Along the Process Chain to Probiotic Tablets: Evaluation of Mechanical Impacts on Microbial Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Viability Assay
2.3. Freeze-Drying
2.4. Milling of Lyophilizates
2.5. Blending with Excipients
2.6. Tablet Preparation
2.7. Tablet Characterization
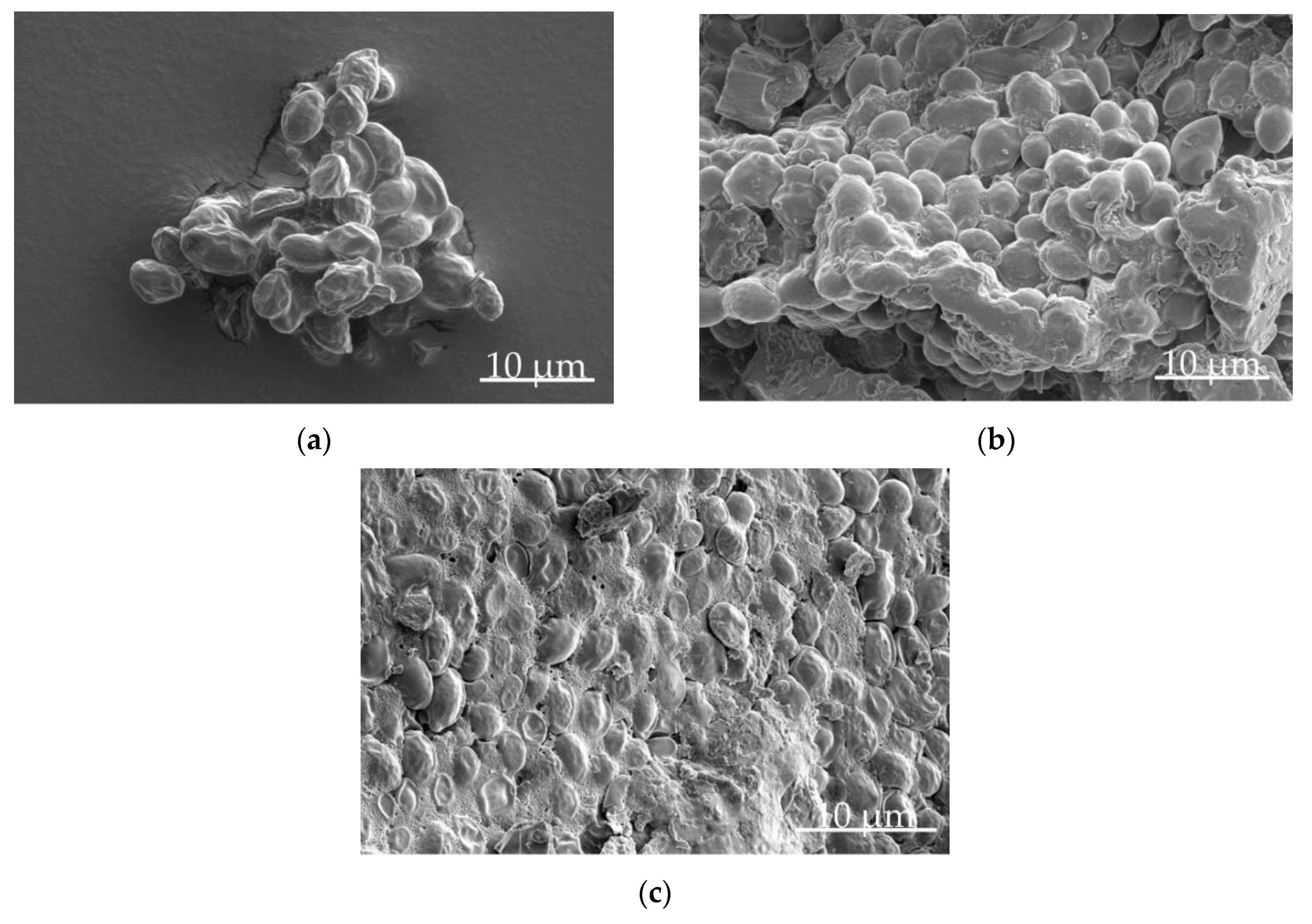
2.8. Scanning Electron Microscopy
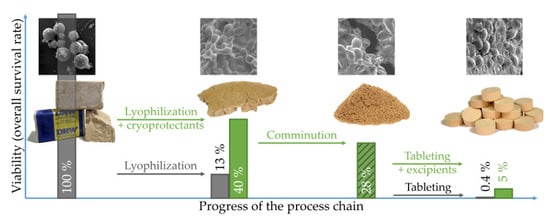
3. Results and Discussion
3.1. Influence of Freeze-Drying on Viability
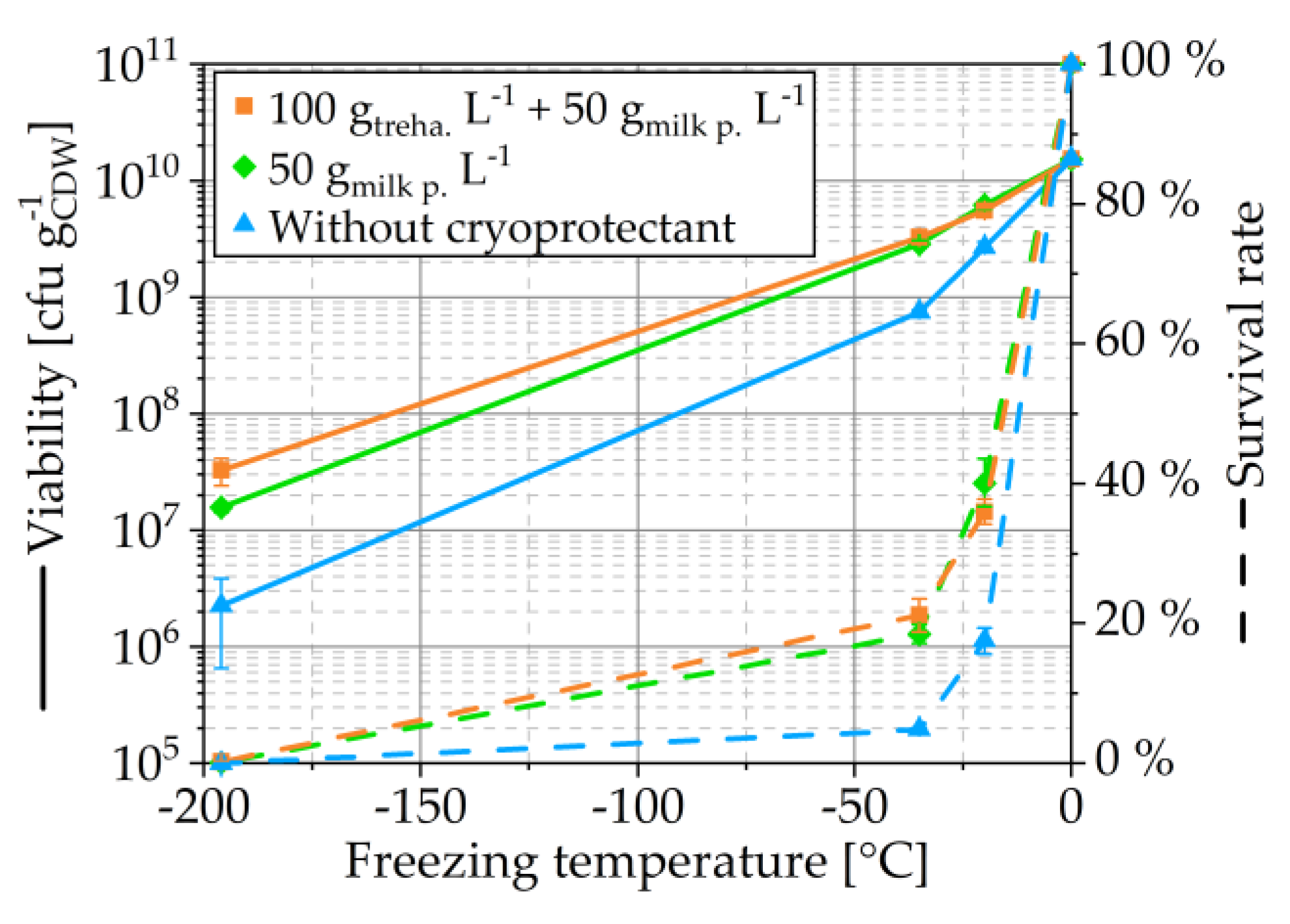
3.1.1. Freezing Temperature
3.1.2. Cryoprotectants
3.2. Influence of Lyophilizate Comminution on Survival
3.3. Influence of Mixing with Excipients on Survival
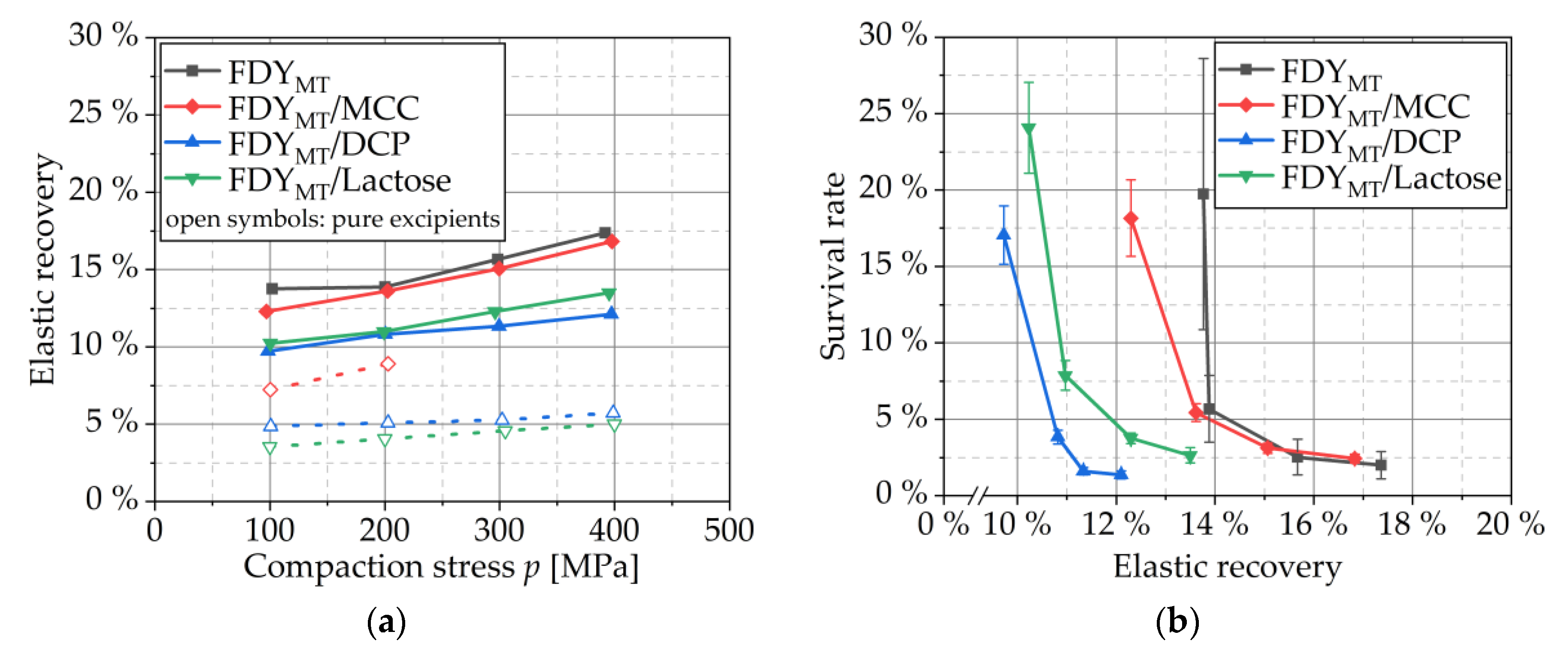
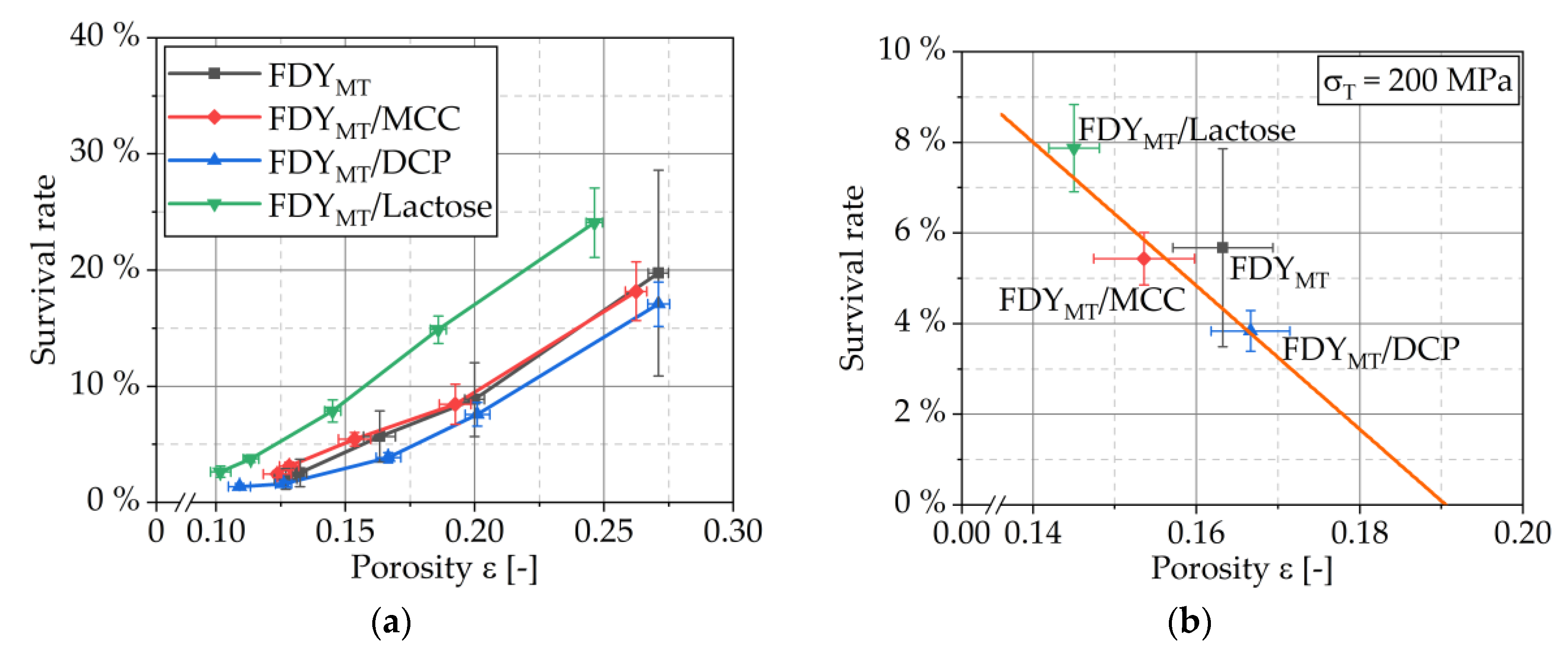
3.4. Influence of Tableting Stress and Formulation on Survival and Physical Tablet Properties
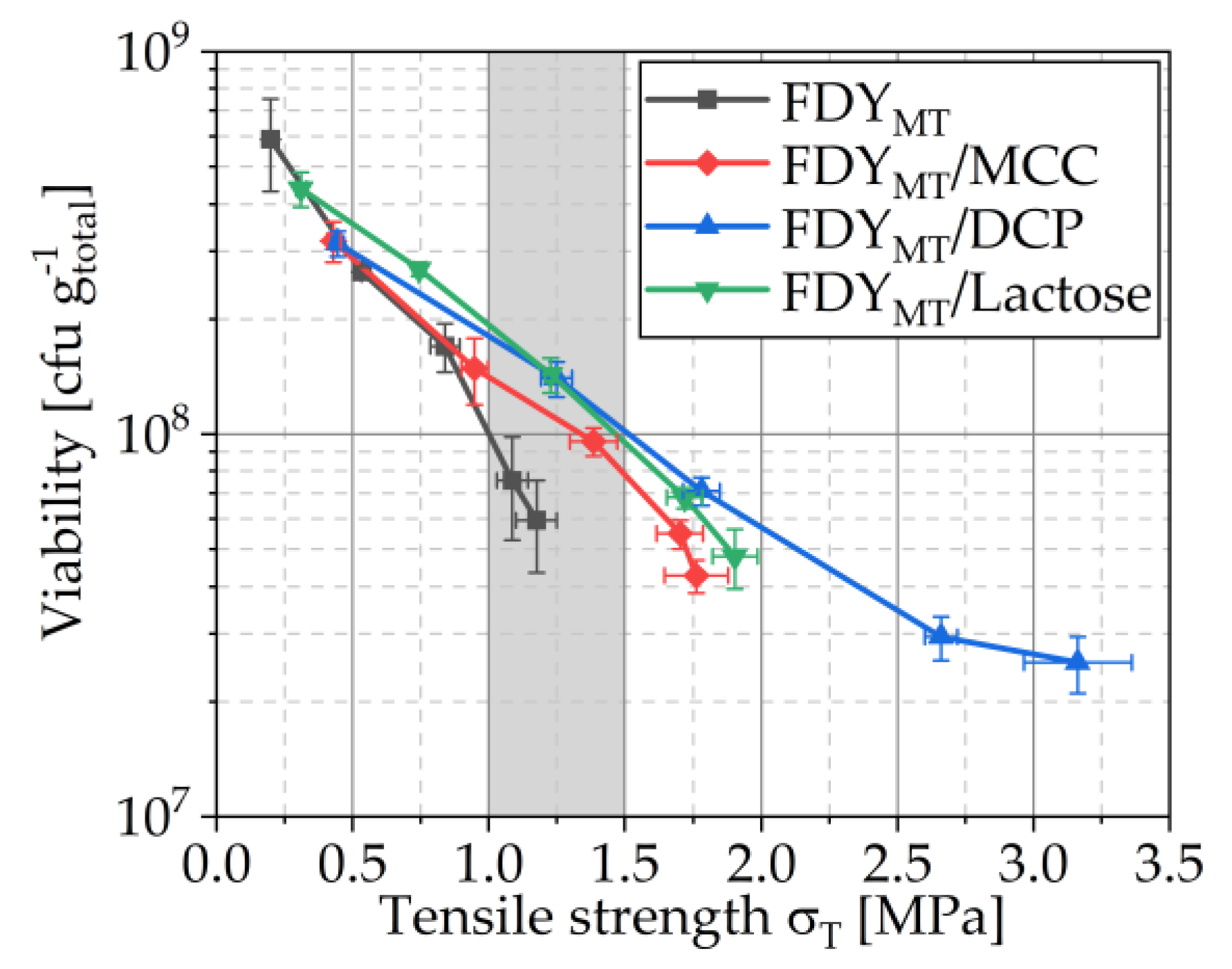
3.4.1. Viability Curves
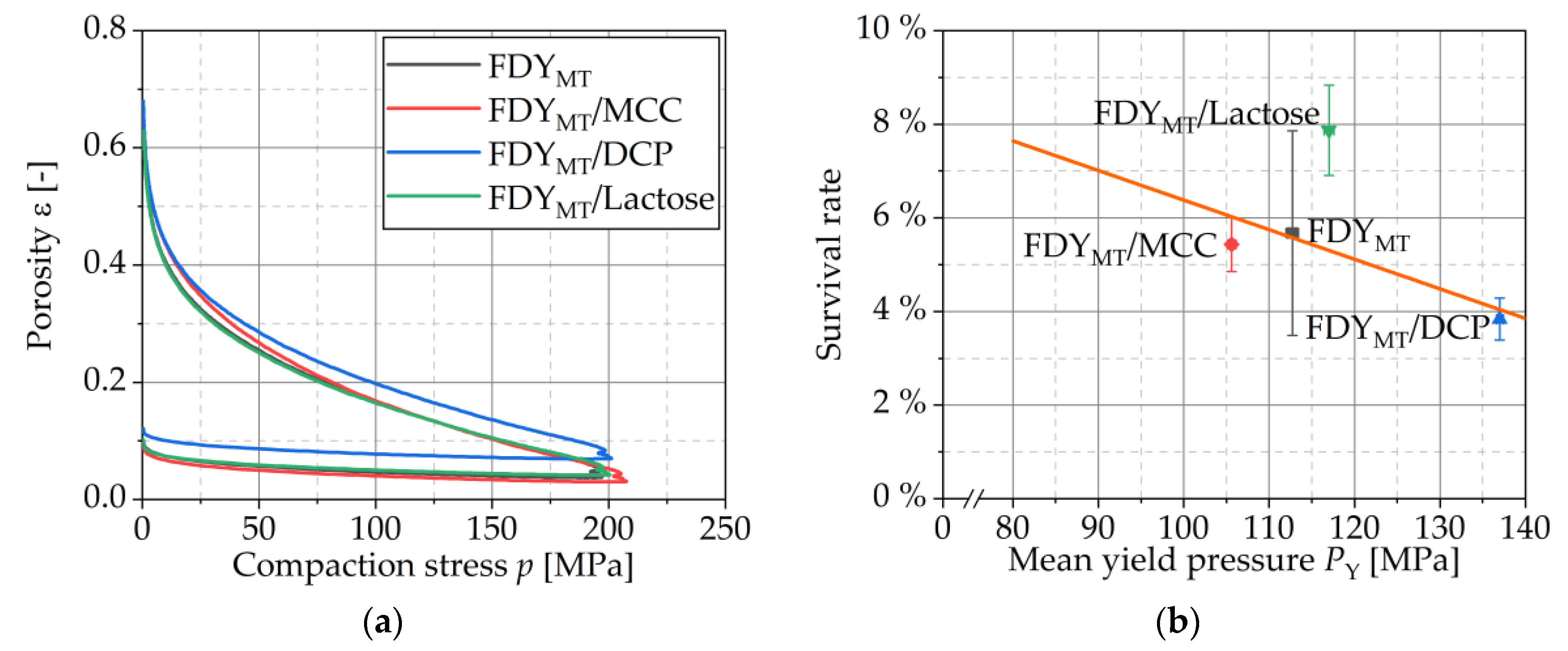
3.4.2. Compression Analysis
3.4.3. Elastic Recovery
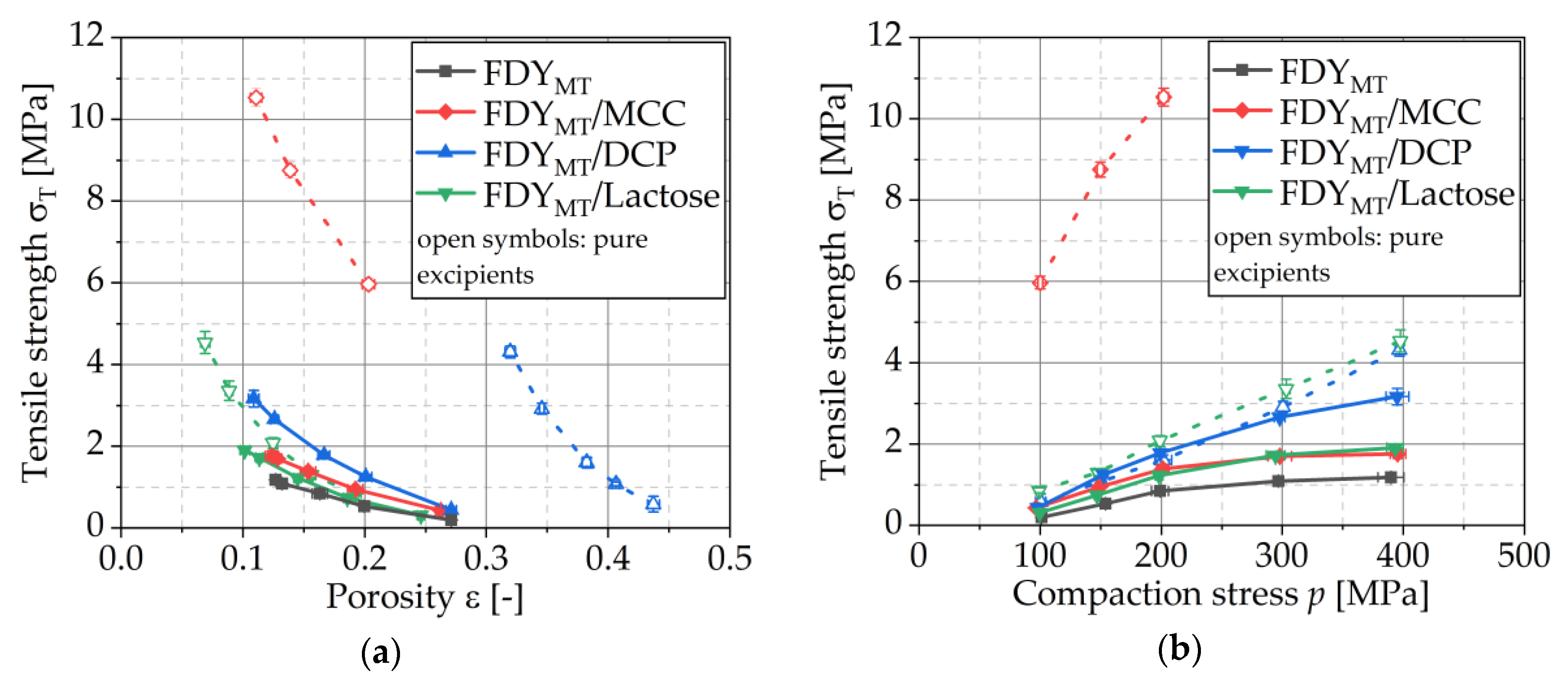
3.4.4. Compressibility
3.4.5. Compactibility and Tabletability
3.4.6. Friability
3.4.7. Disintegration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Santivarangkna, C. Storage Stability of Probiotic Powder. In Advances in Probiotic Technology; Foerst, P., Santivarangkna, C., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Govender, M.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; van Vuuren, S.; Pillay, V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS PharmSciTech 2014, 15, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Klayraung, S.; Viernstein, H.; Okonogi, S. Development of tablets containing probiotics: Effects of formulation and processing parameters on bacterial viability. Int. J. Pharm. 2009, 370, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Blair, T.C.; Buckton, G.; Bloomfield, S.F. On the mechanism of kill of microbial contaminants during tablet compression. Int. J. Pharm. 1991, 72, 111–115. [Google Scholar] [CrossRef]
- Plumpton, E.J.; Gilbert, P.; Fell, J.T. The survival of microorganisms during tabletting. Int. J. Pharm. 1986, 30, 241–246. [Google Scholar] [CrossRef]
- Fassihi, A.R.; Parker, M.S. Inimical Effects of Compaction Speed on Microorganisms in Powder Systems with Dissimilar Compaction Mechanisms. J. Pharm. Sci. 1987, 76, 466–470. [Google Scholar] [CrossRef]
- Plumpton, E.J.; Gilbert, P.; Fell, J.T. Effect of spatial distribution of contaminant microorganisms within tablet formulations on subsequent inactivation through compaction. Int. J. Pharm. 1986, 30, 237–240. [Google Scholar] [CrossRef]
- Byl, E.; Lebeer, S.; Kiekens, F. Elastic recovery of filler-binders to safeguard viability of Lactobacillus rhamnosus GG during direct compression. Eur. J. Pharm. Biopharm. 2018. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia (Ph. Eur.) 9.3; Deutscher Apotheker: Stuttgart, Germany, 2017; ISBN 978-3-7692-6641-2.
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Meryman, H.T. Freezing injury and its prevention in living cells. Annu. Rev. Biophys. Bioeng. 1974, 3, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Benabarre, A.; Teixidó, N.; Usall, J.; Viñas, I. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int. J. Food Microbiol. 2001, 65, 173–182. [Google Scholar] [CrossRef]
- Blanquet, S.; Garrait, G.; Beyssac, E.; Perrier, C.; Denis, S.; Hébrard, G.; Alric, M. Effects of cryoprotectants on the viability and activity of freeze dried recombinant yeasts as novel oral drug delivery systems assessed by an artificial digestive system. Eur. J. Pharm. Biopharm. 2005, 61, 32–39. [Google Scholar] [CrossRef]
- Miyamoto-Shinohara, Y.; Imaizumi, T.; Sukenobe, J.; Murakami, Y.; Kawamura, S.; Komatsu, Y. Survival rate of microbes after freeze-drying and long-term storage. Cryobiology 2000, 41, 251–255. [Google Scholar] [CrossRef]
- Lodato, P.; Segovia de Huergo, M.; Buera, M.P. Viability and thermal stability of a strain of Saccharomyces cerevisiae freeze-dried in different sugar and polymer matrices. Appl. Microbiol. Biotechnol. 1999, 52, 215–220. [Google Scholar] [CrossRef]
- Berny, J.-F.; Hennebert, G.L. Viability and Stability of Yeast Cells and Filamentous Fungus Spores during Freeze-Drying: Effects of Protectants and Cooling Rates. Mycologia 1991, 83, 805. [Google Scholar] [CrossRef]
- Chen, H.; Tian, M.; Chen, L.; Cui, X.; Meng, J.; Shu, G. Optimization of composite cryoprotectant for freeze-drying Bifidobacterium bifidum BB01 by response surface methodology. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1559–1569. [Google Scholar] [CrossRef]
- Nag, A.; Das, S. Effect of trehalose and lactose as cryoprotectant during freeze-drying, in vitro gastro-intestinal transit and survival of microencapsulated freeze-dried Lactobacillus casei 431 cells. Int. J. Dairy Technol. 2013, 66, 162–169. [Google Scholar] [CrossRef]
- Guowei, S.; Yang, X.; Li, C.; Huang, D.; Lei, Z.; He, C. Comprehensive optimization of composite cryoprotectant for Saccharomyces boulardii during freeze-drying and evaluation of its storage stability. Prep. Biochem. Biotechnol. 2019, 49, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B 2005, 40, 107–113. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirsh, A.; Erbe, E.; Williams, R.J. Mechanism of cryoprotection by extracellular polymeric solutes. Biophys. J. 1988, 54, 509–518. [Google Scholar] [CrossRef]
- Seville, J.; Wu, C.-Y. Particle Technology and Engineering. An Engineer’s Guide to Particles and Powders: Fundamentals and Computational Approaches; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nagashima, A.I.; Pansiera, P.E.; Baracat, M.M.; Gómez, R.J.H.C. Development of effervescent products, in powder and tablet form, supplemented with probiotics Lactobacillus acidophilus and Saccharomyces boulardii. Food Sci. Technol. 2013, 33, 605–611. [Google Scholar] [CrossRef]
- Allouche, R.; Dupont, S.; Charriau, A.; Gervais, P.; Beney, L.; Chambin, O. Optimized tableting for extremely oxygen-sensitive probiotics using direct compression. Int. J. Pharm. 2018, 538, 14–20. [Google Scholar] [CrossRef]
- Chan, E.S.; Zhang, Z. Encapsulation of Probiotic Bacteria Lactobacillus Acidophilus by Direct Compression. Food Bioprod. Process. 2002, 80, 78–82. [Google Scholar] [CrossRef]
- Wünsch, I.; Finke, J.H.; John, E.; Juhnke, M.; Kwade, A. A Mathematical Approach to Consider Solid Compressibility in the Compression of Pharmaceutical Powders. Pharmaceutics 2019, 11, 121. [Google Scholar] [CrossRef]
- Hooper, D.; Clarke, F.C. A Modern Approach to the Heckel Equation: The Effect of Compaction Pressure on the Yield Pressure of Ibuprofen and its Sodium Salt. J. Nanomed. Nanotechnol. 2016, 7. [Google Scholar] [CrossRef]
- Ilkka, J.; Paronen, P. Prediction of the compression behaviour of powder mixtures by the Heckel equation. Int. J. Pharm. 1993, 94, 181–187. [Google Scholar] [CrossRef]
- Heckel, R.W. Density-Pressure Relationships in Powder Compaction. Tran. Metall. AIME 1961, 221, 671–675. [Google Scholar]
- Paronen, P.; Juslin, M. Compressional characteristics of four starches. J. Pharm. Pharmacol. 1983, 35, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Malamataris, S.; Pilpel, N. Tensile strength and compression of coated pharmaceutical powders: Tablets. J. Pharm. Pharmacol. 1983, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hersey, J.A.; Rees, J.E. Deformation of Particles during Briquetting. Nat. Phys. Sci. 1971, 230, 96. [Google Scholar] [CrossRef]
- Ryshkewitch, E. Compression Strength of Porous Sintered Alumina and Zirconia. J. Am. Ceram. Soc. 1953, 36, 65–68. [Google Scholar] [CrossRef]
- The United States Pharmacopeia and National Formulary (USP 40-NF 35); The United States Pharmacopeial: North Bethesda, MD, USA, 2017; ISBN 9783769268218.
- Higuchi, T.; Rao, A.N.; Busse, L.W.; Swintosky, J.V. The physics of tablet compression. II. The influence of degree of compression on properties of tablets. J. Am. Pharm. Assoc. 1953, 42, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.J.; Parrott, E.L. Effect of Lubricants on Tensile Strengths of Tablets. Drug Dev. Ind. Pharm. 1984, 10, 259–273. [Google Scholar] [CrossRef]
- Quodbach, J.; Kleinebudde, P. A critical review on tablet disintegration. Pharm. Dev. Technol. 2016, 21, 763–774. [Google Scholar] [CrossRef]



| Cryoprotectant | Viability | Survival Rate (%) | Viability |
|---|---|---|---|
| 40% milk powder | 7.50 ± 1.73 | 42.9 | 4.66 ± 1.08 |
| 25% trehalose + 25% milk powder | 7.04 ± 0.09 | 40.2 | 4.39 ± 0.06 |
| 25% trehalose + 15% milk powder | 7.00 ± 0.10 | 40.0 | 4.88 ± 0.07 |
| 40% trehalose | 6.63 ± 0.49 | 37.9 | 4.19 ± 0.31 |
| 15% dextran | 6.33 ± 1.85 | 36.2 | 5.70 ± 1.67 |
| 25% milk powder | 6.15 ± 1.66 | 35.2 | 4.81 ± 1.30 |
| 15% milk powder | 5.78 ± 0.65 | 33.0 | 5.23 ± 0.59 |
| 25% dextran | 5.58 ± 1.59 | 31.9 | 4.41 ± 1.29 |
| 15% glycerin | 5.36 ± 0.16 | 30.7 | 4.80 ± 0.14 |
| 40% maltose | 4.61 ± 0.56 | 26.3 | 2.97 ± 0.36 |
| 15% trehalose + 15% milk powder | 4.50 ± 0.40 | 25.7 | 3.21 ± 0.28 |
| 40% dextran | 4.42 ± 0.13 | 25.3 | 2.78 ± 0.09 |
| 25% lactose | 4.21 ± 0.28 | 24.1 | 3.29 ± 0.22 |
| 40% lactose | 3.65 ± 0.09 | 20.9 | 2.27 ± 0.06 |
| 25% trehalose | 3.50 ± 0.25 | 20.0 | 2.81 ± 0.20 |
| 15% lactose | 3.48 ± 0.41 | 19.9 | 3.09 ± 0.36 |
| 15% trehalose | 3.33 ± 0.13 | 19.1 | 3.01 ± 0.12 |
| 15% glutamic acid | 2.37 ± 0.47 | 13.5 | 2.21 ± 0.44 |
| 1/5 × PBS | 2.37 ± 0.47 | 13.5 | 2.44 ± 0.48 |
| Purified water | 2.16 ± 0.22 | 12.4 | 2.28 ± 0.23 |
| 25% glycerin | 1.88 ± 0.15 | 10.7 | 1.50 ± 0.16 |
| 25% maltose | 1.50 ± 0.13 | 8.6 | 1.21 ± 0.10 |
| 15% maltose | 1.08 ± 0.10 | 6.2 | 1.21 ± 0.11 |
| 40% glycerin | 0.15 ± 0.01 | 0.8 | 1.21 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorländer, K.; Kampen, I.; Finke, J.H.; Kwade, A. Along the Process Chain to Probiotic Tablets: Evaluation of Mechanical Impacts on Microbial Viability. Pharmaceutics 2020, 12, 66. https://doi.org/10.3390/pharmaceutics12010066
Vorländer K, Kampen I, Finke JH, Kwade A. Along the Process Chain to Probiotic Tablets: Evaluation of Mechanical Impacts on Microbial Viability. Pharmaceutics. 2020; 12(1):66. https://doi.org/10.3390/pharmaceutics12010066
Chicago/Turabian StyleVorländer, Karl, Ingo Kampen, Jan Henrik Finke, and Arno Kwade. 2020. "Along the Process Chain to Probiotic Tablets: Evaluation of Mechanical Impacts on Microbial Viability" Pharmaceutics 12, no. 1: 66. https://doi.org/10.3390/pharmaceutics12010066
APA StyleVorländer, K., Kampen, I., Finke, J. H., & Kwade, A. (2020). Along the Process Chain to Probiotic Tablets: Evaluation of Mechanical Impacts on Microbial Viability. Pharmaceutics, 12(1), 66. https://doi.org/10.3390/pharmaceutics12010066