Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms that Improve Adherence and Acceptance in Older People
Abstract
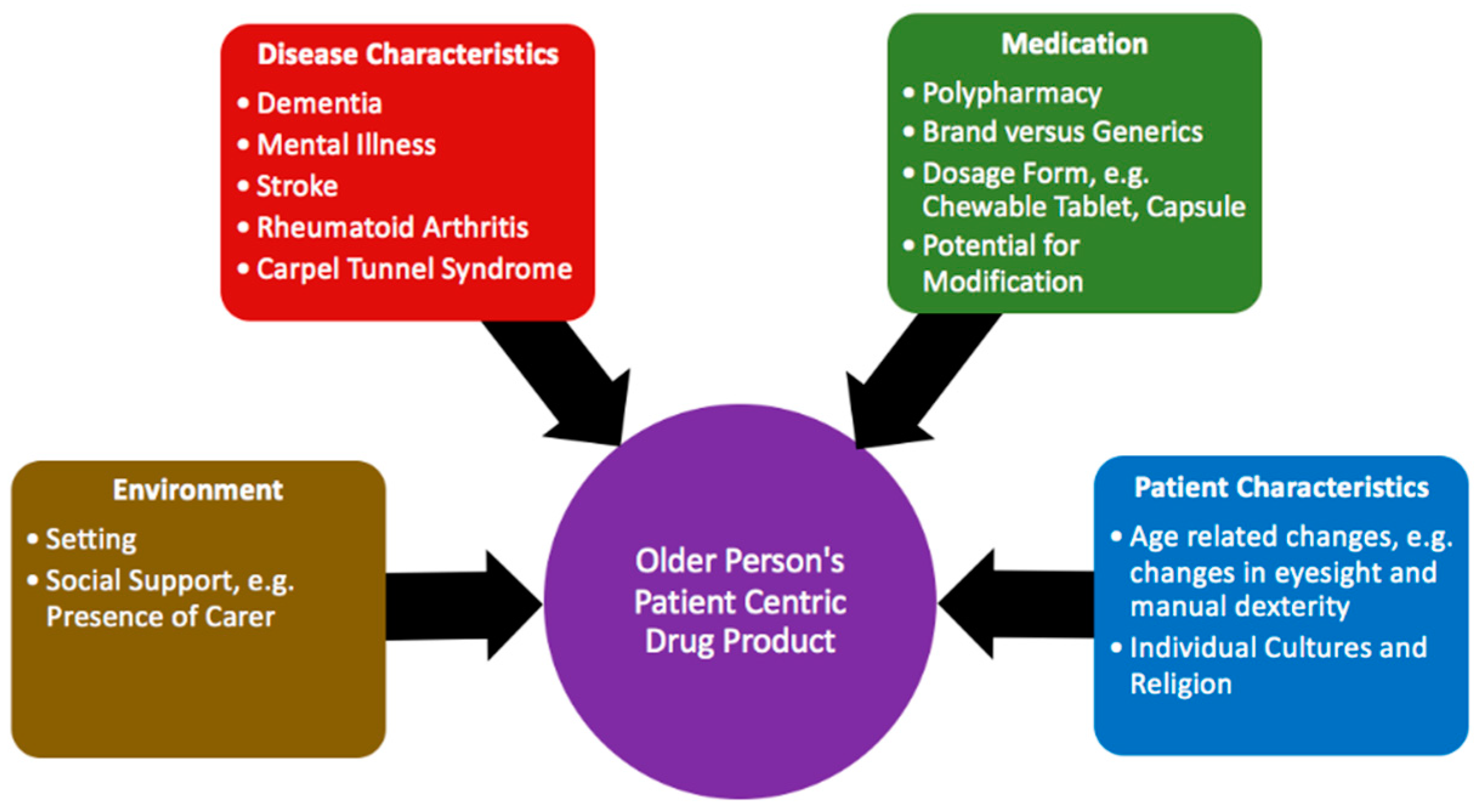
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Qualitative Analysis
2.3. Ethics
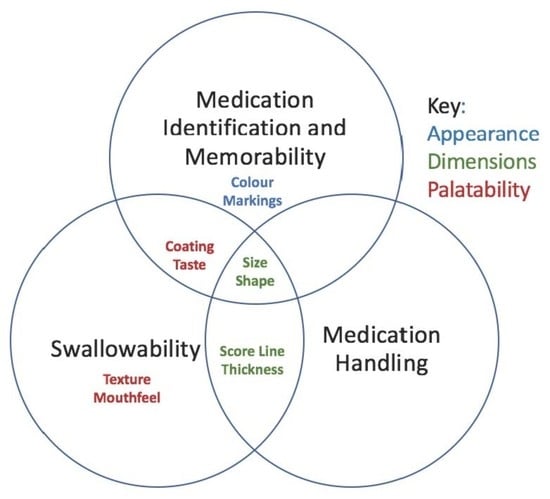
3. Results
3.1. Medication Identification and Memorability
3.1.1. Colour
“I think colour and I think bright colour. I would say bright colour. If they’re wishy-washy pale pink, pale yellow and white, they’re all a bit similar. I think colour would be good and reasonably bright colour.”(P5)
“Is there something that maybe needs to be brighter because they’ve got, maybe, macular degeneration or they’ve got problems with cataracts.”(HCP11)
“If there are a few tablets that are really important, for example anticoagulants … if they have a specific colour maybe it’s easier even, even for the clinician to say “you know the brown tablet or the red tablet, you need to take.”(HCP25)
“Now, these particular ones, without opening it, I think are only white, though I have had some that are white and red, and that, actually, would be a lot better because then I would remember better, I think, when it comes to relating what I’ve done with the Paracetamol … if there was a query in my head I might be able to remember better if it had more of a distinctive combination.”(P3)
3.1.2. Dimensions and Markings
“The larger they are, the easier. I mean I’m not sure about how that would be for my dad, he doesn’t seem to have any problem swallowing them but in terms of, you know, seeing them and making sure that they’re there in his hand”(C5)
“… I always recognise the Amlodipine… because it’s sort of got one, two, three, four, about six or eight sided it is. It’s quite small but it’s an interesting shape, so I do notice that”(P5)
“I am just thinking, for instance, sort of like a Statin and a Doxazosin, the imprint on the tablet’s not very defined. And that’s where some mistakes have actually happened.”(HCP12)
3.1.3. Impact of Changes in Appearance on Identification and Memorability
“They’re quite difficult sometimes because when I get my medication, it’s obviously generic so a different manufacturer will give a different colour, or no colour at all for that matter or a different size and you know if you’re taking, like I say, Memantine, if it changes colour you think, well I wonder what that is I’ve got there.”(P2)
3.2. Medication Handling
3.2.1. Difficulties Removing and Handling
“I mean it’d make it easier for her if she ever needed … couldn’t have a blister pack, and there wasn’t somebody to administer them, if they’re easier to get out of the packaging, because some aren’t”(C3)
“It’s about asking about them … do you feel that you can take it, can you pick it up, and it might be even asking, you know, samples, so can you show me whether or not you can pop it out of the blister pack or out of the packaging.”(HCP11)
“Half the time I probably think I’ve put two in my mouth, and I probably only put one and lost one on the floor”(P4)
3.2.2. Dimensions and Scoring to Improve Handling
“And I think if they can make them so that they don’t roll all over the place if you drop them. And those are the tinier ones obviously, they’re the flat-sided ones. The pillow ones, they’re fine, it’s just the tiny ones seem to roll”(C4)
“The scoring, yes, it’s a marker to actually cut the tablet, however it provides that grip that’s needed to control the tablet.”(HCP5)
3.3. Swallowability
3.3.1. Dimensions
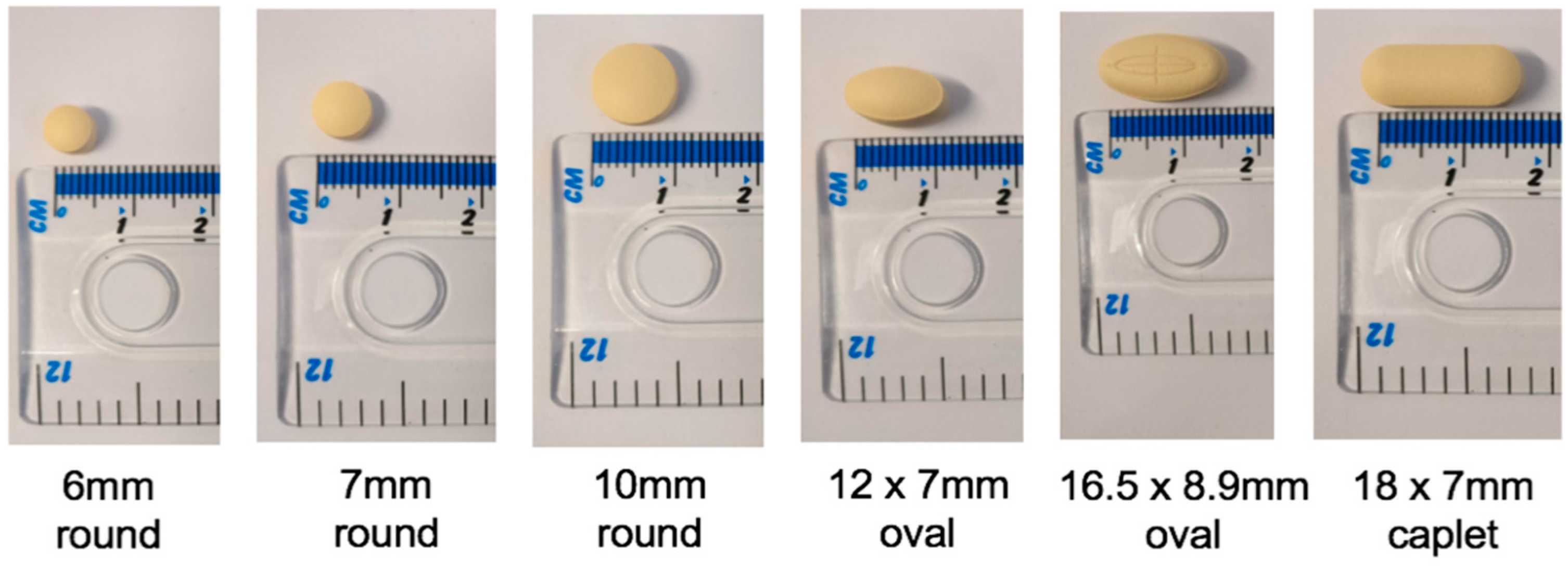
Size: A Balancing Act
“He talks about also, the small tablet, which I think is the rivaroxaban, he talks about that as being difficult for him to swallow and I wonder if it’s because he can’t really feel it in his mouth.”(C2)
“And then when you get to these, this bigger size, (18 × 7 mm caplet) it’s more about, you know, the thought of trying to swallow that bigger size can sometimes put patients off”(HCP8)
The Relationship between Size and Shape
“Do you know what I used to do, I used to break them into four and that was horrendous having to do”(P4)
3.3.2. Palatability: Coating, Texture, Mouthfeel and Taste
“If I’ve not tasted it, I wouldn’t have known that the purpose of you making that coating is for me to have the sugary part of it … You say, oh try, it’s nice taste but you are trying to convince me to take it, but my eyes have already said that is not right, why is it pink instead of white?”(HCP24)
“And they’re not coated so they’re chalky you know; you need a good swallow of water to be able to get them down”(P7)
“If people were, didn’t like taking medication, if there was some neutral but pleasant flavour that they might have … it would have to be something very neutral, because obviously different people like different things”(P18)
4. Discussion
4.1. Main Findings
4.2. Comparison to Other Studies
4.3. Strengths and Limitations
4.4. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stegemann, S.; Ternik, R.L.; Onder, G.; Khan, M.A.; van Riet-Nales, D.A. Defining Patient Centric Pharmaceutical Drug Product Design. AAPS J. 2016, 18, 1047–1055. [Google Scholar] [CrossRef]
- EMA. Good Practice Guide on Risk Minimisation and Prevention of Medication Errors. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2015/11/WC500196981.pdf (accessed on 1 December 2018).
- FDA. Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules: Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm377938.pdf (accessed on 1 December 2017).
- EMA. Reflection Paper on the Pharmaceutical Development of Medicines for Use in the Older Population. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/08/WC500232782.pdf (accessed on 1 December 2018).
- Kozarewicz, P. Regulatory perspectives on acceptability testing of dosage forms in children. Int. J. Pharm. 2014, 469, 245–248. [Google Scholar] [CrossRef]
- NICE. Medicines adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence. Available online: https://www.nice.org.uk/guidance/cg76/resources/medicines-adherence-involving-patients-in-decisions-about-prescribed-medicines-and-supporting-adherence-pdf-975631782085 (accessed on 20 November 2017).
- Wahlich, J.; Stegemann, S.; Orlu-Gul, M. Meeting commentary—“Medicines for older adults: Learning from practice to develop patient centric drug products”. Int. J. Pharm. 2013, 456, 251–257. [Google Scholar] [CrossRef]
- Notenboom, K.; Beers, E.; Riet-Nales, D.A.; Egberts, T.C.; Leufkens, H.G.; Jansen, P.A.; Bouvy, M.L. Practical problems with medication use that older people experience: A qualitative study. J. Am. Geriatr. Soc. 2014, 62, 2339–2344. [Google Scholar] [CrossRef]
- Aston, L.; Hilton, A.; Moutela, T.; Shaw, R.; Maidment, I. Exploring the evidence base for how people with dementia and their informal carers manage their medication in the community: A mixed studies review. BMC Geriatr. 2017, 17, 242. [Google Scholar] [CrossRef]
- Mc Gillicuddy, A.; Kelly, M.; Sweeney, C.; Carmichael, A.; Crean, A.M.; Sahm, L.J. Modification of oral dosage forms for the older adult: An Irish prevalence study. Int. J. Pharm. 2016, 510, 386–393. [Google Scholar] [CrossRef]
- Mercovich, N.; Kyle, G.J.; Naunton, M. Safe to crush? A pilot study into solid dosage form modification in aged care. Australas. J. Ageing 2014, 33, 180–184. [Google Scholar] [CrossRef]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-Centred Pharmaceutical Design to Improve Acceptability of Medicines: Similarities and Differences in Paediatric and Geriatric Populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef]
- Hanning, S.M.; Lopez, F.L.; Wong, I.C.K.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Patient centric formulations for paediatrics and geriatrics: Similarities and differences. Int. J. Pharm. 2016, 512, 355–359. [Google Scholar] [CrossRef]
- Stegemann, S.; Ecker, F.; Maio, M.; Kraahs, P.; Wohlfart, R.; Breitkreutz, J.; Zimmer, A.; Bar-Shalom, D.; Hettrich, P.; Broegmann, B. Geriatric drug therapy: Neglecting the inevitable majority. Ageing Res. Rev. 2010, 9, 384–398. [Google Scholar] [CrossRef]
- Shariff, Z.B.; Dahmash, D.T.; Kirby, D.J.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I.D. Does the Formulation of Oral Solid Dosage Forms Affect Acceptance and Adherence in Older Patients? A Mixed Methods Systematic Review. J. Am. Med. Direct. Assoc. 2020, 21, 1015–1023.e8. [Google Scholar] [CrossRef]
- Eggenberger, S.K.; Nelms, T.P. Family interviews as a method for family research. J. Adv. Nurs. 2007, 58, 282–292. [Google Scholar] [CrossRef]
- Darlington, Y.; Scott, D. Qualitative Research in Practice-Stories from the Field; Open University Press: Buckingham, UK, 2002. [Google Scholar]
- Guest, G.; Bunce, A.; Johnson, L. How Many Interviews Are Enough?:An Experiment with Data Saturation and Variability. Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Channer, K.S.; Virjee, J.P. The effect of size and shape of tablets on their esophageal transit. J. Clin. Pharm. 1986, 26, 141–146. [Google Scholar] [CrossRef]
- Hey, H.; Jørgensen, F.; Sørensen, K.; Hasselbalch, H.; Wamberg, T. Oesophageal transit of six commonly used tablets and capsules. Br. Med. J. 1982, 285, 1717–1719. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Hofmanová, J.; Rajabi-Siahboomi, A.; Haque, S.; Mason, J.; Teckoe, J.; To, D.; Batchelor, H. Developing methodology to evaluate the oral sensory features of pharmaceutical tablet coatings. Int. J. Pharm. 2019, 562, 212–217. [Google Scholar] [CrossRef]
- Gao, L.; Maidment, I.; Matthews, F.E.; Robinson, L.; Brayne, C. Medication usage change in older people (65+) in England over 20 years: Findings from CFAS I and CFAS II. Age Ageing 2018, 47, 220–225. [Google Scholar] [CrossRef]
- Fasto, M.M.; Genina, N.; Kaae, S.; Kalvemark Sporrong, S. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: A pilot study. Int. J. Clin. Pharm. 2019, 41, 1290–1298. [Google Scholar] [CrossRef]
- McCann, R.M.; Jackson, A.J.; Stevenson, M.; Dempster, M.; McElnay, J.C.; Cupples, M.E. Help needed in medication self-management for people with visual impairment: Case-control study. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2012, 62, e530–e537. [Google Scholar] [CrossRef]
- Stegemann, S.; Riedl, R.; Sourij, H. Identification of different shapes, colors and sizes of standard oral dosage forms in diabetes type 2 patients—A pilot study. Int. J. Pharm 2017, 517, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kesselheim, A.S.; Misono, A.S.; Shrank, W.H.; Greene, J.A.; Doherty, M.; Avorn, J.; Choudhry, N.K. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern. Med. 2013, 173, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Babar, Z.U.; Stewart, J.; Reddy, S.; Alzaher, W.; Vareed, P.; Yacoub, N.; Dhroptee, B.; Rew, A. An evaluation of consumers’ knowledge, perceptions and attitudes regarding generic medicines in Auckland. Pharm. World Sci. PWS 2010, 32, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Maidment, I.D.; Aston, L.; Moutela, T.; Fox, C.G.; Hilton, A. A qualitative study exploring medication management in people with dementia living in the community and the potential role of the community pharmacist. Health Expect. 2017, 20, 929–942. [Google Scholar] [CrossRef]
- Yamamoto, S.; Taniguchi, H.; Hayashi, H.; Hori, K.; Tsujimura, T.; Nakamura, Y.; Sato, H.; Inoue, M. How do tablet properties influence swallowing behaviours? J. Pharm. Pharmacol. 2014, 66, 32–39. [Google Scholar] [CrossRef]
- Marquis, J.; Schneider, M.-P.; Payot, V.; Cordonier, A.-C.; Bugnon, O.; Hersberger, K.E.; Arnet, I. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int. J. Clin. Pharm. 2013, 35, 1130–1136. [Google Scholar] [CrossRef]
- Mc Gillicuddy, A.; Crean, A.M.; Sahm, L.J. Older adults with difficulty swallowing oral medicines: A systematic review of the literature. Eur. J. Clin. Pharmacol. 2016, 72, 141–151. [Google Scholar] [CrossRef]
- Channer, K.S.; Virjee, J.P. The effect of formulation on oesophageal transit. J. Pharm. Pharmacol. 1985, 37, 126–129. [Google Scholar] [CrossRef]
- Kurczewska-Michalak, M.; Kardas, P.; Czajkowski, M. Patients’ Preferences and Willingness to Pay for Solid Forms of Oral Medications-Results of the Discrete Choice Experiment in Polish Outpatients. Pharmaceutics 2020, 12, 236. [Google Scholar] [CrossRef]
- Fields, J.; Go, J.T.; Schulze, K.S. Pill properties that cause dysphagia and treatment failure. Curr. Ther. Res. 2015, 77, 79–82. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Izham, M.; Al-Haddad, M. Preferences of color, size, shape, and taste of oral solid dosage forms. Pharmacologyonline 2010, 2, 754–762. [Google Scholar]
- Elliott, J.L. Swallowing disorders in the elderly: A guide to diagnosis and treatment. Geriatrics 1988, 43, 95–100, 104, 113. [Google Scholar]
- Rautamo, M.; Kvarnström, K.; Sivén, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed]

| Older People |
| Background Information |
| Details regarding Current Medication |
| Impact of Characteristics on ability to take Medication as directed |
| Changes to help make Medication easier to take |
| Carers |
| Background Information |
| Details regarding Current Medication administered |
| Impact of Characteristics on ability to administer Medication as directed |
| Changes to help make Medication easier to administer |
| Health and Social Care Professionals |
| Background Information |
| Experience with Older People/their Carers, their Medication and the Impact of the Physical Characteristics |
| Changes to help make Medication easier for older people or their carers to take/administer |
| Role of Health and Social Care Professionals in relation to Optimising the Physical Characteristics of Medication |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariff, Z.; Kirby, D.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I. Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms that Improve Adherence and Acceptance in Older People. Pharmaceutics 2020, 12, 905. https://doi.org/10.3390/pharmaceutics12100905
Shariff Z, Kirby D, Missaghi S, Rajabi-Siahboomi A, Maidment I. Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms that Improve Adherence and Acceptance in Older People. Pharmaceutics. 2020; 12(10):905. https://doi.org/10.3390/pharmaceutics12100905
Chicago/Turabian StyleShariff, Zakia, Daniel Kirby, Shahrzad Missaghi, Ali Rajabi-Siahboomi, and Ian Maidment. 2020. "Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms that Improve Adherence and Acceptance in Older People" Pharmaceutics 12, no. 10: 905. https://doi.org/10.3390/pharmaceutics12100905
APA StyleShariff, Z., Kirby, D., Missaghi, S., Rajabi-Siahboomi, A., & Maidment, I. (2020). Patient-Centric Medicine Design: Key Characteristics of Oral Solid Dosage Forms that Improve Adherence and Acceptance in Older People. Pharmaceutics, 12(10), 905. https://doi.org/10.3390/pharmaceutics12100905