Application of Multiple Linear Regression and Artificial Neural Networks for the Prediction of the Packing and Capsule Filling Performance of Coated and Plain Pellets Differing in Density and Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pellets
Coating Process
2.3. Characterization of the Pellets
2.3.1. Size, Shape, Moisture Content, and Density
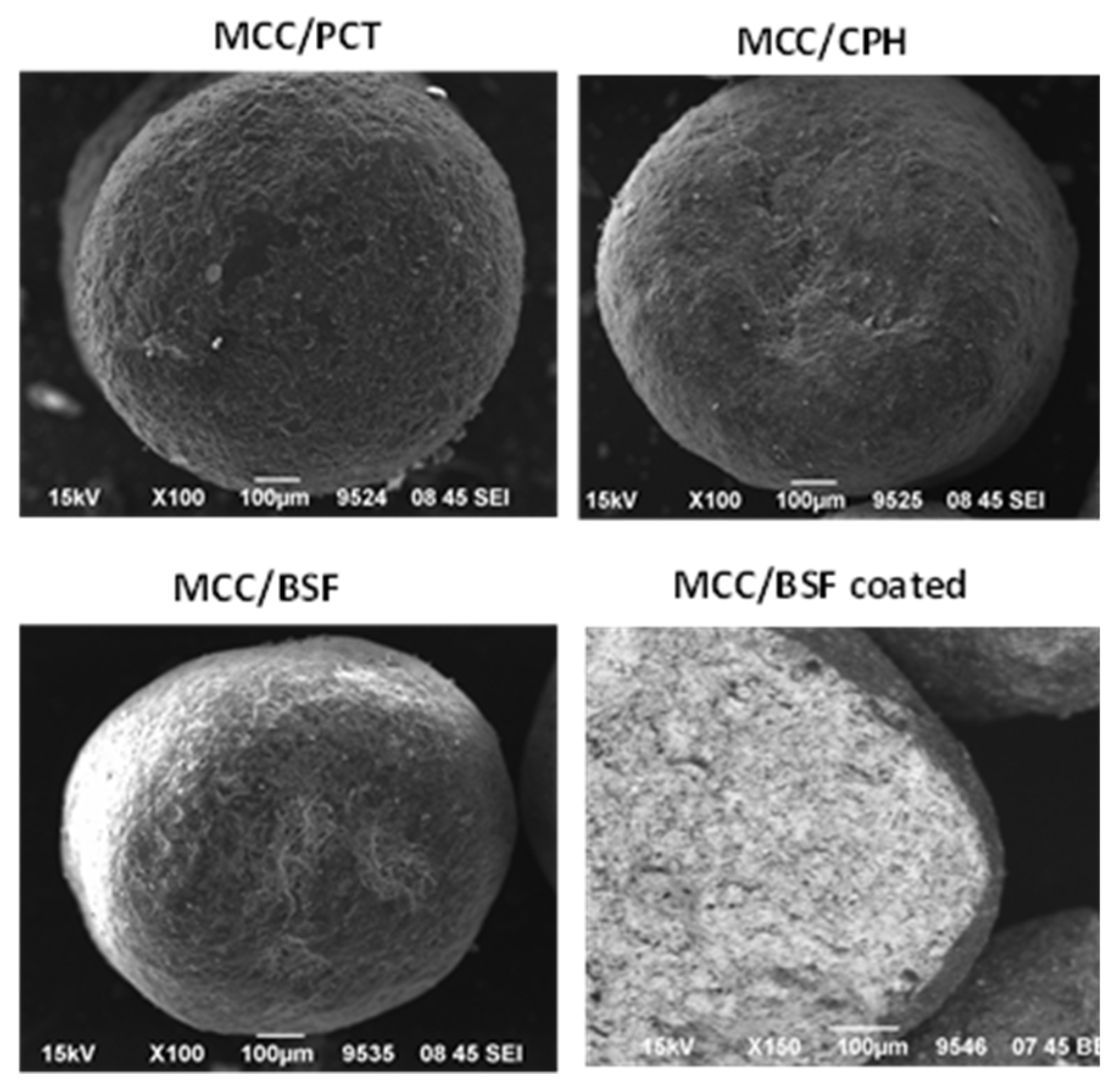
2.3.2. SEM Microphotographs
2.4. Evaluation of the Packing Ability of Pellets
2.5. Capsule Filling
2.6. Design of Experiments (DoE)
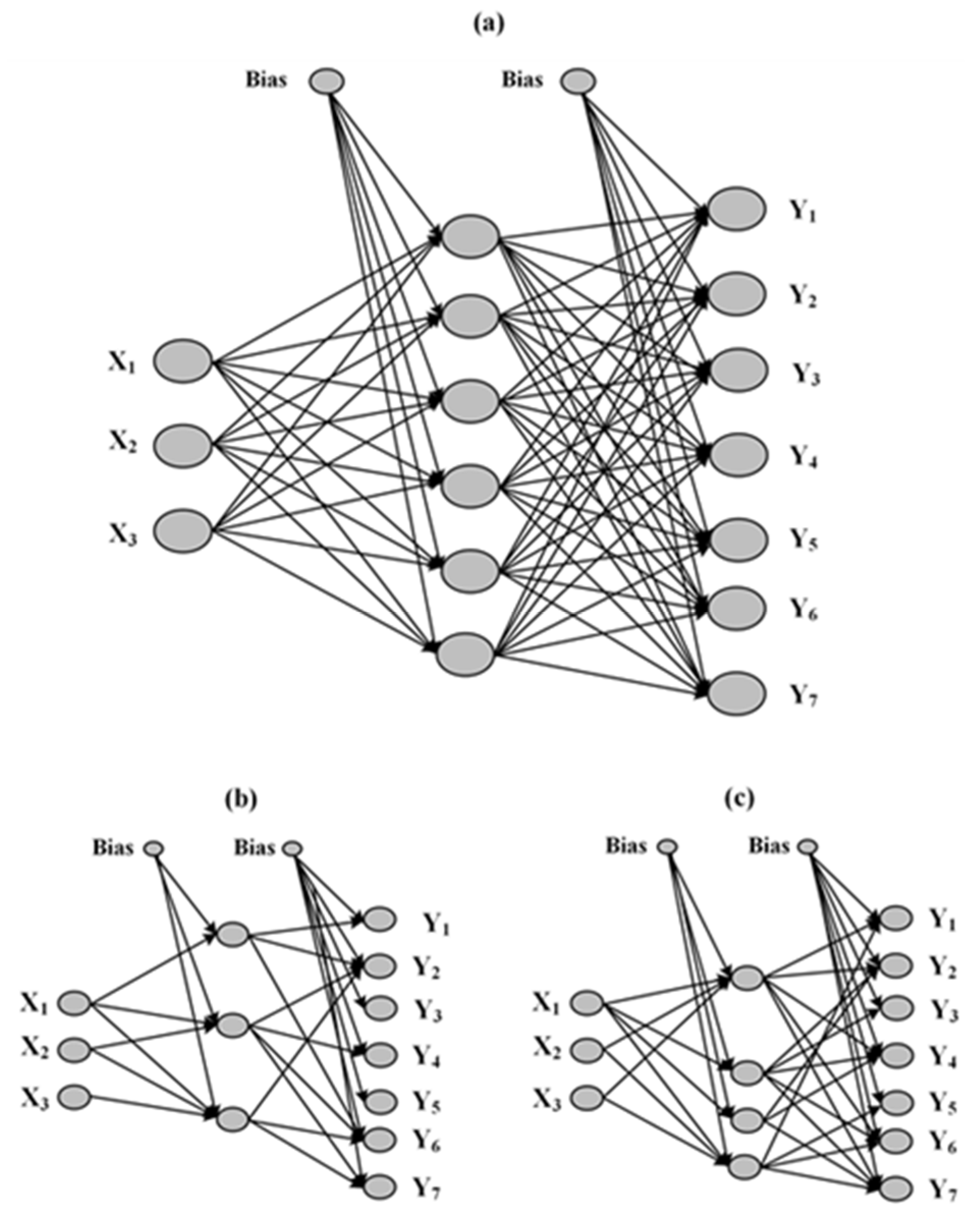
2.7. Artificial Neural Networks (ANNs)
External Validation of MLR and ANN Models
3. Results and Discussion
3.1. Preparation and Characteristics of the Pellets
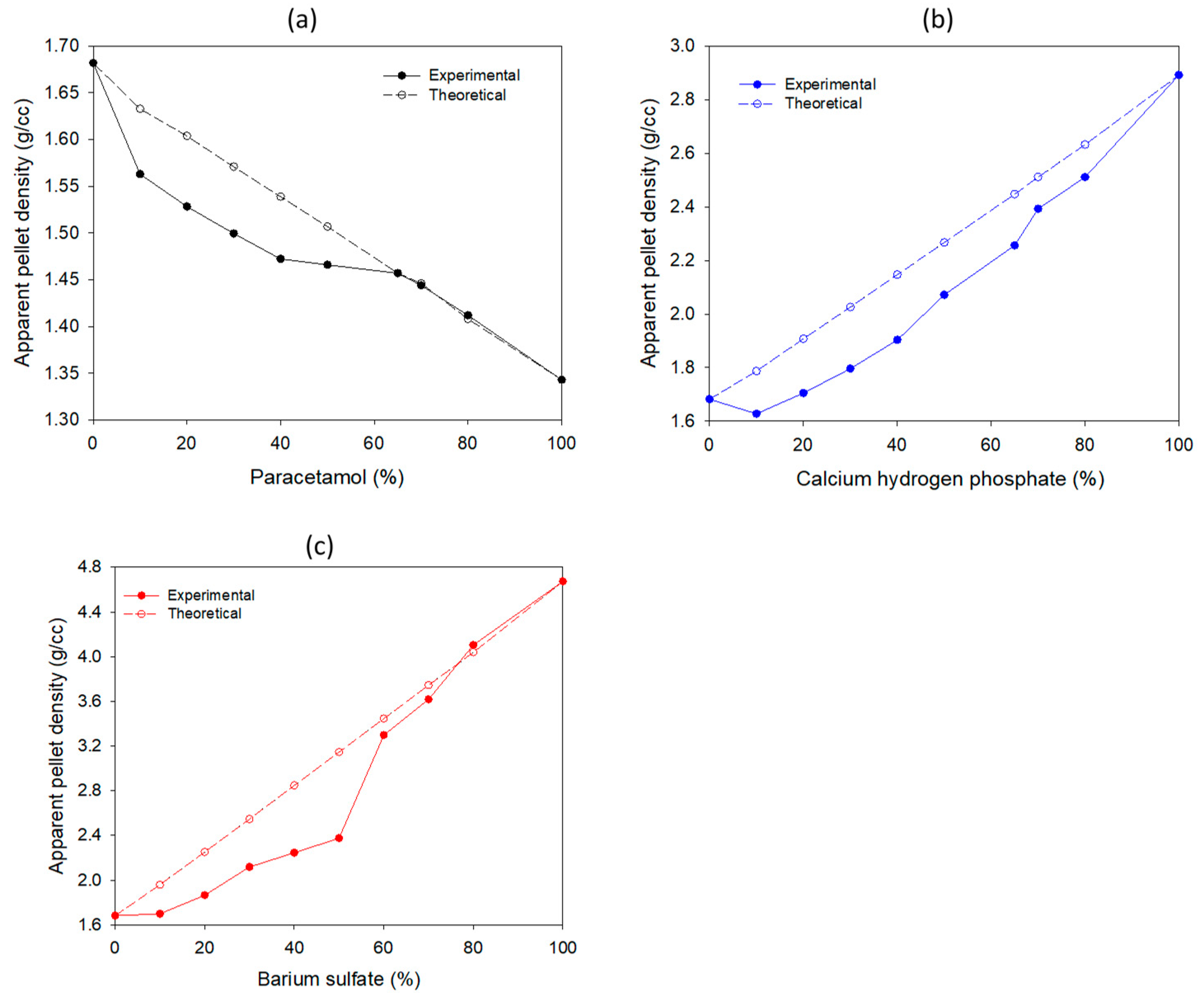
3.2. Selection of “Apparent” Density Levels
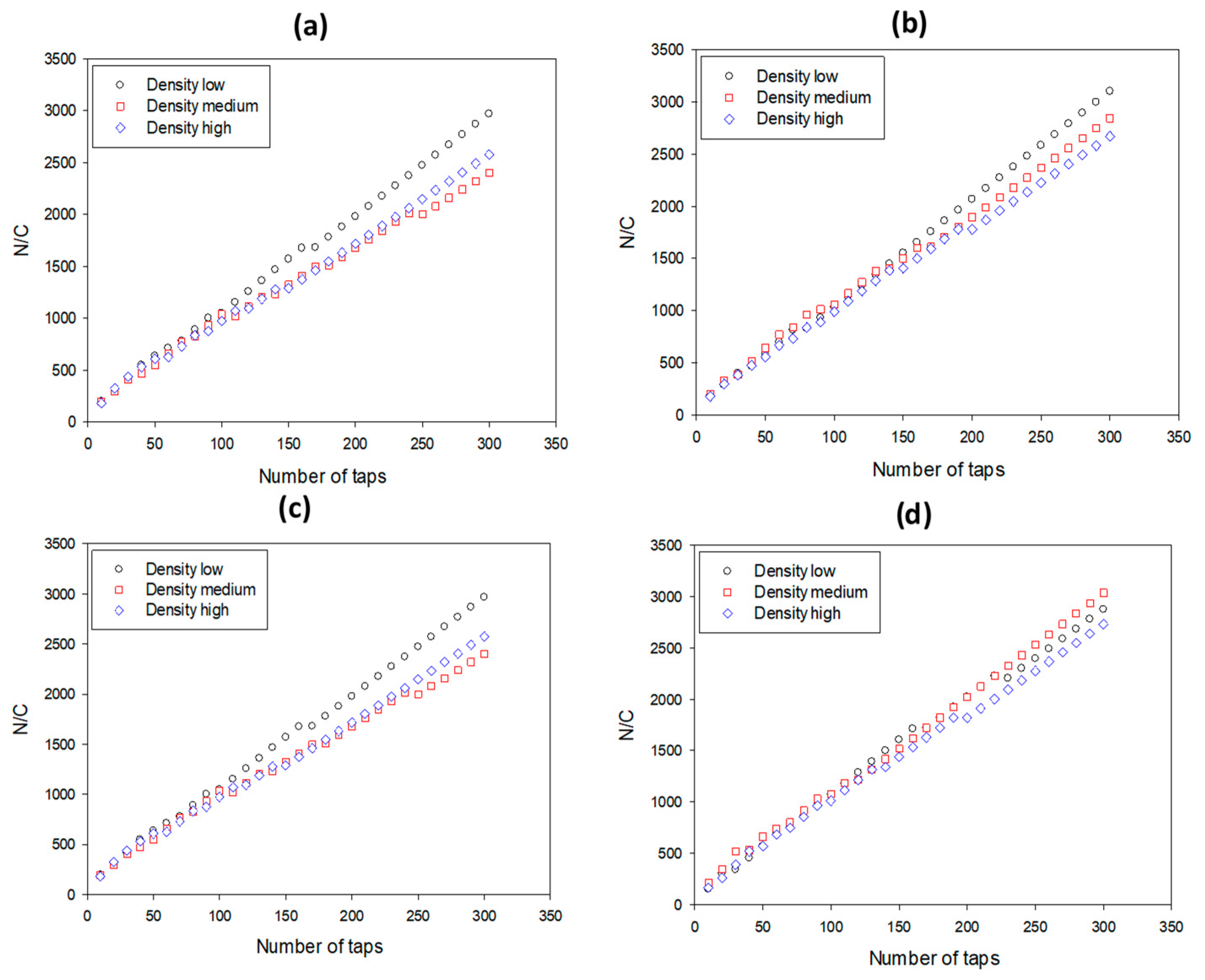
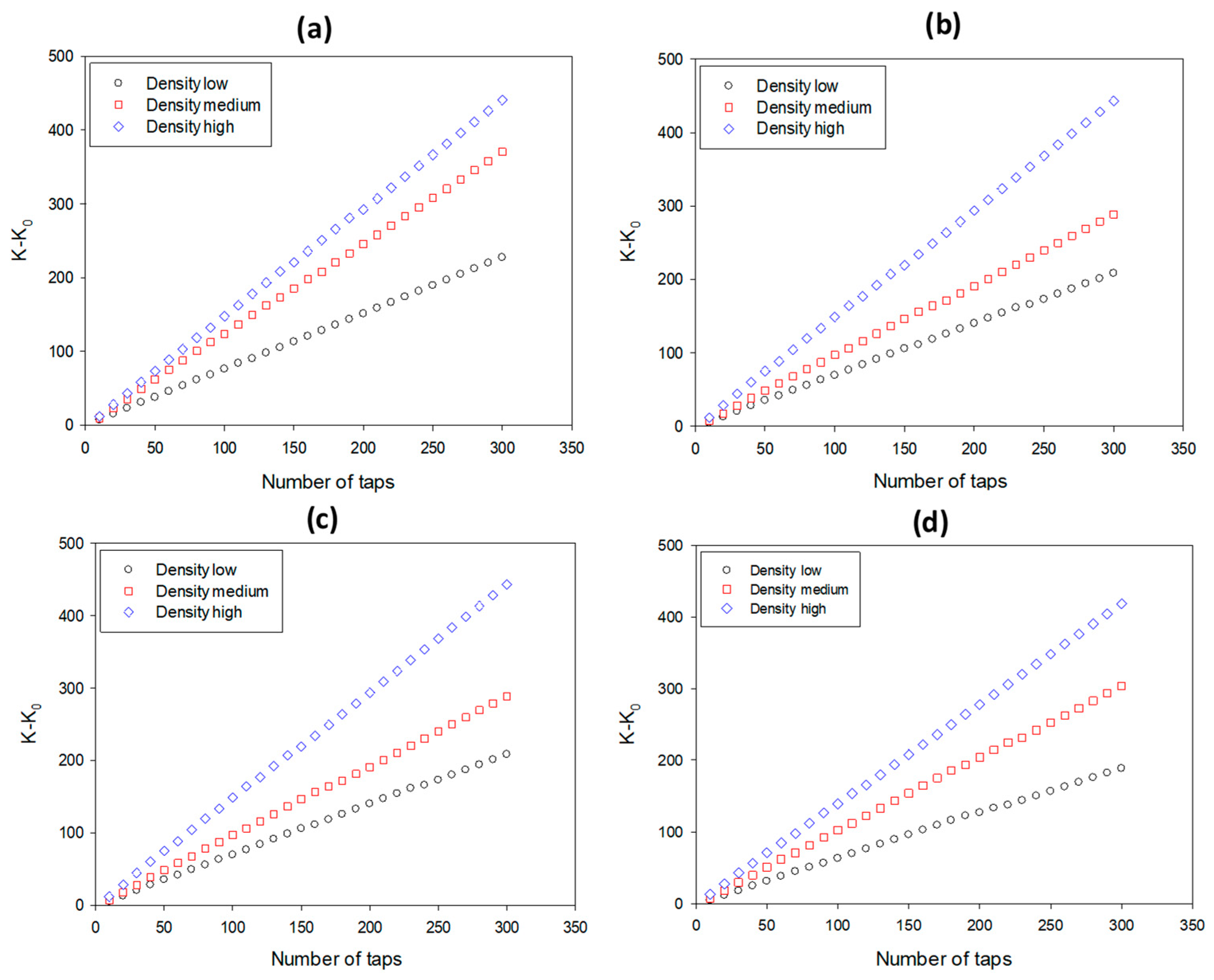
3.3. Dynamic Packing
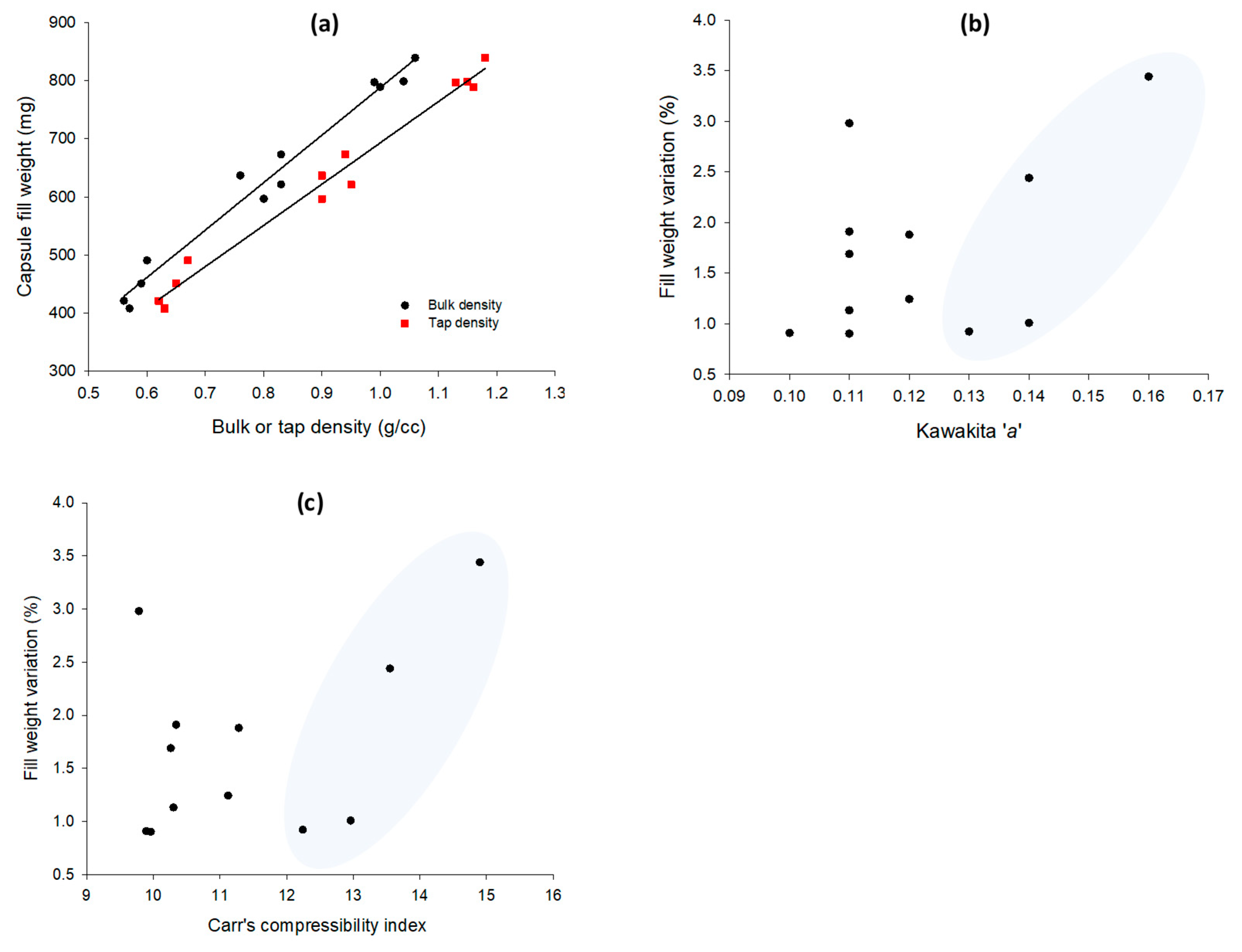
3.4. Correlations between Packing and Capsule Filling Parameters
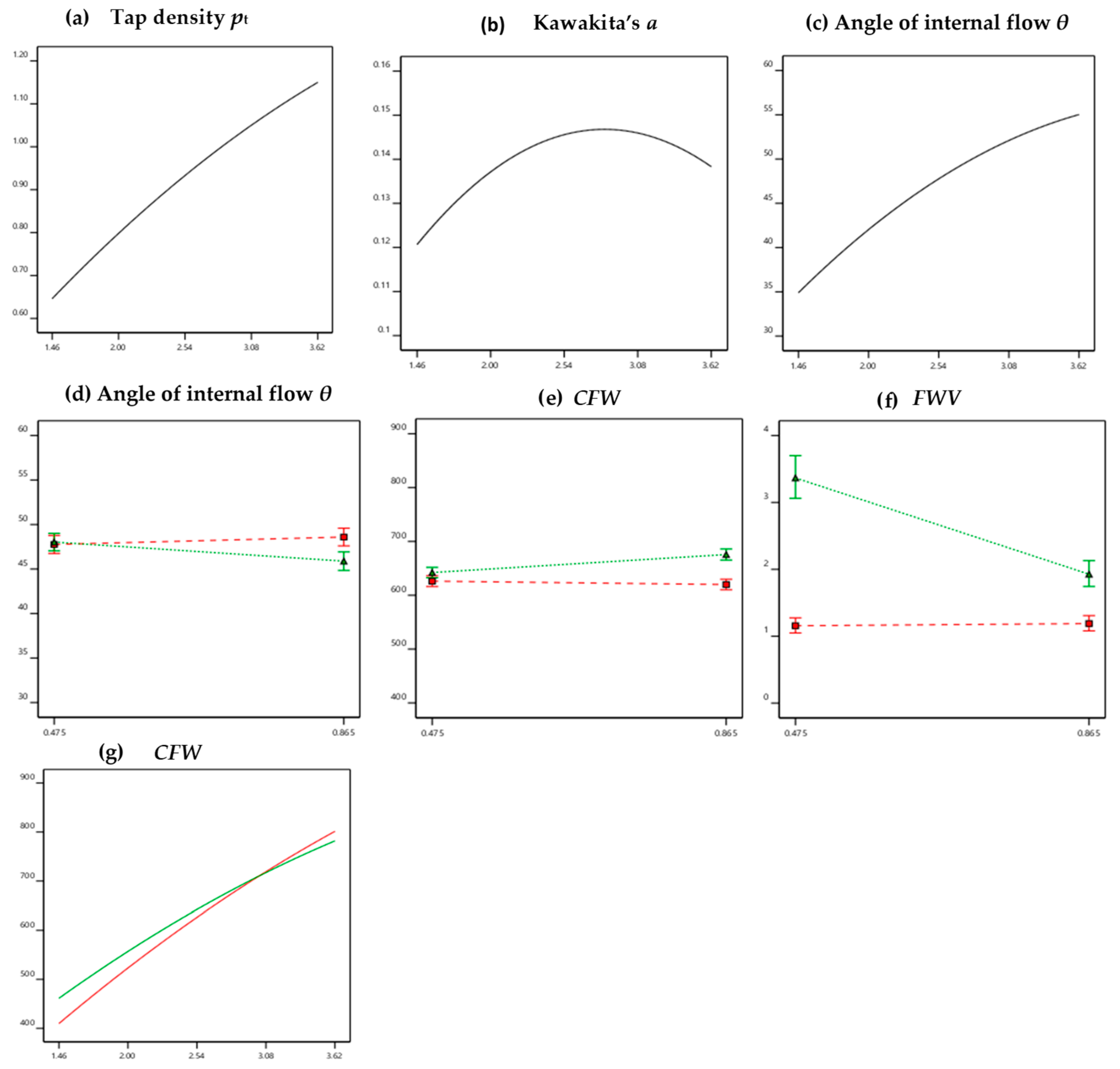
3.5. Multiple Linear Regression Analysis
3.6. Artificial Neural Networks
3.7. Results of Validation with External Set
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chopra, R.; Podczeck, F.; Newton, J.M.; Alderborn, G. The influence of pellet shape and film coating on the filling of pellets into hard shell capsules. Eur. J. Pharm. Biopharm. 2002, 53, 327–333. [Google Scholar] [CrossRef]
- Rowe, R.C.; York, P.; Colbourn, E.A.; Roskilly, S.J. The influence of pellet shape, size and distribution on capsule filling--a preliminary evaluation of three-dimensional computer simulation using a Monte-Carlo technique. Int. J. Pharm. 2005, 300, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Bader, F. The prediction of the bulk densities of powder mixtures, and its relationship to the filling of hard gelatin capsules. J. Pharm. Pharmacol. 1981, 33, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Varthalis, S.; Pilpel, N. Anomalies in some properties of powder mixtures. J. Pharm. Pharmacol. 1976, 28, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F.; Blackwell, S.; Gold, M.; Newton, J.M. The filling of granules into hard gelatin capsules. Int. J. Pharm. 1999, 188, 59–69. [Google Scholar] [CrossRef]
- Ali, A.M.; de Matas, M.; York, P.; Rowe, R.C. Role of pellet size, shape, and filling method in achieving fill weight uniformity for encapsulated pelletized systems: A comparison of experiment and computer simulation. J. Pharm. Sci. 2010, 99, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Stranzinger, S.; Faulhammer, E.; Li, J.; Dong, R.; Zeitler, J.A.; Biserni, S.; Calzolari, V.; Khinast, J.K.; Markle, D. Predicting capsule fill weight from in-situ powder density measurements using terahertz reflection technology. Int. J. Pharm. 2019, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.; Colombo, P.; Colombo, G.; Rekkas, D. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Partheniadis, I.; Gkogkou, P.; Kantiranis, N.; Nikolakakis, I. Modulation of the Release of a Non-Interacting Low Solubility Drug from Chitosan Pellets Using Different Pellet Size, Composition and Numerical Optimization. Pharmaceutics 2019, 11, 175. [Google Scholar] [CrossRef]
- Toziou, P.M.; Barmpalexis, P.; Boukouvala, P.; Verghese, S.; Nikolakakis, I. Quantification of live Lactobacillus acidophilus in mixed populations of live and killed by application of attenuated reflection Fourier transform infrared spectroscopy combined with chemometrics. J. Pharm. Biomed. Anal. 2018, 154, 16–22. [Google Scholar] [CrossRef]
- Kachrimanis, K.; Karamyan, V.; Malamataris, S. Artificial neural networks (ANNs) and modeling of powder flow. Int. J. Pharm. 2003, 250, 13–23. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Kanaze, F.I.; Kachrimanis, K.; Georgarakis, E. Artificial neural networks in the optimization of a nimodipine controlled release tablet formulation. Eur. J. Pharm. Biopharm. 2010, 74, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Balaxi, M.; Nikolakakis, I.; Malamataris, S. Preparation of Porous Microcrystalline Cellulose Pellets by Freeze-Drying: Effects of Wetting Liquid and Initial Freezing Conditions. J. Pharm. Sci. 2010, 99, 2104–2113. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating Flow Properties of Solids. Chem. Eng. J. 1965, 72, 163–168. [Google Scholar]
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 4th ed.; Churchill Livingstone: Edinburgh, UK, 2013; p. 196. [Google Scholar]
- Kawakita, K.; Ludde, K.H. Some Considerations on Powder Compression Equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Riedmiller, M.; Braun, H. Direct adaptive method for faster backpropagation learning: The RPROP algorithm. In Proceedings of the IEEE International Conference on Neural Networks, San Francisco, CA, USA, 28 March–1 April 1993; pp. 586–591. [Google Scholar]
- Yu, C.C.; Liu, B.D. A backpropagation algorithm with adaptive learning rate and momentum coefficient. In Proceedings of the 2002 International Joint Conference on Neural Networks IJCNN’02, Honolulu, HI, USA, 12–17 May 2002; pp. 1218–1223. [Google Scholar]
- Barmpalexis, P.; Kachrimanis, K.; Georgarakis, E. Solid dispersions in the development of a nimodipine floating tablet formulation and optimization by artificial neural networks and genetic programming. Eur. J. Pharm. Biopharm. 2011, 77, 122–131. [Google Scholar] [CrossRef] [PubMed]

| Pelletcode | Composition/Ratio | Liquid Binder (mL) | Extruder Screen (mm) | Coating | Moisture Content (%) | Apparent Pellet Density (g/cc) | Pellet Size Distribution | |||
|---|---|---|---|---|---|---|---|---|---|---|
| D10 (μm) | D50 (μm) | D90 (μm) | Span | |||||||
| F1 | MCC/PRC/35:65 | 8.3 | 0.5 | Yes | 1.59 | 1.45 | 356 | 485 | 606 | 0.52 |
| F2 | MCC/PRC/35:65 | 8.3 | 1.0 | Yes | 1.60 | 1.45 | 715 | 842 | 986 | 0.32 |
| F3 * | MCC/PRC/35:65 | 8.3 | 0.5 | No | 1.69 | 1.45 | 358 | 471 | 560 | 0.43 |
| F4 * | MCC/PRC/35:65 | 8.3 | 0.5 | No | 1.70 | 1.45 | 357 | 470 | 559 | 0.43 |
| F5 | MCC/PRC/35:65 | 8.3 | 1.0 | No | 1.73 | 1.45 | 690 | 713 | 867 | 0.25 |
| F6 + | MCC/CaP/20:80 | 7.2 | 0.5 | Yes | 1.39 | 2.53 | 460 | 503 | 625 | 0.33 |
| F7 + | MCC/CaP/20:80 | 7.2 | 0.5 | Yes | 1.40 | 2.53 | 458 | 501 | 623 | 0.33 |
| F8 # | MCC/CaP/20:80 | 7.2 | 1.0 | Yes | 1.37 | 2.53 | 850 | 955 | 988 | 0.14 |
| F9 # | MCC/CaP/20:80 | 7.2 | 1.0 | Yes | 1.36 | 2.53 | 847 | 952 | 985 | 0.14 |
| F10 ** | MCC/CaP/20:80 | 7.2 | 0.5 | No | 1.35 | 2.53 | 455 | 518 | 613 | 0.31 |
| F11 ** | MCC/CaP/20:80 | 7.2 | 0.5 | No | 1.35 | 2.53 | 457 | 520 | 616 | 0.30 |
| F12 ++ | MCC/CaP/20:80 | 7.2 | 1.0 | No | 1.37 | 2.53 | 854 | 901 | 948 | 0.10 |
| F13 ++ | MCC/CaP/20:80 | 7.2 | 1.0 | No | 1.36 | 2.53 | 860 | 907 | 954 | 0.10 |
| F14 ++ | MCC/CaP/20:80 | 7.2 | 1.0 | No | 1.37 | 2.53 | 856 | 903 | 949 | 0.10 |
| F15 | MCC/BaS/30:70 | 7.5 | 0.5 | Yes | 1.61 | 3.61 | 343 | 469 | 524 | 0.39 |
| F16 ## | MCC/BaS/30:70 | 7.5 | 1.0 | Yes | 1.64 | 3.61 | 771 | 895 | 1010 | 0.27 |
| F17 ## | MCC/BaS/30:70 | 7.5 | 1.0 | Yes | 1.62 | 3.61 | 763 | 887 | 1002 | 0.27 |
| F18 | MCC/BaS/30:70 | 7.5 | 0.5 | No | 1.91 | 3.61 | 285 | 402 | 515 | 0.57 |
| F19 | MCC/BaS/30:70 | 7.5 | 1.0 | No | 1.87 | 3.61 | 740 | 885 | 986 | 0.28 |
| Factors | Formulation Code | ||||
| T1 | T2 | T3 | T4 | T5 | |
| X1: apparent pellet density (g/cc) | 2.0715 | 2.0715 | 2.376 | 1.500 | 3.619 |
| X2: pellet size (mm) | 0.865 | 0.475 | 0.475 | 0.865 | 0.865 |
| X3: pellet coating | No | No | No | Yes | No |
| Responses | Formulation Code | ||||
| T1 | T2 | T3 | T4 | T5 | |
| Y1: pellet’s bulk density (g/cc) a | 0.81 | 0.81 | 0.95 | 0.57 | 1.06 |
| Y2: pellet’s tap density (g/cc) b | 0.92 | 0.95 | 1.09 | 0.63 | 1.18 |
| Y3: Carr’s index (%) c | 11.94 | 14.16 | 12.09 | 9.52 | 10.26 |
| Y4: Kawakita’s parameter a d | 0.13 | 0.15 | 0.13 | 0.10 | 0.11 |
| Y5: angle of internal friction (deg) e | 34.13 | 32.43 | 32.58 | 36.49 | 54.01 |
| Y6: capsule fill weight (mg) f | 649.15 | 632.90 | 748.93 | 415.28 | 839.45 |
| Y7: capsule weight variation (%) g | 1.81 | 1.88 | 1.51 | 1.19 | 1.69 |
| Code | Factors | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 (g/cc) | X2 (mm) | X3 | pb (g/cc) | pt (g/cc) | CC% (%) | a | θ (deg) | CFW (mg) | FWV (%) | |
| F1 | 1.457 | 0.475 | Yes | 0.57 | 0.63 | 10.30 | 0.11 | 36.21 | 407.8 | 1.13 |
| F2 | 1.457 | 0.865 | Yes | 0.57 | 0.62 | 9.89 | 0.10 | 37.71 | 417.4 | 0.91 |
| F3 * | 1.457 | 0.475 | No | 0.59 | 0.65 | 9.78 | 0.11 | 33.88 | 450.7 | 2.98 |
| F4 * | 1.457 | 0.475 | No | 0.60 | 0.66 | 11.04 | 0.13 | 34.20 | 464.1 | 3.61 |
| F5 | 1.457 | 0.865 | No | 0.60 | 0.67 | 10.34 | 0.11 | 31.97 | 490.4 | 1.91 |
| F6 + | 2.512 | 0.475 | Yes | 0.85 | 0.93 | 12.47 | 0.14 | 46.74 | 627.2 | 1.25 |
| F7 + | 2.512 | 0.475 | Yes | 0.83 | 0.95 | 12.24 | 0.13 | 45.92 | 621.4 | 0.92 |
| F8 # | 2.512 | 0.865 | Yes | 0.80 | 0.90 | 11.8 | 0.12 | 48.86 | 596.9 | 1.24 |
| F9 # | 2.512 | 0.865 | Yes | 0.83 | 0.93 | 11.0 | 0.13 | 46.67 | 604.3 | 1.28 |
| F10 ** | 2.512 | 0.475 | No | 0.76 | 0.90 | 14.90 | 0.16 | 48.66 | 636.8 | 3.44 |
| F11 ** | 2.512 | 0.475 | No | 0.78 | 0.91 | 16.05 | 0.16 | 49.38 | 658.7 | 3.49 |
| F12 ++ | 2.512 | 0.865 | No | 0.83 | 0.94 | 11.28 | 0.12 | 45.99 | 673.1 | 1.88 |
| F13 ++ | 2.512 | 0.865 | No | 0.82 | 0.93 | 10.83 | 0.11 | 45.42 | 660.5 | 1.73 |
| F14 ++ | 2.512 | 0.865 | No | 0.84 | 0.95 | 11.73 | 0.13 | 46.56 | 685.8 | 2.03 |
| F15 | 3.619 | 0.475 | Yes | 0.99 | 1.13 | 12.96 | 0.14 | 56.08 | 797.5 | 1.01 |
| F16 ## | 3.619 | 0.865 | Yes | 1.07 | 1.16 | 11.68 | 0.12 | 55.70 | 806.3 | 1.07 |
| F17 ## | 3.619 | 0.865 | Yes | 1.04 | 1.15 | 9.96 | 0.11 | 55.20 | 799.1 | 0.90 |
| F18 | 3.619 | 0.475 | No | 1.00 | 1.16 | 13.55 | 0.14 | 55.05 | 770.6 | 2.29 |
| F19 | 3.619 | 0.865 | No | 1.06 | 1.18 | 10.26 | 0.11 | 54.01 | 829.4 | 1.69 |
| Response | Intercept # | X1 | X2 | X3 | X1X2 | X1X3 | X2X3 | X12 | Model Equations with Actual Values for Small or Large, Plain or Coated Pellets | R2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Y1(pb) | <0.010 | <0.010 | 0.296 + 0.204X1 | 0.971 | ||||||
| B | Y2(pt) | <0.010 | <0.010 | <0.010 | 0.149 + 0.385X1 − 0.029X12 | 0.991 | |||||
| C | Y3(CC%) | 0.021 | 0.023 | <0.01 | 0.013 | Small: 2.716 + 7.647X1 − 1.328X12 Large: 0.795 + 7.647X1 − 1.328X12 | 0.639 | ||||
| D | Y4(a) | <0.010 | 0.023 | <0.01 | <0.010 | Small: 0.036 + 0.078X1 − 0.014 X12 Large: 0.014 + 0.078X1 − 0.014 X12 | 0.705 | ||||
| E | Y5(θ) | <0.010 | <0.010 | 0.328 * | 0.089 * | 0.04 | <0.010 | Small/coated: 8.688 + 21.482X1 − 2.398 X12 Small/plain: 8.952 + 21.482X1 − 2.398 X12 Large/coated: 9.521 + 21.482X1 − 2.398 X12 Large/plain: 6.813 + 21.482X1 − 2.398 X12 | 0.979 | ||
| F | Y6(CFW) | <0.010 | <0.010 | 0.053 * | <0.010 | <0.010 | <0.010 | 0.010 | Small/coated: 52.711 + 270.793X1 − 17.662 X12 Small/plain: 152.052 + 237.960X1 − 17.662 X12 Large/coated: 46.443 + 270.794X1 − 17.662 X12 Large/plain: 185.482 + 237.960X1 − 17.662 X12 | 0.994 | |
| G | Y7(FWV) | <0.010 | 0.018 | <0.01 | <0.010 | 0.020 | <0.001 | 0.012 | Small/coated: −0.680 + 1.522X1 – 0.299X12 Small/plain: 2.397 + 1.107X1 − 0.299X12 Large/coated: -0.643 + 1.522X1 − 0.299X12 Large/plain: 0.974 + 1.107X1 − 0.299X12 | 0.964 |
| Input Variable | Sensitivity | Saliency (×102) |
|---|---|---|
| X1: “apparent” pellet density | 7.05 | 8.37 |
| X2: pellet size | 2.32 | 1.71 |
| X3: pellet coating | 2.48 | 2.05 |
| Responses | R2 of Predicted vs. Experimental Results Achieved by the Models | |||||
|---|---|---|---|---|---|---|
| MLR | ANN | |||||
| StBack | BackMom | Rprop | OBD | MBP | ||
| Y1: pellet’s bulk density | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| Y2: pellet’s tap density | 0.998 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| Y3: Carr’s index | 0.659 | 0.923 | 0.937 | 0.916 | 0.920 | 0.908 |
| Y4: Kawakita’s parameter a | 0.611 | 0.947 | 0.954 | 0.969 | 0.951 | 0.923 |
| Y5: angle of internal flow | 0.877 | 0.950 | 0.936 | 0.907 | 0.942 | 0.935 |
| Y6: capsule fill weight | 0.948 | 0.991 | 0.966 | 0.980 | 0.959 | 0.971 |
| Y7: capsule weight variation | 0.334 | 0.920 | 0.853 | 0.922 | 0.918 | 0.910 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barmpalexis, P.; Partheniadis, I.; Mitra, K.-S.; Toskas, M.; Papadopoulou, L.; Nikolakakis, I. Application of Multiple Linear Regression and Artificial Neural Networks for the Prediction of the Packing and Capsule Filling Performance of Coated and Plain Pellets Differing in Density and Size. Pharmaceutics 2020, 12, 244. https://doi.org/10.3390/pharmaceutics12030244
Barmpalexis P, Partheniadis I, Mitra K-S, Toskas M, Papadopoulou L, Nikolakakis I. Application of Multiple Linear Regression and Artificial Neural Networks for the Prediction of the Packing and Capsule Filling Performance of Coated and Plain Pellets Differing in Density and Size. Pharmaceutics. 2020; 12(3):244. https://doi.org/10.3390/pharmaceutics12030244
Chicago/Turabian StyleBarmpalexis, Panagiotis, Ioannis Partheniadis, Konstantina-Sepfora Mitra, Miltiadis Toskas, Labrini Papadopoulou, and Ioannis Nikolakakis. 2020. "Application of Multiple Linear Regression and Artificial Neural Networks for the Prediction of the Packing and Capsule Filling Performance of Coated and Plain Pellets Differing in Density and Size" Pharmaceutics 12, no. 3: 244. https://doi.org/10.3390/pharmaceutics12030244
APA StyleBarmpalexis, P., Partheniadis, I., Mitra, K.-S., Toskas, M., Papadopoulou, L., & Nikolakakis, I. (2020). Application of Multiple Linear Regression and Artificial Neural Networks for the Prediction of the Packing and Capsule Filling Performance of Coated and Plain Pellets Differing in Density and Size. Pharmaceutics, 12(3), 244. https://doi.org/10.3390/pharmaceutics12030244








