Novel Population Pharmacokinetic Approach to Explain the Differences between Cystic Fibrosis Patients and Healthy Volunteers via Protein Binding
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design and Drug Administration
2.3. Blood Sampling
2.4. Urine Collections
2.5. Drug Analysis
2.6. Population Pharmacokinetic Analysis
2.6.1. Population Model
2.6.2. Modeling Approach
2.6.3. Body Size and Composition
2.6.4. Between-Subject Variability Model
2.6.5. Observation Model
2.6.6. Estimation and Computation
2.6.7. Monte Carlo Simulations
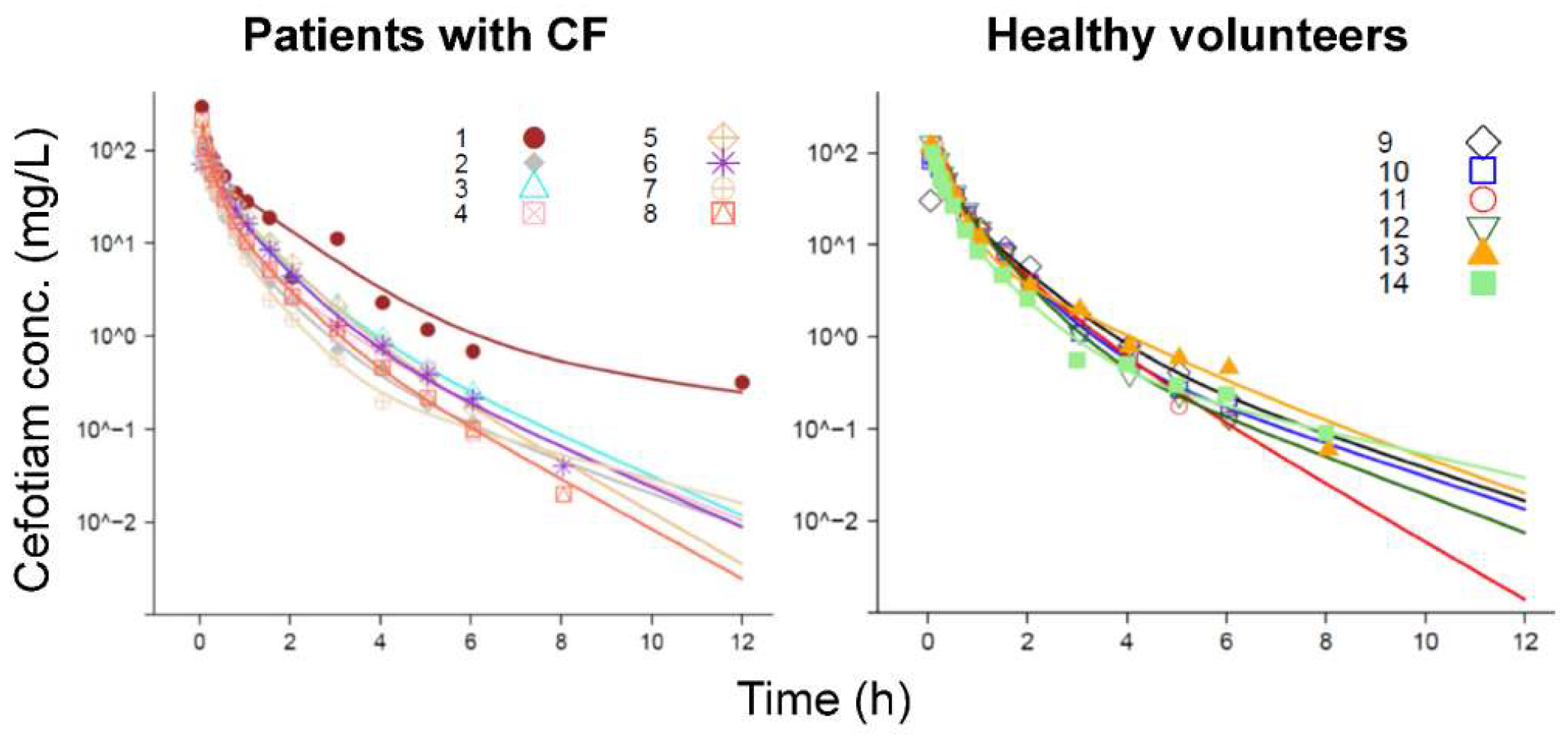
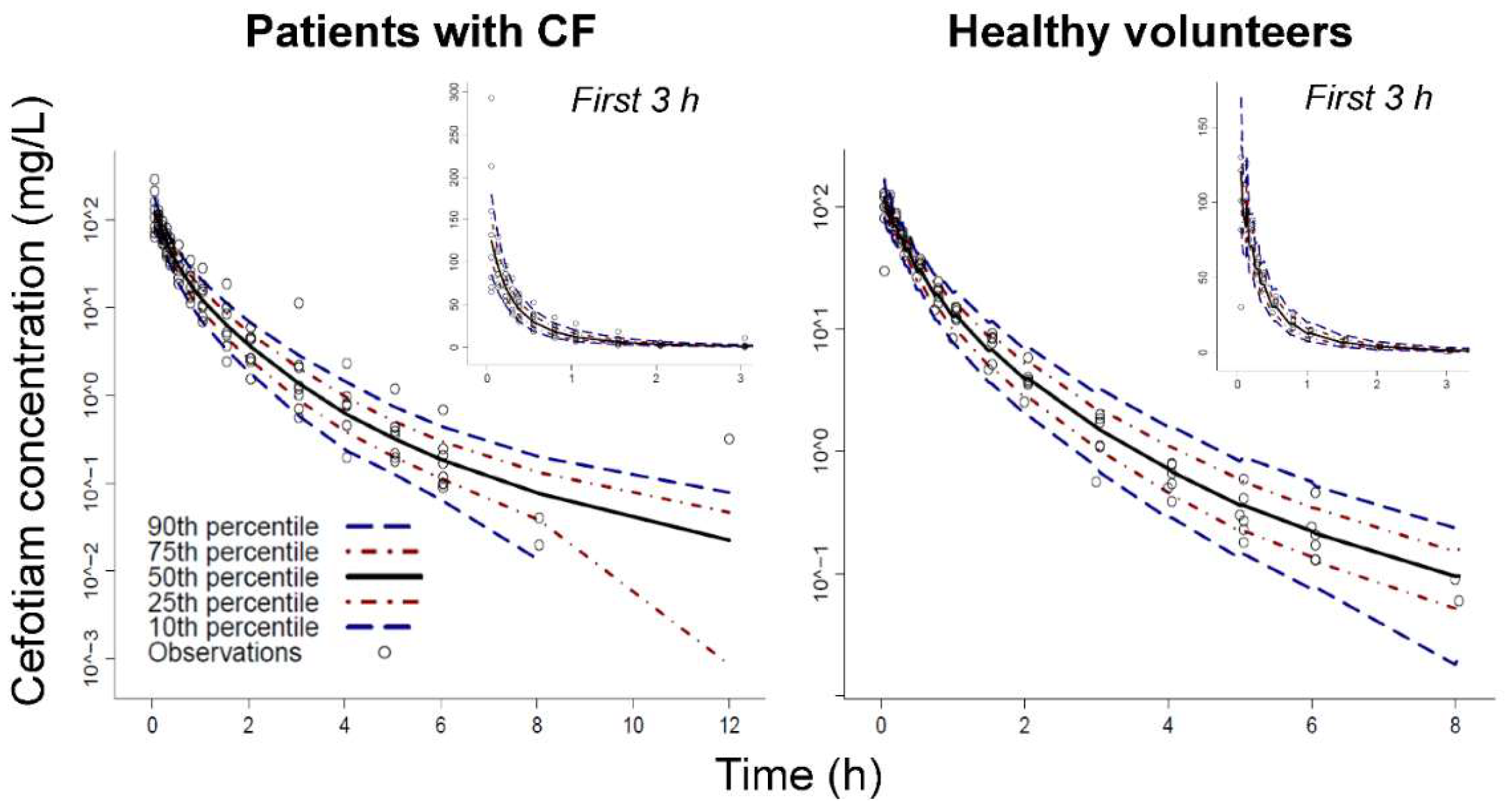
3. Results
Population Pharmacokinetic Modeling
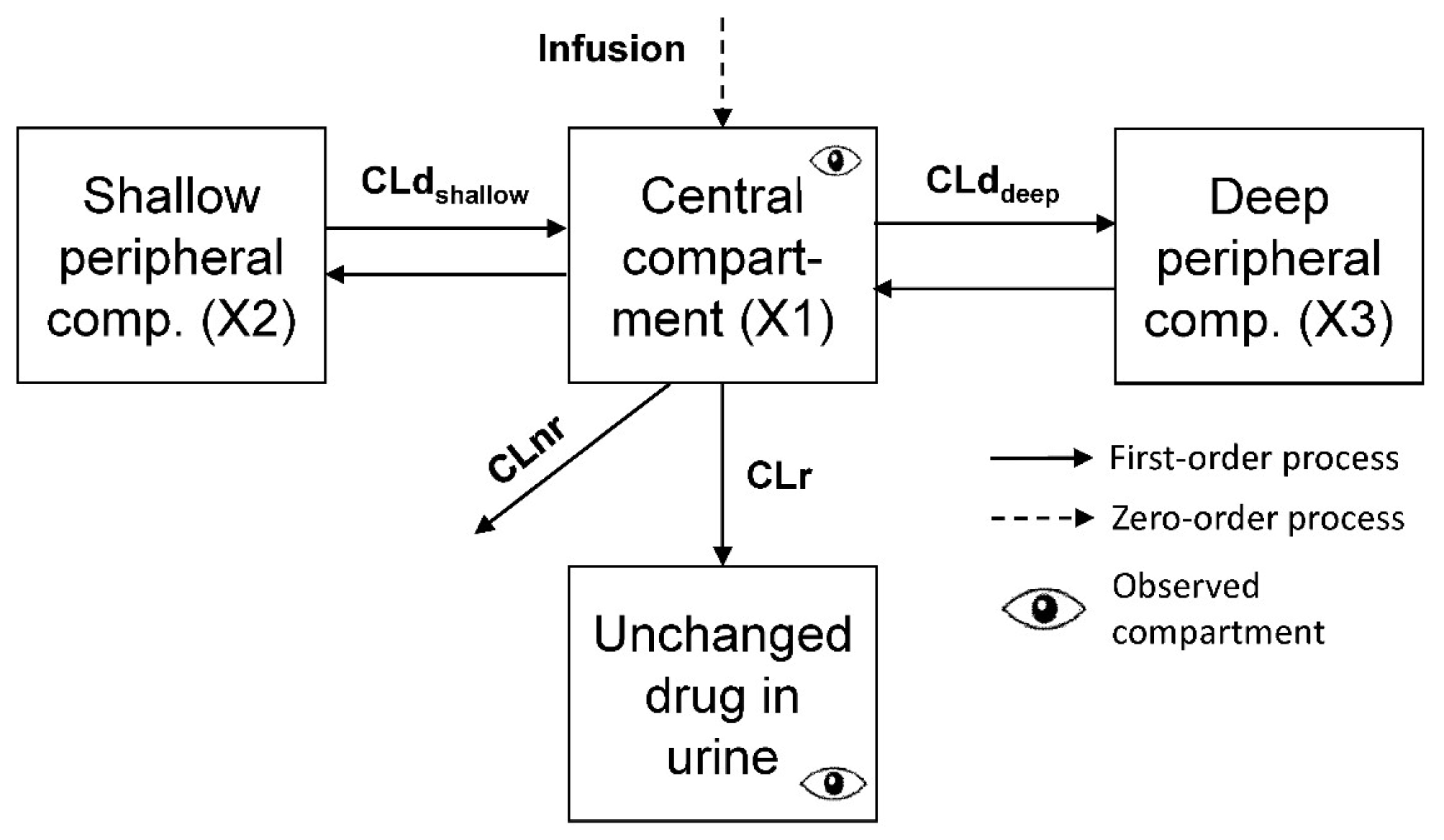
Structural Model
Body Size Models
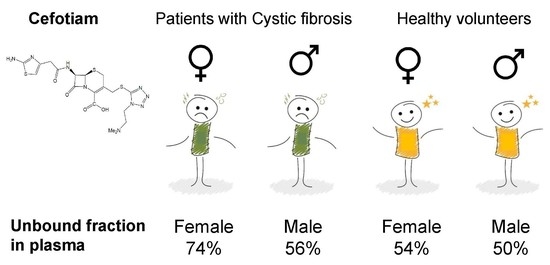
Estimating Unbound Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Touw, D.J.; Vinks, A.A.; Mouton, J.W.; Horrevorts, A.M. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis. Current practice and suggestions for future directions. Clin. Pharmacokinet. 1998, 35, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Rey, E.; Treluyer, J.M.; Pons, G. Drug disposition in cystic fibrosis. Clin. Pharmacokinet. 1998, 35, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Jiao, Y.; Drescher, S.K.; Oliver, A.; Louie, A.; Moya, B.; Tao, X.; Wittau, M.; Tsuji, B.T.; Zavascki, A.P.; et al. Four decades of beta-lactam antibiotic pharmacokinetics in cystic fibrosis. Clin. Pharmacokinet. 2019, 58, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Prandota, J. Clinical pharmacology of antibiotics and other drugs in cystic fibrosis. Drugs 1988, 35, 542–578. [Google Scholar] [CrossRef]
- Jusko, W.J.; Mosovich, L.L.; Gerbracht, L.M.; Mattar, M.E.; Yaffe, S.J. Enhanced renal excretion of dicloxacillin in patients with cystic fibrosis. Pediatrics 1975, 56, 1038–1044. [Google Scholar]
- Spino, M.; Chai, R.P.; Isles, A.F.; Thiessen, J.J.; Tesoro, A.; Gold, R.; MacLeod, S.M. Cloxacillin absorption and disposition in cystic fibrosis. J. Pediatr. 1984, 105, 829–835. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Duffull, S.B.; Landersdorfer, C.B.; Kinzig, M.; Holzgrabe, U.; Stephan, U.; Drusano, G.L.; Sorgel, F. Comparison of the pharmacokinetics and pharmacodynamic profile of carumonam in cystic fibrosis patients and healthy volunteers. Diagn. Microbiol. Infect. Dis. 2009, 65, 130–141. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Landersdorfer, C.B.; Huttner, S.J.; Drusano, G.L.; Kinzig, M.; Holzgrabe, U.; Stephan, U.; Sorgel, F. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2010, 54, 1275–1282. [Google Scholar] [CrossRef]
- Vinks, A.A.; van Rossem, R.N.; Mathot, R.A.; Heijerman, H.G.; Mouton, J.W. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation. Antimicrob. Agents Chemother. 2007, 51, 3049–3055. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Kinzig, M.; Landersdorfer, C.B.; Holzgrabe, U.; Stephan, U.; Sorgel, F. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2011, 55, 2927–2936. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Duffull, S.B.; Kinzig-Schippers, M.; Holzgrabe, U.; Stephan, U.; Drusano, G.L.; Sorgel, F. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2007, 51, 2497–2507. [Google Scholar] [CrossRef]
- Spino, M.; Chai, R.P.; Isles, A.F.; Balfe, J.W.; Brown, R.G.; Thiessen, J.J.; MacLeod, S.M. Assessment of glomerular filtration rate and effective renal plasma flow in cystic fibrosis. J. Pediatr. 1985, 107, 64–70. [Google Scholar] [CrossRef]
- Hedman, A.; Adan-Abdi, Y.; Alvan, G.; Strandvik, B.; Arvidsson, A. Influence of the glomerular filtration rate on renal clearance of ceftazidime in cystic fibrosis. Clin. Pharmacokinet. 1988, 15, 57–65. [Google Scholar] [CrossRef]
- Wang, J.P.; Unadkat, J.D.; al-Habet, S.M.; O’Sullivan, T.A.; Williams-Warren, J.; Smith, A.L.; Ramsey, B. Disposition of drugs in cystic fibrosis. Iv. Mechanisms for enhanced renal clearance of ticarcillin. Clin. Pharmacol. Ther. 1993, 54, 293–302. [Google Scholar] [CrossRef]
- Brogard, J.M.; Jehl, F.; Willemin, B.; Lamalle, A.M.; Blickle, J.F.; Monteil, H. Clinical pharmacokinetics of cefotiam. Clin. Pharmacokinet. 1989, 17, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, T.; Sakamoto, H.; Fukada, S.; Nakamoto, S.; Hirose, T.; Itoh, N.; Nishida, M. Pharmacokinetics of ceftizoxime in animals after parenteral dosing. Antimicrob. Agents Chemother. 1980, 17, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Querol-Ferrer, V.; Zini, R.; Tillement, J.P. The blood binding of cefotiam and cyclohexanol, metabolites of the prodrug cefotiam hexetil, in-vitro. J. Pharm. Pharmacol. 1991, 43, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Andrews, J.M.; Bedford, K.A. Cefoperazone and cefotiam--two new cephalosporins: An in-vitro comparison. J. Antimicrob. Chemother. 1981, 7, 343–352. [Google Scholar] [CrossRef]
- Ikawa, K.; Morikawa, N.; Sakamoto, K.; Ikeda, K.; Ohge, H.; Takesue, Y.; Sueda, T. Pharmacokinetics and pharmacodynamic assessment of imipenem in the intraperitoneal fluid of abdominal surgery patients. Chemotherapy 2008, 54, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Holford, N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Hope, W.; Eakin, A.E.; Guina, T.; Tam, V.H.; Louie, A.; Drusano, G.L.; Hoover, J.L. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob. Agents Chemother. 2019, 63, e02307–e02318. [Google Scholar] [CrossRef] [PubMed]
- Kees, F.; Raasch, W.; Steger, M.; Grobecker, H. High-performance liquid chromatographic assay for cefotiam and d3-cefotiam in human serum. J. Chromatogr. 1990, 525, 484–489. [Google Scholar] [CrossRef][Green Version]
- Bulitta, J.B.; Okusanya, O.O.; Forrest, A.; Bhavnani, S.M.; Clark, K.; Still, J.G.; Fernandes, P.; Ambrose, P.G. Population pharmacokinetics of fusidic acid: Rationale for front-loaded dosing regimens due to autoinhibition of clearance. Antimicrob. Agents Chemother. 2013, 57, 498–507. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentre, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Paik, S.H.; Chi, Y.H.; Kim, T.H.; Shin, S.; Landersdorfer, C.B.; Jiao, Y.; Yadav, R.; Shin, B.S. Characterizing the time-course of antihypertensive activity and optimal dose range of fimasartan via mechanism-based population modeling. Eur. J. Pharm. Sci. 2017, 107, 32–44. [Google Scholar] [CrossRef]
- Riccardi, K.; Cawley, S.; Yates, P.D.; Chang, C.; Funk, C.; Niosi, M.; Lin, J.; Di, L. Plasma protein binding of challenging compounds. J. Pharm. Sci. 2015, 104, 2627–2636. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. The fourth dimension of life: Fractal geometry and allometric scaling of organisms. Science 1999, 284, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H. A size standard for pharmacokinetics. Clin. Pharmacokinet. 1996, 30, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- James, W. Research on Obesity; Her Majesty’s Stationery Office: London, UK, 1976.
- Bauer, R.J.; Guzy, S.; Ng, C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J. 2007, 9, E60–E83. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Bingolbali, A.; Shin, B.S.; Landersdorfer, C.B. Development of a new pre- and post-processing tool (sadapt-tran) for nonlinear mixed-effects modeling in s-adapt. AAPS J. 2011, 13, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Landersdorfer, C.B. Performance and robustness of the monte carlo importance sampling algorithm using parallelized s-adapt for basic and complex mechanistic models. AAPS J. 2011, 13, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Wittau, M.; Scheele, J.; Kurlbaum, M.; Brockschmidt, C.; Wolf, A.M.; Hemper, E.; Henne-Bruns, D.; Bulitta, J.B. Population pharmacokinetics and target attainment of meropenem in plasma and tissue of morbidly obese patients after laparoscopic intraperitoneal surgery. Antimicrob. Agents Chemother. 2015, 59, 6241–6247. [Google Scholar] [CrossRef]
- Barbato, F.; La Rotonda, M.I.; Morrica, P. pH-dependence of hydrophobic parameters in a set of cephaloporin antibiotics. Pharmacochemical Lib. 1991, 16, 99–102. [Google Scholar]
- Meulemans, A.; Vicart, P.; Mohler, J.; Vulpillat, M. Determination of antibiotic lipophilicity with a micromethod: Application to brain permeability in man and rats. Chemotherapy 1988, 34, 90–95. [Google Scholar] [CrossRef]
- Tawara, S.; Matsumoto, S.; Kamimura, T.; Goto, S. Effect of protein binding in serum on therapeutic efficacy of cephem antibiotics. Antimicrob. Agents Chemother. 1992, 36, 17–24. [Google Scholar] [CrossRef][Green Version]
- Perl, T.M.; Pfaller, M.A.; Houston, A.; Wenzel, R.P. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrob. Agents Chemother. 1990, 34, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Rolinson, G.N.; Sutherland, R. The binding of antibiotics to serum proteins. Br. J. Pharmacol. 1965, 25, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, S.J.; Gerbracht, L.M.; Mosovich, L.L.; Mattar, M.E.; Danish, M.; Jusko, W.J. Pharmacokinetics of methicillin in patients with cystic fibrosis. J. Infect. Dis. 1977, 135, 828–831. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.; Hack, B.D.; Weber, A.; Chaffin, D.; Ramsey, B.; Smith, A.L. Pharmacokinetics of ticarcillin in patients with cystic fibrosis: A controlled prospective study. Clin. Pharmacol. Ther. 1990, 47, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Beringer, P.M.; Kriengkauykiat, J.; Zhang, X.; Hidayat, L.; Liu, S.; Louie, S.; Synold, T.; Burckart, G.J.; Rao, P.A.; Shapiro, B.; et al. Lack of effect of p-glycoprotein inhibition on renal clearance of dicloxacillin in patients with cystic fibrosis. Pharmacotherapy 2008, 28, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.R.; Arcasoy, S.M.; Shah, A.; Schulze, P.C.; Sze, J.; Sonett, J.R.; Lederer, D.J. Hypoalbuminemia and early mortality after lung transplantation: A cohort study. Am. J. Transplant. 2012, 12, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Sörgel, F.; Stephan, U.; Wiesemann, H.-G.; Gottschalk, B.; Stehr, C.; Rey, M.; Böwing, H.B.; Dominick, H.C.; Geldmacher von Mallinckrodt, M. High dose treatment with antibiotics in cystic fibrosis--a reappraisal with special reference to the pharmacokinetics of beta-lactams and new fluoroquinolones in adult CF-patients. Infection 1987, 15, 385–396. [Google Scholar] [CrossRef]
- Brisson, A.M.; Bryskier, A.; Millerioux, L.; Fourtillan, J.B. Pharmacokinetics of cefotiam administered intravenously and intramuscularly to healthy adults. Antimicrob. Agents Chemother. 1984, 26, 513–518. [Google Scholar] [CrossRef]
- Daschner, F.D.; Hemmer, K.A.; Offermann, P.; Slanicka, J. Pharmacokinetics of cefotiam in normal humans. Antimicrob. Agents Chemother. 1982, 22, 958–960. [Google Scholar] [CrossRef]
- Chiba, K.; Tsuchiya, M.; Kato, J.; Ochi, K.; Kawa, Z.; Ishizaki, T. Cefotiam disposition in markedly obese athlete patients, japanese sumo wrestlers. Antimicrob. Agents Chemother. 1989, 33, 1188–1192. [Google Scholar] [CrossRef]
- Rouan, M.C.; Lecaillon, J.B.; Guibert, J.; Modai, J.; Schoeller, J.P. Pharmacokinetics of cefotiam in humans. Antimicrob. Agents Chemother. 1985, 27, 177–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, Y.; Shen, H.; Hu, Y.; Feng, M.R.; Smith, D.E. Population pharmacokinetic modeling of cefadroxil renal transport in wild-type and pept2 knockout mice. Xenobiotica 2016, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Kinzig, M.; Jakob, V.; Holzgrabe, U.; Sorgel, F.; Holford, N.H. Nonlinear pharmacokinetics of piperacillin in healthy volunteers--implications for optimal dosage regimens. Br. J. Clin. Pharmacol. 2010, 70, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Landersdorfer, C.B.; Bulitta, J.B.; Kirkpatrick, C.M.; Kinzig, M.; Holzgrabe, U.; Drusano, G.L.; Stephan, U.; Sorgel, F. Population pharmacokinetics of piperacillin at two dose levels: Influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob. Agents Chemother. 2012, 56, 5715–5723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Landersdorfer, C.B.; Kirkpatrick, C.M.; Kinzig, M.; Bulitta, J.B.; Holzgrabe, U.; Sorgel, F. Inhibition of flucloxacillin tubular renal secretion by piperacillin. Br. J. Clin. Pharmacol. 2008, 66, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Bow, D.A.; Perry, J.L.; Simon, J.D.; Pritchard, J.B. The impact of plasma protein binding on the renal transport of organic anions. J. Pharmacol. Exp. Ther. 2006, 316, 349–355. [Google Scholar] [CrossRef]
- Rodriguez, C.A.; Smith, D.E. Influence of the unbound concentration of cefonicid on its renal elimination in isolated perfused rat kidneys. Antimicrob. Agents. Chemother. 1991, 35, 2395–2400. [Google Scholar] [CrossRef]
- Benabdeslam, H.; Garcia, I.; Bellon, G.; Gilly, R.; Revol, A. Biochemical assessment of the nutritional status of cystic fibrosis patients treated with pancreatic enzyme extracts. Am. J. Clin. Nutr. 1998, 67, 912–918. [Google Scholar] [CrossRef]
- Stonebraker, J.R.; Ooi, C.Y.; Pace, R.G.; Corvol, H.; Knowles, M.R.; Durie, P.R.; Ling, S.C. Features of severe liver disease with portal hypertension in patients with cystic fibrosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1207–1215.e3. [Google Scholar] [CrossRef]
- Shinagawa, N.; Tachi, Y.; Ishikawa, S.; Yura, J. Prophylactic antibiotics for patients undergoing elective biliary tract surgery: A prospective randomized study of cefotiam and cefoperazone. Jpn. J. Surg. 1987, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, K.; Kobayashi, S.; Machida, T.; Yoshida, K. Randomized prospective comparison of fosfomycin and cefotiam for prevention of postoperative infection following urological surgery. J. Infect. Chemother. 2007, 13, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wildfeuer, A.; Mallwitz, J.; Gotthardt, H.; Hille, E.; Gruber, H.; Dahmen, G.; Pfaff, G.; Gobel, C. Pharmacokinetics of ampicillin, sulbactam and cefotiam in patients undergoing orthopedic surgery. Infection 1997, 25, 258–262. [Google Scholar] [CrossRef]
- Knoop, M.; Schutze, M.; Piek, J.; Drewelow, B.; Mundkowski, R. Antibiotic prophylaxis in cerebrospinal fluid shunting: Reassessment of cefotiam penetration into human csf. Zentralbl. Neurochir. 2007, 68, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, T.; Gilsbach, J.M. Intra-operative antibiotic prophylaxis in neurosurgery. A prospective, randomized, controlled study on cefotiam. Acta Neurochir. 1991, 113, 103–109. [Google Scholar] [CrossRef]
- Shinagawa, N. Antimicrobial prophylaxis in surgery. Jpn. J. Antibiot. 2004, 57, 11–32. [Google Scholar]
- Hedrick, T.L.; Smith, P.W.; Gazoni, L.M.; Sawyer, R.G. The appropriate use of antibiotics in surgery: A review of surgical infections. Curr. Probl. Surg. 2007, 44, 635–675. [Google Scholar] [CrossRef]
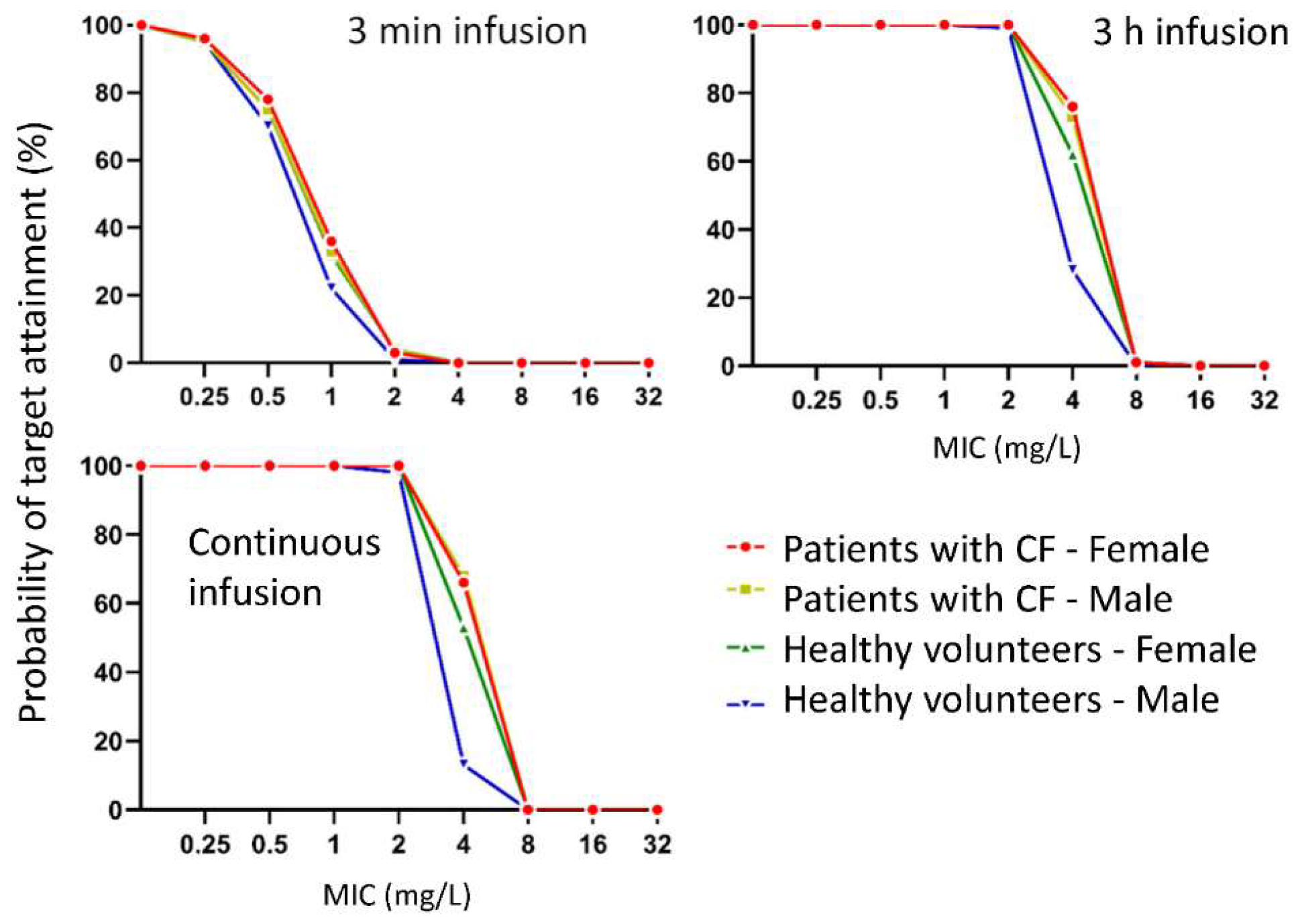
| Demographic Variable | Patients with CF | Healthy Volunteers |
|---|---|---|
| Number of subjects (males/females) | 8 (4/4) | 6 (3/3) |
| Age (yr) | 19 [17–24] | 23.5 [21–26] |
| Height (cm) | 167 [157–173] | 169 [164–190] |
| Total body weight (WT) (kg) | 45.5 [33.0–59.0] | 68.5 [58.0–80.0] |
| WT in females | 48.0 [33.0–59.0] | 58.0 [58.0–62.0] |
| WT in males | 44.9 [44.6–53.5] | 80.0 [75.0–80.0] |
| Lean body mass (LBM) a (kg) | 40.3 [28.8–46.2] | 50.6 [44.6–65.4] |
| LBM in females | 38.8 [28.8–45.7] | 44.6 [44.6–45.4] |
| LBM in males | 40.4 [39.6–46.2] | 62.8 [55.8–65.4] |
| Body mass index (kg m−2) | 17.0 [13.4–19.9] | 22.5 [20.3–27.9] |
| Pharmacokinetic Parameter | Patients with CF | Healthy Volunteers |
|---|---|---|
| Total clearance (L/h) | 17.1 [8.97–27.8] | 17.7 [16.2–24.0] |
| in females | 22.1 [15.0–27.8] a | 16.2 [16.2–18.5] a |
| in males | 15.9 [8.97–21.1] a | 19.1 [16.9 - 24.0] a |
| Renal clearance (L/h) | 12.0 [4.27–19.5] | 11.6 [10.6–17.0] |
| in females | 15.5 [10.7–19.5] a | 11.8 [10.6–12.5] a |
| in males | 11.7 [4.27–12.6] a | 11.3 [10.9–17.0] a |
| Non-renal clearance (L/h) | 5.08 [3.19–8.47] | 5.97 [4.41–7.76] |
| in females | 6.52 [4.22–8.26] a | 5.65 [4.41–6.01] a |
| in males | 4.96 [3.19–8.47] a | 7.04 [5.93–7.76] a |
| Volume of distribution at steady-state (L) | 12.4 [8.80–18.1] | 12.8 [10.5–16.7] |
| in females | 13.3 [8.80–18.1] a | 10.7 [10.5–13.3] a |
| in males | 12.3 [10.6–13.6] a | 16.7 [12.4–16.7] a |
| Peak concentration (mg/L) | 124 [74.1–293] | 111 [81.7–130] |
| Terminal half-life (h) | 0.931 [0.881–1.91] | 1.08 [0.753–1.66] |
| Mean residence time (h) | 0.699 [0.527–1.22] | 0.707 [0.646–0.874] |
| Fraction of dose excreted unchanged into urine (%) | 70.3% [47.6–77.8%] | 66.3% [59.4–72.7%] |
| Body Size Model a | FCYFCLr | FCYFCLnr | FCYFVss |
|---|---|---|---|
| 1) No body size model | 0.99 (22.7%) | 0.90 (11.4%) | 1.03 (53.1%) |
| 2) WT linear scaling | 1.43 (12.4%) | 1.31 (22.7%) | 1.52 (12.4%) |
| 3) WT allometric | 1.31 (10.4%) | 1.19 (7.7%) | 1.52 (14.0%) |
| 4) LBM linear scaling | 1.29 (12.4%) | 1.19 (22.9%) | 1.38 (14.8%) |
| 5) LBM allometric | 1.21 (7.5%) | 1.11 (14.7%) | 1.38 (6.9%) |
| Pharmacokinetic Parameter | Symbol | Unit | Population Mean (SE%) | BSV a (SE%) |
|---|---|---|---|---|
| Unbound renal clearance | CLru | L/h | 23.8 (6.9%) | 0.237 (52.7%) |
| Unbound nonrenal clearance | CLnru | L/h | 11.0 (7.0%) | 0.237 (50.2%) |
| Unbound total clearance | CLtotu | L/h | 34.8 b | |
| Unbound volume of distribution of central compartment | V1u | L | 15.6 (6.5%) | 0.189 (74.0%) |
| Unbound volume of distribution of shallow peripheral compartment | V2u | L | 6.91 (14.1%) | 0.256 (88.2%) |
| Unbound volume of distribution of deep peripheral compartment | V3u | L | 4.56 (16.4%) | 0.451 (131%) |
| Unbound volume of distribution at steady-state | Vssu | L | 27.1 c | |
| Unbound distribution clearance for shallow peripheral compartment | CLdshallow,u | L/h | 13.8 (15.0%) | 0.416 (183%) |
| Unbound distribution clearance for deep peripheral compartment | CLddeep,u | L/h | 1.84 (26.1%) | 0.309 (83.8%) |
| Unbound fraction in plasma for females with CF | fuCF,F | 0.744 (4.5%) d | ||
| Unbound fraction in plasma for males with CF | fuCF,M | 0.563 (13.5%) d | ||
| Unbound fraction in plasma for female healthy volunteers | fuHV,F | 0.545 (13.6%) d | ||
| Unbound fraction in plasma for male healthy volunteers | fuHV,M | 0.50 (fixed) | ||
| SD of additive residual error for plasma concentrations | SDin | mg/L | 0.0186 (53.7%) | |
| Proportional residual error for plasma concentrations | SDsl | 0.166 (7.8%) | ||
| SD of additive residual error for fraction of dose in urine | UDin | % | 0.384 (76.1%) |
| Dosage Regimen | Bacteriostasis Target (40% fT>MIC) | |||
| Patients with CF | Healthy Volunteers | |||
| Female | Male | Female | Male | |
| Continuous infusion of 3000 mg/day (with a 500 mg loading dose at 0 h) | 2 | 2 | 2 | 2 |
| Prolonged (3 h) infusions of 1000 mg every 8 h | 2 | 2 | 2 | 2 |
| Short-term (3 min) infusions of 1000 mg every 8 h | 0.25 | 0.25 | 0.25 | 0.25 |
| Near-Maximal Killing Target (65% fT>MIC) | ||||
| Continuous infusion of 3000 mg/day (with a 500 mg loading dose at 0 h) | 2 | 2 | 2 | 2 |
| Prolonged (3 h) infusions of 1000 mg every 8 h | 0.25 | 0.25 | 0.25 | 0.25 |
| Short-term (3 min) infusions of 1000 mg every 8 h | 0.0625 | 0.0625 | 0.0625 | 0.0625 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.R.; Bulitta, J.B.; Kinzig, M.; Landersdorfer, C.B.; Jiao, Y.; Sutaria, D.S.; Tao, X.; Höhl, R.; Holzgrabe, U.; Kees, F.; et al. Novel Population Pharmacokinetic Approach to Explain the Differences between Cystic Fibrosis Patients and Healthy Volunteers via Protein Binding. Pharmaceutics 2019, 11, 286. https://doi.org/10.3390/pharmaceutics11060286
Shah NR, Bulitta JB, Kinzig M, Landersdorfer CB, Jiao Y, Sutaria DS, Tao X, Höhl R, Holzgrabe U, Kees F, et al. Novel Population Pharmacokinetic Approach to Explain the Differences between Cystic Fibrosis Patients and Healthy Volunteers via Protein Binding. Pharmaceutics. 2019; 11(6):286. https://doi.org/10.3390/pharmaceutics11060286
Chicago/Turabian StyleShah, Nirav R., Jürgen B. Bulitta, Martina Kinzig, Cornelia B. Landersdorfer, Yuanyuan Jiao, Dhruvitkumar S. Sutaria, Xun Tao, Rainer Höhl, Ulrike Holzgrabe, Frieder Kees, and et al. 2019. "Novel Population Pharmacokinetic Approach to Explain the Differences between Cystic Fibrosis Patients and Healthy Volunteers via Protein Binding" Pharmaceutics 11, no. 6: 286. https://doi.org/10.3390/pharmaceutics11060286
APA StyleShah, N. R., Bulitta, J. B., Kinzig, M., Landersdorfer, C. B., Jiao, Y., Sutaria, D. S., Tao, X., Höhl, R., Holzgrabe, U., Kees, F., Stephan, U., & Sörgel, F. (2019). Novel Population Pharmacokinetic Approach to Explain the Differences between Cystic Fibrosis Patients and Healthy Volunteers via Protein Binding. Pharmaceutics, 11(6), 286. https://doi.org/10.3390/pharmaceutics11060286