In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms
Abstract
1. Introduction
2. Physiological Considerations for Gastroretentive Dosage Forms
2.1. Gastric Motility and Transit Times
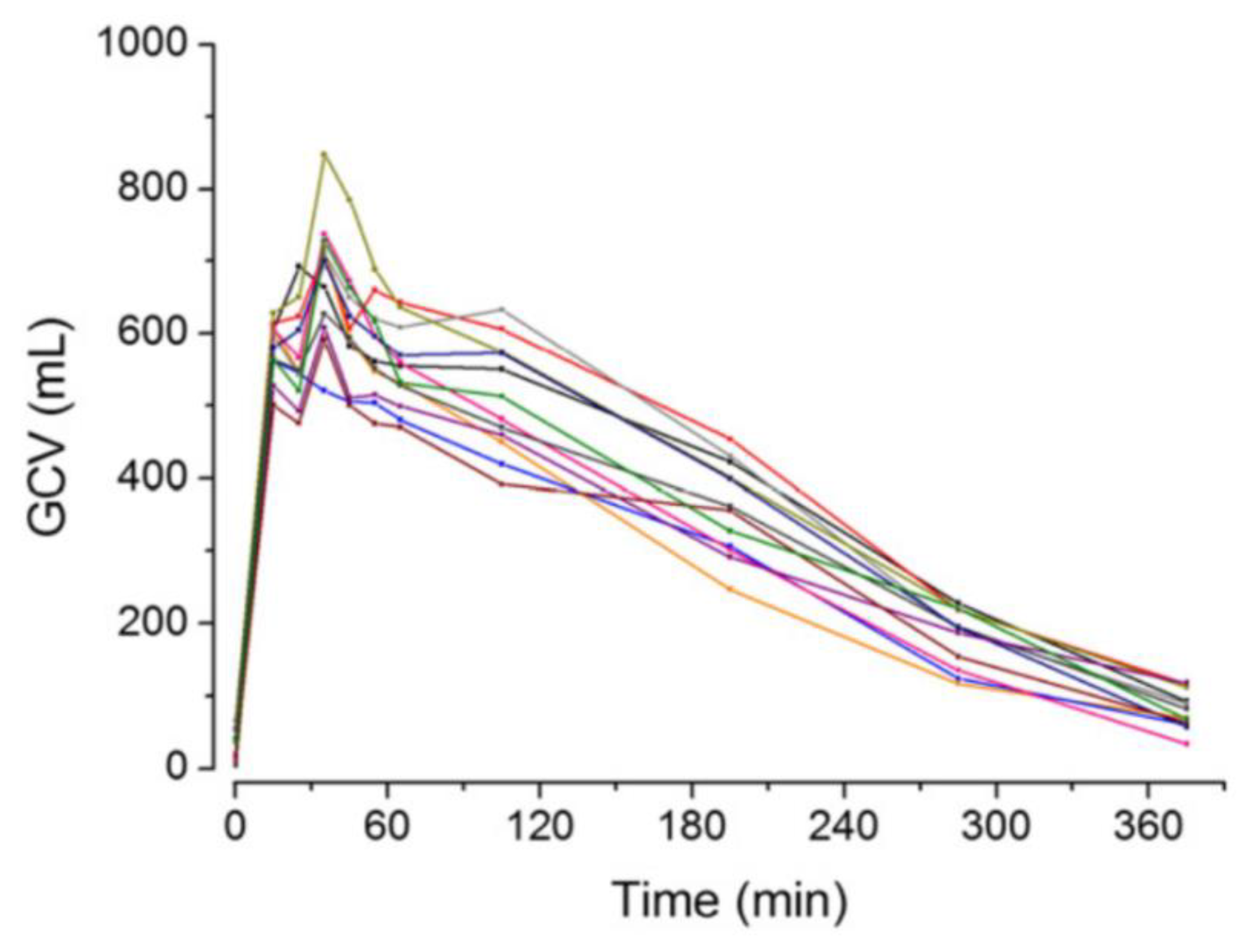
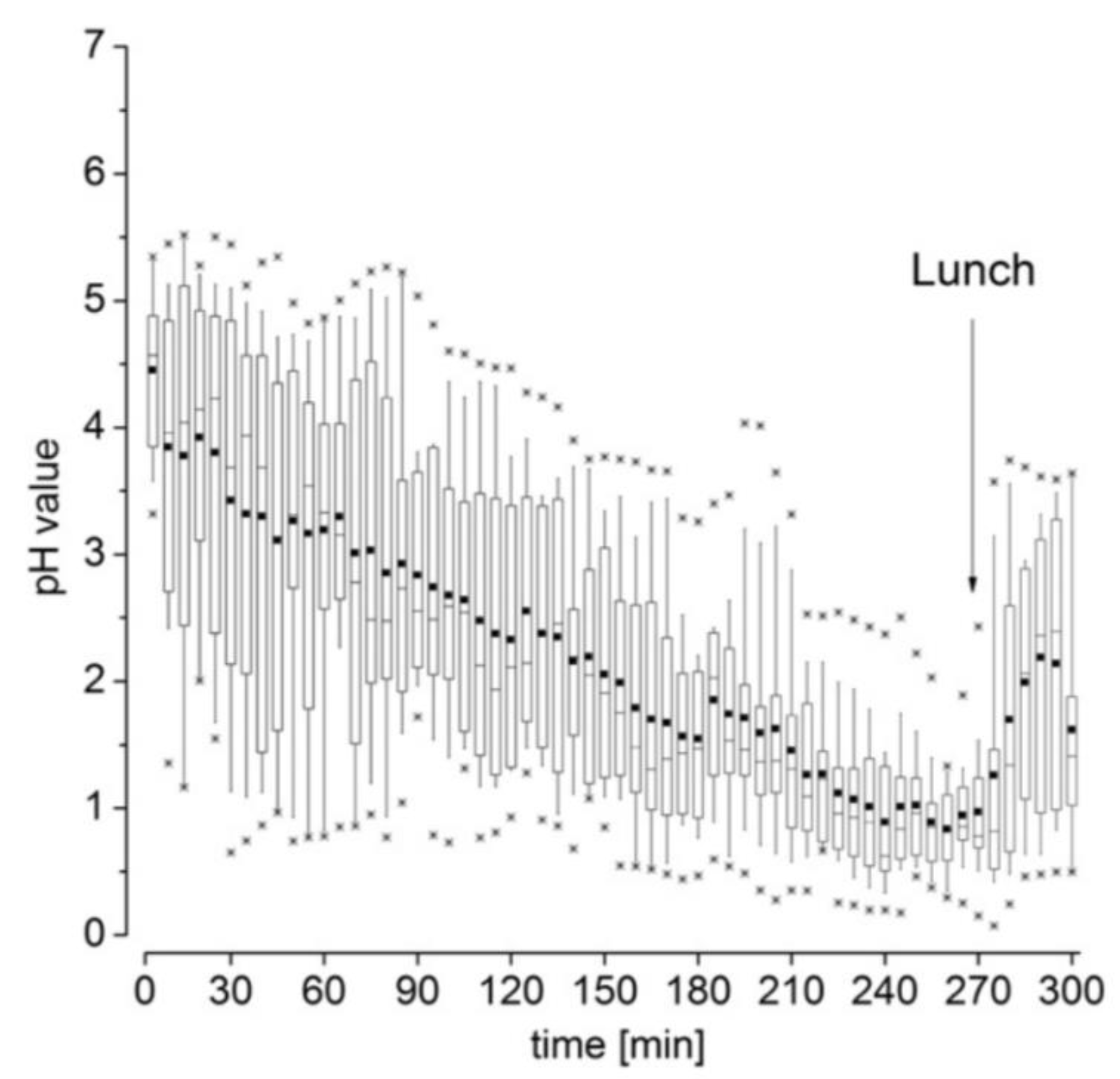
2.2. Gastric Volume and pH
3. Formulation Strategies
3.1. Floating
3.2. Sedimentation
3.3. Expansion
3.4. Mucoadhesion
4. Characterization of Gastroretentive Dosage Forms
- In vitro assessment of drug release behavior;
- In vitro and ex vivo assessment of gastroretentive properties;
- In vivo assessment of gastroretentive properties.
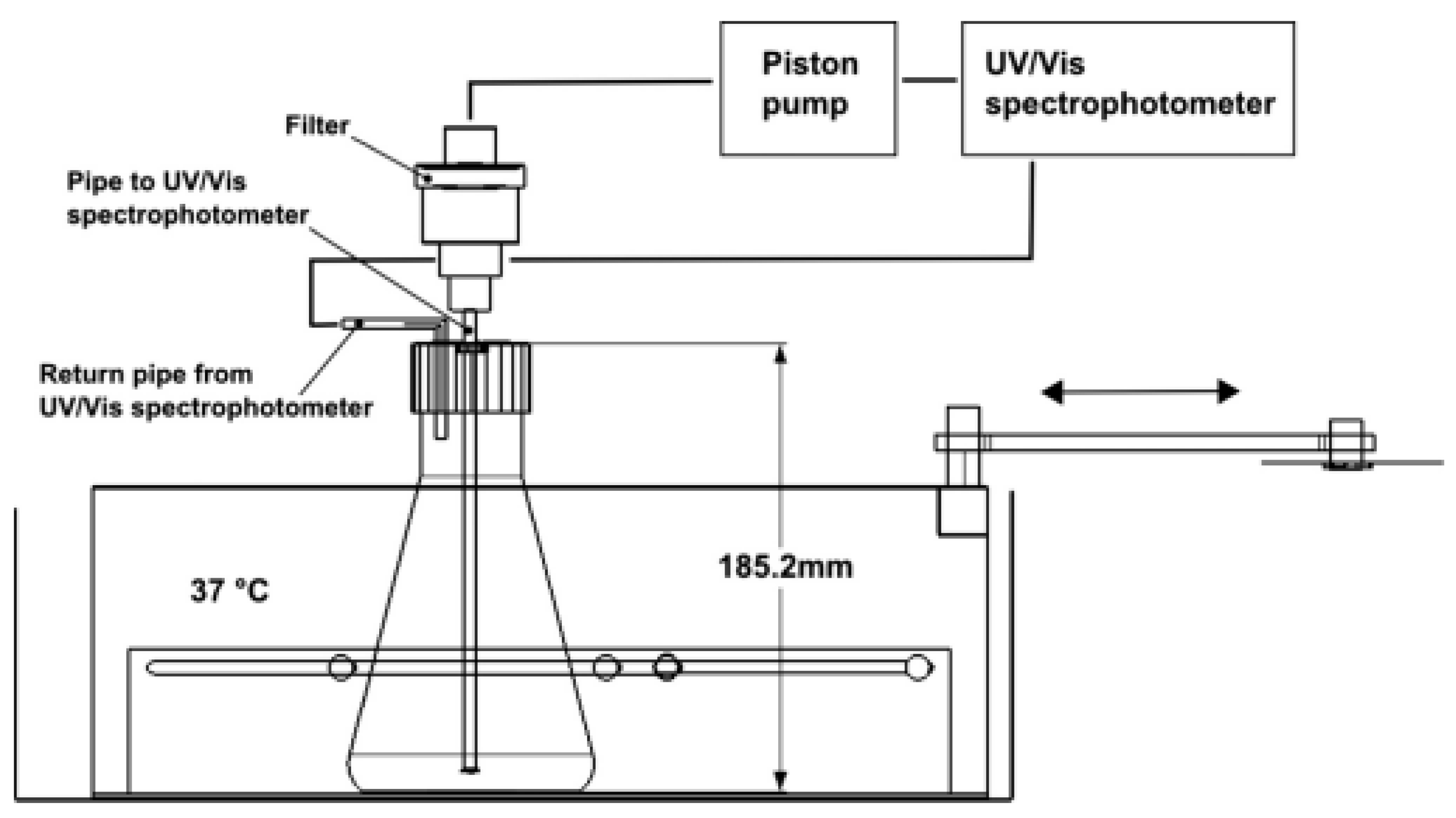
4.1. In Vitro Assessment of Drug Release Behavior
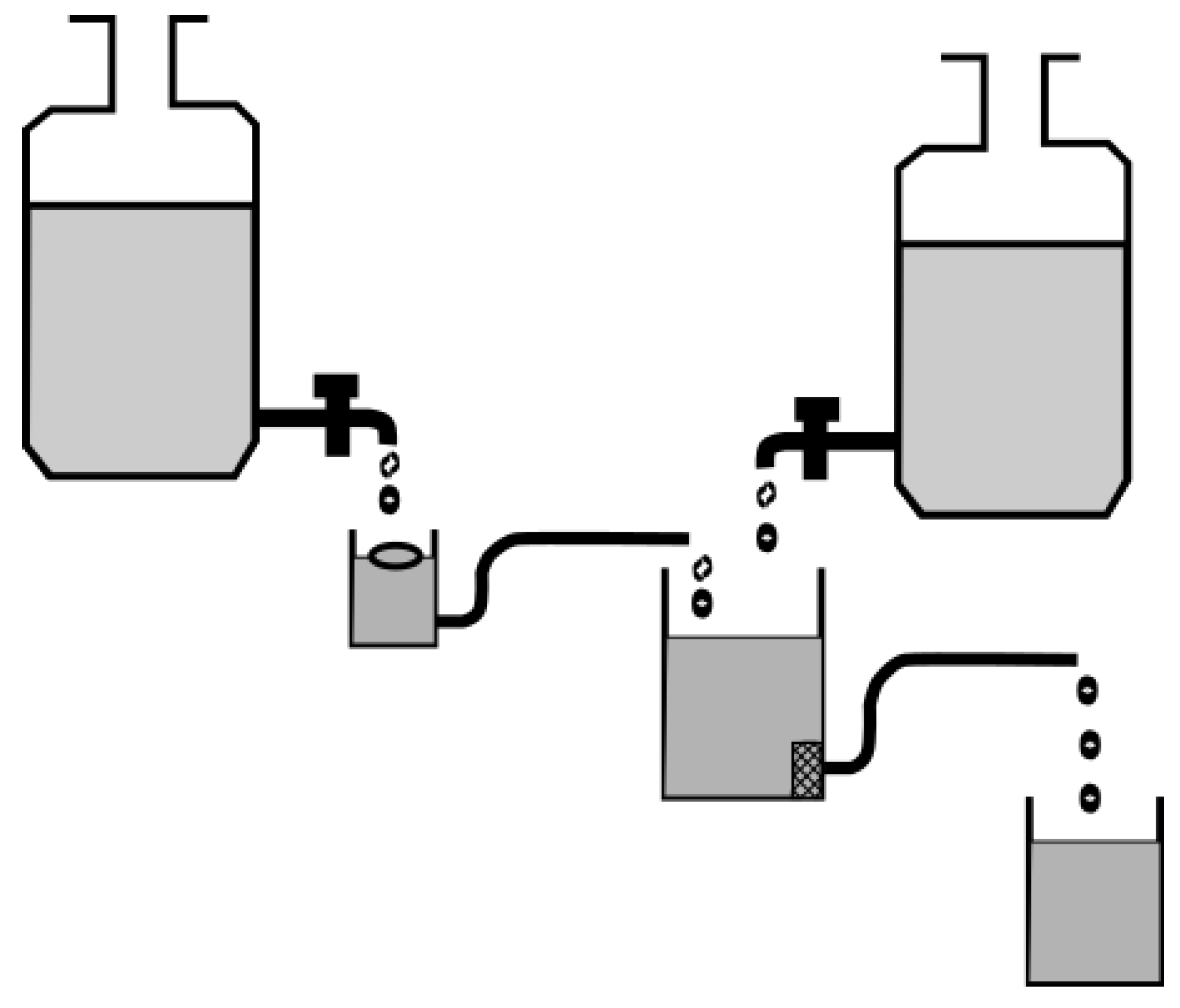
4.2. In Vitro and Ex Vivo Assessment of Gastroretentive Properties
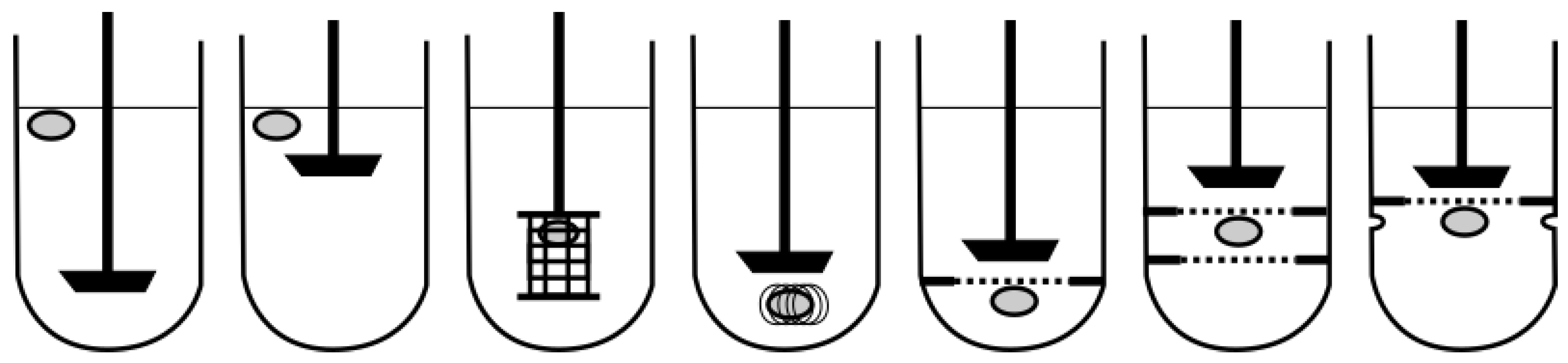
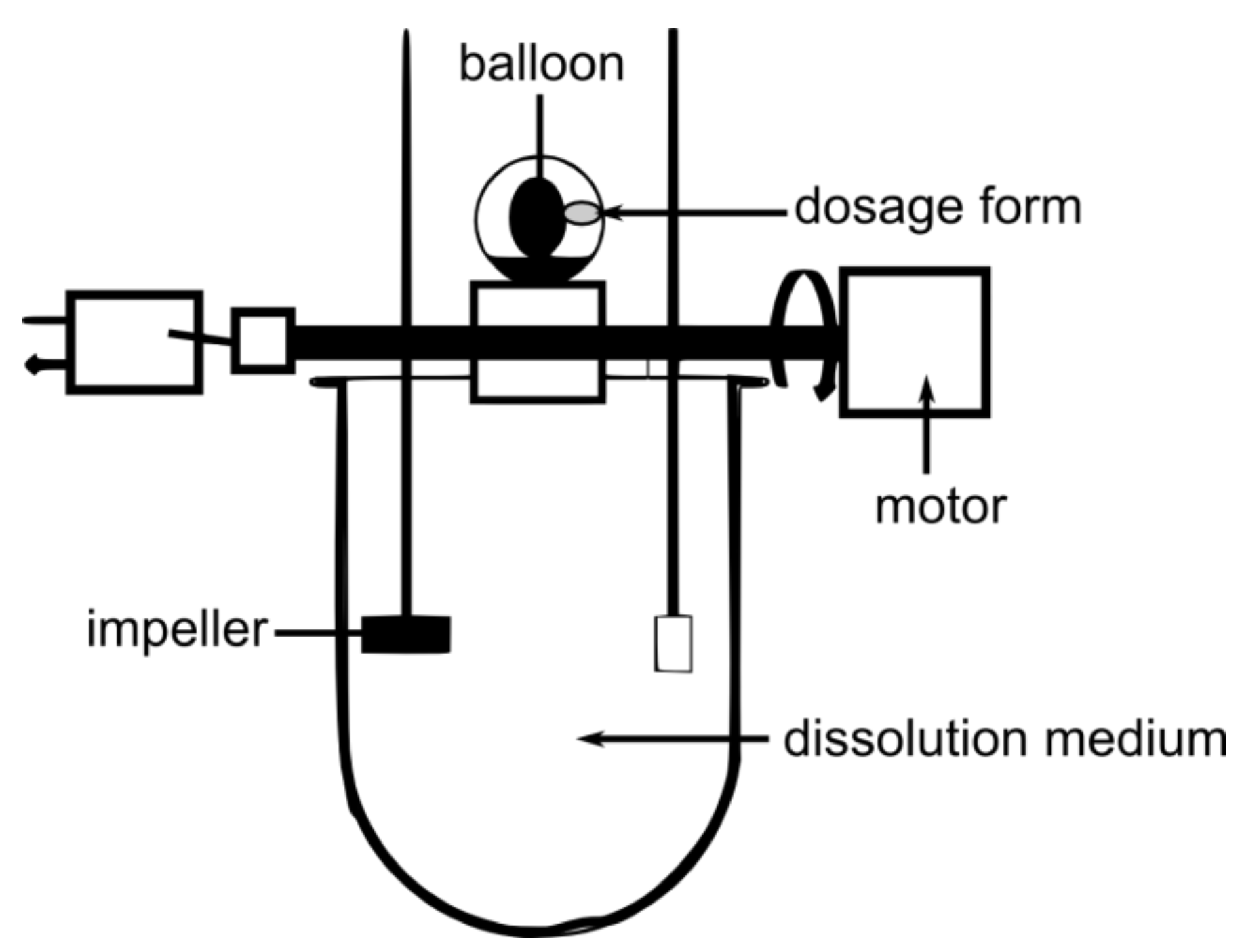
4.2.1. Floating Dosage Forms
4.2.2. Sinking Dosage Forms
4.2.3. Expanding Dosage Forms
4.2.4. Mucoadhesive Dosage Forms
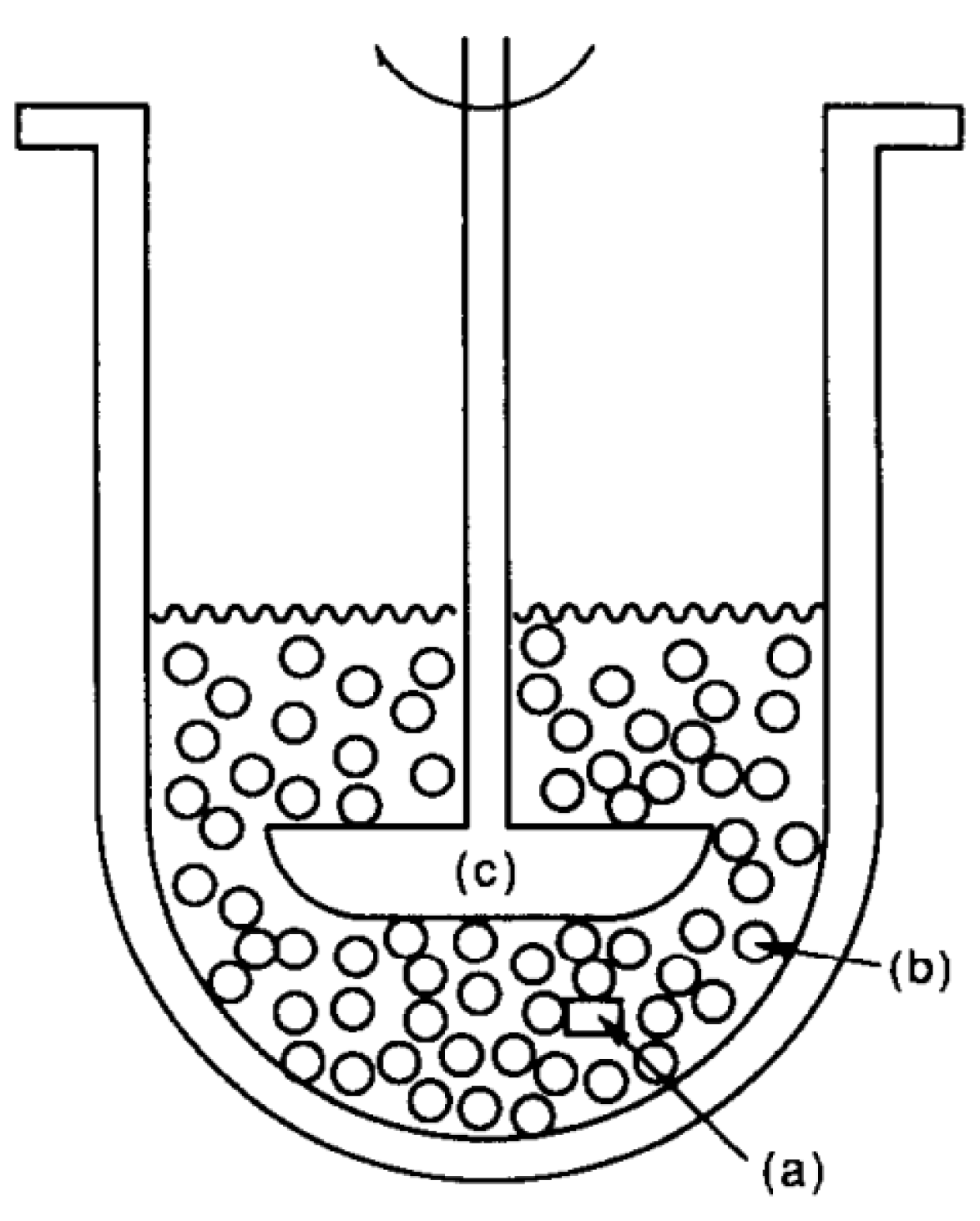
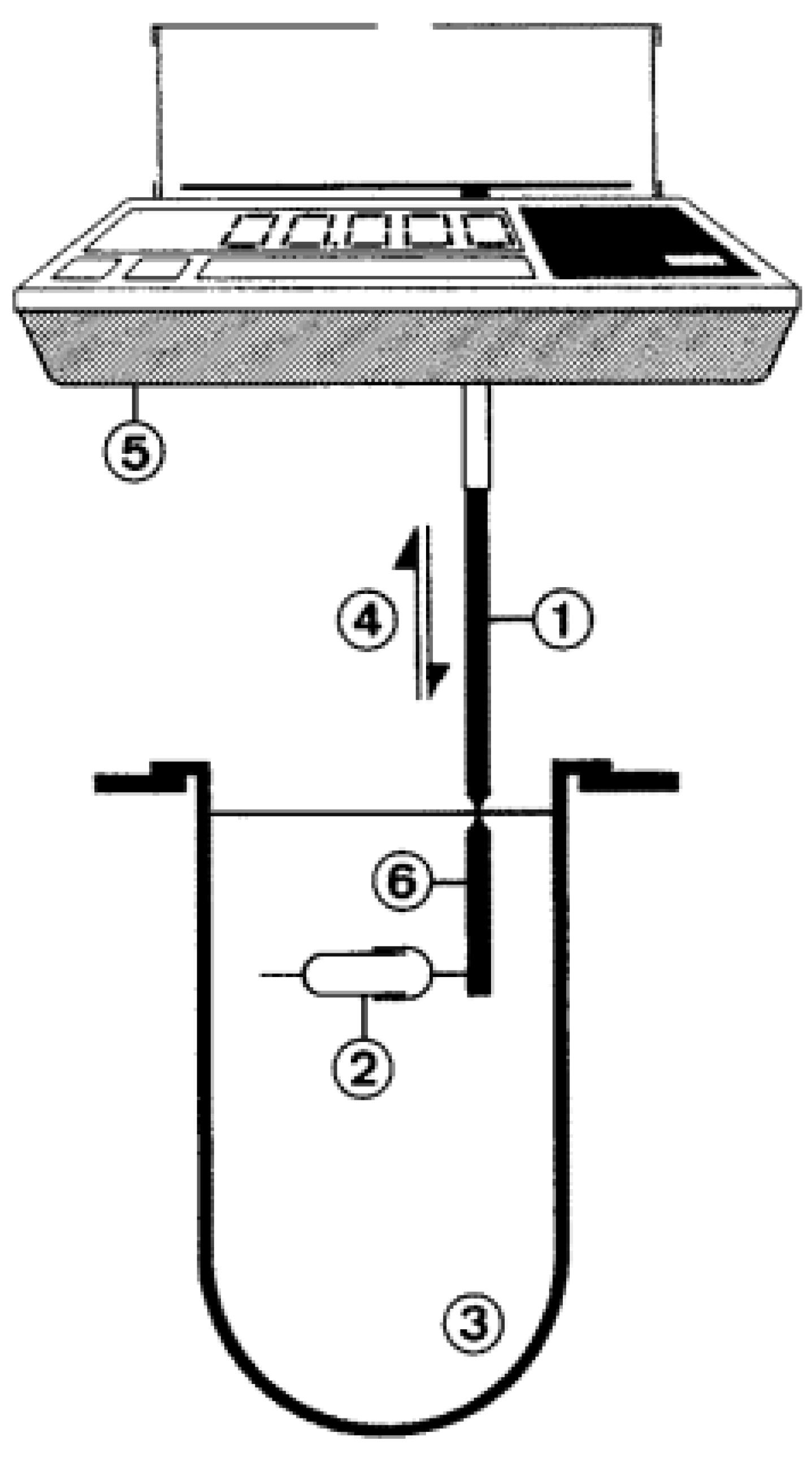
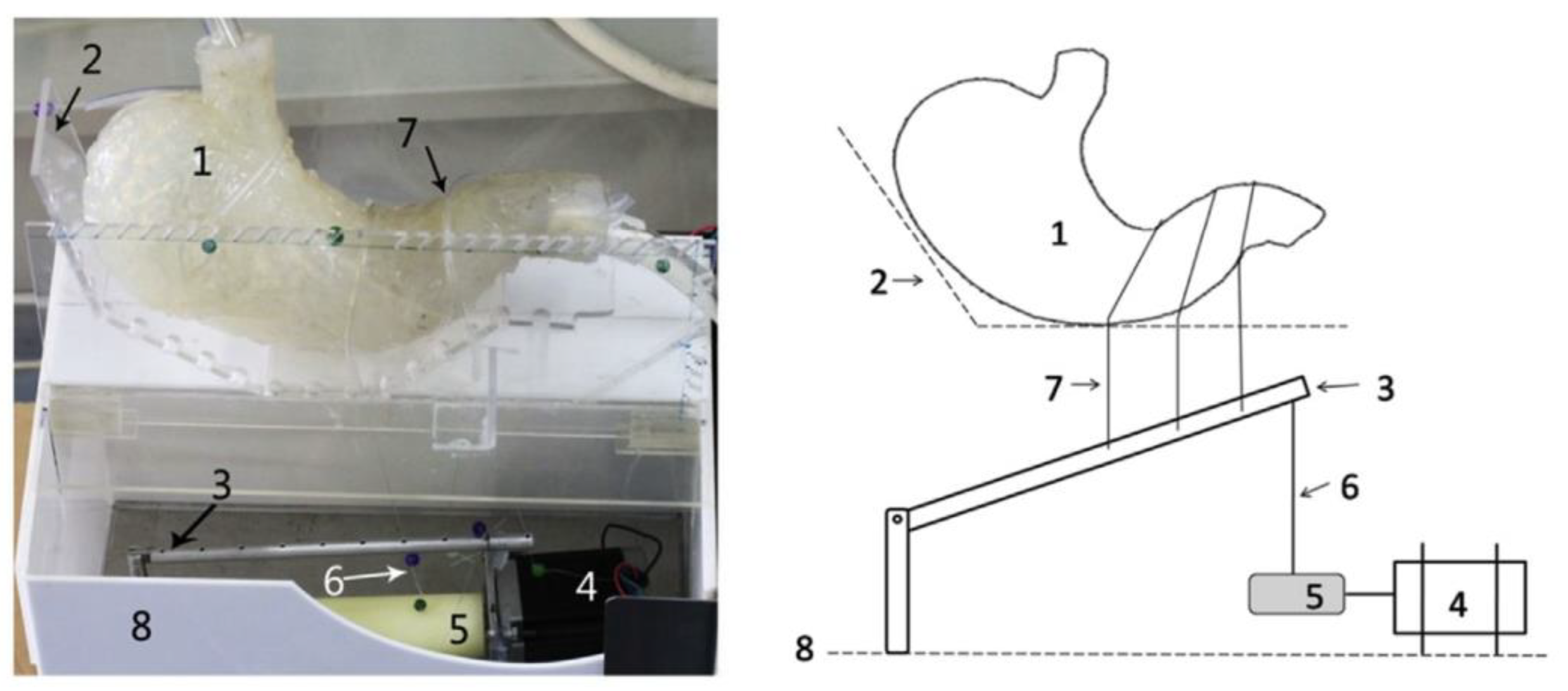
4.2.5. General Models
4.3. In Vivo Assessment of Gastroretentive Properties
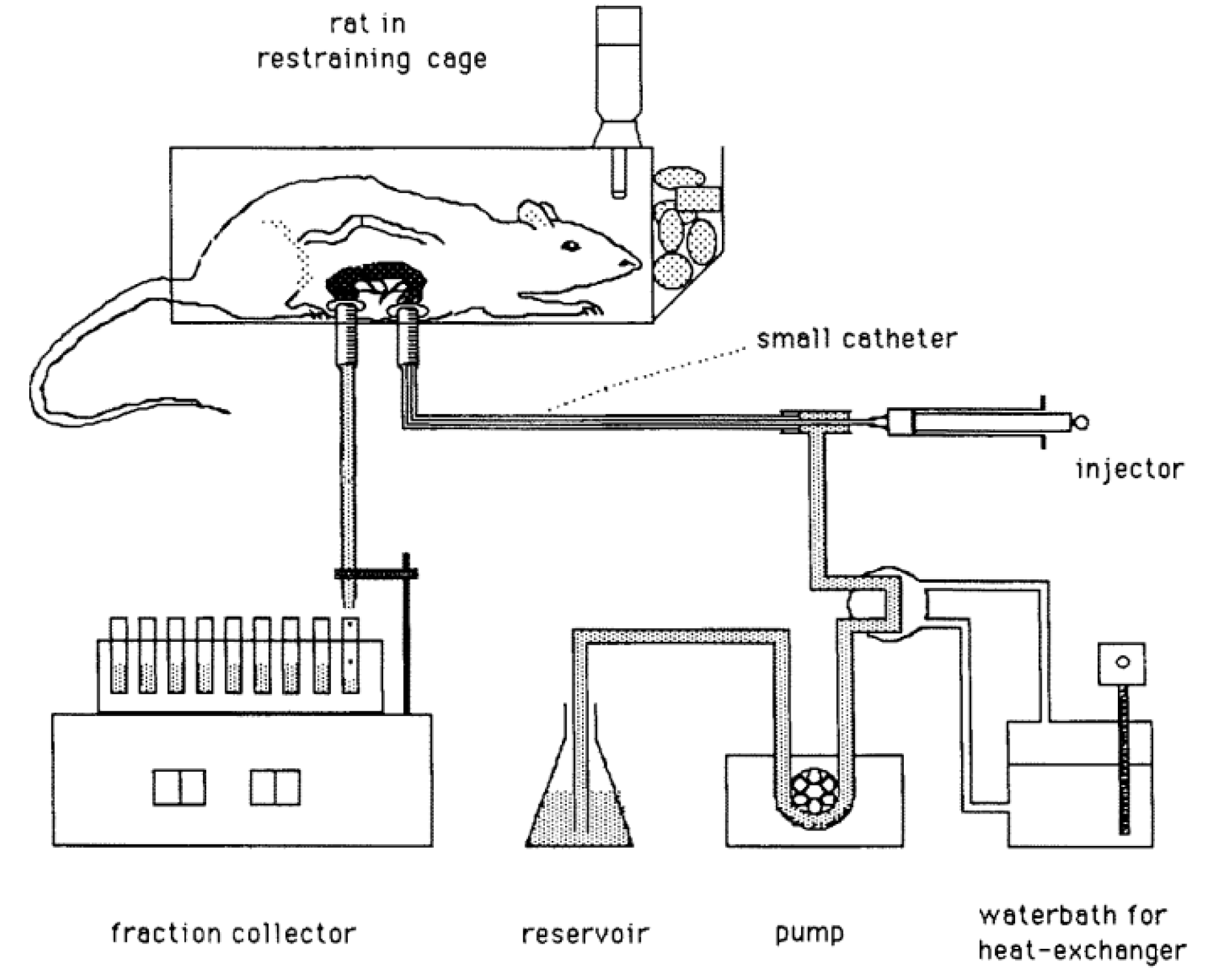
4.3.1. Considerations for Animal Studies on Gastroretentive Dosage Forms
4.3.2. Considerations for Human Studies on Gastroretentive Dosage Forms
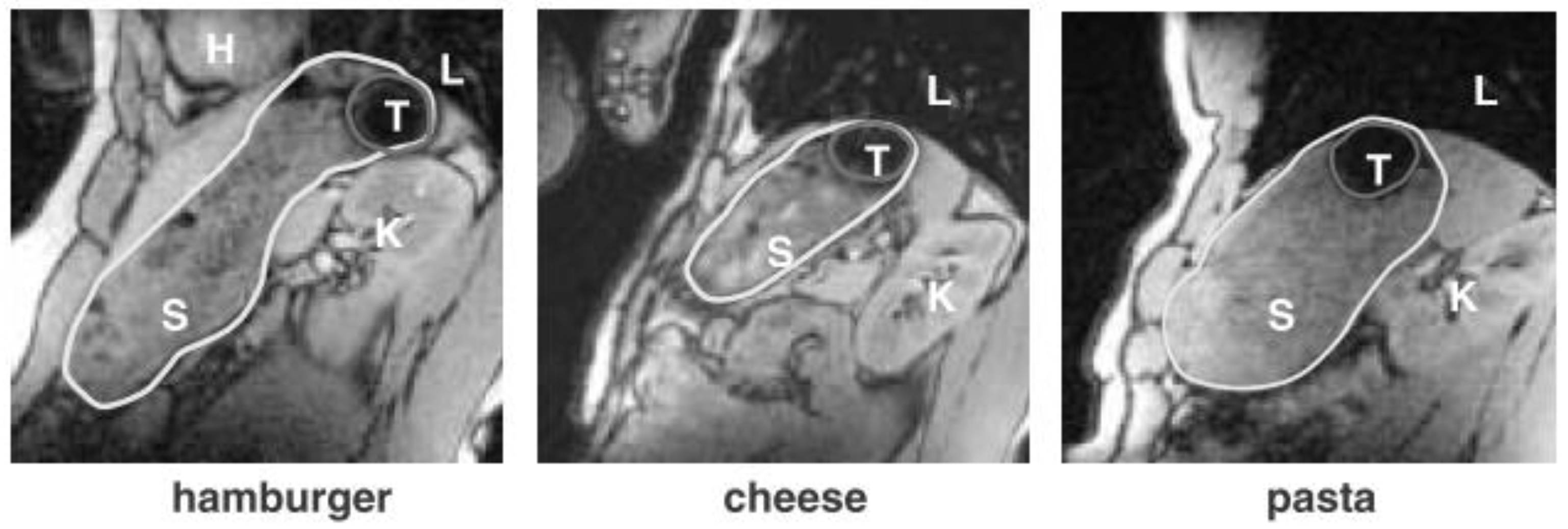
To Feed or Not to Feed?
Choosing the Right Control Formulation
Choosing the Right Model Drug
Choosing the Right Imaging Technique
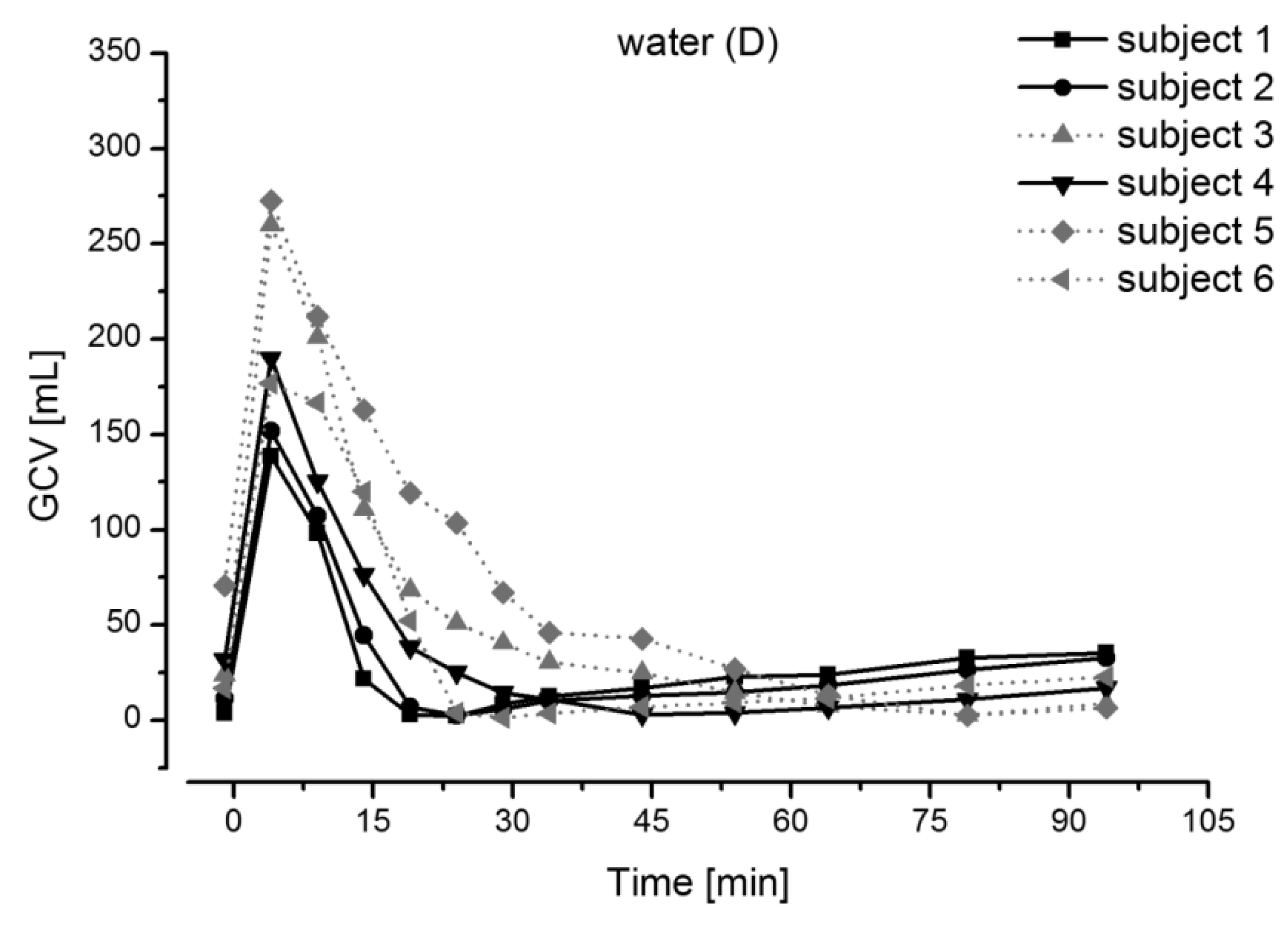
Magnetic Resonance Imaging
Magnetic Moment Imaging
Gastroscopy
Ultrasonography
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Awasthi, R.; Kulkarni, G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Drug Deliv. 2014, 23, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 2006, 3, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Bardonnet, P.L.; Faivre, V.; Pugh, W.J.; Piffaretti, J.C.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Stepensky, D.; Lavy, E.; Eyal, S.; Klausner, E.; Friedman, M. Pharmacokinetic and pharmacodynamic aspects of gastroretentive dosage forms. Int. J. Pharm. 2004, 277, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.A.; Rhodes, C.T.; Shah, N.H.; Malick, A.W. Controlled-release drug delivery systems for prolonged gastric residence: An overview. Drug Dev. Ind. Pharm. 1996, 22, 531–539. [Google Scholar] [CrossRef]
- Levy, G. Pharmacokinetic approaches to the study of drug interactions. Ann. N. Y. Acad. Sci. 1976, 281, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Fowle, A.; Bittiner, S.; Bye, A.; Isaacs, P. Human gastrointestinal absorption of acyclovir from tablet duodenal infusion and sipped solution. Br. J. Clin. Pharmacol. 1986, 21, 459–462. [Google Scholar] [CrossRef]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef]
- Park, K.; Robinson, J.R. Bioadhesive polymers as platforms for oral-controlled drug delivery: Method to study bioadhesion. Int. J. Pharm. 1984, 19, 107–127. [Google Scholar] [CrossRef]
- Waterman, K.C. A critical review of gastric retentive controlled drug delivery. Pharm. Dev. Technol. 2007, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The migrating motot complex: Control mechanism and its role in health and disease. Nat. Rev. Gastrorenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Mechanism of interdigestive migrating motor complex. J. Neurogastroenterol. Motil. 2012, 18, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.P.; Di Lorenzo, C.; Valenzuela, J.E. Variability of migrating motor complex in humans. Dig. Dis. Sci. 1992, 37, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; van der Reijden, A.C.; van Berge Henegouwen, G.P.; Akkermans, L.M.A. Migrating motor complex cycle duration is determined by gastric or duodenal origin of phase III. Am. J. Physiol. Liver Physiol. 1998, 275, G1246–G1251. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Garbacz, G.; Neumann, M.; Weitschies, W. Simulating the postprandial stomach: Physiological considerations for dissolution and release testing. Mol. Pharm. 2013, 10, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Mojaverian, P.; Ferguson, R.K.; Vlasses, P.H.; Rocci, M.L.; Oren, A.; Fix, J.A.; Caldwell, L.J.; Gardner, C. Estimation of gastric residence time of the heidelberg capsule in humans: Effect of varying food composition. Gastroenterology 1985, 89, 392–397. [Google Scholar] [CrossRef]
- Ewe, K.; Press, G.; Bollen, S.; Schuhn, I. Gastric emptying of indigestible tablets in relation to composition and time of ingestion of meals studied by metal detector. Dig. Dis. Sci. 1991, 36, 146–152. [Google Scholar] [CrossRef]
- Abrahamsson, B.; Alpsten, M.; Jonsson, U.E.; Lundberg, P.J.; Sandberg, A.; Sundgren, M.; Svenheden, A.; Tölli, J. Gastro-intestinal transit of a multiple-unit formulation (metoprolol CR/ZOK) and a non-disintegrating tablet with the emphasis on colon. Int. J. Pharm. 1996, 140, 229–235. [Google Scholar] [CrossRef]
- Coupe, A.J.; Davis, S.S.; Wilding, I.R. Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm. Res. 1991, 8, 360–364. [Google Scholar] [CrossRef]
- Cassilly, D.; Kantor, S.; Knight, L.C.; Maurer, A.H.; Fisher, R.S.; Semler, J.; Parkman, H.P. Gastric emptying of a non-digestible solid: Assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol. Motil. 2008, 20, 311–319. [Google Scholar] [CrossRef]
- Koziolek, M.; Schneider, F.; Grimm, M.; Modeß, C.; Seekamp, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 2015, 220, 71–78. [Google Scholar] [CrossRef]
- Devereux, J.E.; Newton, J.M.; Short, M.B. The influence of density on the gastrointestinal transit of pellets. J. Pharm. Pharmacol. 1990, 42, 500–501. [Google Scholar] [CrossRef]
- Podczeck, F.; Course, N.C.; Newton, J.M.; Short, M.B. The influence of non-disintegrating tablet dimensions and density on their gastric emptying in fasted volunteers. J. Pharm. Pharmacol. 2007, 59, 23–27. [Google Scholar] [CrossRef]
- Takeuchi, H.; Thongborisute, J.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1583–1594. [Google Scholar] [CrossRef]
- Grimm, M.; Koziolek, M.; Saleh, M.; Schneider, F.; Garbacz, G.; Kühn, J.-P.; Weitschies, W. Gastric emptying and small bowel water content after administration of grapefruit juice compared to water and isocaloric solutions of glucose and fructose: A four-way crossover MRI pilot study in healthy subjects. Mol. Pharm. 2018, 15, 548–559. [Google Scholar] [CrossRef]
- Schiller, C.; Fröhlich, C.-P.; Giessmann, T.; Siegmund, W.; Mönnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef]
- Fidler, J.; Bharucha, A.E.; Camillieri, M.; Camp, J.; Burton, D.; Grimm, R.; Riederer, S.J.; Robb, R.A.; Zinsmeister, A.R. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol. Motil. 2009, 21, 42–51. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the intellicap system. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Garbacz, G.; Kühn, J.; Weitschies, W. Intragastric volume changes after intake of a high-caloric, high-fat standard breakfast in healthy human subjects investigated by MRI. Mol. Pharm. 2014, 11, 1632–1639. [Google Scholar] [CrossRef]
- Cecil, J.E.; Francis, J.; Read, N.W. Comparison of the effects of a high-fat and high-carbohydrate soup delivered orally and intragastrically on gastric emptying, appetite, and eating behaviour. Physiol. Behav. 1999, 67, 299–306. [Google Scholar] [CrossRef]
- Weitschies, W.; Friedrich, C.; Wedemeyer, R.S.; Schmidtmann, M.; Kosch, O.; Kinzig, M.; Trahms, L.; Sörgel, F.; Siegmund, W.; Horkovics-Kovats, S.; et al. Bioavailability of amoxicillin and clavulanic acid from extended release tablets depends on intragastric tablet deposition and gastric emptying. Eur. J. Pharm. Biopharm. 2008, 70, 641–648. [Google Scholar] [CrossRef]
- Weitschies, W.; Wedemeyer, R.-S.; Kosch, O.; Fach, K.; Nagel, S.; Söderlind, E.; Trahms, L.; Abrahamsson, B.; Mönnikes, H. Impact of the intragastric location of extended release tablets on food interactions. J. Control. Release 2005, 108, 375–385. [Google Scholar] [CrossRef]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.P.; Barnett, J.L.; Jarvenpaa, M.K. Upper gastrointestinal (GI) pH in young healthy men and women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef]
- Malagelada, J.-R.; Go, V.L.W.; Summerskill, W.H.J. Different gastric, pancreatic, and biliary responses to solid-liquid or homogenized meals. Dig. Dis. Sci. 1979, 24, 101–110. [Google Scholar] [CrossRef]
- Sauter, M.M.; Steingoetter, A.; Curcic, J.; Treier, R.; Kuyumcu, S.; Fired, M.; Boesiger, P.; Schwizer, W.; Goetze, O. Quantification of meal induced gastric secretion and its effect on caloric emptying by magnetic resonance imaging (MRI). Gastroenterology 2011, 140, S-297. [Google Scholar] [CrossRef]
- Hoad, C.L.; Parker, H.; Hudders, N.; Costigan, C.; Cox, E.F.; Perkins, A.C.; Blackshaw, P.E.; Marciani, L.; Spiller, R.C.; Fox, M.R.; et al. Measurement of gastric meal and secretion volumes using magnetic resonance imaging. Phys. Med. Biol. 2015, 60, 1367–1383. [Google Scholar] [CrossRef]
- Watanabe, S.; Masanori, K.; Ishino, Y.; Miyao, K. Solid Therapeutic Preparation Remaining in Stomach. U.S. Patent US3976764A, 24 August 1976. [Google Scholar]
- Bennett, C.E.; Hardy, J.G.; Wilson, C.G. The influence of posture on the gastric emptying of antacids. Int. J. Pharm. 1984, 21, 341–347. [Google Scholar] [CrossRef]
- Steingoetter, A.; Kunz, P.; Weishaupt, D.; Mäder, K.; Lengsfeld, H.; Thumshirn, M.; Boesiger, P.; Fried, M.; Schwizer, W. Analysis of the meal-dependent intragastric performance of a gastric-retentive tablet assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2003, 18, 713–720. [Google Scholar] [CrossRef]
- Hoelzel, F. The rate of passage of inert materials through the digestive tract. Am. J. Physiol. 1930, 92, 466–497. [Google Scholar] [CrossRef]
- Clarke, G.M.; Newton, J.M.; Short, M.B. Comparative gastrointestinal transit of pellet systems of varying density. Int. J. Pharm. 1995, 114, 1–11. [Google Scholar] [CrossRef]
- Davis, S.S.; Stockwell, A.F.; Taylor, M.J.; Hardy, J.G.; Whalley, D.R.; Wilson, C.G.; Bechgaard, H.; Christensen, F.N. The Effect of Density on the Gastric Emptying of Single- and Multi-Unit Dosage Forms. Pharm. Res. 1986, 3, 208–213. [Google Scholar] [CrossRef]
- Burdan, F.; Rozylo-Kalinowska, I.; Szumilo, J.; Zinkiewicz, K.; Dworzanski, W.; Krupski, W.; Dabrowski, A. Anatomical classification of the shape and topography of the stomach. Surg. Radiol. Anat. 2012, 34, 171–178. [Google Scholar] [CrossRef]
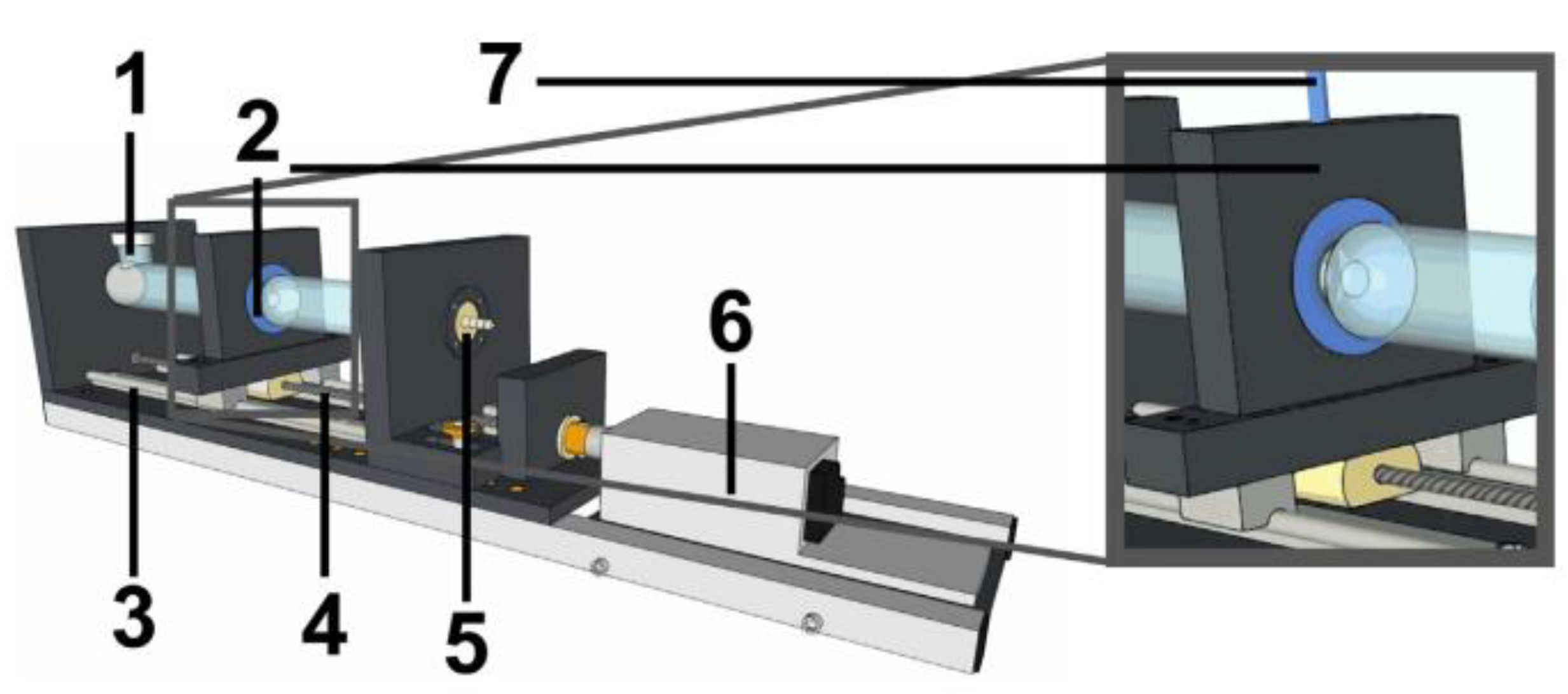
- Neumann, M.; Schneider, F.; Koziolek, M.; Garbacz, G.; Weitschies, W. A novel mechanical antrum model for the prediction of the gastroretentive potential of dosage forms. Int. J. Pharm. 2017, 530, 63–70. [Google Scholar] [CrossRef]
- Coffin, M.D.; Burke, M.D. Controlling release by gastroretention. In Controlled Release in Oral Drug Delivery; Wilson, C.G., Crowley, P.J., Eds.; Springer: New York, NY, USA, 2011; pp. 361–383. [Google Scholar]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Kagan, L.; Lapidot, N.; Afargan, M.; Kirmayer, D.; Moor, E.; Mardor, Y.; Friedman, M.; Hoffman, A. Gastroretentive Accordion Pill: Enhancement of riboflavin bioavailability in humans. J. Control. Release 2006, 113, 208–215. [Google Scholar] [CrossRef]
- Fix, J.A.; Cargill, R.; Engle, K. Controlled gastric emptying. III. Gastric residence time of a nondisintegrating. Pharm. Res. 1993, 10, 1087–1089. [Google Scholar] [CrossRef]
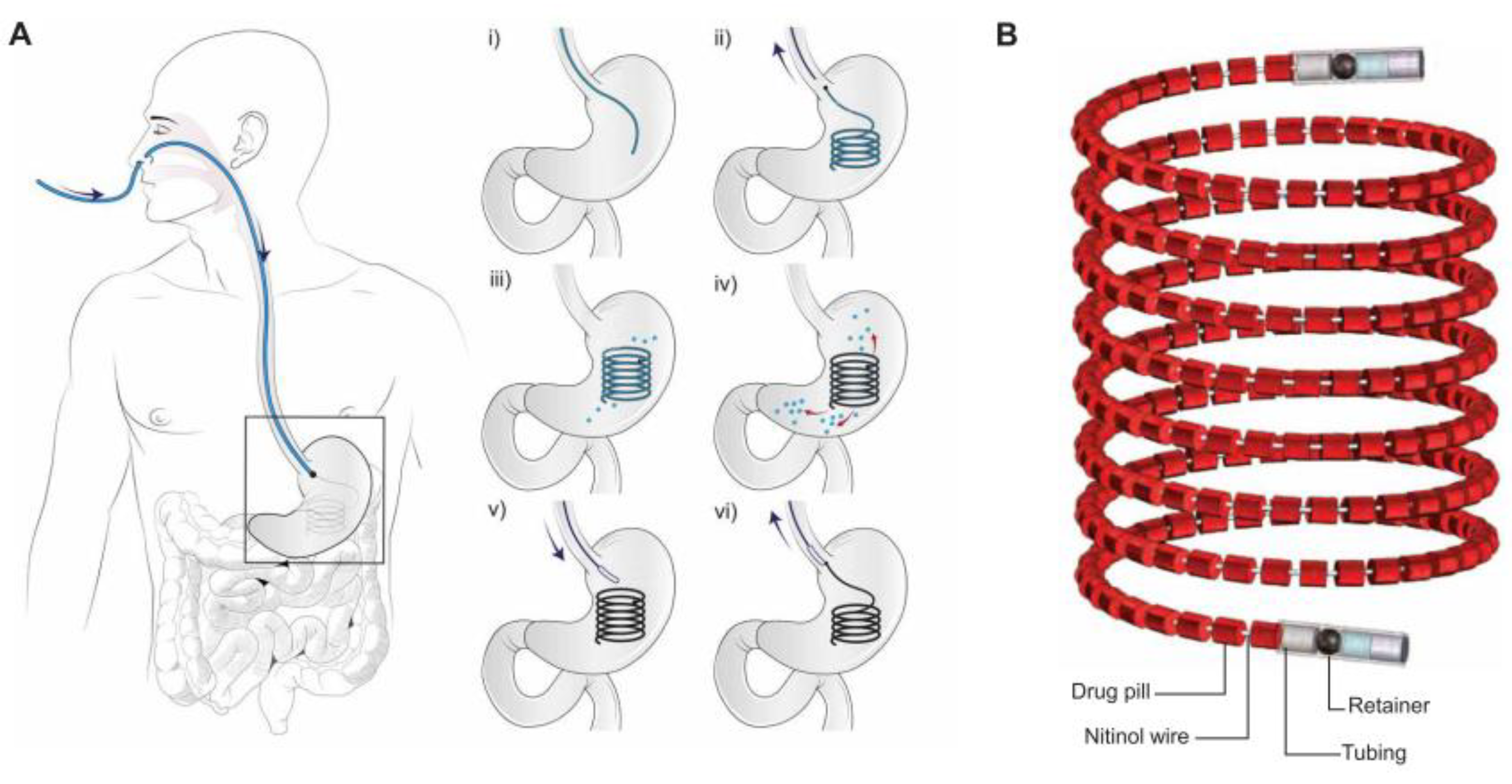
- Verma, M.; Vishwanath, K.; Eweje, F.; Roxhed, N.; Grant, T.; Castaneda, M.; Steiger, C.; Mazdiyasni, H.; Bensel, T.; Minahan, D.; et al. A gastric resident drug delivery system for prolonged gram-level dosing of tuberculosis treatment. Sci. Transl. Med. 2019, 11, 1–8. [Google Scholar] [CrossRef]
- Koziolek, M.; Garbacz, G.; Neumann, M.; Weitschies, W. Simulating the postprandial stomach: Biorelevant test methods for the estimation of intragastric drug dissolution. Mol. Pharm. 2013, 10, 2211–2221. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Abrahamsson, B.; Brewster, M.; Brouwers, J.; Butler, J.; Carlert, S.; Dickinson, P.A.; Dressman, J.; Holm, R.; Klein, S. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 342–366. [Google Scholar] [CrossRef]
- Parikh, D.C.; Amin, A.F. In vitro and in vivo techniques to assess the performance of gastro-retentive drug delivery systems: A review. Expert Opin. Drug Deliv. 2008, 5, 951–965. [Google Scholar] [CrossRef]
- Saab, M.; Issa, M.; Samy, W.; El-Maradny, H. Alternative approaches in formulating floating hollow tablets via sublimation technique; A platform tailored drug release profile. Pharmazie 2016, 71, 701–708. [Google Scholar]
- Abouelatta, S.M.; Aboelwafa, A.A.; El-Gazayerly, O.N. Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug. Drug Deliv. 2018, 25, 1161–1174. [Google Scholar] [CrossRef]
- Pillay, V.; Fassihi, R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: An alternative method. J. Control. Release 1998, 55, 45–55. [Google Scholar] [CrossRef]
- Dürig, T.; Fassihi, R. Evaluation of floating and sticking extended release delivery systems: An unconventional dissolution test. J. Control. Release 2000, 67, 37–44. [Google Scholar] [CrossRef]
- Kong, Y.L.; Zou, X.; McCandler, C.A.; Kirtane, A.R.; Ning, S.; Zhou, J.; Abid, A.; Jafari, M.; Rogner, J.; Minahan, D.; et al. 3D-printed gastric resident electronics. Adv. Mater. Technol. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Eberle, V.A.; Schoelkopf, J.; Gane, P.A.C.; Alles, R.; Huwyler, J.; Puchkov, M. Floating gastroretentive drug delivery systems: Comparison of experimental and simulated dissolution profiles and floatation behavior. Eur. J. Pharm. Sci. 2014, 58, 34–43. [Google Scholar] [CrossRef]
- Bai, G.E.; Armenante, P.M.; Plank, R.V.; Gentzler, M.; Ford, K.; Harmon, P. Hydrodynamic investigation of USP dissolution test apparatus II. J. Pharm. Sci. 2007, 396, 2327–2349. [Google Scholar] [CrossRef]
- Baxter, J.L.; Kukura, J.; Muzzio, F.J. Hydrodynamics-induced variability in the USP apparatus II dissolution test. Int. J. Pharm. 2005, 292, 17–28. [Google Scholar] [CrossRef]
- Parikh, R.K.; Parikh, D.C.; Delvadia, R.R.; Patel, S.M. A novel multicompartment dissolution apparatus for evaluation of floating dosage form containing poorly soluble weakly basic drug. Dissolution Technol. 2006, 13, 14–19. [Google Scholar] [CrossRef]
- Llabot, J.M.; Manzo, R.H.; Allemandi, D.A. Drug release from carbomer:carbomer sodium salt matrices with potential use as mucoadhesive drug delivery system. Int. J. Pharm. 2004, 276, 59–66. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kondo, S.-I.; Sasai, Y.; Kuzuya, M. Preparation of floating drug delivery system by plasma technique. Chem. Pharm. Bull. 2006, 54, 514–518. [Google Scholar] [CrossRef][Green Version]
- Aoki, S.; Ando, H.; Tatsuishi, K.; Uesugi, K.; Ozawa, H. Determination of the mechanical impact force in the in vitro dissolution test and evaluation of the correlation between in vivo and in vitro release. Int. J. Pharm. 1993, 95, 67–75. [Google Scholar] [CrossRef]
- Aoki, S.; Uesugi, K.; Tatsuishi, K.; Ozawa, H.; Kayano, M. Evaluation of the correlation between in vivo and in vitro release of phenylpropanolamine HCl from controlled-release tablets. Int. J. Pharm. 1992, 85, 65–73. [Google Scholar] [CrossRef]
- Schneider, F.; Hoppe, M.; Koziolek, M.; Weitschies, W. Influence of postprandial intragastric pressures on drug release from gastroretentive dosage forms. AAPS PharmSciTech 2018, 19, 2843–2850. [Google Scholar] [CrossRef]
- Garbacz, G.; Klein, S.; Weitschies, W. A biorelevant dissolution stress test device—Background and experiences. Expert Opin. Drug Deliv. 2010, 7, 1251–1261. [Google Scholar] [CrossRef]
- Vardakou, M.; Mercuri, A.; Naylor, T.A.; Rizzo, D.; Butler, J.M.; Connolly, P.C.; Wickham, M.S.J.; Faulks, R.M. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. Int. J. Pharm. 2011, 419, 192–199. [Google Scholar] [CrossRef]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The design, operation, and application of a dynamic gastric model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Marteau, P.; Minekus, M.; Havenaar, R.; Huis in’t Veld, J.H.J. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: Validation and the effects of Bile. J. Dairy Sci. 1997, 80, 1031–1037. [Google Scholar] [CrossRef]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.-P.; Denis, S.; Havenaar, R.; Alric, M. A dynamic arificial gastrointestinal system for studying the behavior of orally administrated drug dosage forms under various physiological conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef]
- Timmermans, J.; Moës, A.J. How well do floating dosage forms float? Int. J. Pharm. 1990, 62, 207–216. [Google Scholar] [CrossRef]
- Sheth, P.R.; Tossounian, J. The hydrodynamically balanced system (HbsTM): A novel drug delivery system for oral use. Drug Dev. Ind. Pharm. 1984, 10, 313–339. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Wang, B. Sinking-magnetic microparticles prepared by the electrospray method for enhanced gastric antimicrobial delivery. Mol. Pharm. 2014, 11, 1640–1650. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, T.G.; Choi, H.K. Development of oral drug delivery system using floating microspheres. J. Microencapsul. 1999, 16, 715–729. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Agnihotri, S.A.; Mallikarjuna, N.N.; Kulkarni, P.V. Effect of coexcipients on drug release and floating property of nifedipine hollow microspheres: A novel gastro retentive drug delivery system. J. Appl. Polym. Sci. 2006, 100, 486–494. [Google Scholar] [CrossRef]
- Ichikawa, M.; Watanabe, S.; Miyake, Y. A new multiple-unit oral floating dosage system. I: Preparation and in vitro evaluation of floating and sustained-release characteristics. J. Pharm. Sci. 1991, 80, 1062–1066. [Google Scholar] [CrossRef]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: A review. AAPS PharmSciTech 2005, 6, 372–390. [Google Scholar] [CrossRef]
- Rajinikanth, P.S.; Mishra, B. Floating in situ gelling system for stomach site-specific delivery of clarithromycin to eradicate H. pylori. J. Control. Release 2008, 125, 33–41. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, L.; Nie, S.; Yan, T.; Tang, X.; Pan, W. A novel gastric-resident osmotic pump tablet: In vitro and in vivo evaluation. Int. J. Pharm. 2010, 383, 30–36. [Google Scholar] [CrossRef]
- Gröning, R.; Cloer, C.; Georgarakis, M.; Müller, R.S. Compressed collagen sponges as gastroretentive dosage forms: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2007, 30, 1–6. [Google Scholar] [CrossRef]
- Doroyński, P.; Kulinowski, P.; Mendyk, A.; Jachowicz, R. Gastroretentive drug delivery systems with l-dopa based on carrageenans and hydroxypropylmethylcellulose. Int. J. Pharm. 2011, 404, 169–175. [Google Scholar] [CrossRef]
- Matharu, A.S.; Motto, M.G.; Patel, M.R.; Simonelli, A.P.; Dave, R.H. Evaluation of hydroxypropyl methylcellulose matrix systems as swellable gastro-retentive drug delivery systems (GRDDS). J. Pharm. Sci. 2011, 100, 150–163. [Google Scholar] [CrossRef]
- Mostafavi, A.; Emami, J.; Varshosaz, J.; Davies, N.M.; Rezazadeh, M. Development of a prolonged-release gastroretentive tablet formulation of ciprofloxacin hydrochloride: Pharmacokinetic characterization in healthy human volunteers. Int. J. Pharm. 2011, 409, 128–136. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Kassem, A.A.; El-Kamel, A.H. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrate. Int. J. Pharm. 2014, 464, 10–18. [Google Scholar] [CrossRef]
- Chen, J.; Park, H.; Park, K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J. Biomed. Mater. Res. 1999, 44, 53–62. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K.; Rocca, J.G. Recent developments in superporous hydrogels. J. Pharm. Pharmacol. 2007, 59, 317–327. [Google Scholar] [CrossRef]
- Klausner, E.A.; Eyal, S.; Lavy, E.; Friedman, M.; Hoffman, A. Novel levodopa gastroretentive dosage form: In-vivo evaluation in dogs. J. Control. Release 2003, 88, 117–126. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gan, Y.; Zhang, X.X. Novel gastroretentive sustained-release tablet of tacrolimus based on self-microemulsifying mixture: In vitro evaluation and in vivo bioavailability test. Acta Pharmacol. Sin. 2011, 32, 1294–1302. [Google Scholar] [CrossRef]
- Burgalassi, S.; Panichi, L.; Saettone, M.F.; Jacobsen, J.; Rassing, M.R. Development and in vitro/in vivo testing of mucoadhesive buccal patches releasing benzydamine and lidocaine. Int. J. Pharm. 1996, 133, 1–7. [Google Scholar] [CrossRef]
- Pund, S.; Joshi, A.; Vasu, K.; Nivsarkar, M.; Shishoo, C. Gastroretentive delivery of rifampicin: In vitro mucoadhesion and in vivo gamma scintigraphy. Int. J. Pharm. 2011, 411, 106–112. [Google Scholar] [CrossRef]
- Patil, S.; Talele, G.S. Gastroretentive mucoadhesive tablet of lafutidine for controlled release and enhanced bioavailability. Drug Deliv. 2015, 22, 312–319. [Google Scholar] [CrossRef]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Diacerein-Loaded novel gastroretentive nanofiber system using PLLA: Development and in vitro characterization. Artif. Cells Nanomed. Biotechnol. 2016, 44, 928–936. [Google Scholar]
- Ponchel, G.; Touchard, F.; Duchene, D.; Peppas, N.A. Bioadhesive analysis of controlled-release systems. I. Fracture and interpenetration analysis in poly(acrylic acid)-containing systems. J. Control. Release 1987, 5, 129–141. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef]
- Varum, F.J.O.; Veiga, F.; Sousa, J.S.; Basit, A.W. An investigation into the role of mucus thickness on mucoadhesion in the gastrointestinal tract of pig. Eur. J. Pharm. Sci. 2010, 40, 335–341. [Google Scholar] [CrossRef]
- Laulicht, B.; Cheifetz, P.; Tripathi, A.; Mathiowitz, E. Are in vivo gastric bioadhesive forces accurately reflected by in vitro experiments? J. Control. Release 2009, 134, 103–110. [Google Scholar] [CrossRef]
- Santos, C.A.; Jacob, J.S.; Hertzog, B.A.; Freedman, B.D.; Press, D.L.; Harnpicharnchai, P.; Mathiowitz, E. Correlation of two bioadhesion assays: The everted sac technique and the CAHN microbalance. J. Control. Release 1999, 61, 113–122. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Bowstra, J.A.; Tukker, J.J.; Junginger, H.E. Intestinal transit of bioadhesive microspheres in an in situ loop in the rat. J. Control. Release 1990, 13, 51–62. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, W.; Qian, L.; Zhang, X.; Zeng, P.; Pan, J. In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J. Control. Release 2005, 102, 135–144. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Jain, S.; Singh, H.P.; Tiwary, A.K. Mucoadhesive Microspheres for Gastroretentive Delivery of Acyclovir: In Vitro and In Vivo Evaluation. AAPS J. 2008, 10, 322–330. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Tripathi, P.; Ubaidulla, U.; Anand, V. Design and development of gliclazide mucoadhesive microcapsules: In vitro and in vivo evaluation. AAPS PharmSciTech 2008, 9, 224–230. [Google Scholar] [CrossRef]
- Mortazavi, S.A. An in vitro assessment of mucus/mucoadhesive interactions. Int. J. Pharm. 1995, 124, 173–182. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Fan, T.; Liao, Z.; Wu, P.; Wu, X.; Chen, X.D. Gastric emptying and morphology of a ‘near real’ in vitro human stomach model (RD-IV-HSM). J. Food Eng. 2016, 183, 1–8. [Google Scholar] [CrossRef]
- Shalaby, W.S.W.; Blevins, W.E.; Park, K. Use of ultrasound imaging and fluoroscopic imaging to study gastric retention of enzyme-digestible hydrogels. Biomaterials 1992, 13, 289–296. [Google Scholar] [CrossRef]
- Gökbulut, E.; Vural, İ.; Aşıkoğlu, M.; Özdemir, N. Floating drug delivery system of itraconazole: Formulation, in vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2019, 49, 491–501. [Google Scholar] [CrossRef]
- Mitra, A.; Kesisoglou, F. Impaired drug absorption due to high stomach pH: A review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol. Pharm. 2013, 10, 3970–3979. [Google Scholar] [CrossRef]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal farm: Considerations in animal gastrointestinal physiology and relevance to drug delivery in humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Bollmann, T.; Schäfer, K.J.; Blattner, S.M.; Lotz, R.; Boeck, G.; Weitschies, W. Characterization of the GI transit conditions in beagle dogs with a telemetric motility capsule. Eur. J. Pharm. Biopharm. 2019, 136, 221–230. [Google Scholar] [CrossRef]
- Sagawa, K.; Li, F.; Liese, R.; Sutton, C.S. Fed and fasted gastric pH and gastric residence time in conscious beagle dogs. J. Pharm. Sci. 2009, 98, 2494–2500. [Google Scholar] [CrossRef]
- McInnes, F.; Clear, N.; Humphrey, M.; Stevens, H.N.E. In vivo performance of an oral MR matrix tablet formulation in the beagle dog in the fed and fasted state: Assessment of mechanical weakness. Pharm. Res. 2008, 25, 1075–1084. [Google Scholar] [CrossRef]
- Aoyagi, N.; Ogata, H.; Kaniwa, N.; Uchiyama, M.; Yasuda, Y.; Tanioka, Y. Gastric Emptying of Tablets and Granules in Humans, Dogs, Pigs, and Stomach-Emptying-Controlled Rabbits. J. Pharm. Sci. 1992, 81, 1170–1174. [Google Scholar] [CrossRef]
- Kaniwa, N.; Aoyagi, N.; Ogata, H.; Ejima, A. Gastric emptying rates of drug preparations. I. Effects of size of dosage forms, food and species on gastric emptying rates. J. Pharmacobiodyn. 1988, 11, 563–570. [Google Scholar] [CrossRef]
- Dressman, J.B. Comparison of Canine and Human Gastrointestinal Physiology. Pharm. Res. 1986, 3, 123–131. [Google Scholar] [CrossRef]
- Cargill, R.; Caldwell, L.J.; Engle, K.; Fix, J.A.; Porter, P.A.; Gardner, C.R. Controlled Gastric Emptying. 1. Effects of Physical Properties on Gastric Residence Times of Nondisintegrating Geometric Shapes in Beagle Dogs. Pharm. Res. 1988, 5, 533–536. [Google Scholar] [CrossRef]
- Kesisoglou, F. Use of preclinical dog studies and absorption modeling to facilitate late stage formulation bridging for a BCS II drug candidate. AAPS PharmSciTech 2014, 15, 20–28. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Abouzid, O.; Minahan, D.; Bensel, T.; Hill, A.L.; Selinger, C.; Bershteyn, A.; Craig, M.; Mo, S.S; Mazdiyasni, H. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Hossain, M.; Abramowitz, W.; Watrous, B.J.; Szpunar, G.J.; Ayres, J.W. Gastrointestinal transit of nondisintegrating, nonerodible oral dosage forms in pigs. Pharm. Res. 1990, 7, 1163–1166. [Google Scholar] [CrossRef]
- Davis, S.S.; Illum, L.; Hinchcliffe, M. Gastrointestinal transit of dosage forms in the pig. J. Pharm. Pharmacol. 2001, 53, 33–39. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Scheuch, E.; Weitschies, W.; Grimm, M.; Schneider, F.; Bachmann, L.; Vervuert, I. Measurement of abomasal conditions (pH, pressure and temperature) in healthy and diarrheic dairy calves using a wireless ambulatory capsule. Livest. Sci. 2017, 203, 41–47. [Google Scholar] [CrossRef]
- Berner, B.; Cowles, V.E. Case studies in swelling polymeric gastric retentive tablets. Expert Opin. Drug Deliv. 2006, 3, 541–548. [Google Scholar] [CrossRef]
- Gusler, G.; Gorsline, J.; Levy, G.; Zhang, S.Z.; Weston, I.E.; Naret, D.; Berner, B. Pharmacokinetics of metformin gastric-retentive tablets. J. Clin. Pharmacol. 2001, 41, 655–661. [Google Scholar] [CrossRef]
- Ingani, H.M.; Timmermans, J.; Moës, A.J. Conception and in vivo investigation of peroral sustained release floating dosage forms with enhanced gastrointestinal transit. Int. J. Pharm. 1987, 35, 157–164. [Google Scholar] [CrossRef]
- Hilton, A.K.; Deasy, P.B. In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. Int. J. Pharm. 1992, 86, 79–88. [Google Scholar] [CrossRef]
- Garbacz, G.; Wedemeyer, R.-S.; Nagel, S.; Giessmann, T.; Mönnikes, H.; Wilson, C.G.; Siegmund, W.; Weitschies, W. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur. J. Pharm. Biopharm. 2008, 70, 421–428. [Google Scholar] [CrossRef]
- Van Gansbeke, B.; Timmermans, J.; Schoutens, A.; Moës, A.J. Intragastric positioning of two concurrently ingested pharmaceutical matrix dosage forms. Int. J. Radiat. Appl. Instrum. 1991, 18, 711–718. [Google Scholar] [CrossRef]
- Klausner, E.A.; Lavy, E.; Barta, M.; Cserepes, E.; Friedman, M.; Hoffman, A. Novel gastroretentive dosage forms: Evaluation of gastroretentivity and its effect on levodopa absorption in humans. Pharm. Res. 2003, 20, 1466–1473. [Google Scholar] [CrossRef]
- Sharma, O.P.; Shah, M.V.; Parikh, D.C.; Mehta, T.A. Formulation optimization of gastroretentive drug delivery system for allopurinol using experimental design. Expert Opin. Drug Deliv. 2015, 12, 513–524. [Google Scholar] [CrossRef]
- Vemula, S.K.; Veerareddy, P.R. Development, evaluation and pharmacokinetics of time-dependent ketorolac tromethamine tablets. Expert Opin. Drug Deliv. 2013, 10, 33–45. [Google Scholar] [CrossRef]
- Otsuka, M.; Yamanaka, A.; Uchino, T.; Otsuka, K.; Sadamoto, K.; Ohshima, H. Quantitative Evaluation of the Disintegration of Orally Rapid Disintegrating Tablets by X-ray Computed Tomography. Chem. Pharm. Bull. 2012, 60, 1502–1507. [Google Scholar] [CrossRef][Green Version]
- Curley, L.; Hinton, J.; Marjoribanks, C.; Mirjalili, A.; Kennedy, J.; Svirskis, D. Magnetic Resonance Imaging to Visualize Disintegration of Oral Formulations. J. Pharm. Sci. 2017, 106, 745–750. [Google Scholar] [CrossRef]
- Marciani, L.; Young, P.; Wright, J.; Moore, R.J.; Evans, D.F.; Spiller, R.C.; Gowland, P.A. Echo-planar magnetic resonance imaging of Gaviscon alginate rafts in-vivo. J. Pharm. Pharmacol. 2002, 54, 1351–1356. [Google Scholar] [CrossRef]
- Grimm, M.; Ball, K.; Scholz, E.; Schneider, F.; Sivert, A.; Benameur, H.; Kromrey, M.L.; Kühn, J.-P.; Weitschies, W. Characterization of the gastrointestinal transit and disintegration behavior of floating and sinking acid-resistant capsules using a novel MRI labeling technique. Eur. J. Pharm. Sci. 2019, 129, 163–172. [Google Scholar] [CrossRef]
- Weitschies, W.; Wilson, C.G. In vivo imaging of drug delivery systems in the gastrointestinal tract. Int. J. Pharm. 2011, 417, 216–226. [Google Scholar] [CrossRef]
- Weitschies, W.; Blume, H.; Mönnikes, H. Magnetic Marker Monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef]
- Laulicht, B.; Tripathi, A.; Schlageter, V.; Kucera, P.; Mathiowitz, E. Understanding gastric forces calculated from high-resolution pill tracking. Proc. Natl. Acad. Sci. USA 2010, 107, 8201–8206. [Google Scholar] [CrossRef]
- Laulicht, B.; Gidmark, N.J.; Tripathi, A.; Mathiowitz, E. Localization of magnetic pills. Proc. Natl. Acad. Sci. USA 2011, 108, 2252–2257. [Google Scholar] [CrossRef]
- Stathopoulos, E.; Schlageter, V.; Meyrat, B.; De Ribaupierre, Y.; Kucera, P. Magnetic pill tracking: A novel non-invasive tool for investigation of human digestive motility. Neurogastroenterol. Motil. 2005, 17, 148–154. [Google Scholar] [CrossRef]
- Jain, A.K.; Söderlind, E.; Viriden, A.; Schug, B.; Abrahamsson, B.; Knopke, C.; Tajarobi, F.; Blume, H.; Anschütz, M.; Welinder, A.; et al. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J. Control. Release 2014, 187, 50–58. [Google Scholar] [CrossRef]
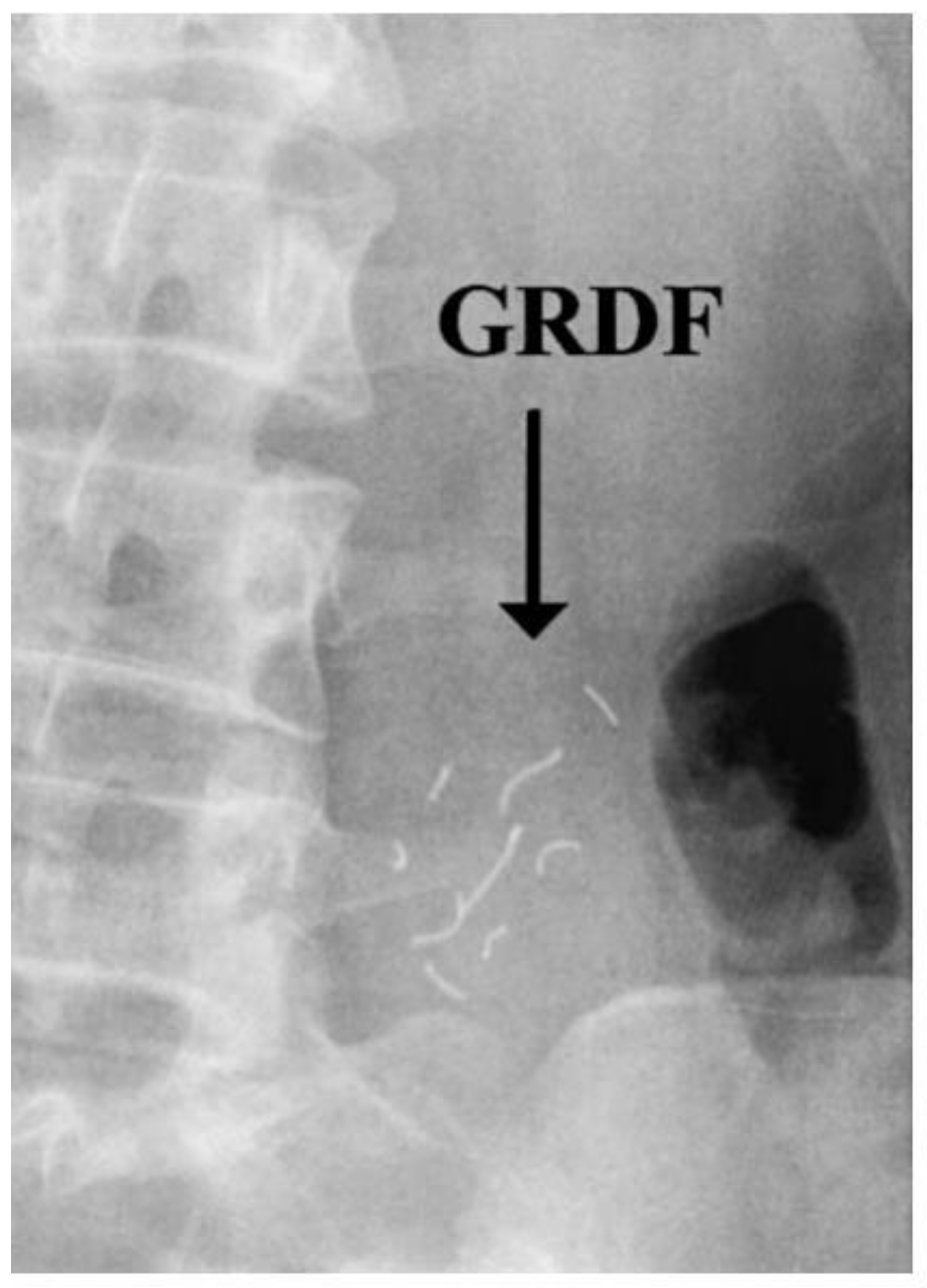
- Graham, D.Y.; Smith, L.J.; Jones, R.D.; Rakhit, A.; Tipnis, V.; Hurley, M.E. Gastroscopic localization of a microencapsulated KCI preparation in the human stomach. Gastrointest. Endosc. 1987, 33, 220–223. [Google Scholar] [CrossRef]
- Pedersen, P.B.; Bar-Shalom, D.; Baldursdottir, S.; Vilmann, P.; Müllertz, A. Feasibility of capsule endoscopy for direct imaging of drug delivery systems in the fasted upper-gastrointestinal tract. Pharm. Res. 2014, 31, 2044–2053. [Google Scholar] [CrossRef]
- Seo, J.; Kim, Y. Ultrasound imaging and beyond: Recent advances in medical ultrasound. Biomed. Eng. Lett. 2017, 7, 57–58. [Google Scholar] [CrossRef]
- Taftachi, F.; Sanaei-Zadeh, H.; Zamani, N.; Emamhadi, M. The role of ultrasound in the visualization of the ingested medications in acute poisoning—A literature review. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2175–2177. [Google Scholar]
- Nordt, S.P.; Campbell, C.; Medak, A.; Tomaszweski, C.; Clark, R.F. Ultrasound visualization of ingested tablets: A pilot study. Pharmacotherapy 2011, 31, 273–276. [Google Scholar] [CrossRef]
- Amital, Y.; Silver, B.; Leikin, J.B.; Frischer, H. Visualization of ingested medications in the stomach by ultrasound. Am. J. Emerg. Med. 1992, 10, 18–23. [Google Scholar] [CrossRef]
- Maublant, J.C.; Hassine, H.; Sournac, M.; Dapogny, M.; Veyre, A.; Aiache, J.M.; Goutay, E. Ultrasonic Visualization of Tablets in the Gastrointestinal Tract. J. Nucl. Med. 1988, 29, 129. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, F.; Koziolek, M.; Weitschies, W. In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms. Pharmaceutics 2019, 11, 416. https://doi.org/10.3390/pharmaceutics11080416
Schneider F, Koziolek M, Weitschies W. In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms. Pharmaceutics. 2019; 11(8):416. https://doi.org/10.3390/pharmaceutics11080416
Chicago/Turabian StyleSchneider, Felix, Mirko Koziolek, and Werner Weitschies. 2019. "In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms" Pharmaceutics 11, no. 8: 416. https://doi.org/10.3390/pharmaceutics11080416
APA StyleSchneider, F., Koziolek, M., & Weitschies, W. (2019). In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms. Pharmaceutics, 11(8), 416. https://doi.org/10.3390/pharmaceutics11080416






