Development of an Orodispersible Film Containing Stabilized Influenza Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Virus Preparation
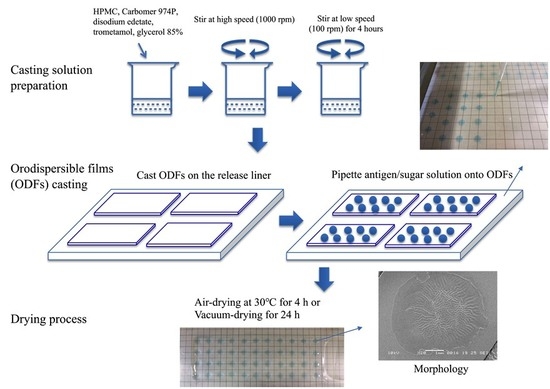
2.3. Preparation of the Casting Solution and ODFs
2.4. Preparation of Sugar Solutions with Antigen and Antigen Incorporated ODFs
2.5. Uniformity of Mass and Thickness
2.6. Disintegration Time
2.7. Mechanical Properties
2.8. Process and Storage Stability Testing
2.9. β-Galactosidase Enzymatic Activity Assay
2.10. Hemagglutination Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. ODFs with β-Galactosidase Incorporated
3.1.1. Characterizations of ODFs
- (a)
- Uniformity of Mass and Thickness and DisintegrationAs shown in Table 3 and Table 4, incorporation of β-galactosidase together with sugars in ODFs showed acceptable uniformity of mass and thickness with low standard deviations. All ODFs disintegrated within 30 s, which is recommended by U.S. Food and Drug Administration (FDA) for orally disintegrating tablets [28]. Furthermore, vacuum-dried ODFs showed slightly shorter (but not significantly different) disintegration time than air-dried ODFs.
- (b)
- Mechanical PropertiesAs can be seen in Table 3 and Table 4, by the incorporation of sugars, the ODFs had slightly lower tensile strength and elongation at break than plain ODFs (p < 0.0001), which means they became more brittle and thus less flexible. Furthermore, ODFs with the highest trehalose concentration (Trehalose (1.0) and BSA-Trehalose (1.0)) had the lowest tensile strength and elongation at break in both air- and vacuum-dried ODFs, which means they were the most brittle and fragile. Additionally, in a previous study, we found that incorporation of increasing amounts of trehalose in ODFs resulted in increasing deterioration of the mechanical properties [18]. This phenomenon can be explained by the low molecular weight of trehalose, which makes it a poor film former. Vacuum-dried ODFs showed a slightly lower (but not significantly different) tensile strength and elongation at break than air-dried ODFs.
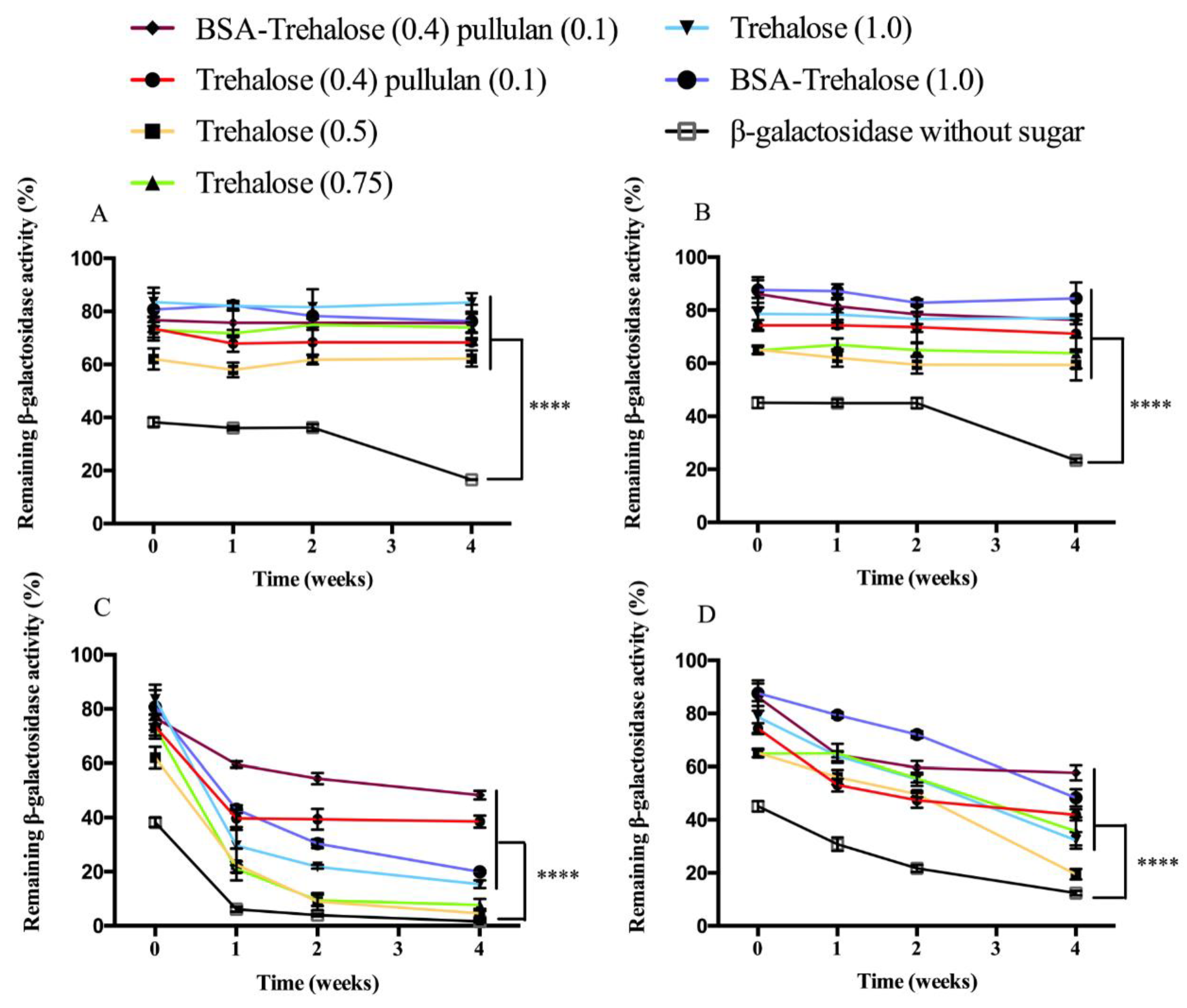
3.1.2. Enzymatic Activity of β-Galactosidase
3.2. ODFs with WIV Incorporated
3.2.1. Biological Activity of WIV Incorporated into ODFs
3.2.2. Biological Activity of WIV without ODFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somes, M.P.; Turner, R.M.; Dwyer, L.J.; Newall, A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine 2018, 36, 3199–3207. [Google Scholar] [CrossRef]
- Vemula, S.V.; Sayedahmed, E.E.; Sambhara, S.; Suresh, K. Vaccine approaches conferring cross-protection against influenza viruses. Expert Rev. Vaccines 2017, 16, 1141–1154. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Wang, B.; Zeng, S.; Qi, F.; Lu, C.; Kimura, Y.; Liu, B. Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J. Med. Virol. 2014, 86, 886–894. [Google Scholar] [CrossRef]
- Mann, J.F.S.; Tregoning, J.S.; Aldon, Y.; Shattock, R.J.; Mckay, P.F. CD71 targeting boosts immunogenicity of sublingually delivered in fl uenza haemagglutinin antigen and protects against viral challenge in mice. J. Control. Release 2016, 232, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Shim, B.; Choi, Y.K.; Yun, C.; Lee, E.; Jeon, Y.S.; Park, S.; Cheon, I.S.; Joo, D.; Cho, C.H.; Song, M.; et al. Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza. PLoS ONE 2011, 6, e27953. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.N. Cytokine Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine 2011, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Nguyen, H.H.; Cuburu, N.; Horimoto, T.; Ko, S.; Park, S.; Czerkinsky, C.; Kweon, M. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 1644–1649. [Google Scholar] [CrossRef]
- Lee, Y.; Youn, H.; Kwon, J.; Lee, D.; Park, J.; Yuk, S.; Erdene-ochir, T.; Kim, K.; Lee, J.; Park, S.; et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antivir. Res. 2013, 98, 284–290. [Google Scholar] [CrossRef]
- Jones, A.T.; Shen, X.; Walter, K.L.; Labranche, C.C.; Wyatt, L.S.; Tomaras, G.D.; Montefiori, D.C.; Moss, B.; Barouch, D.H.; Clements, J.D.; et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 2019, 10, 798. [Google Scholar] [CrossRef]
- Kraan, H.; Vrieling, H.; Czerkinsky, C.; Jiskoot, W.; Kersten, G.; Amorij, J. Buccal and sublingual vaccine delivery. J. Control. Release 2014, 190, 580–592. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Yorgensen, Y.M.; Morasse, A.; Evans, J.T.; Burkhart, D.J. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to in fluenza vaccination. J. Control. Release 2016, 223, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, S.; Patil, H.P.; Frijlink, H.W.; Huckriede, A.; Hinrichs, W.L.J. Simplifying influenza vaccination during pandemics: Sublingual priming and intramuscular boosting of immune responses with heterologous whole inactivated influenza vaccine. AAPS J. 2014, 16, 342–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Visser, J.C.; Weggemans, O.A.F.; Boosman, R.J.; Loos, K.U.; Frijlink, H.W.; Woerdenbag, H.J. Increased drug load and polymer compatibility of bilayered orodispersible films. Eur. J. Pharm. Sci. 2017, 107, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; De Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior immunogenicity of inactivated whole virus h5n1 influenza vaccine is primarily controlled by toll-like receptor signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef]
- Bhide, Y.; Dong, W.; Gribonika, I.; Voshart, D.; Meijerhof, T.; de Vries-Idema, J.; Norley, S.; Guilfoyle, K.; Skeldon, S.; Engelhardt, O.G.; et al. Cross-protective potential and protection-relevant immune mechanisms of whole inactivated influenza virus vaccines are determined by adjuvants and route of immunization. Front. Immunol. 2019, 10, 646. [Google Scholar] [CrossRef]
- Tonnis, W.F.; Mensink, M.A.; de Jager, A.; van der Voort Maarschalk, K.; Frijlink, H.W.; Hinrichs, W.L.J. Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol. Pharm. 2015, 12, 684–694. [Google Scholar] [CrossRef]
- Teekamp, N.; Tian, Y.; Visser, J.C.; Olinga, P.; Frijlink, H.W.; Woerdenbag, H.J.; Hinrichs, W.L.J. Addition of pullulan to trehalose glasses improves the stability of β-galactosidase at high moisture conditions. Carbohydr.Polym. 2017, 176, 374–380. [Google Scholar] [CrossRef]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef]
- Geeraedts, F.; Saluja, V.; ter Veer, W.; Amorij, J.; Frijlink, H.W.; Wilschut, J.; Hinrichs, W.L.J.; Huckriede, A. Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010, 12, 215–222. [Google Scholar] [CrossRef]
- Ebensen, T.; Schulze, K.; Riese, P.; Link, C.; Morr, M.; Guzmán, C.A. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 2007, 25, 1464–1469. [Google Scholar] [CrossRef]
- Tomar, J.; Patil, H.P.; Bracho, G.; Tonnis, W.F.; Frijlink, H.W.; Petrovsky, N.; Vanbever, R.; Huckriede, A.; Hinrichs, W.L.J. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J. Control. Release 2018, 288, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Tomar, J.; Biel, C.; de Haan, C.A.M.; Rottier, P.J.M.; Petrovsky, N.; Frijlink, H.W.; Huckriede, A.; Hinrichs, W.L.J.; Peeters, B. Passive inhalation of dry powder influenza vaccine formulations completely protects chickens against H5N1 lethal viral challenge. Eur. J. Pharm. Biopharm. 2018, 133, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Dohmen, W.M.C.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kraan, H.; van Herpen, P.; Kersten, G.; Amorij, J. Development of thermostable lyophilized inactivated polio vaccine. Pharm. Res. 2014, 31, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Bhide, Y.; Tomar, J.; Dong, W.; de Vries-idema, J.; Frijlink, H.W.; Huckriede, A.; Hinrichs, W.L.J. Pulmonary delivery of influenza vaccine formulations in cotton rats: Site of deposition plays a minor role in the protective efficacy against clinical isolate of H1N1pdm virus. Drug Deliv. 2018, 25, 533–545. [Google Scholar] [CrossRef]
- Orally Disintegrating Tablets-FDA. Available online: https://www.fda.gov/media/70877/download (accessed on 1 December 2008).
- Lipiäinen, T.; Räikkönen, H.; Kolu, A.; Peltoniemi, M.; Juppo, A. Comparison of melibiose and trehalose as stabilising excipients for spray- dried β -galactosidase formulations. Int. J. Pharm. 2018, 543, 21–28. [Google Scholar] [CrossRef]
- Murugappan, S.; Patil, H.P.; Kanojia, G.; ter Veer, W.; Meijerhof, T.; Frijlink, H.W.; Huckriede, A.; Hinrichs, W.L.J. Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: Comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. Eur. J. Pharm. Biopharm. 2013, 85, 716–725. [Google Scholar] [CrossRef]
- Shetty, G.R.; Rao, B.L.; Asha, S.; Wang, Y.; Sangappa, Y. Preparation and characterization of silk fibroin/hydroxypropyl methyl cellulose (HPMC) blend films. Fiber Polym. 2015, 16, 1734–1741. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, M.; Li, G. Preparation and characterization of collagen/hydroxypropyl methylcellulose (HPMC) blend film. Carbohydr. Polym. 2015, 119, 194–201. [Google Scholar] [CrossRef] [PubMed]
| Formulation Code | Trehalose (mg) | Pullulan (mg) | BSA (mg) | β-Galactosidase (mg) | Methylene Blue (mg) | Total Volume of HEPES Buffer (μL) |
|---|---|---|---|---|---|---|
| BSA-Trehalose (0.4) pullulan (0.1) | 200 | 50 | 10 | 1 | 0.02 | 500 |
| Trehalose (0.4) pullulan (0.1) | 200 | 50 | / | 1 | 0.02 | 500 |
| Trehalose (0.5) | 250 | / | / | 1 | 0.02 | 500 |
| Trehalose (0.75) | 375 | / | / | 1.5 | 0.02 | 500 |
| Trehalose (1.0) | 500 | / | / | 2 | 0.02 | 500 |
| BSA-Trehalose (1.0) | 500 | / | 10 | 2 | 0.02 | 500 |
| Without sugar | / | / | / | 2 | 0.02 | 500 |
| Formulation Code | Trehalose (mg) | Pullulan (mg) | BSA (mg) | WIV (μg) | Methylene Blue (mg) | Total Volume of HEPES Buffer (μL, 2 mM, pH 7.4) | * Density of the Solution (mg/mL) | ** WIV Content in Each Dot (6 μL) (μg) |
|---|---|---|---|---|---|---|---|---|
| BSA-Trehalose (0.4) pullulan (0.1) | 120 | 30 | 3.2 | 320 | 0.02 | 300 | 1170 | 5 |
| Trehalose (0.4) pullulan (0.1) | 120 | 30 | / | 332 | 0.02 | 300 | 1130 | 5 |
| BSA-Trehalose (1.0) | 200 | / | 2.7 | 270 | 0.02 | 200 | 1250 | 5 |
| Without sugar | / | / | / | 250 | 0.02 | 300 | 1000 | 5 |
| Sugar/β-Galactosidase Solutions | BSA-Trehalose (0.4) Pullulan (0.1) | Trehalose (0.4) Pullulan (0.1) | Trehalose (0.5) | Trehalose (0.75) | Trehalose (1.0) | BSA-Trehalose (1.0) | Without Sugar | Plain Films # |
|---|---|---|---|---|---|---|---|---|
| Weight (mg) | 33.96 ± 0.94 **** | 33.82 ± 1.25 **** | 34.43 ± 0.48 **** | 37.67 ± 0.85 **** | 38.87 ± 0.83 **** | 38.83 ± 1.32 **** | 26.08 ± 1.24 ** | 24.24 ± 1.23 |
| Thickness (μm) | 118.56 ± 5.66 **** | 119.04 ± 3.49 **** | 109.09 ± 3.07 **** | 135.69 ± 4.35 **** | 143.82 ± 5.13 **** | 147.47 ± 6.03 **** | 71.6 ± 2.81 **** | 59.71 ± 3.25 |
| Disintegration time (s) | 22.67 ± 1.24 **** | 21.40 ± 0.72 **** | 23.81 ± 1.28 **** | 25.48 ± 1.77 **** | 26.68 ± 1.63 **** | 27.31 ± 2.58 **** | 16.81 ± 2.59 | 16.52 ± 1.66 |
| Tensile strength (N/mm2) | 2.37 ± 1.03 **** | 1.97 ± 0.50 **** | 1.47 ± 0.61 **** | 1.23 ± 0.34 **** | 1.17 ± 0.27 **** | 1.10 ± 0.50 **** | 4.80 ± 1.06 | 5.93 ± 1.57 |
| Elongation at break (%) | 6.24 ± 1.58 **** | 6.24 ± 1.20 **** | 6.14 ± 2.39 **** | 5.41 ± 1.67 **** | 3.5 ± 1.91 **** | 4.82 ± 1.76 **** | 10.72 ± 1.44 ** | 15.28 ± 2.84 |
| Sugar/β-Galactosidase Solutions | BSA-Trehalose (0.4) Pullulan (0.1) | Trehalose (0.4) Pullulan (0.1) | Trehalose (0.5) | Trehalose (0.75) | Trehalose (1.0) | BSA-Trehalose (1.0) | Without Sugar | Plain Films # |
|---|---|---|---|---|---|---|---|---|
| Weight (mg) | 33.61 ± 0.91 **** | 32.43 ± 0.81 **** | 33.76 ± 0.70 **** | 36.40 ± 1.19 **** | 38.48 ± 2.04 **** | 38.94 ± 0.43 **** | 27.6 ± 0.78 **** | 23.31 ± 1.32 |
| Thickness (μm) | 117.6 ± 2.37 **** | 116.5 ± 3.17 **** | 108 ± 5.45 **** | 113.67 ± 3.81 **** | 149.53 ± 7.34 **** | 150.47 ± 12.78 **** | 75.40 ± 2.95 **** | 59.93 ± 3.65 |
| Disintegration time (s) | 21.82 ± 1.16 **** | 21.99 ± 1.47 **** | 22.03 ±1.06 **** | 23.63 ± 1.51 **** | 25.13 ± 0.82 **** | 24.55 ± 1.90 **** | 15.63 ± 0.79 | 15.87± 1.47 |
| Tensile strength (N/mm2) | 2.04 ± 1.25 **** | 1.92 ± 1.23 **** | 1.85 ± 0.80 **** | 1.07 ± 0.25 **** | 1.05 ± 0.45 **** | 0.71 ± 0.15 **** | 4.22 ± 1.58 ** | 5.43 ± 1.96 |
| Elongation at break (%) | 4.70 ± 2.21 **** | 6.51 ± 3.53 **** | 4.96 ± 1.73 **** | 5.05 ± 1.03 **** | 4.73 ± 1.62 **** | 3.66 ± 1.83 **** | 10.13 ± 1.44 ** | 14.59 ± 2.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Bhide, Y.C.; Woerdenbag, H.J.; Huckriede, A.L.W.; Frijlink, H.W.; Hinrichs, W.L.J.; Visser, J.C. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics 2020, 12, 245. https://doi.org/10.3390/pharmaceutics12030245
Tian Y, Bhide YC, Woerdenbag HJ, Huckriede ALW, Frijlink HW, Hinrichs WLJ, Visser JC. Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics. 2020; 12(3):245. https://doi.org/10.3390/pharmaceutics12030245
Chicago/Turabian StyleTian, Yu, Yoshita C. Bhide, Herman J. Woerdenbag, Anke L. W. Huckriede, Henderik W. Frijlink, Wouter L. J. Hinrichs, and J. Carolina Visser. 2020. "Development of an Orodispersible Film Containing Stabilized Influenza Vaccine" Pharmaceutics 12, no. 3: 245. https://doi.org/10.3390/pharmaceutics12030245
APA StyleTian, Y., Bhide, Y. C., Woerdenbag, H. J., Huckriede, A. L. W., Frijlink, H. W., Hinrichs, W. L. J., & Visser, J. C. (2020). Development of an Orodispersible Film Containing Stabilized Influenza Vaccine. Pharmaceutics, 12(3), 245. https://doi.org/10.3390/pharmaceutics12030245