Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Microcapsules Carrying NGF
2.2. Materials
2.3. Encapsulation Efficiency
2.4. Scanning Electron Microscopy (SEM)
2.5. Primary Nerve Cell Cultures
2.6. Introducing Encapsulated NGF to Neuronal Cultures
2.7. Assessment of Neuronal Morphology and the Neurite Outgrowth Rate
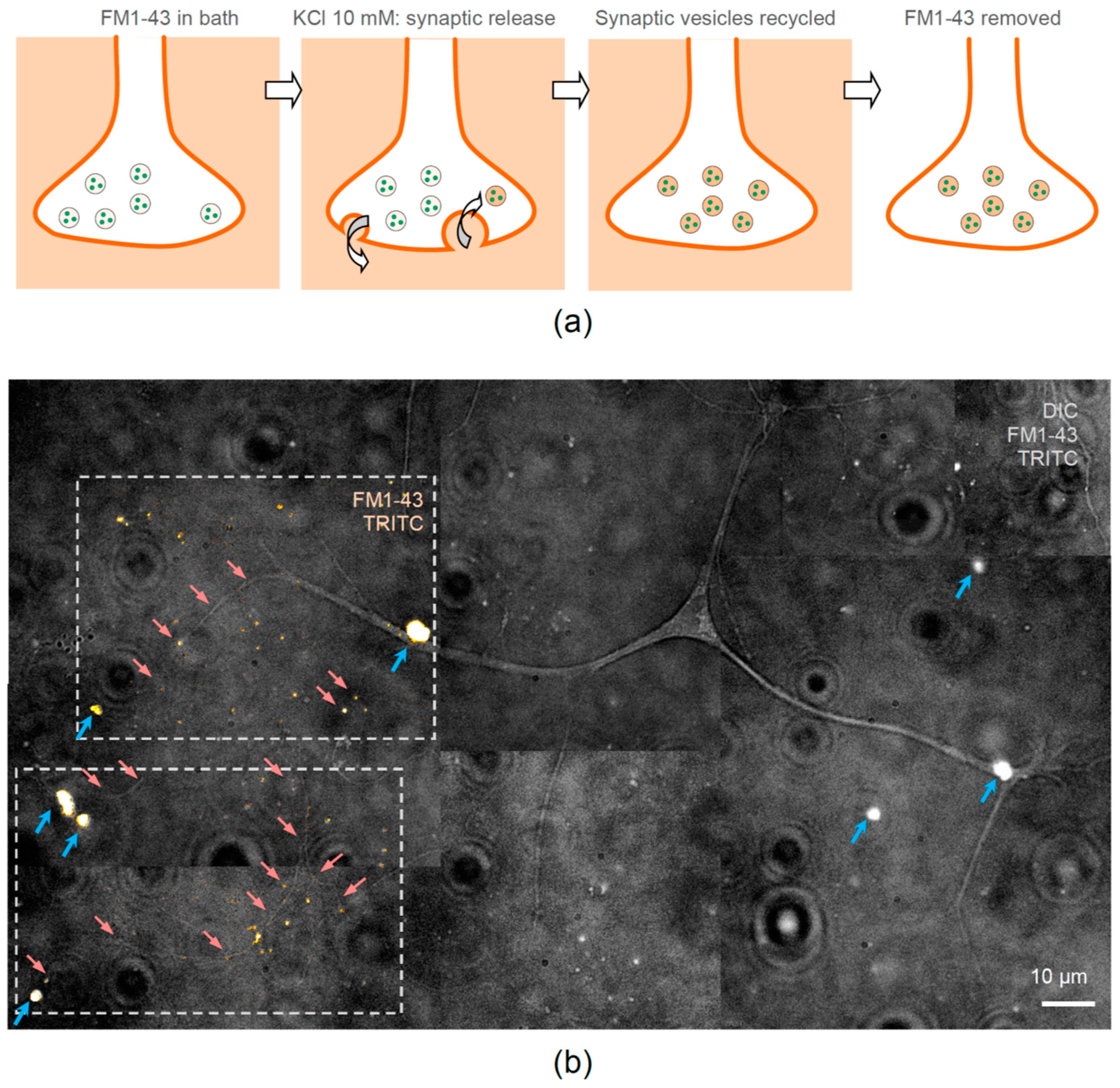
2.8. Visualizing Functional Synapses with FM1-43 Dye
2.9. Statistics
3. Results
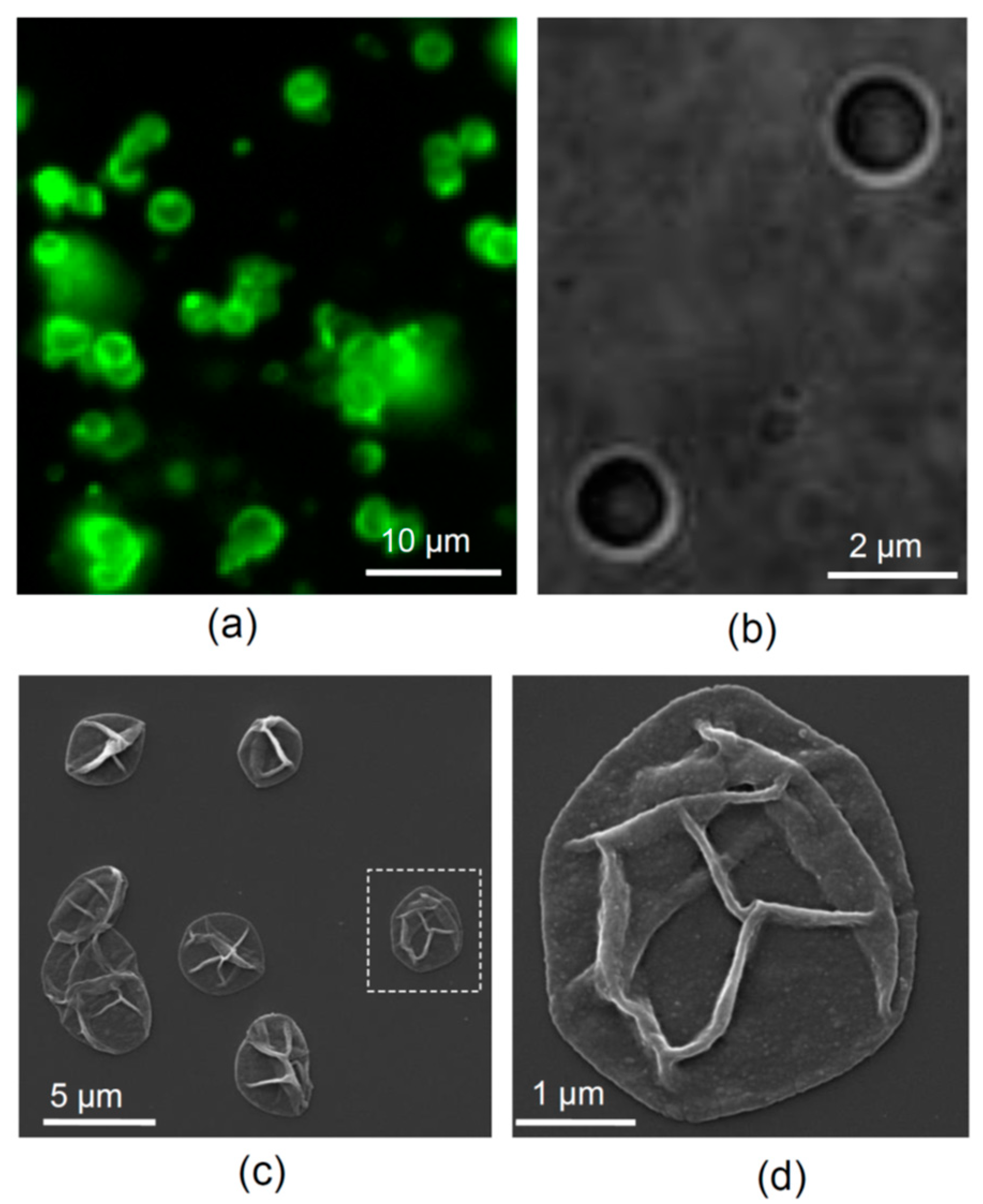
3.1. Microcapsules
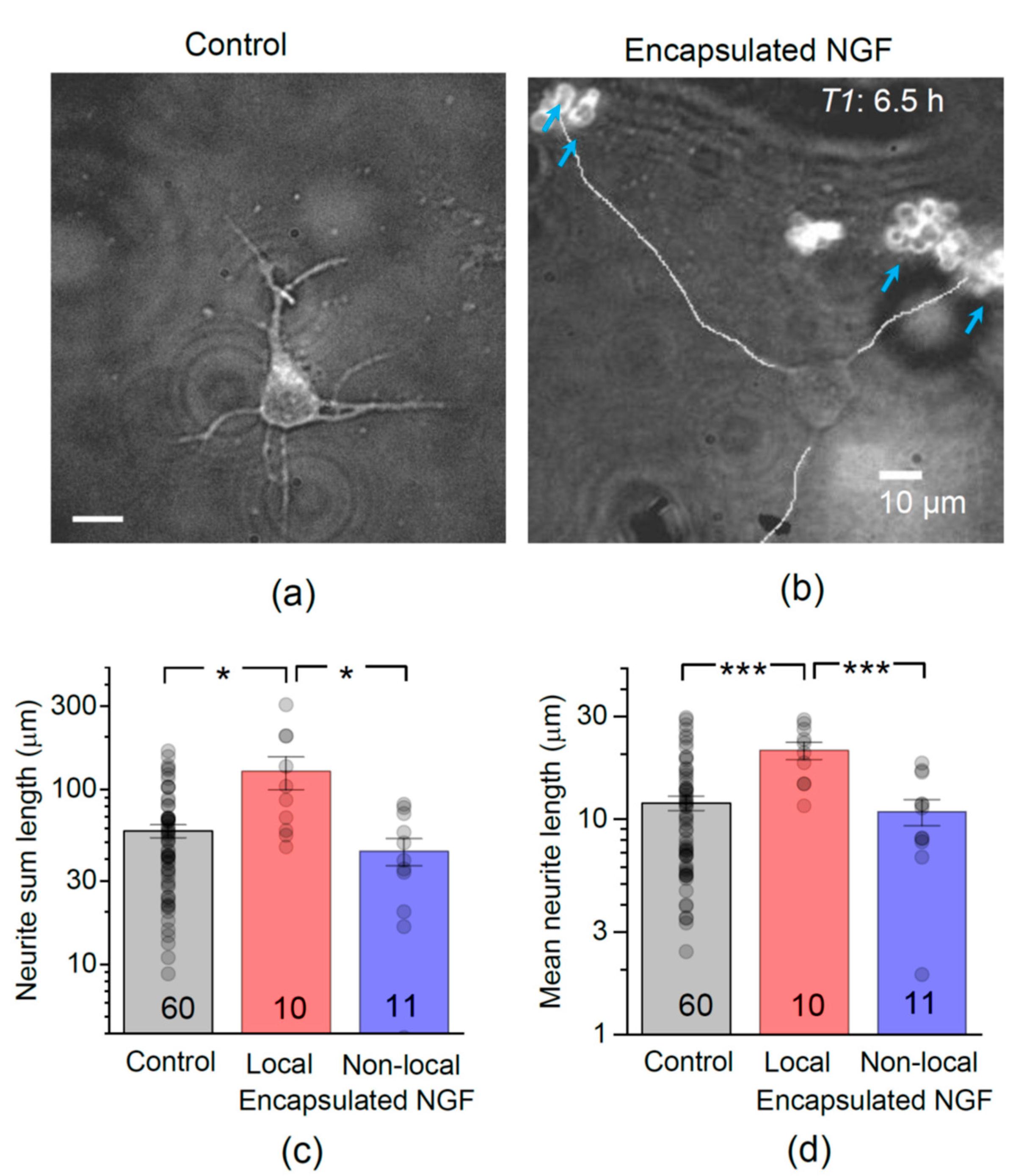
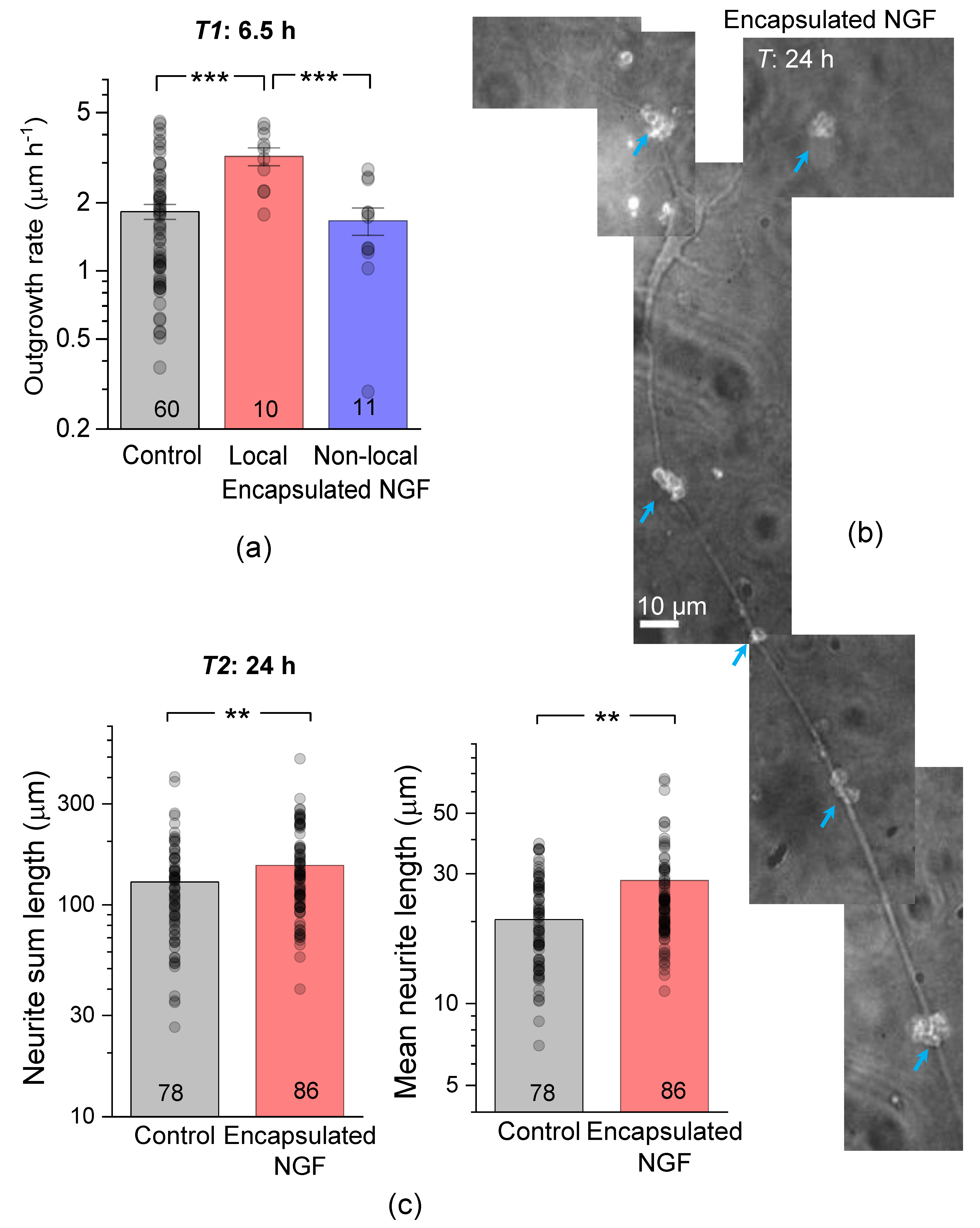
3.2. Encapsulated NGF Boosts the Neurite Outgrowth Rate
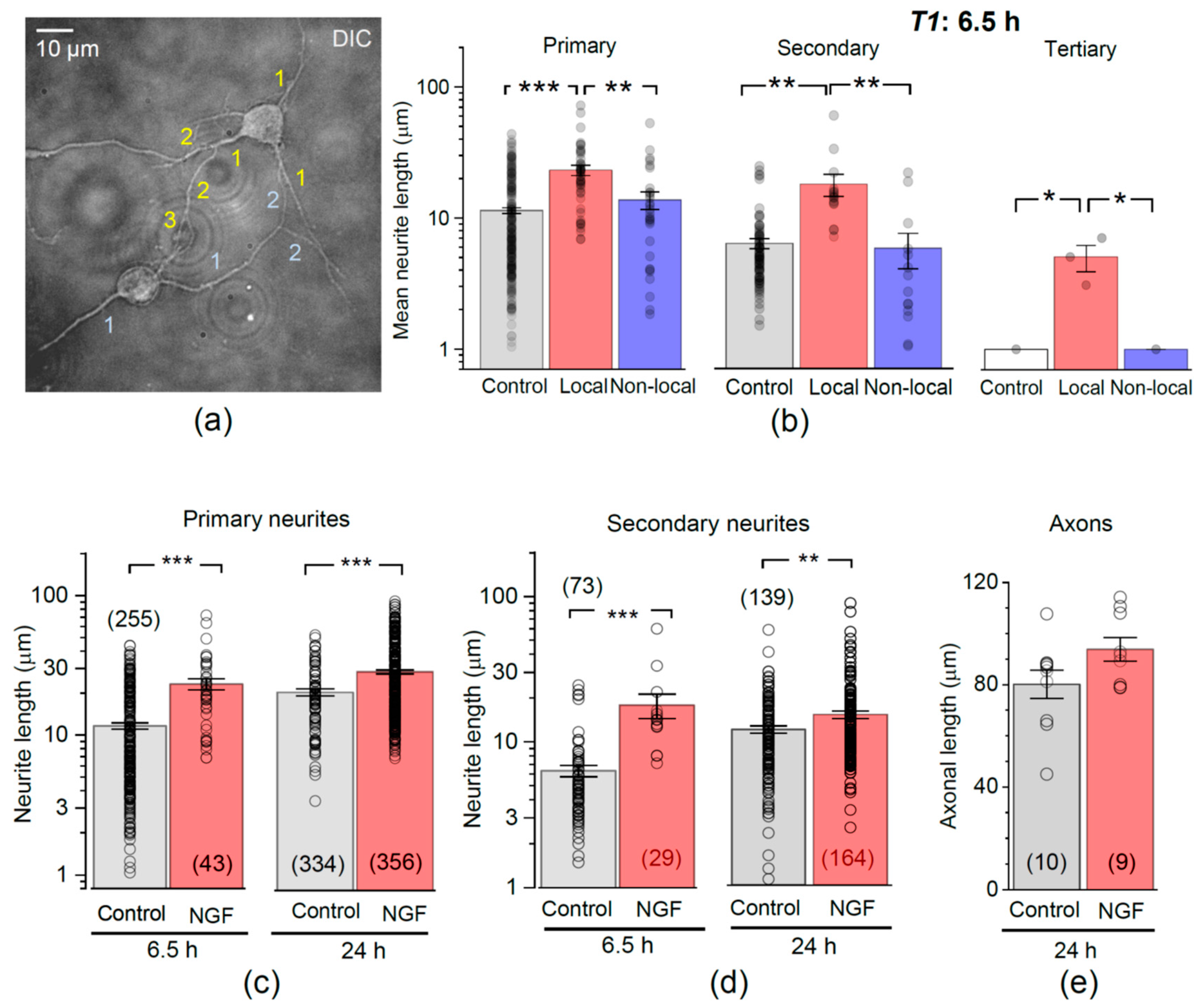
3.3. Encapsulated NGF Enhances Neurite Branching
3.4. Neurons Cultured with Encapsulated NGF Form Functional Synapses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic facto |
| BSA | bovine serum albumin |
| DIC | differential interference contrast microscopy |
| DS | dextran sulfate sodium salt |
| FITC | fluorescein isothiocyanate |
| FM1-43 dye | N-(3-Triethylammoniumpropyl)-4-(4-(Dibutylamino) Styryl) Pyridinium Dibromide |
| GDNF | glial-cell-derived neurotrophic factor |
| iPSC | induced pluripotent stem cells |
| KCl | high-potassium medium |
| LbL | layer-by-layer technique |
| NGF | nerve growth factor |
| Parg | poly-L-arginine |
| PLGA | poly-lactic-co-glycolic-acid |
| ROI | region of interest |
| SEM | scanning electron microscopy |
| s.e.m. | standard error of the mean |
| TRITC | tetramethylrhodamine |
References
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, F.; Alleva, E. The NGF saga: From animal models of psychosocial stress to stress-related psychopathology. Front. Neuroendocrinol. 2009, 30, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Blesch, A. Nerve growth factor: From animal models of cholinergic neuronal degeneration to gene therapy in Alzheimer’s disease. Prog. Brain Res. 2004, 146, 441–449. [Google Scholar] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Mohammadi, R.; Noruzi, S.; Mohamadi, Y.; Azizian, M.; Mousavy, S.M.; Ghasemi, F.; Hesari, A.; Sahebkar, A.; Salarinia, R.; et al. Stem cell- and gene-based therapies as potential candidates in Alzheimer’s therapy. J. Cell. Biochem. 2018, 119, 8723–8736. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; U, H.S.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Yang, J.H.; Barba, D.; U, H.S.; Bakay, R.A.; Pay, M.M.; Masliah, E.; Conner, J.M.; Kobalka, P.; Roy, S.; et al. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol. 2015, 72, 1139–1147. [Google Scholar] [CrossRef]
- Lindvall, O.; Wahlberg, L.U. Encapsulated cell biodelivery of GDNF: A novel clinical strategy for neuroprotection and neuroregeneration in Parkinson’s disease? Exp. Neurol. 2008, 209, 82–88. [Google Scholar] [CrossRef]
- Winn, S.R.; Lindner, M.D.; Lee, A.; Haggett, G.; Francis, J.M.; Emerich, D.F. Polymer-encapsulated genetically modified cells continue to secrete human nerve growth factor for over one year in rat ventricles: Behavioral and anatomical consequences. Exp. Neurol. 1996, 140, 126–138. [Google Scholar] [CrossRef]
- Fjord-Larsen, L.; Kusk, P.; Emerich, D.F.; Thanos, C.; Torp, M.; Bintz, B.; Tornoe, J.; Johnsen, A.H.; Wahlberg, L.U. Increased encapsulated cell biodelivery of nerve growth factor in the brain by transposon-mediated gene transfer. Gene Ther. 2012, 19, 1010–1017. [Google Scholar] [CrossRef][Green Version]
- He, H.; Luedke, E.; Zhang, X.; Yu, B.; Schmitt, A.; McClarren, B.; Grignol, V.; Carson, W.E., 3rd; Lee, L.J. A naonoporous cell-therapy device with controllable biodegradation for long-term drug release. J. Control. Release 2013, 165, 226–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wahlberg, L.U.; Lind, G.; Almqvist, P.M.; Kusk, P.; Tornoe, J.; Juliusson, B.; Soderman, M.; Sellden, E.; Seiger, A.; Eriksdotter-Jonhagen, M.; et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: A technology platform for restorative neurosurgery. J. Neurosurg. 2012, 117, 340–347. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.; Knight, A.M.; Spinner, R.J.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J. In vitro and in vivo release of nerve growth factor from biodegradable poly-lactic-co-glycolic-acid microspheres. J. Biomed. Mater. Res. A 2010, 95, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Menei, P.; Pean, J.M.; Nerriere-Daguin, V.; Jollivet, C.; Brachet, P.; Benoit, J.P. Intracerebral implantation of NGF-releasing biodegradable microspheres protects striatum against excitotoxic damage. Exp. Neurol. 2000, 161, 259–272. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.; Borntraeger, A.; Knight, A.M.; Hebert-Blouin, M.N.; Spinner, R.J.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J. Short- and long-term peripheral nerve regeneration using a poly-lactic-co-glycolic-acid scaffold containing nerve growth factor and glial cell line-derived neurotrophic factor releasing microspheres. J. Biomed. Mater. Res. Part A 2012, 100, 2139–2146. [Google Scholar] [CrossRef]
- Santos, D.; Giudetti, G.; Micera, S.; Navarro, X.; del Valle, J. Focal release of neurotrophic factors by biodegradable microspheres enhance motor and sensory axonal regeneration in vitro and in vivo. Brain Res. 2016, 1636, 93–106. [Google Scholar] [CrossRef]
- Mili, B.; Das, K.; Kumar, A.; Saxena, A.C.; Singh, P.; Ghosh, S.; Bag, S. Preparation of NGF encapsulated chitosan nanoparticles and its evaluation on neuronal differentiation potentiality of canine mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2017, 29, 4. [Google Scholar] [CrossRef]
- Rajam, M.; Pulavendran, S.; Rose, C.; Mandal, A.B. Chitosan nanoparticles as a dual growth factor delivery system for tissue engineering applications. Int. J. Pharm. 2011, 410, 145–152. [Google Scholar] [CrossRef]
- Habraken, W.J.; Boerman, O.C.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J. Biomed. Mater. Res. A 2009, 91, 614–622. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hwang, J.Y.; Shin, U.S. Preparation of nano/macroporous polycaprolactone microspheres for an injectable cell delivery system using room temperature ionic liquid and camphene. J. Colloid Interface Sci. 2016, 465, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kraskiewicz, H.; Breen, B.; Sargeant, T.; McMahon, S.; Pandit, A. Assembly of protein-based hollow spheres encapsulating a therapeutic factor. ACS Chem. Neurosci. 2013, 4, 1297–1304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- She, Z.; Antipina, M.N.; Li, J.; Sukhorukov, G.B. Mechanism of protein release from polyelectrolyte multilayer microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Zheng, K.; Dong, L.; Sapelkin, A.; Voitenko, N.; Sukhorukov, G.B.; Rusakov, D.A. Nano-engineered microcapsules boost the treatment of persistent pain. Drug Deliv. 2018, 25, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Epifanovskaya, O.S.; Shakirova, A.I.; Mock, U.; Riecken, K.; Okilova, M.V.; Sergeev, V.S.; Afanasyev, B.V.; et al. Efficient gene editing via non-viral delivery of CRISPR-Cas9 system using polymeric and hybrid microcarriers. Nanomedicine 2018, 14, 97–108. [Google Scholar] [CrossRef]
- Balabushevitch, N.G.; Sukhorukov, G.B.; Moroz, N.A.; Volodkin, D.V.; Larionova, N.I.; Donath, E.; Mohwald, H. Encapsulation of proteins by layer-by-layer adsorption of polyelectrolytes onto protein aggregates: Factors regulating the protein release. Biotechnol. Bioeng. 2001, 76, 207–213. [Google Scholar] [CrossRef]
- Shenoy, D.B.; Antipov, A.A.; Sukhorukov, G.B.; Möhwald, H. Layer-by-layer engineering of biocompatible, decomposable core-shell structures. Biomacromolecules 2003, 4, 265–272. [Google Scholar] [CrossRef]
- Stuart, M.A.; Huck, W.T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Mohwald, H. Multifunctional cargo systems for biotechnology. Trends Biotechnol. 2007, 25, 93–98. [Google Scholar] [CrossRef]
- Zhu, X.H.; Wang, C.H.; Tong, Y.W. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res. A 2009, 89, 411–423. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Tian, L.; He, X.; Gao, Q.; Wu, T.; Ramakrishna, S.; Zheng, J.; Mo, X. Evaluation of the potential of rhTGF- beta3 encapsulated P(LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton’s jelly of human umbilical cord. Mater. Sci Eng. C Mater. Biol. Appl. 2017, 70, 637–645. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Wang, C.; Li, J.; Sukhorukov, G.B.; Antipina, M.N. Encapsulation of basic fibroblast growth factor by polyelectrolyte multilayer microcapsules and its controlled release for enhancing cell proliferation. Biomacromolecules 2012, 13, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef]
- Henneberger, C.; Bard, L.; Panatier, A.; Reynolds, J.P.; Kopach, O.; Medvedev, N.I.; Minge, D.; Herde, M.K.; Anders, S.; Kraev, I.; et al. LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron 2020, 108, 919–936.e911. [Google Scholar] [CrossRef]
- Jensen, T.P.; Zheng, K.Y.; Cole, N.; Marvin, J.S.; Looger, L.L.; Rusakov, D.A. Multiplex imaging relates quantal glutamate release to presynaptic Ca2+ homeostasis at multiple synapses in situ. Nat. Commun. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Craig, A.M.; Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 1994, 17, 267–310. [Google Scholar] [CrossRef]
- Ermolyuk, Y.S.; Alder, F.G.; Henneberger, C.; Rusakov, D.A.; Kullmann, D.M.; Volynski, K.E. Independent regulation of basal neurotransmitter release efficacy by variable Ca2+ influx and bouton size at small central synapses. PLoS Biol. 2012, 10, e1001396. [Google Scholar] [CrossRef]
- Gaffield, M.A.; Betz, W.J. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat. Protoc. 2006, 1, 2916–2921. [Google Scholar] [CrossRef]
- Jareb, M.; Banker, G. Inhibition of axonal growth by brefeldin A in hippocampal neurons in culture. J. Neurosci. 1997, 17, 8955–8963. [Google Scholar] [CrossRef] [PubMed]
- Brann, A.B.; Scott, R.; Neuberger, Y.; Abulafia, D.; Boldin, S.; Fainzilber, M.; Futerman, A.H. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 8199–8206. [Google Scholar] [CrossRef]
- Novak, P.; Gorelik, J.; Vivekananda, U.; Shevchuk, A.I.; Ermolyuk, Y.S.; Bailey, R.J.; Bushby, A.J.; Moss, G.W.; Rusakov, D.A.; Klenerman, D.; et al. Nanoscale-targeted patch-clamp recordings of functional presynaptic ion channels. Neuron 2013, 79, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Kupikowska-Stobba, B.; Lewinska, D. Polymer microcapsules and microbeads as cell carriers for in vivo biomedical applications. Biomater. Sci. 2020, 8, 1536–1574. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Sindeeva, O.A.; Lomova, M.V.; Gusliakov, O.I.; Tarakanchikova, Y.V.; Tyutyaev, E.V.; Pinyaev, S.I.; Kulikov, O.A.; German, S.V.; Pyataev, N.A.; et al. Endovascular addressing improves the effectiveness of magnetic targeting of drug carrier. Comparison with the conventional administration method. Nanomed. Nanotechnol. Biol. Med. 2020, 102184. [Google Scholar] [CrossRef]
- Navolokin, N.A.; German, S.V.; Bucharskaya, A.B.; Godage, O.S.; Zuev, V.V.; Maslyakova, G.N.; Pyataev, N.A.; Zamyshliaev, P.S.; Zharkov, M.N.; Terentyuk, G.S.; et al. Systemic Administration of Polyelectrolyte Microcapsules: Where Do They Accumulate and When? In Vivo and Ex Vivo Study. Nanomaterials 2018, 8, 812. [Google Scholar] [CrossRef]
- Novoselova, M.V.; German, S.V.; Sindeeva, O.A.; Kulikov, O.A.; Minaeva, O.V.; Brodovskaya, E.P.; Ageev, V.P.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; et al. Submicron-Sized Nanocomposite Magnetic-Sensitive Carriers: Controllable Organ Distribution and Biological Effects. Polymers 2019, 11, 1082. [Google Scholar] [CrossRef]
- Pavlov, A.M.; Sapelkin, A.V.; Huang, X.; P’Ng K, M.; Bushby, A.J.; Sukhorukov, G.B.; Skirtach, A.G. Neuron cells uptake of polymeric microcapsules and subsequent intracellular release. Macromol. Biosci. 2011, 11, 848–854. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Petrova, A.V.; Lepik, K.V.; Okilova, M.V.; Vasin, A.V.; Afanasyev, B.V.; Sukhorukov, G.B. Hybrid inorganic-organic capsules for efficient intracellular delivery of novel siRNAs against influenza A (H1N1) virus infection. Sci. Rep. 2017, 7, 102. [Google Scholar] [CrossRef]
- Ribeiro, C.; Borges, J. Preparation of Well-Dispersed Chitosan/Alginate Hollow Multilayered Microcapsules for Enhanced Cellular Internalization. Molecules 2018, 23, 625. [Google Scholar] [CrossRef]
- Kumar, A.; Pareek, V.; Faiq, M.A.; Kumar, P.; Raza, K.; Prasoon, P.; Dantham, S.; Mochan, S. Regulatory role of NGFs in neurocognitive functions. Rev. Neurosci. 2017, 28, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef]
- Meakin, S.O.; Shooter, E.M. The nerve growth factor family of receptors. Trends Neurosci. 1992, 15, 323–331. [Google Scholar] [CrossRef]
- Ascano, M.; Bodmer, D.; Kuruvilla, R. Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 2012, 22, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Secondo, A.; Esposito, A.; Sirabella, R.; Boscia, F.; Pannaccione, A.; Molinaro, P.; Cantile, M.; Ciccone, R.; Sisalli, M.J.; Scorziello, A.; et al. Involvement of the Na+/Ca2+ exchanger isoform 1 (NCX1) in neuronal growth factor (NGF)-induced neuronal differentiation through Ca2+-dependent Akt phosphorylation. J. Biol. Chem. 2015, 290, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Huang, J.S.; Chen, A.; Skalka, N.; Rosin-Arbesfeld, R.; Loh, Y.P. Neurotrophic factor-alpha1 modulates NGF-induced neurite outgrowth through interaction with Wnt-3a and Wnt-5a in PC12 cells and cortical neurons. Mol. Cell. Neurosci. 2015, 68, 222–233. [Google Scholar] [CrossRef]
- Sato, Y.; Suzuki, S.; Kitabatake, M.; Hara, T.; Kojima, M. Generation of TrkA/TrkB chimeric receptor constructs reveals molecular mechanisms underlying BDNF-induced dendritic outgrowth in hippocampal neurons. Cell. Mol. Neurobiol. 2011, 31, 605–614. [Google Scholar] [CrossRef]
- Tatebayashi, Y.; Haque, N.; Tung, Y.C.; Iqbal, K.; Grundke-Iqbal, I. Role of tau phosphorylation by glycogen synthase kinase-3beta in the regulation of organelle transport. J. Cell Sci. 2004, 117, 1653–1663. [Google Scholar] [CrossRef]
- Shamloo, A.; Heibatollahi, M.; Mofrad, M.R. Directional migration and differentiation of neural stem cells within three-dimensional microenvironments. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 335–344. [Google Scholar] [CrossRef]
- Curley, J.L.; Catig, G.C.; Horn-Ranney, E.L.; Moore, M.J. Sensory axon guidance with semaphorin 6A and nerve growth factor in a biomimetic choice point model. Biofabrication 2014, 6, 035026. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shoichet, M.S. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience 2001, 103, 831–840. [Google Scholar] [CrossRef]
- Munoz Javier, A.; del Pino, P.; Bedard, M.F.; Ho, D.; Skirtach, A.G.; Sukhorukov, G.B.; Plank, C.; Parak, W.J. Photoactivated release of cargo from the cavity of polyelectrolyte capsules to the cytosol of cells. Langmuir ACS J. Surf. Colloids 2008, 24, 12517–12520. [Google Scholar] [CrossRef] [PubMed]
- Tanbour, R.; Martins, A.M.; Pitt, W.G.; Husseini, G.A. Drug Delivery Systems Based on Polymeric Micelles and Ultrasound: A Review. Curr. Pharm. Des. 2016, 22, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Weingart, L.; Ketschek, A.; Sainath, R.; Pacheco, A.; Smith, G.M.; Gallo, G. Neurotrophins induce fission of mitochondria along embryonic sensory axons. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cory, S. BDNF modulates, but does not mediate, activity-dependent branching and remodeling of optic axon arbors in vivo. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 9996–10003. [Google Scholar] [CrossRef]
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF is essential for hippocampal plasticity and learning. J. Neurosci. 2009, 29, 10883–10889. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, Y.; Yang, F.; Shen, W.; Tang, T.T.; Feng, L.; Duan, S.; Lu, B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010, 13, 302–309. [Google Scholar] [CrossRef]
- Rauti, R.; Cellot, G.; D’Andrea, P.; Colliva, A.; Scaini, D.; Tongiorgi, E.; Ballerini, L. BDNF impact on synaptic dynamics: Extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol. Brain 2020, 13, 43. [Google Scholar] [CrossRef]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef]
- Fahnestock, M.; Michalski, B.; Xu, B.; Coughlin, M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci. 2001, 18, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Long, D.; Song, C.; Li, X. Recombinant human NGF-loaded microspheres promote survival of basal forebrain cholinergic neurons and improve memory impairments of spatial learning in the rat model of Alzheimer’s disease with fimbria-fornix lesion. Neurosci. Lett. 2009, 453, 204–209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopach, O.; Pavlov, A.M.; Sindeeva, O.A.; Sukhorukov, G.B.; Rusakov, D.A. Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation. Pharmaceutics 2021, 13, 25. https://doi.org/10.3390/pharmaceutics13010025
Kopach O, Pavlov AM, Sindeeva OA, Sukhorukov GB, Rusakov DA. Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation. Pharmaceutics. 2021; 13(1):25. https://doi.org/10.3390/pharmaceutics13010025
Chicago/Turabian StyleKopach, Olga, Anton M. Pavlov, Olga A. Sindeeva, Gleb B. Sukhorukov, and Dmitri A. Rusakov. 2021. "Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation" Pharmaceutics 13, no. 1: 25. https://doi.org/10.3390/pharmaceutics13010025
APA StyleKopach, O., Pavlov, A. M., Sindeeva, O. A., Sukhorukov, G. B., & Rusakov, D. A. (2021). Biodegradable Microcapsules Loaded with Nerve Growth Factor Enable Neurite Guidance and Synapse Formation. Pharmaceutics, 13(1), 25. https://doi.org/10.3390/pharmaceutics13010025








