Acceptability in the Older Population: The Importance of an Appropriate Tablet Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- the results of intake (the required dose fully, partly or not taken at all);
- the patient reaction during the administration using a 3-point hedonic face scale (positive, neutral or negative reaction);
- the time needed to prepare (from opening any packaging to having a required dose of medication ready to use, including all handling and modifications) and to administer the required dose of medication (from a required dose of medication ready to use to the end of the intake). The sum of the times of preparation and administration were both recorded with a timer and reported using 10 s intervals, classified as short (20 s and less), medium (from 30 s to 1 min), or long (more than 1 min).
- dividing the intake of a dose which cannot be taken as a whole;
- altering the intended use (modifying the dosage form such as opening a capsule or crushing a tablet; using another route/mode of administration);
- using food/drink to mask a bad taste or ease swallowing;
- using a device not provided;
- using restraint (the patient had to be made to take it).
2.2. Data Analysis
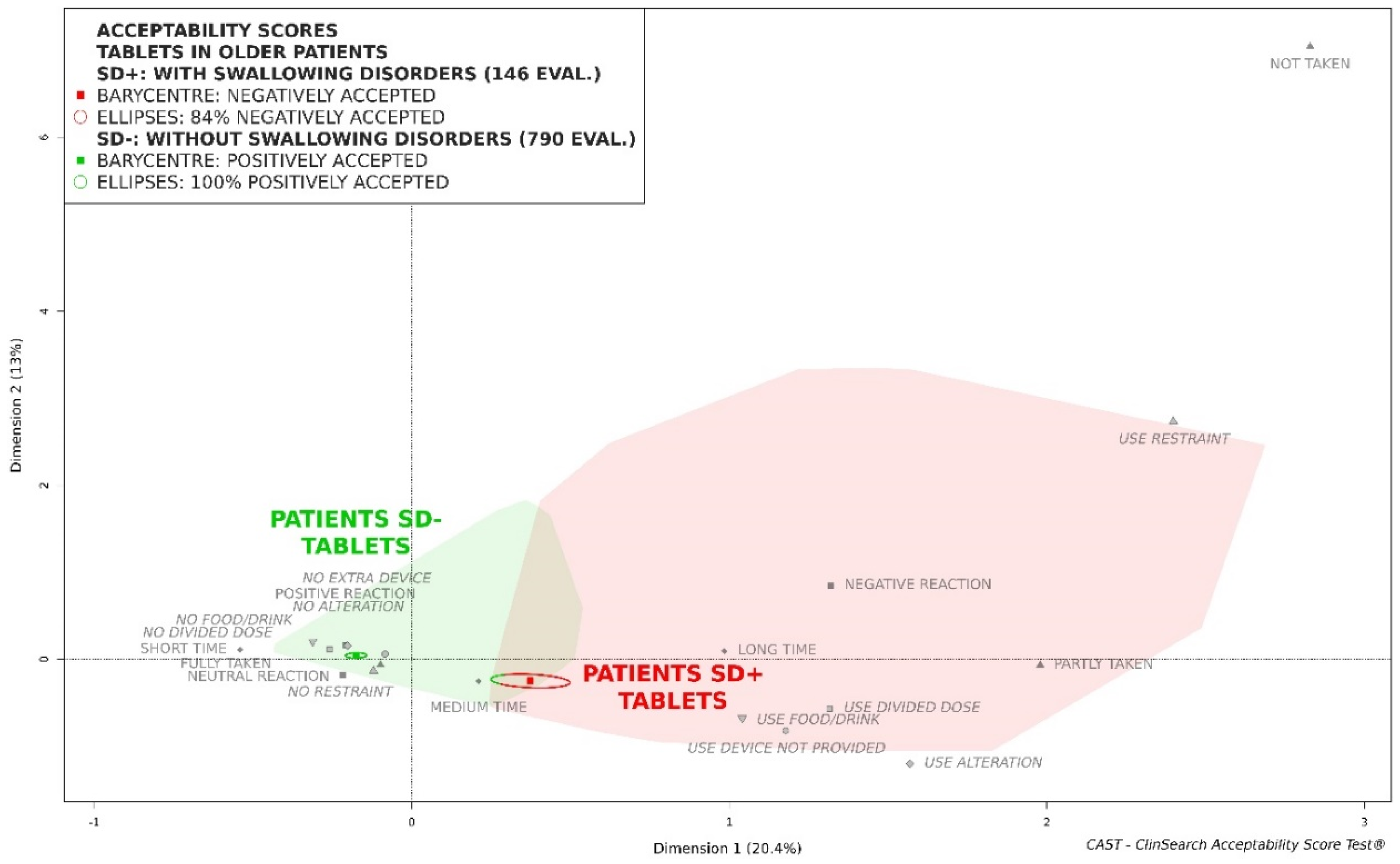
- the older patients without swallowing disorders (SD−) and the older patients with swallowing disorders (SD+) who had taken tablets, regardless of their size;
- the older patients SD+ had taken tablets smaller than a given threshold and the older patients SD+ had taken tablets which are equal and larger than the threshold. Size thresholds increasing by steps of 0.5 mm were explored from 6 mm to 10 mm.
3. Results
3.1. Patients and Medicines
3.2. Acceptability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Population Division, Department of Economic and Social Affairs, United Nations. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); United Nations: New York, NY, USA, 2019. [Google Scholar]
- European Medicine Agency. Reflection Paper on the Pharmaceutical Development of Medicines for Use in the Older Population; Draft. EMA/CHMP/QWP/292439/2017 Rev.: 4.0; EMA: London, UK, 2017. [Google Scholar]
- Puisieux, F.; D’Andrea, C.; Baconnier, P.; Bui-Dinh, D.; Castaings-Pelet, S.; Crestani, B.; Desrues, B.; Ferron, C.; Franco, A.; Gaillat, J. Swallowing disorders, pneumonia and respiratory tract infectious disease in the elderly. Rev. Mal. Respir. 2011, 28, e76–e93. [Google Scholar] [CrossRef]
- Kelly, J.; Wright, D. Administering medication to adult patients with dysphagia. Nurs. Stand. 2009, 23, 62–68. [Google Scholar] [CrossRef]
- Stegemann, S.; Gosch, M.; Breitkreutz, J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int. J. Pharm. 2012, 430, 197–206. [Google Scholar] [CrossRef]
- Schiele, J.T.; Penner, H.; Schneider, H.; Quinzler, R.; Reich, G.; Wezler, N.; Micol, W.; Oster, P.; Haefeli, W.E. Swallowing tablets and capsules increases the risk of penetration and aspiration in patients with stroke-induced dysphagia. Dysphagia 2015, 30, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef] [PubMed]
- Mc Gillicuddy, A.; Crean, A.M.; Sahm, L.J. Older adults with difficulty swallowing oral medicines: A systematic review of the literature. Eur. J. Clin. Pharmacol. 2016, 72, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Marquis, J.; Schneider, M.-P.; Payot, V.; Cordonier, A.-C.; Bugnon, O.; Hersberger, K.E.; Arnet, I. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int. J. Clin. Pharm. 2013, 35, 1130–1136. [Google Scholar] [CrossRef]
- Sestili, M.; Logrippo, S.; Cespi, M.; Bonacucina, G.; Ferrara, L.; Busco, S.; Grappasonni, I.; Palmieri, G.F.; Ganzetti, R.; Blasi, P. Potentially inappropriate prescribing of oral solid medications in elderly dysphagic patients. Pharmaceutics 2018, 10, 280. [Google Scholar] [CrossRef]
- Logrippo, S.; Ricci, G.; Sestili, M.; Cespi, M.; Ferrara, L.; Palmieri, G.F.; Ganzetti, R.; Bonacucina, G.; Blasi, P. Oral drug therapy in elderly with dysphagia: Between a rock and a hard place! Clin. Interv. Aging 2017, 12, 241. [Google Scholar] [CrossRef]
- Fodil, M.; Fillette, A.; Trivalle, C. Considérations portant sur l’écrasement des comprimés en gériatrie. NPG Neurol.-Psychiatr.-Gériatr. 2013, 13, 35–40. [Google Scholar] [CrossRef]
- Yamamoto, S.; Taniguchi, H.; Hayashi, H.; Hori, K.; Tsujimura, T.; Nakamura, Y.; Sato, H.; Inoue, M. How do tablet properties influence swallowing behaviours? J. Pharm. Pharmacol. 2014, 66, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.; Zweidorff, O.; Hjelde, T.; Rødland, E. Problems when swallowing tablets. A questionnaire study from general practice. J. Nor. Med. Assoc. 1995, 115, 947–949. [Google Scholar]
- Fields, J.; Go, J.T.; Schulze, K.S. Pill properties that cause dysphagia and treatment failure. Curr. Ther. Res. 2015, 77, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ghaffur, A.; Bains, J.; Hamdy, S. Acceptability of oral solid medicines in older adults with and without dysphagia: A nested pilot validation questionnaire based observational study. Int. J. Pharm. 2016, 512, 374–381. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Pense-Lheritier, A.M.; Aoussat, A. Standardized method to assess medicines’ acceptability: Focus on paediatric population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Vallet, T.; Ruiz, F.; Lavarde, M.; Pense-Lheritier, A.M.; Aoussat, A. Standardized evaluation of medicine acceptability in paediatric population: Reliability of a model. J. Pharm. Pharmacol. 2018, 70, 42–50. [Google Scholar] [CrossRef]
- Vallet, T.; Belissa, E.; Laribe-Caget, S.; Chevallier, A.; Chedhomme, F.X.; Leglise, P.; Piccoli, M.; Michelon, H.; Bloch, V.; Meaume, S.; et al. A Decision Support Tool Facilitating Medicine Design for Optimal Acceptability in The Older Population. Pharm. Res. 2018, 35, 136. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Dufay Wojcicki, A.; Belissa, É.; Fontan, J.-E.; de Pontual, L.; Nathanson, S.; Chevallier, A.; Laribe-Caget, S.; Boudy, V. Dosage form suitability in vulnerable populations: A focus on paracetamol acceptability from infants to centenarians. PLoS ONE 2019, 14, e0221261. [Google Scholar] [CrossRef]
- Belissa, E.; Vallet, T.; Laribe-Caget, S.; Chevallier, A.; Chedhomme, F.X.; Abdallah, F.; Bachalat, N.; Belbachir, S.A.; Boulaich, I.; Bloch, V.; et al. Acceptability of oral liquid pharmaceutical products in older adults: Palatability and swallowability issues. BMC Geriatr. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Ruiz, F.; Keeley, A.; Léglise, P.; Tuleu, C.; Lachuer, C.; Rwabihama, J.-P.; Bachalat, N.; Boulaich, I.; Abdallah, F.; Rabus, M.; et al. Sex Differences in Medicine Acceptability: A New Factor to Be Considered in Medicine Formulation. Pharmaceutics 2019, 11, 368. [Google Scholar] [CrossRef]
- Food & Drug Administration. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. Fed. Regist. 2009, 74, 65132–65133. [Google Scholar]
- Food and Drug Administration, U.S. Department of Health and Human Services. Size, shape, and other physical attributes of generic tablets and capsules guidance for industry. Fed. Regist. 2015, 80, 35366–35367. [Google Scholar]
- Brotherman, D.P.; Bayraktaroglu, T.O.; Garofalo, R.J. Comparison of ease of swallowing of dietary supplement products for age-related eye disease. J. Am. Pharm. Assoc. 2004, 44, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hofmanová, J.; Rajabi-Siahboomi, A.; Haque, S.; Mason, J.; Teckoe, J.; To, D.; Batchelor, H. Developing methodology to evaluate the oral sensory features of pharmaceutical tablet coatings. Int. J. Pharm. 2019, 562, 212–217. [Google Scholar] [CrossRef]
- Overgaard, A.B.; Hojsted, J.; Hansen, R.; Moller-Sonnergaard, J.; Christrup, L.L. Patients’ evaluation of shape, size and colour of solid dosage forms. Pharm. World Sci. 2001, 23, 185–188. [Google Scholar] [CrossRef]
- Kurczewska-Michalak, M.; Kardas, P.; Czajkowski, M. Patients’ Preferences and Willingness to Pay for Solid Forms of Oral Medications—Results of the Discrete Choice Experiment in Polish Outpatients. Pharmaceutics 2020, 12, 236. [Google Scholar] [CrossRef]
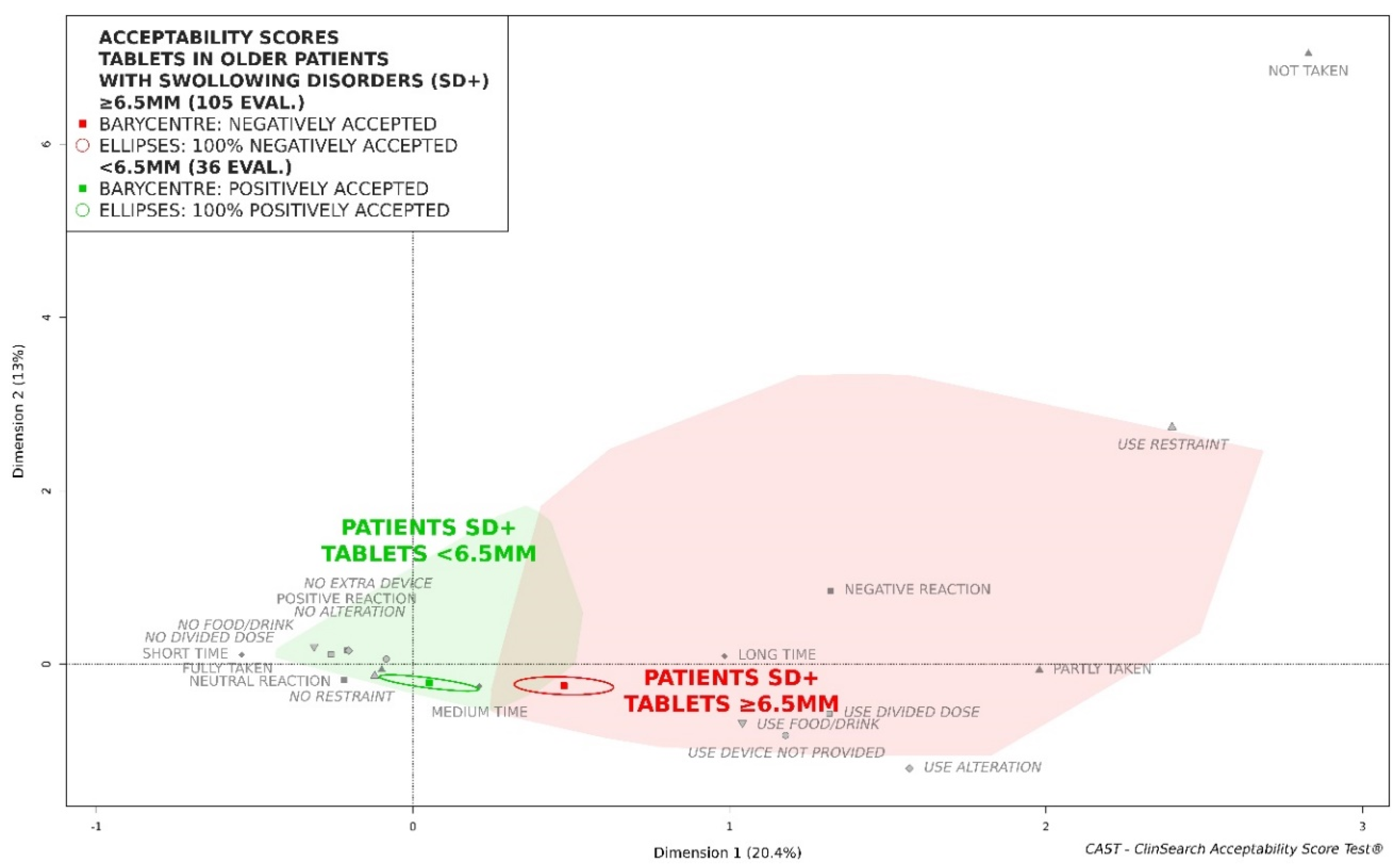

| Tablets ≥ 6.5 mm (n = 105) | Tablets < 6.5 mm (n = 36) | Statistical Test | |
|---|---|---|---|
| Result intake | |||
| fully taken | 91 (89) a | 35 (97) | F b: p = 0.47 |
| partly taken | 10 (10) | 1 (3) | |
| not taken | 1 (1) | 0 (0) | |
| md c: 3 | |||
| Patient reaction | |||
| positive | 5 (5) | 0 (0) | F: p = 0.016 |
| neutral | 69 (67) | 32 (91) | |
| negative | 29 (28) | 3 (9) | |
| md: 2 | md: 1 | ||
| Preparation and administration time | |||
| short time | 36 (35) | 17 (47) | χ2 d: p = 0.09 |
| medium time | 38 (37) | 15 (42) | |
| long time | 30 (29) | 4 (11) | |
| md: 1 | |||
| Divided dose | |||
| use divided dose | 31 (30) | 2 (6) | χ2: p = 0.007 |
| Food/drink | |||
| use food/drink | 62 (59) | 20 (56) | χ2: p = 0.86 |
| Restraint | |||
| use restraint | 7 (7) | 0 (0) | χ2: p = 0.25 |
| Alteration | |||
| use alteration | 54 (51) | 8 (22) | χ2: p = 0.004 |
| Extra device | |||
| use device not provided | 12 (11) | 4 (11) | χ2: p = 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet, T.; Michelon, H.; Orlu, M.; Jani, Y.; Leglise, P.; Laribe-Caget, S.; Piccoli, M.; Le Fur, A.; Liu, F.; Ruiz, F.; et al. Acceptability in the Older Population: The Importance of an Appropriate Tablet Size. Pharmaceutics 2020, 12, 746. https://doi.org/10.3390/pharmaceutics12080746
Vallet T, Michelon H, Orlu M, Jani Y, Leglise P, Laribe-Caget S, Piccoli M, Le Fur A, Liu F, Ruiz F, et al. Acceptability in the Older Population: The Importance of an Appropriate Tablet Size. Pharmaceutics. 2020; 12(8):746. https://doi.org/10.3390/pharmaceutics12080746
Chicago/Turabian StyleVallet, Thibault, Hugues Michelon, Mine Orlu, Yogini Jani, Patrick Leglise, Sandra Laribe-Caget, Matthieu Piccoli, Aurélie Le Fur, Fang Liu, Fabrice Ruiz, and et al. 2020. "Acceptability in the Older Population: The Importance of an Appropriate Tablet Size" Pharmaceutics 12, no. 8: 746. https://doi.org/10.3390/pharmaceutics12080746
APA StyleVallet, T., Michelon, H., Orlu, M., Jani, Y., Leglise, P., Laribe-Caget, S., Piccoli, M., Le Fur, A., Liu, F., Ruiz, F., & Boudy, V. (2020). Acceptability in the Older Population: The Importance of an Appropriate Tablet Size. Pharmaceutics, 12(8), 746. https://doi.org/10.3390/pharmaceutics12080746






