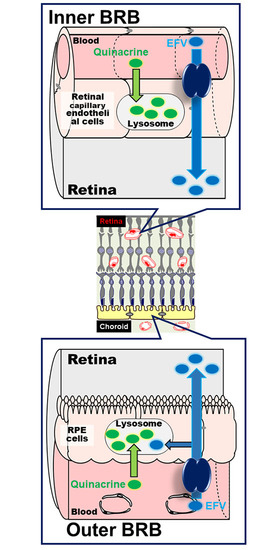
Uptake Study in Lysosome-Enriched Fraction: Critical Involvement of Lysosomal Trapping in Quinacrine Uptake but Not Fluorescence-Labeled Verapamil Transport at Blood-Retinal Barrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cellular Uptake Analysis
2.2.2. Confocal Microscopy of Fluorescent Compounds
2.2.3. Preparation of Lysosome-Enriched Fraction
2.2.4. Uptake Assay of Cationic Drugs in Lysosome-Enriched Fraction
/(fluorescence intensity in the medium)
/(fraction/medium ratio in the absence of inhibitor)
2.2.5. Statistical Analysis
3. Results
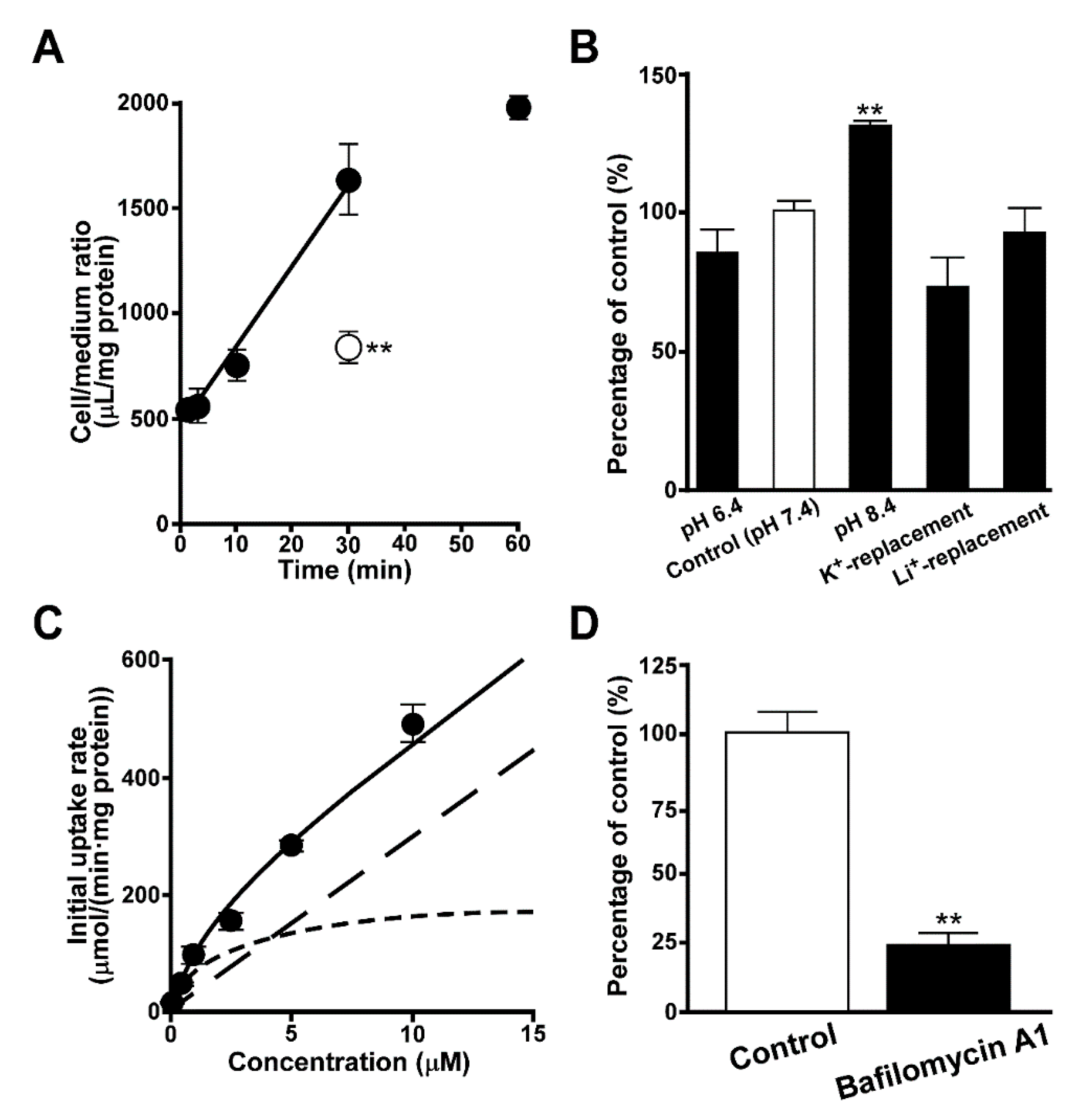
3.1. Uptake of Quinacrine by TR-iBRB2 Cells
3.2. Effect of Intracellular pH on Quinacrine Uptake by TR-iBRB2 Cells
3.3. Inhibition of Quinacrine Uptake by TR-iBRB2 Cells
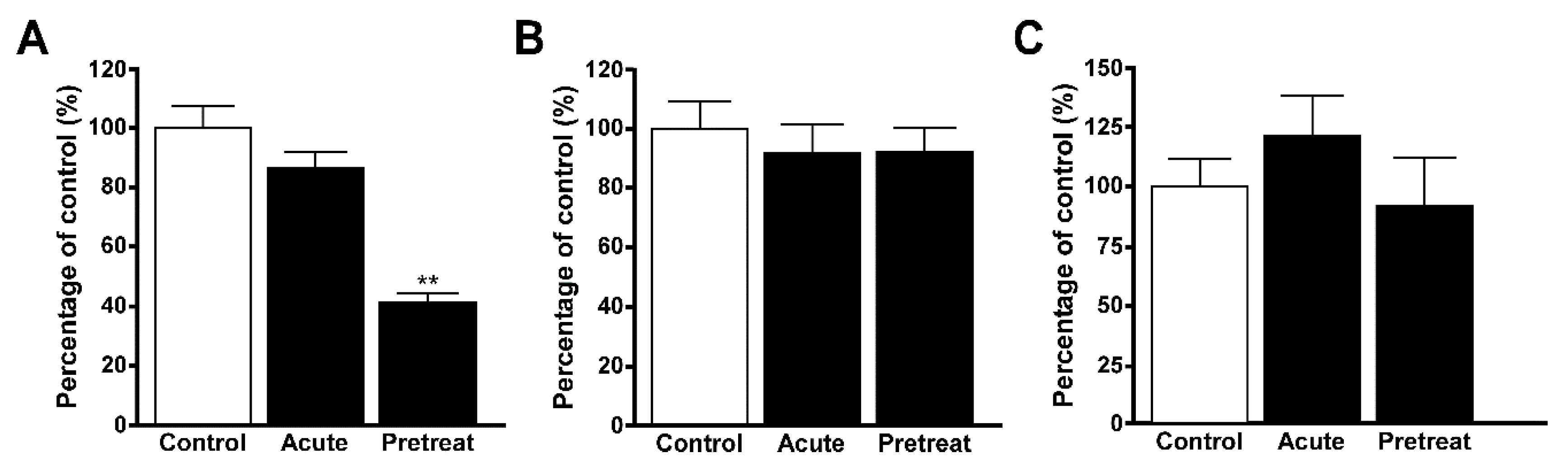
3.4. Uptake of Quinacrine in Lysosome-Enriched Fraction
3.5. Uptake of EFV in Lysosomal-Enriched Fraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cunha-Vaz, J.G.; Shakib, M.; Ashton, N. Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br. J. Ophthalmol. 1966, 50, 441453. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Tomi, M.; Tachikawa, M. Strategies for therapy of retinal diseases using systemic drug delivery: Relevance of transporters at the blood-retinal barrier. Expert. Opin. Drug. Deliv. 2011, 8, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Akanuma, S.; Hosoya, K. Recent advances in drug and nutrient transport across the blood-retinal barrier. Expert. Opin. Drug. Metab. Toxicol. 2018, 14, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kasahara, T.; Kasahara, M.; Ezaki, O.; Hirano, H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat. Investig. Ophthalmol. Vis. Sci. 1992, 33, 377–383. [Google Scholar]
- Kubo, Y.; Akanuma, S.; Hosoya, K. Impact of SLC6A transporters in physiological taurine transport at the bloodretinal barrier and in the liver. Biol. Pharm. Bull. 2016, 39, 1903–1911. [Google Scholar] [CrossRef]
- Kubo, Y.; Obata, A.; Akanuma, S.; Hosoya, K. Impact of cationic amino acid transporter 1 on blood-retinal barrier transport of L-ornithine. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5925–5932. [Google Scholar] [CrossRef]
- Kubo, Y.; Yahata, S.; Miki, S.; Akanuma, S.; Hosoya, K. Blood-to-retina transport of riboflavin via RFVTs at the inner blood-retinal barrier. Drug. Metab. Pharmacokinet. 2017, 32, 92–99. [Google Scholar] [CrossRef]
- Kubo, Y.; Miki, S.; Akanuma, S.; Hosoya, K. Riboflavin transport mediated by riboflavin transporters (RFVTs/SLC52A) at the rat outer blood-retinal barrier. Drug. Metab. Pharmacokinet. 2019, 34, 380–386. [Google Scholar] [CrossRef]
- Nagase, K.; Tomi, M.; Tachikawa, M.; Hosoya, K. Functional and molecular characterization of adenosine transport at the rat inner blood-retinal barrier. Biochim. Biophys. Acta. 2006, 1758, 13–19. [Google Scholar] [CrossRef]
- Nakashima, T.; Tomi, M.; Katayama, K.; Tachikawa, M.; Watanabe, M.; Terasaki, T.; Hosoya, K.I. Blood-to-retina transport of creatine via creatine transporter (CRT) at the rat inner blood-retinal barrier. J. Neurochem. 2004, 89, 1454–1461. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X. Roles of Drug Transporters in Blood-Retinal Barrier. In Drug Transporters in Drug Disposition, Effects and Toxicity. Advances in Experimental Medicine and Biology; Liu, X., Pan, G., Eds.; Springer: Singapore, 2019; p. 1140. [Google Scholar]
- Kubo, Y.; Akanuma, S.; Hosoya, K. Influx transport of cationic drug at the blood-retinal barrier: Impact on the retinal delivery of neuroprotectants. Biol. Pharm. Bull. 2017, 40, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Tega, Y.; Kubo, Y.; Yuzurihara, C.; Akanuma, S.; Hosoya, K. Carrier-mediated transport of nicotine across the inner blood-retinal barrier: Involvement of a novel organic cation transporter driven by an outward H(+) gradient. J. Pharm. Sci. 2015, 104, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Tsuchiyama, A.; Shimizu, Y.; Akanuma, S.; Hosoya, K. Involvement of carrier-mediated transport in the retinal uptake of clonidine at the inner blood-retinal barrier. Mol. Pharm. 2014, 11, 3747–3753. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kusagawa, Y.; Tachikawa, M.; Akanuma, S.; Hosoya, K. Involvement of a novel organic cation transporter in verapamil transport across the inner blood-retinal barrier. Pharm. Res. 2013, 30, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Shimizu, Y.; Kusagawa, Y.; Akanuma, S.; Hosoya, K. Propranolol transport across the inner blood-retinal barrier: Potential involvement of a novel organic cation transporter. J. Pharm. Sci. 2013, 102, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Sweet, D.H.; Hu, D.N.; Pritchard, J.B. Characterization of a novel cationic drug transporter in human retinal pigment epithelial cells. J. Pharmacol. Exp. Ther. 2001, 296, 450–457. [Google Scholar]
- Molinuevo, J.L.; Lladó, A.; Rami, L. Memantine: Targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am. J. Alzheimers Dis. Other Demen. 2005, 20, 77–85. [Google Scholar] [CrossRef]
- Martini, D.; Monte, M.D.; Ristori, C.; Cupisti, E.; Mei, S.; Fiorini, P.; Filippi, L.; Bagnoli, P. Antiangiogenic effects of β2 -adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011, 119, 1317–1329. [Google Scholar] [CrossRef]
- Yoles, E.; Wheeler, L.A.; Schwartz, M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 65–73. [Google Scholar]
- Nadanaciva, S.; Lu, S.; Gebhard, D.F.; Jessen, B.A.; Pennie, W.D.; Will, Y. A high content screening assay for identifying lysosomotropic compounds. Toxicol, In Vitro. 2011, 25, 715–723. [Google Scholar] [CrossRef]
- Kazmi, F.; Hensley, T.; Pope, C.; Funk, R.S.; Loewen, G.J.; Buckley, D.B.; Parkinson, A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug. Metab. Dispos. 2013, 41, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, B.; Percival, M.D.; Falgueyret, J.P. Quantitation of the lysosomotropic character of cationic amphiphilic drugs using the fluorescent basic amine Red DND-99. Anal. Biochem. 2004, 327, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yoshimori, T.; Yamamoto, A.; Moriyama, Y.; Futai, M.; Tashiro, Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 1991, 266, 17707–17712. [Google Scholar] [PubMed]
- Ohkuma, S.; Moriyama, Y.; Takano, T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc. Natl. Acad. Sci. USA. 1982, 79, 2758–2762. [Google Scholar] [CrossRef]
- Kubo, Y.; Seko, N.; Usui, T.; Akanuma, S.; Hosoya, K. Lysosomal trapping is present in retinal capillary endothelial cells: Insight into its influence on cationic drug transport at the inner blood-retinal barrier. Biol. Pharm. Bull. 2016, 39, 1319–1324. [Google Scholar] [CrossRef]
- Kubo, Y.; Nakazawa, A.; Akanuma, S.; Hosoya, K. Blood-to-retina transport of fluorescence-labeled verapamil at the blood-retinal Barrier. Pharm. Res. 2018, 35, 93. [Google Scholar] [CrossRef]
- Daniel, W.A.; Wójcikowski, J. Contribution of lysosomal trapping to the total tissue uptake of psychotropic drugs. Pharmacol. Toxicol. 1997, 80, 62–68. [Google Scholar] [CrossRef]
- Ishizaki, J.; Yokogawa, K.; Ichimura, F.; Ohkuma, S. Uptake of imipramine in rat liver lysosomes in vitro and its inhibition by basic drugs. J. Pharmacol. Exp. Ther. 2000, 294, 1088–1098. [Google Scholar]
- Logan, R.; Kong, A.C.; Krise, J.P. Time-dependent effects of hydrophobic amine-containing drugs on lysosome structure and biogenesis in cultured human fibroblasts. J. Pharm. Sci. 2014, 103, 3287–3296. [Google Scholar] [CrossRef]
- Marceau, F.; Bawolak, M.T.; Bouthillier, J.; Morissette, G. Vacuolar ATPase-mediated cellular concentration and retention of quinacrine: A model for the distribution of lipophilic cationic drugs to autophagic vacuoles. Drug. Metab. Dispos. 2009, 37, 2271–2274. [Google Scholar] [CrossRef]
- Roy, C.; Gagné, V.; Fernandes, M.J.; Marceau, F. High affinity capture and concentration of quinacrine in polymorphonuclear neutrophils via vacuolar ATPase-mediated ion trapping: Comparison with other peripheral blood leukocytes and implications for the distribution of cationic drugs. Toxicol. Appl. Pharmacol. 2013, 270, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.; Charest-Morin, X.; Boivin-Welch, M.; Bouthillier, J.; Marceau, F. Autophagic flux inhibition and lysosomogenesis ensuing cellular capture and retention of the cationic drug quinacrine in murine models. PeerJ. 2015, 3, e1314. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Tomi, M.; Ohtsuki, S.; Takanaga, H.; Ueda, M.; Yanai, N.; Obinata, M.; Terasaki, T. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp. Eye Res. 2001, 72, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Mathews, A.P.; Cohen-Gould, L.; Gundersen, D.; Rodriguez-Boulan, E. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 1993, 104, 37–49. [Google Scholar] [PubMed]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; Uno, T. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharmacobiodyn. 1981, 4, 879–885. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Nogami, K.; Jomura, R.; Akanuma, S.-I.; Abe, H.; Inouye, M.; Kubo, Y.; Hosoya, K.-I. Investigation of receptor-mediated cyanocobalamin (vitamin B12) transport across the inner blood-retinal barrier using fluorescence-labeled cyanocobalamin. Mol. Pharm. 2018, 15, 3583–3594. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Okamoto, T.; Morita, M.; Imanaka, T. Translocation of the ABC transporter ABCD4 from the endoplasmic reticulum to lysosomes requires the escort protein LMBD1. Sci. Rep. 2016, 6, 30183. [Google Scholar] [CrossRef]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.-J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Hosoya, K.; Tomi, M. Advances in the cell biology of transport via the inner blood-retinal barrier: Establishment of cell lines and transport functions. Biol. Pharm. Bull. 2005, 28, 1–8. [Google Scholar] [CrossRef]
- Hosoya, K.; Yamamoto, A.; Akanuma, S.; Tachikawa, M. Lipophilicity and transporter influence on blood-retinal barrier permeability: A comparison with blood-brain barrier permeability. Pharm. Res. 2010, 27, 2715–2724. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Relative Uptake (% of Control) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR-iBRB2 Cells | Lysosome-Enriched Fraction of TR-iBRB2 Cells | Lysosome-Enriched Fraction of RPE-J Cells | ||||||||||
| Control | 100 | ± | 3 | 100 | ± | 3 | 100 | ± | 11 | |||
| Desipramine | 47.9 | ± | 2.2 ** | 47.2 | ± | 1.9 ** | 64.4 | ± | 0.9 ** | |||
| Imipramine | 55.2 | ± | 2.7 ** | 49.6 | ± | 5.0 ** | 61.8 | ± | 2.9 ** | |||
| Propranolol | 55.4 | ± | 2.5 ** | 65.8 | ± | 1.8 ** | 76.8 | ± | 3.8 * | |||
| Verapamil | 64.7 | ± | 1.2 ** | 42.4 | ± | 1.3 ** | 57.3 | ± | 2.1 ** | |||
| Pyrilamine | 72.6 | ± | 2.3 ** | 87.1 | ± | 5.0 | 66.0 | ± | 2.4 ** | |||
| Clonidine | 90.3 | ± | 6.6 | 106 | ± | 11 | 89.4 | ± | 6.8 | |||
| Nicotine | 79.5 | ± | 6.0 ** | 106 | ± | 2 | 97.1 | ± | 7.1 | |||
| Choline | 98.7 | ± | 3.3 | N.D. | N.D. | |||||||
| Cimetidine | 88.3 | ± | 1.9 | N.D. | N.D. | |||||||
| L-Carnitine | 98.5 | ± | 8.9 | N.D. | N.D. | |||||||
| PAH | 97.6 | ± | 8.2 | N.D. | N.D. | |||||||
| Compounds | Relative Uptake (% of Control) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR-iBRB2 Cells [27] | Lysosome-Enriched Fraction of TR-iBRB2 Cells | Lysosome-Enriched Fraction of RPE-J Cells | ||||||||||
| Control | 100 | ± | 3 | 100 | ± | 3 | 100 | ± | 7 | |||
| Desipramine | 47.9 | ± | 2.2 ** | 61.8 | ± | 1.9 * | 65.5 | ± | 5.8 * | |||
| Imipramine | 55.2 | ± | 2.7 ** | 68.5 | ± | 2.8 | 82.5 | ± | 11.0 | |||
| Propranolol | 55.4 | ± | 2.5 ** | 108 | ± | 13 | 114 | ± | 7 | |||
| Verapamil | 64.7 | ± | 1.2 ** | 71.5 | ± | 3.0 | 51.0 | ± | 3.8 ** | |||
| Pyrilamine | 72.6 | ± | 2.3 ** | 143 | ± | 1 | 122 | ± | 12 | |||
| Clonidine | 90.3 | ± | 6.6 | 132 | ± | 7 | 98.6 | ± | 4.1 | |||
| Nicotine | 79.5 | ± | 6.0 ** | 147 | ± | 23 | 110 | ± | 10 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, Y.; Yamada, M.; Konakawa, S.; Akanuma, S.-i.; Hosoya, K.-i. Uptake Study in Lysosome-Enriched Fraction: Critical Involvement of Lysosomal Trapping in Quinacrine Uptake but Not Fluorescence-Labeled Verapamil Transport at Blood-Retinal Barrier. Pharmaceutics 2020, 12, 747. https://doi.org/10.3390/pharmaceutics12080747
Kubo Y, Yamada M, Konakawa S, Akanuma S-i, Hosoya K-i. Uptake Study in Lysosome-Enriched Fraction: Critical Involvement of Lysosomal Trapping in Quinacrine Uptake but Not Fluorescence-Labeled Verapamil Transport at Blood-Retinal Barrier. Pharmaceutics. 2020; 12(8):747. https://doi.org/10.3390/pharmaceutics12080747
Chicago/Turabian StyleKubo, Yoshiyuki, Miki Yamada, Saki Konakawa, Shin-ichi Akanuma, and Ken-ichi Hosoya. 2020. "Uptake Study in Lysosome-Enriched Fraction: Critical Involvement of Lysosomal Trapping in Quinacrine Uptake but Not Fluorescence-Labeled Verapamil Transport at Blood-Retinal Barrier" Pharmaceutics 12, no. 8: 747. https://doi.org/10.3390/pharmaceutics12080747
APA StyleKubo, Y., Yamada, M., Konakawa, S., Akanuma, S.-i., & Hosoya, K.-i. (2020). Uptake Study in Lysosome-Enriched Fraction: Critical Involvement of Lysosomal Trapping in Quinacrine Uptake but Not Fluorescence-Labeled Verapamil Transport at Blood-Retinal Barrier. Pharmaceutics, 12(8), 747. https://doi.org/10.3390/pharmaceutics12080747