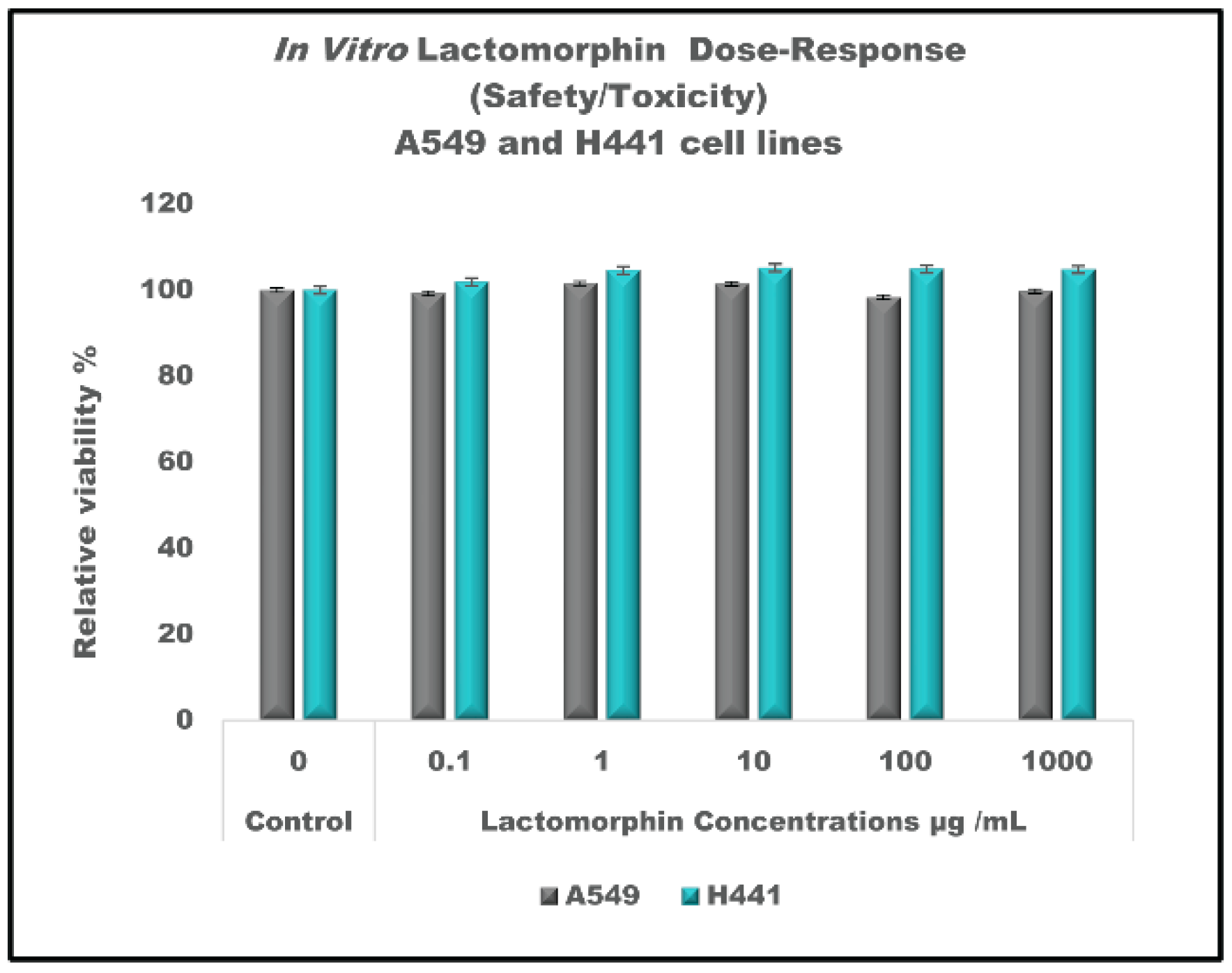
3.14. In Vitro Cell Culture, Development of Cell Monolayer and Cell Viability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lowery, J.J.; Raymond, T.J.; Giuvelis, D.; Bidlack, J.M.; Polt, R.; Bilsky, E.J. In Vivo Characterization of MMP-2200, a Mixed δ/μ Opioid Agonist, in Mice. J. Pharmacol. Exp. Ther. 2011, 336, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Mosberg, I.; Arbor, A. Roles of Mu, Delta and Kappa Oploid Receptors in Spinal and Supraspinal Mediation of Gastrointestinal Transit Effects and Hot-Plate Analgesia in the Mouse. J. Pharmacol. Exp. Ther. 1984, 230, 341–348. [Google Scholar]
- Stevenson, G.W.; Luginbuhl, A.; Dunbar, C.; LaVigne, J.; Dutra, J.; Atherton, P.; Bell, B.; Cone, K.; Giuvelis, D.; Polt, R.; et al. The mixed-action delta/mu opioid agonist MMP-2200 does not produce conditioned place preference but does maintain drug self-administration in rats, and induces in vitro markers of tolerance and dependence. Pharmacol. Biochem. Behavior 2015, 132, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Elmagbari, N.O. Antinociceptive Structure-Activity Studies with Enkephalin-Based Opioid Glycopeptides. J. Pharmacol. Exp. Ther. 2004, 311, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.K.; Nema, R.K. A Review on Novel Techniques for Drug Delivery to the Brain. Curr. Res. Pharm. Sci. 2012, 3, 134–141. [Google Scholar]
- Hawkins, B.T. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Eixarch, H.; Haltner-Ukomadu, E.; Beisswenger, C.; Bock, U. Drug delivery to the lung: Permeability and physicochemical characteristics of drugs as the basis for a pulmonary biopharmaceutical classification system (pBCS). J. Epithel. Biol. Pharmacol. 2010, 3, 1–14. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Hickey, J. Targeting Inhaled Therapy beyond the Lungs. Respiration 2014, 88, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef]
- Currier, G.; Walsh, P. Safety and efficacy review of inhaled loxapine for treatment of agitation. Clin. Schizophr. Relat. Psychoses 2013, 7, 25–32. [Google Scholar] [CrossRef]
- Alexander-Williams, J.M.; Rowbotham, D.J. Novel routes of opioid administration. Br. J. Anaesth. 1998, 81, 3–7. [Google Scholar] [CrossRef]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules Formation by Nano Spray Drying; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128094365. [Google Scholar]
- Duan, J.; Vogt, F.G.; Li, X.; Hayes, D.; Mansour, H.M. Design, characterization, and aerosolization of organic solution advanced spray-dried moxifloxacin and ofloxacin dipalmitoylphosphatidylcholine (DPPC) microparticulate/nanoparticulate powders for pulmonary inhalation aerosol delivery. Int. J. Nanomed. 2013, 8, 3489–3505. [Google Scholar] [CrossRef]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Design, Characterization, and Aerosol Dispersion Performance Modeling of Advanced Spray-Dried Microparticulate/Nanoparticulate Mannitol Powders for Targeted Pulmonary Delivery as Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 81–93. [Google Scholar] [CrossRef]
- Park, C.W.; Li, X.; Vogt, F.G.; Hayes, D.; Zwischenberger, J.B.; Park, E.S.; Mansour, H.M. Advanced spray-dried design, physicochemical characterization, and aerosol dispersion performance of vancomycin and clarithromycin multifunctional controlled release particles for targeted respiratory delivery as dry powder inhalation aerosols. Int. J. Pharm. 2013, 455, 374–392. [Google Scholar] [CrossRef]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. AAPS PharmSciTech 2014, 15, 1574–1587. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Hayes, D.; Mansour, H.M. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried cyclosporine A multifunctional particles for dry powder inhalation aerosol delivery. Int. J. Nanomed. 2013, 8, 1269–1283. [Google Scholar] [CrossRef]
- Muralidharan, P.; Hayes, D.; Black, S.M.; Mansour, H.M. Microparticulate/nanoparticulate powders of a novel Nrf2 activator and an aerosol performance enhancer for pulmonary delivery targeting the lung Nrf2/Keap-1 pathway. Mol. Syst. Des. Eng. 2016, 1, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Meenach, S.A.; Vogt, F.G.; Anderson, K.W.; Zach Hilt, J.; McGarry, R.C.; Mansour, H.M. Design, Physicochemical Characterization, and Optimization of Organic Solution Advanced Spray-Dried Inhalable Dipalmitoylphosphatidylcholine (DPPC) and Dipalmitoylphosphatidylethanolamine Poly(Ethylene Glycol) (DPPE-PEG) Microparticles and Nanoparticles for Targeted Respiratory Nanomedicine Delivery as Dry Powder Inhalation Aerosols. Int. J. Nanomed. 2013, 8, 275–293. [Google Scholar] [CrossRef]
- Wu, X.; Hayes, D.; Zwischenberger, J.B.; Kuhn, R.J.; Mansour, H.M. Design and physicochemical characterization of advanced spray-dried tacrolimus multifunctional particles for inhalation. Drug Des. Devel. Ther. 2013, 7, 59–72. [Google Scholar]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Design, Characterization, and Aerosol Dispersion Performance Modeling of Advanced Co-Spray Dried Antibiotics with Mannitol as Respirable Microparticles / Nanoparticles for Targeted Pulmonary Delivery as Dry Powder Inhalers. J. Pharm. Sci. 2014, 103, 2937–2949. [Google Scholar] [CrossRef]
- Baka, E.; Comer, J.E.A.; Takács-Novák, K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Glomme, A.; März, J.; Dressman, J.B. Comparison of a miniaturized shake-flask solubility method with automated potentiometric acid/base titrations and calculated solubilities. J. Pharm. Sci. 2005, 94, 1–16. [Google Scholar] [CrossRef]
- Apley, M.; Crist, G.B.; Fellner, V.; Gonzalez, M.A.; Hunter, R.P.; Martinez, M.N.; Messenheimer, J.R.; Modric, S.; Papich, M.G.; Parr, A.F.; et al. Determination of thermodynamic solubility of active pharmaceutical ingredients for veterinary species: A new USP general chapter. Dissolut. Technol. 2017, 24, 36–39. [Google Scholar] [CrossRef]
- Bergström, C.A.S.; Norinder, U.; Luthman, K.; Artursson, P. Experimental and computational screening models for prediction of aqueous drug solubility. Pharm. Res. 2002, 19, 182–188. [Google Scholar] [CrossRef]
- Tan, H.; Semin, D.; Wacker, M.; Cheetham, J. An automated screening assay for determination of aqueous equilibrium solubility enabling SPR study during drug lead optimization. J. Assoc. Lab. Autom. 2005, 10, 364–373. [Google Scholar] [CrossRef]
- Işık, M.; Levorse, D.; Mobley, D.L.; Rhodes, T.; Chodera, J.D. Octanol-water partition coe ffi cient measurements for the SAMPL6 Blind Prediction Challenge. bioRxiv 2019. [Google Scholar] [CrossRef]
- Wenlock, M.C.; Potter, T.; Barton, P.; Austin, R.P. A method for measuring the lipophilicity of compounds in mixtures of 10. J. Biomol. Screen. 2011, 16, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Schönsee, C.D.; Bucheli, T.D. Experimental Determination of Octanol-Water Partition Coefficients of Selected Natural Toxins. J. Chem. Eng. Data 2020, 65, 1946–1953. [Google Scholar] [CrossRef]
- Kempińska, D.; Chmiel, T.; Kot-Wasik, A.; Mróz, A.; Mazerska, Z.; Namieśnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. Trends Anal. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef]
- Chakole, R.D.; Charde, M.S.; Kumar, J.; Welankiwar, A.S. Review: Development of forced degradation studies of drugs. Int. J. Adv. Pharm. 2013, 2, 34–39. [Google Scholar]
- Venkataraman, S.; Manasa, M. Forced degradation studies: Regulatory guidance, characterization of drugs, and their degradation products—A review. Drug Invent. Today 2018, 10, 137–146. [Google Scholar]
- Singh, R.; Rehman, Z. Current trends in forced degradation study for pharmaceutical product development. J. Pharm. Educ. Res. 2012, 3, 54–64. [Google Scholar]
- Sharma, M.K.; Murugesan, M. Forced Degradation Study an Essential Approach to Develop Stability Indicating Method. J. Chromatogr. Sep. Tech. 2017, 08, 10–12. [Google Scholar] [CrossRef]
- Hasin, F. Forced Degradation and Stability Indicating Studies of Imatinib Tablet. World J. Pharm. Res. 2017, 6, 55–61. [Google Scholar] [CrossRef]
- Prekodravac, B.; Damm, M.; Kappe, C.O. Microwave-assisted forced degradation using high-throughput microtiter platforms. J. Pharm. Biomed. Anal. 2011, 56, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Scc, C. Blood-Brain Barrier hCMEC/D3 Cell Line. 2011, Volume 19. Available online: https://www.emdmillipore.com/US/en/product/Blood-Brain-Barrier-hCMEC-D3-Cell-Line,MM_NF-SCC066#documentation (accessed on 24 December 2020).
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Bénardais, K.; Pul, R.; Singh, V.; Skripuletz, T.; Lee, D.H.; Linker, R.A.; Gudi, V.; Stangel, M. Effects of fumaric acid esters on blood-brain barrier tight junction proteins. Neurosci. Lett. 2013, 555, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Sajja, R.K.; Prasad, S.; Cucullo, L. Impact of altered glycaemia on blood-brain barrier endothelium: An in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS 2014, 11, 1–14. [Google Scholar] [CrossRef]
- Laboratories, B. CloneticsTM Normal Human Astrocytes. 2011, pp. 1–4. Available online: https://bioscience.lonza.com/lonza_bs/CH/en/download/product/asset/29377 (accessed on 24 December 2020).
- Li, X.; Mansour, H.M. Physicochemical Characterization and Water Vapor Sorption of Organic Solution Advanced Spray-Dried Inhalable Trehalose Microparticles and Nanoparticles for Targeted Dry Powder Pulmonary Inhalation Delivery. AAPS PharmSciTech 2011, 12, 1420–1430. [Google Scholar] [CrossRef]
- Jones, M.D.; Hooton, J.C.; Dawson, M.L.; Ferrie, A.R.; Price, R. Dehydration of trehalose dihydrate at low relative humidity and ambient temperature. Int. J. Pharm. 2006, 313, 87–98. [Google Scholar] [CrossRef]
- Labute, P.; Williams, C. Application of Hückel Theory to Pharmacophore Discovery. CICSJ Bull. 2015, 33, 33. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Matias, A.A.; Poejo, J.; Serra, A.T.; Duarte, C.M.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int. J. Pharm. 2016, 515, 1–10. [Google Scholar] [CrossRef]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Zebraski, S.E.; Kochenash, S.M.; Raffa, R.B. Lung opioid receptors: Pharmacology and possible target for nebulized morphine in dyspnea. Life Sci. 2000, 66, 2221–2231. [Google Scholar] [CrossRef]
- Madar, I.; Bencherif, B.; Lever, J.; Heitmiller, R.F.; Yang, S.C.; Brock, M.; Brahmer, J.; Ravert, H.; Dannals, R.; Frost, J.J. Imaging δ- and μ-opioid receptors by PET in lung carcinoma patients. J. Nucl. Med. 2007, 48, 207–213. [Google Scholar] [PubMed]
- Lechanteur, A.; Evrard, B. Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Lins, R.D.; Chandrasekhar, I.; Freitas, L.C.G.; Hünenberger, P.H. Interaction of the Disaccharide Trehalose with a Phospholipid Bilayer: A Molecular Dynamics Study. Biophys. J. 2004, 86, 2273–2285. [Google Scholar] [CrossRef]
- Ameri, M.; Maa, Y.F. Spray drying of biopharmaceuticals: Stability and process considerations. Dry. Technol. 2006, 24, 763–768. [Google Scholar] [CrossRef]
- Xi, J.; Si, X.A.; Gaide, R. Electrophoretic particle guidance significantly enhances olfactory drug delivery: A feasibility study. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Garcia, G.J.M.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Dong, J.; Shang, Y.; Inthavong, K.; Chan, H.K.; Tu, J. Numerical Comparison of Nasal Aerosol Administration Systems for Efficient Nose-to-Brain Drug Delivery. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Special Section on Drug Delivery Technologies-Minireview Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef]
- Williams, R.O.; Carvalho, T.C.; Peters, J.I. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung-A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Büchi Training Papers Spray Drying. Order Code 97758. 2002, pp. 1–19. Available online: https://www.buchi.com/sites/default/files/downloads/Set_3_Training_Papers_Spray_Drying_en_01.pdf (accessed on 24 December 2020).
- Egleton, R.D.; Bilsky, E.J.; Tollin, G.; Dhanasekaran, M.; Lowery, J.; Alves, I.; Davis, P.; Porreca, F.; Yamamura, H.I.; Yeomans, L.; et al. Biousian glycopeptides penetrate the blood-brain barrier. Tetrahedron Asymmetry 2005, 16, 65–75. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Palian, M.M.; Alves, I.; Yeomans, L.; Keyari, C.M.; Davis, P.; Bilsky, E.J.; Egleton, R.D.; Yamamura, H.I.; Jacobsen, N.E.; et al. Glycopeptides related to β-endorphin adopt helical amphipathic conformations in the presence of lipid bilayers. J. Am. Chem. Soc. 2005, 127, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; St. Louis, L.; Knapp, B.I.; Muthu, D.; Anglin, B.; Giuvelis, D.; Bidlack, J.M.; Bilsky, E.J.; Polt, R. Can amphipathic helices influence the CNS antinociceptive activity of glycopeptides related to β-endorphin? J. Med. Chem. 2014, 57, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Apostol, C.R.; Hay, M.; Polt, R. Glycopeptide drugs: A pharmacological dimension between “Small Molecules” and “Biologics”. Peptides 2020, 131. [Google Scholar] [CrossRef]
- Bai, S.; Yang, T.; Abbruscato, T.J.; Ahsan, F. Evaluation of human nasal RPMI 2650 cells grown at an air-liquid interface as a model for nasal drug transport studies. J. Pharm. Sci. 2008, 97, 1165–1178. [Google Scholar] [CrossRef]
- Pozzoli, M.; Ong, H.X.; Morgan, L.; Sukkar, M.; Traini, D.; Young, P.M.; Sonvico, F. ucApplication of RPMI 2650 nasal cell model to a 3D printed apparatus for the testing of drug deposition and permeation of nasal prodts. Eur. J. Pharm. Biopharm. 2016, 107, 223–233. [Google Scholar] [CrossRef]
- Vu, K.; Weksler, B.; Romero, I.; Couraud, P.O.; Gelli, A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1803–1807. [Google Scholar] [CrossRef]
- Gibbons, H.M.; Dragunow, M. Adult human brain cell culture for neuroscience research. Int. J. Biochem. Cell Biol. 2010, 42, 844–856. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Griebenow, K.; Sola, R.J. Glycosylation of Therapeutic Proteins An Effective Strategy to Optimize Efficacy. BioDrugs 2010, 24, 9–21. [Google Scholar]



| Formulations (Powder Composition = Molar Ratio) | Solvent | Outlet Temperature (°C) | Pump Rate (%) |
|---|---|---|---|
| SD-Lactomorphin | Methanol | - | High (100%) * |
| SD-Lactomorphin | Methanol | 31 | High (75%) |
| SD-Lactomorphin | Methanol | 47 | Med (50%) |
| SD-Lactomorphin | Methanol | 76 | Low (25%) |
| SD-Lactomorphin | Ethanol | 52 | Low (25%) |
| SD-Lactomorphin | Isopropanol | 51 | Low (25%) |
| co-SD-Lactomorphin:Trehalose = 75:25 | Methanol | 52 | Low (25%) |
| co-SD-Lactomorphin:Trehalose = 50:50 | Methanol | 50 | Low (25%) |
| co-SD-Lactomorphin:Trehalose = 25:75 | Methanol | 44 | Low (25%) |
| SD-Trehalose | Methanol | 48 | Low (25%) |
| Powder Composition (Molar Ratio) | Spray Drying Pump Rate (%) | Solvent | Mean Size (μm) | Size Range (μm) |
|---|---|---|---|---|
| Raw Lactomorphin | NA | NA | 1.51 ± 0.71 | 0.07–3.54 |
| Raw Trehalose ⁑ | NA | NA | NA | NA |
| SD-Trehalose | Low (25%) | Methanol | 0.95 ± 0.28 | 0.49–1.80 |
| SD-Lactomorphin | Low (25%) | Methanol | 0.80 ± 0.25 | 0.21–1.60 |
| Co-SD-Lactomorphin:Trehalose 25: 75 | Low (25%) | Methanol | 1.37 ± 0.56 | 0.11–3.42 |
| Co-SD-Lactomorphin:Trehalose 50: 50 | Low (25%) | Methanol | 1.33 ± 0.54 | 0.43–3.86 |
| Co-SD-Lactomorphin:Trehalose 75: 25 | Low (25%) | Methanol | 1.43 ± 0.37 | 0.55–2.37 |
| SD-Lactomorphin * | Low (50%) | Methanol | NA | NA |
| SD-Lactomorphin ⁑ | Low (75%) | Methanol | NA | NA |
| SD-Lactomorphin * | Low (25%) | Ethanol | NA | NA |
| SD-Lactomorphin * | Low (25%) | Isopropanol | NA | NA |
| Sample/Parameter | Raw Lactomorphin | SD-Lactomorphin 75% PR | SD-Lactomorphin 50% PR | SD-Lactomorphin 25% PR | SD-Lactomorphin in EtOH | SD-Lactomorphin in Isop | Raw Trehalose | SD-Trehalose | co-SD-Lactomorphin-Trehalose (25–75) | co-SD-Lactomorphin-Trehalose (50–50) | co-SD-Lactomorphin-Trehalose (75–25) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg onset (°C) | 82.07 ± 1.53 | 82.59 ± 1.25 | 87.03 ± 0.64 | 91.30 ± 2.25 | - | 98.13 ± 9.84 | - | 50.88 ± 5.49 | 70.70 ± 1.35 | 73.62 ± 6.31 | 77.43 ±5.27 |
| Tg mid (°C) | 84.69 ± 0.72 | 87.18 ± 1.47 | 90.20 ± 4.36 | 93.68 ± 0.95 | - | 100.52 ± 9.50 | - | 53.14 ± 3.89 | 73.05 ± 0.63 | 78.29 ± 6.85 | 82.13 ± 6.21 |
| Tg End (°C) | 87.31 ± 0.09 | 89.99 ± 0.62 | 93.46 ± 0.61 | 96.06 ± 2.02 | - | 104.13 ± 11.62 | - | 56.27 ± 1.63 | 79.84 ± 0.71 | 82.92 ± 7.72 | 85.92 ± 7.73 |
| ∆Cp J/(g·°C) | 0.80 ± 0.22 | 0.72 ± 0.23 | 0.70 ± 0.0918 | 0.80 ± 0.15 | - | 1.20 ± 0.88 | - | 0.51 ± 0.01 | 0.43 ± 0.04 | 0.68 ± 0.02 | 0.66 ± 0.09 |
| Endotherm1 onset (°C) | 175.72 ± 5.98 | 170.88 ± 3.63 | 177.66 ± 5.31 | 180.90 ± 0.44 | 182.08 ± 2.12 | 185.54 ± 1.37 | 94.70 ± 0.08 | 143.11 ± 5.78 | 167.96 ± 4.81 | 176.01 ± 1.59 | 174.00 ± 0.89 |
| Endotherm1 peak (°C) | 175.96 ±6.20 | 171.50 ±3.24 | 177.71 ± 5.38 | 183.10 ± 1.24 | 182.26 ± 2.00 | 192.59 ± 8.00 | 95.85 ± 0.12 | 144.91 ± 4.91 | 173.47 ± 11.99 | 176.46 ± 1.74 | 174.39 ± 0.95 |
| ∆H1 (Enthalpy) J/g | 55.43 ± 12.35 | 43.26 ± 10.80 | 40.91 ± 2.61 | 37.25 ± 3.33 | 2.88 ± 0.52 | 13.79 ± 0.95 | 91.92 ± 7.79 | 1.34 ± 0.10 | 29.92 ± 8.67 | 3.68 ± 1.04 | 3.20 ± 0.43 |
| Endotherm2 onset (°C) | - | - | - | - | 233.96 ± 22.56 | 238.41 ± 17.99 | 141.32 ± 1.20 | 187.89 ± 2.16 | 209.6 5± 8.03 | 208.65 ± 15.48 | 214.66 ± 13.72 |
| Endotherm2 peak (°C) | - | - | - | - | 247.19 ± 2.1 | 243.47 ± 14.33 | 141.01 ± 1.14 | 196.89 ± 2.70 | 213.28 ± 7.12 | 210.53 ± 14.30 | 215.33 ± 14.78 |
| ∆H2 (Enthalpy) J/g | - | - | - | - | 35.7 ± 7.08 | 31.57 ± 4.73 | 106.90 ± 6.15 | 56.07 ± 6.21 | 34.02 ± 9.16 | 47.73 ±10.30 | 39.66 ± 8.07 |
| Endotherm3 onset (°C) | - | - | - | - | - | - | 196.54 ± 1.10 | - | - | - | - |
| Endotherm3 peak (°C) | - | - | - | - | - | - | 204.24 ± 1.10 | - | - | - | - |
| ∆H3 (Enthalpy) J/g | - | - | - | - | - | - | 118.33 ± 4.17 | - | - | - | - |
| Exotherm (TC) °C | - | - | - | - | 150.58 ± 2.28 | 166.23 ± 0.46 | - | 110.01 ± 0.95 | - | - | - |
| System | Water % (w/w) |
|---|---|
| Raw Lactomorphin | 6.07 ± 0.67 |
| Raw Trehalose | 8.11 ± 1.96 |
| SD-Trehalose in methanol at 25% PR | 4.76 ± 0.54 |
| SD-Lactomorphin in methanol at 25% PR | 3.38 ± 0.23 |
| SD-Lactomorphin in methanol at 50% PR | 3.96 ± 0.26 |
| SD-Lactomorphin in methanol at 75% PR | 4.63 ± 0.70 |
| SD-Trehalose in ethanol at 25% PR | 9.67 ± 0.71 |
| SD-Trehalose in isopropanol at 25% PR | 4.22 ± 0.15 |
| Co-SD-Lactomorphin:Trehalose 25:75 in methanol at 25% PR | 4.35 ± 0.56 |
| Co-SD-Lactomorphin:Trehalose 50:50 in methanol at 25% PR | 5.04 ± 1.05 |
| Co-SD-Lactomorphin:Trehalose 75:25 in methanol at 25% PR | 5.59 ± 0.38 |
| Powder Formulation Composition | Emitted Dose | Fine Particle Fraction | Respirable Fraction | MMAD | GSD |
|---|---|---|---|---|---|
| (Molar Ratio) | (ED) (%) | (FPF) (%) | (RF) (%) | (μm) | |
| Raw Lactomorphin | 100.33 ± 2.12 | 27.86 ± 0.94 | 81.06 ± 0.57 | 4.29 ± 0.24 | 4.05 ± 0.74 |
| SD-Trehalose in methanol at 25% PR | 88.19 ± 0.40 | 52.00 ± 0.65 | 99.05 ± 0.20 | 1.74 ± 0.04 | 1.83 ± 0.06 |
| SD-Lactomorphin in methanol at 50% PR | 88.98 ± 1.73 | 52.45 ± 7.47 | 97.70 ± 0.43 | 1.66 ± 0.02 | 1.85 ± 0.10 |
| SD-Lactomorphin in methanol at 75% PR | 94.63 ± 0.78 | 46.15 ± 3.20 | 94.47 ± 3.62 | 2.16 ± 0.12 | 2.02 ± 0.01 |
| SD-Trehalose in ethanol at 25% PR | 97.87 ± 15 | 63.09 ± 2.34 | 98.76 ± 0.09 | 1.98 ± 0.07 | 3.04 ± 0.09 |
| SD-Trehalose in isopropanol at 25% PR | 102.29 ± 5.88 | 66.21 ± 5.17 | 98.64 ± 0.90 | 1.64 ± 0.29 | 3.01 ± 0.27 |
| Co-SD-Lactomorphin:Trehalose 25:75 in methanol at 25% PR | 91.24 ± 2.26 | 50.59 ± 3.88 | 98.68 ± 0.00 | 2.21 ± 0.23 | 2.93 ± 0.96 |
| Co-SD Lactomorphin:Trehalose 50:50 in methanol at 25% PR | 88.86 ± 1.13 | 53.07 ± 2.35 | 99.15 ± 0.50 | 1.89 ± 0.04 | 2.31 ± 0.00 |
| Co-SD Lactomorphin:Trehalose 75:25 in methanol at 25% PR | 89.00 ± 0.67 | 45.92 ± 5.53 | 95.33 ± 3.99 | 2.07 ± 0.07 | 1.84 ± 0.17 |
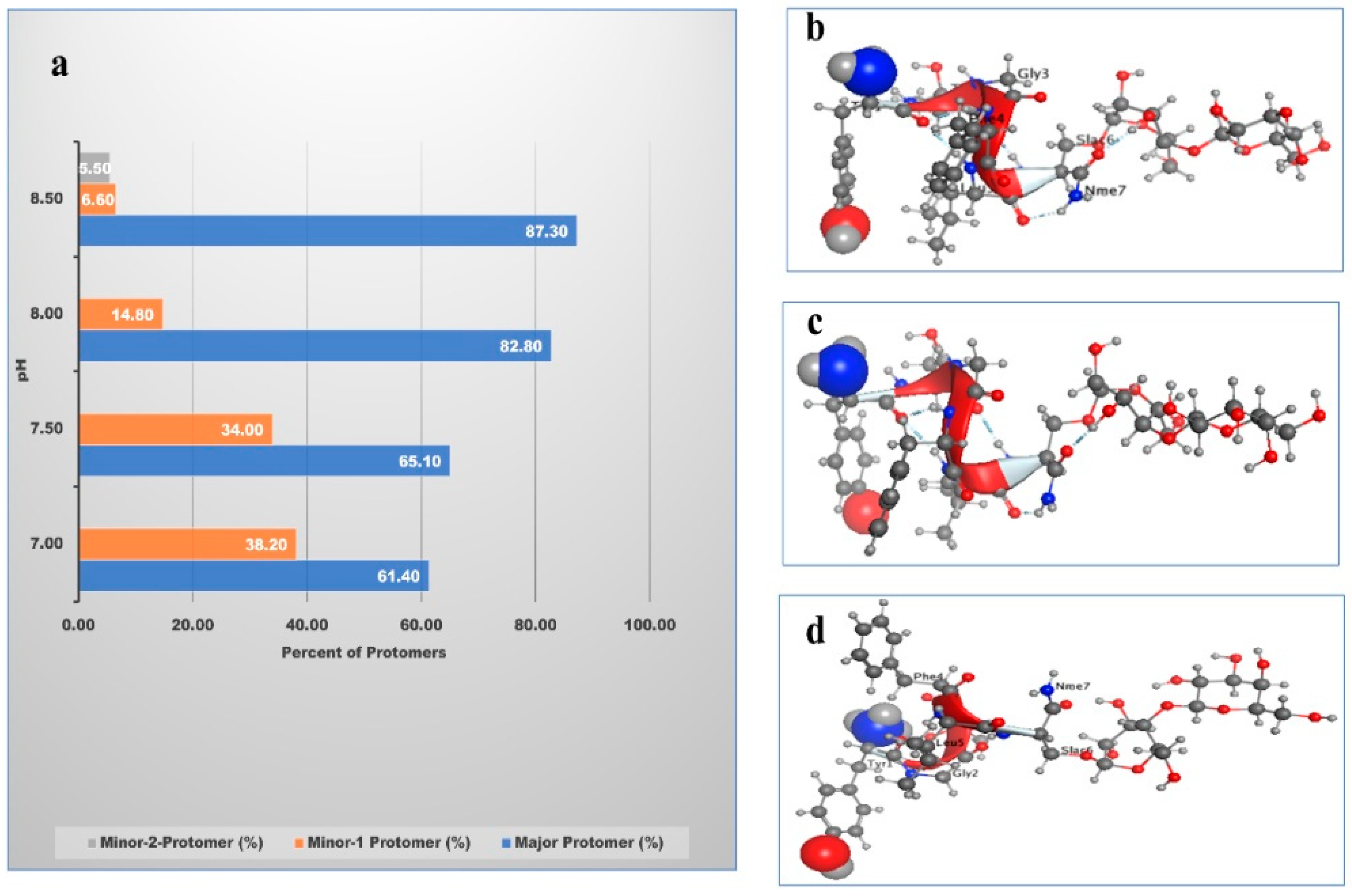
| pH | Major Protomer% | Minor-1 Protomer% | Minor-2 Protomer% |
|---|---|---|---|
| 7.0 | 60.4 | 39.3 | - |
| 7.5 | 66.1 | 33.0 | - |
| 8.0 | 83.4 | 14.3 | - |
| 8.5 | 87.0 | 7.0 | 6.0 |
| 9.0 | 77.3 | 29.4 | - |
| Solubility in: | pH Value | Solubility in mg/mL ± Std dev | Solubility (USP) Definition | Calculated Log S * | MOE Calculated h_logS |
|---|---|---|---|---|---|
| Water | 8.50 (adjusted) | 267.00 ± 6.00 | Freely soluble (Fs) | −0.6 | −0.6565 |
| PBS | 8.50 (adjusted) | 570.33 ± 2.00 | Freely soluble (Fs) | −0.3 | - |
| NS | 8.50 (adjusted) | 140.37 ± 0.32 | Freely soluble (Fs) | −0.9 | - |
| Methanol | 7.27 (measured) | 45.67 ± 1.00 | Soluble (s) | −1.3 | - |
| Ethanol | 7.30 (measured) | 18.00 ± 0.00 | Sparingly soluble (sps) | −1.7 | - |
| Experimental LogP of Lactomorphin at Room Temperature (25 °C) and Physiological Temperature (37 °C) (Mean ± Standard Deviation, n = 3) | Computational LogP of Lactomorphin Protomers Exist at pH 8.5 and at Room Temperature (25 °C) | ||
|---|---|---|---|
| Temperature (°C) | LogP | Protomer | Computational LogP |
| Major Protomer | −2.76 | ||
| 25 °C | −2.40 ± 0.00 | Minor-1 Protomer | −2.67 |
| 37 °C | −2.33 ± 0.02 | Minor-2 Protomer | −7.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabsi, W.; Al-Obeidi, F.A.; Polt, R.; Mansour, H.M. Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. Pharmaceutics 2021, 13, 26. https://doi.org/10.3390/pharmaceutics13010026
Alabsi W, Al-Obeidi FA, Polt R, Mansour HM. Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. Pharmaceutics. 2021; 13(1):26. https://doi.org/10.3390/pharmaceutics13010026
Chicago/Turabian StyleAlabsi, Wafaa, Fahad A. Al-Obeidi, Robin Polt, and Heidi M. Mansour. 2021. "Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies" Pharmaceutics 13, no. 1: 26. https://doi.org/10.3390/pharmaceutics13010026
APA StyleAlabsi, W., Al-Obeidi, F. A., Polt, R., & Mansour, H. M. (2021). Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. Pharmaceutics, 13(1), 26. https://doi.org/10.3390/pharmaceutics13010026






