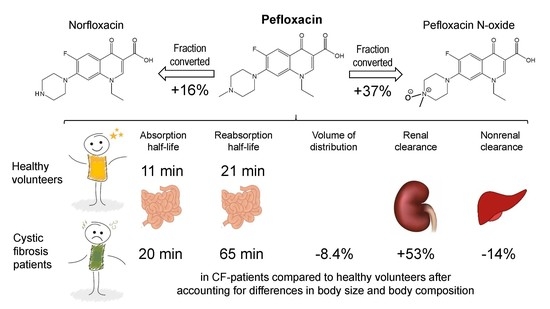
Comparable Bioavailability and Disposition of Pefloxacin in Patients with Cystic Fibrosis and Healthy Volunteers Assessed via Population Pharmacokinetics
Abstract
1. Introduction
2. Methods and Materials
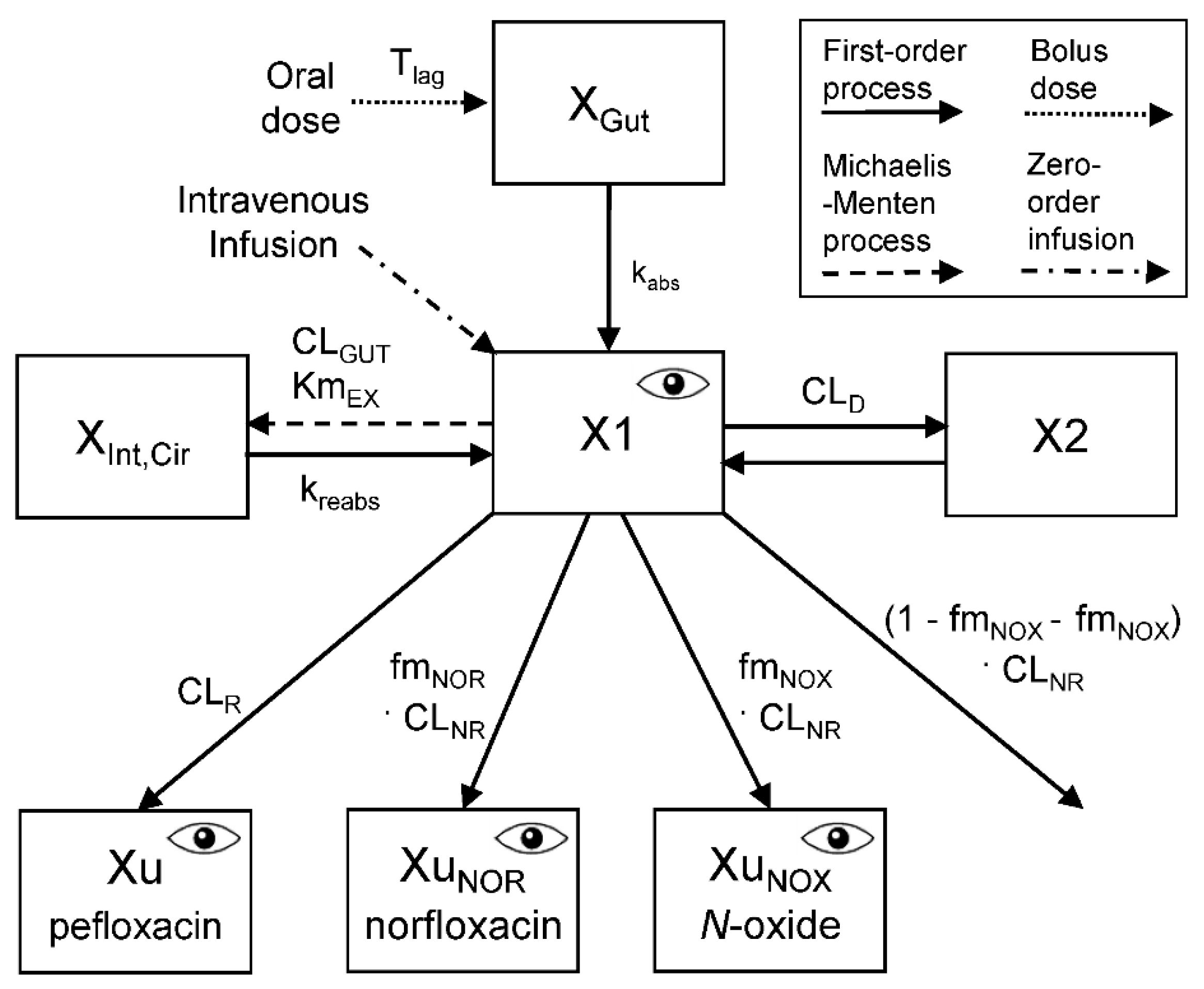
Population Pharmacokinetic Analysis
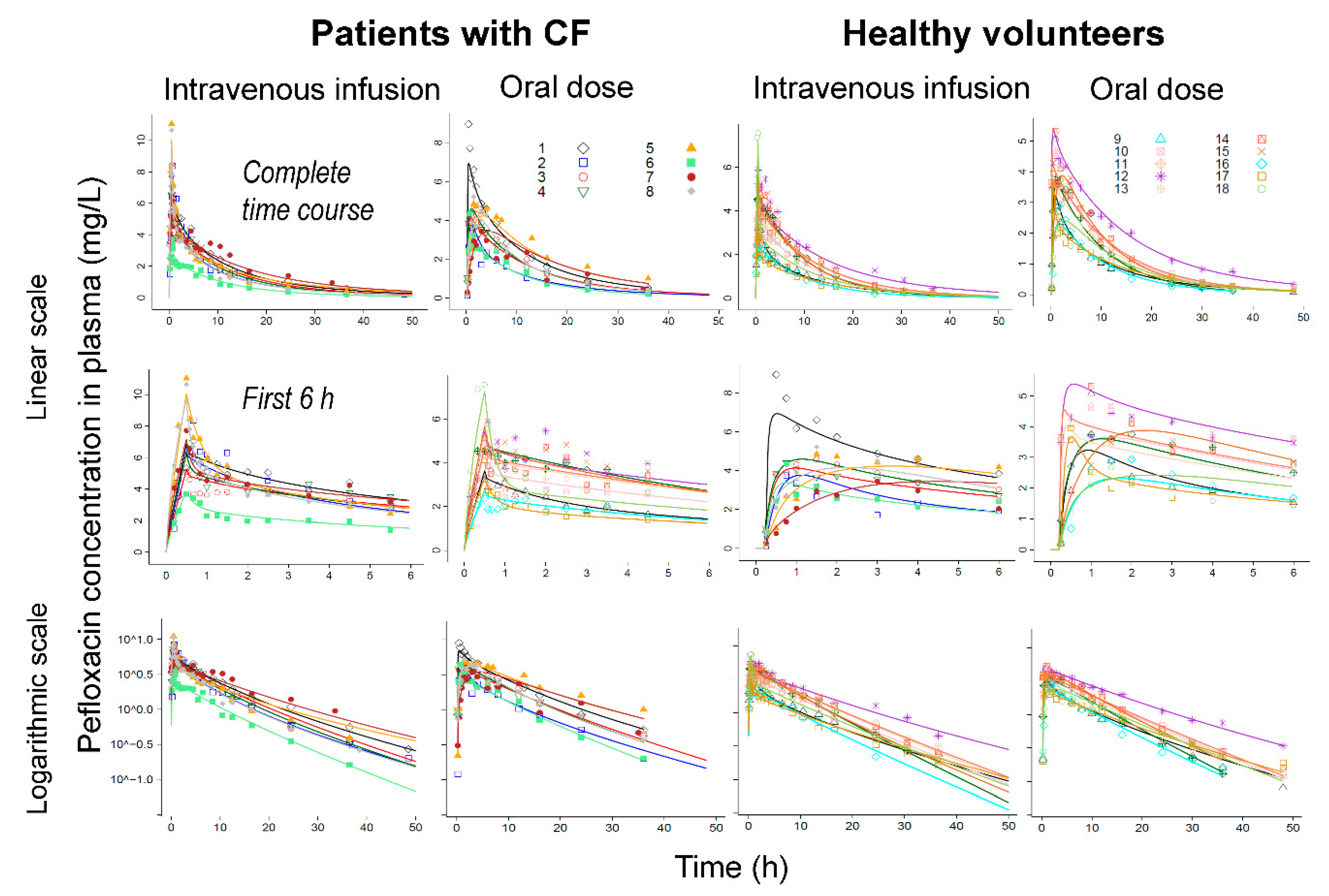
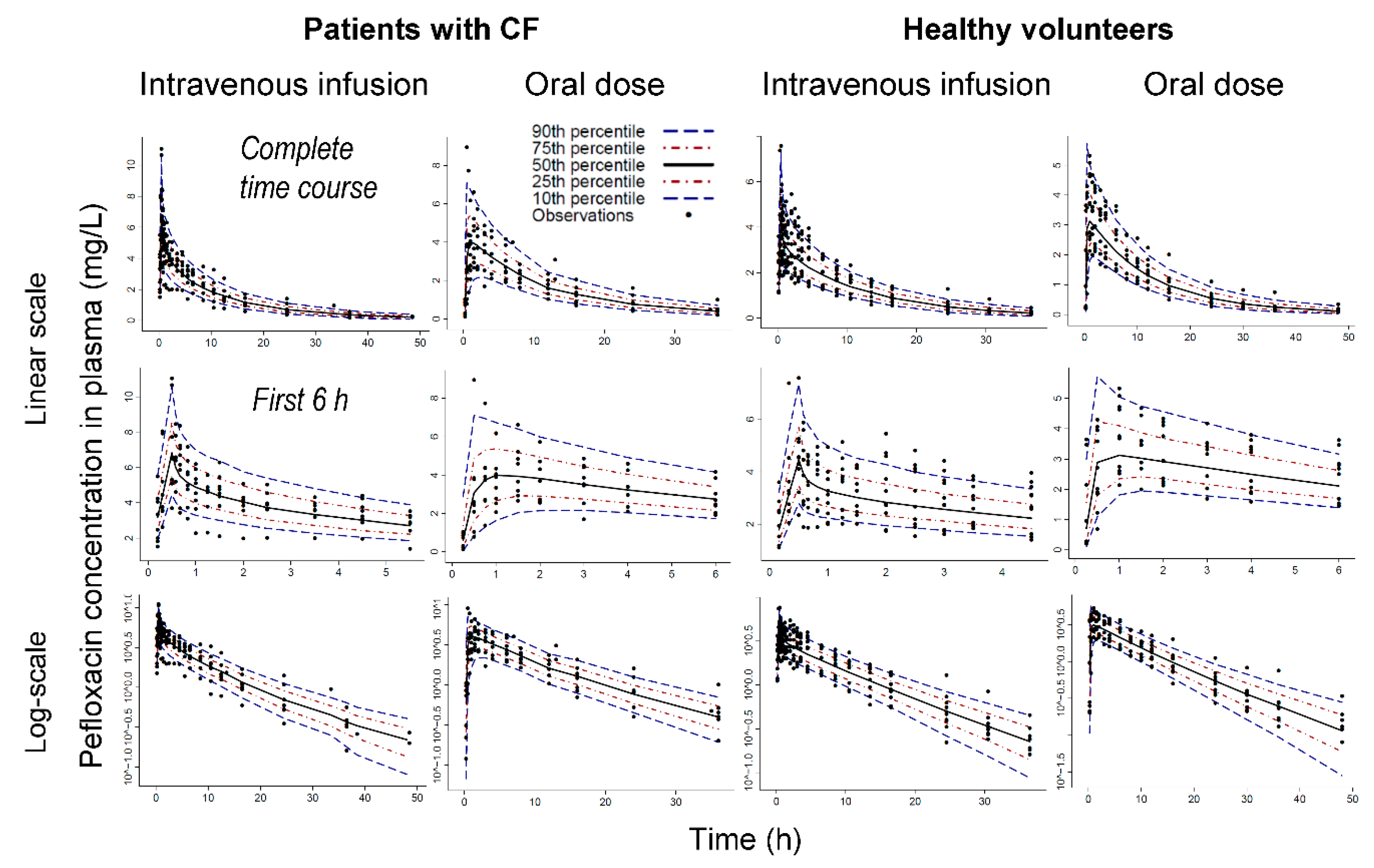
3. Results
Population Pharmacokinetic Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bulitta, J.B.; Jiao, Y.; Drescher, S.K.; Oliver, A.; Louie, A.; Moya, B.; Tao, X.; Wittau, M.; Tsuji, B.T.; Zavascki, A.P.; et al. Four decades of beta-lactam antibiotic pharmacokinetics in cystic fibrosis. Clin. Pharmacokinet. 2019, 58, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Touw, D.J.; Vinks, A.A.; Mouton, J.W.; Horrevorts, A.M. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis. Current practice and suggestions for future directions. Clin. Pharmacokinet. 1998, 35, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Rey, E.; Treluyer, J.M.; Pons, G. Drug disposition in cystic fibrosis. Clin. Pharmacokinet. 1998, 35, 313–329. [Google Scholar] [CrossRef] [PubMed]
- LeBel, M.; Bergeron, M.G.; Vallee, F.; Fiset, C.; Chasse, G.; Bigonesse, P.; Rivard, G. Pharmacokinetics and pharmacodynamics of ciprofloxacin in cystic fibrosis patients. Antimicrob. Agents Chemother. 1986, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Christensson, B.A.; Nilsson-Ehle, I.; Ljungberg, B.; Lindblad, A.; Malmborg, A.S.; Hjelte, L.; Strandvik, B. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrob. Agents Chemother. 1992, 36, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Koup, J.R.; Williams-Warren, J.; Weber, A.; Heggen, L.; Stempel, D.; Smith, A.L. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrob. Agents Chemother. 1987, 31, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Gastonguay, M.R. Population pharmacokinetics of ciprofloxacin in pediatric patients. J. Clin. Pharmacol. 2003, 43, 698–710. [Google Scholar] [CrossRef]
- Payen, S.; Serreau, R.; Munck, A.; Aujard, Y.; Aigrain, Y.; Bressolle, F.; Jacqz-Aigrain, E. Population pharmacokinetics of ciprofloxacin in pediatric and adolescent patients with acute infections. Antimicrob. Agents Chemother. 2003, 47, 3170–3178. [Google Scholar] [CrossRef]
- Reed, M.D.; Stern, R.C.; Myers, C.M.; Yamashita, T.S.; Blumer, J.L. Lack of unique ciprofloxacin pharmacokinetic characteristics in patients with cystic fibrosis. J. Clin. Pharmacol. 1988, 28, 691–699. [Google Scholar] [CrossRef]
- Mimeault, J.; Vallee, F.; Seelmann, R.; Sorgel, F.; Ruel, M.; LeBel, M. Altered disposition of fleroxacin in patients with cystic fibrosis. Clin. Pharmacol. Ther. 1990, 47, 618–628. [Google Scholar] [CrossRef]
- Jiao, Y.; Kim, T.H.; Tao, X.; Kinzig, M.; Landersdorfer, C.B.; Drescher, S.K.; Sutaria, D.S.; Moya, B.; Holzgrabe, U.; Sorgel, F.; et al. First population pharmacokinetic analysis showing increased quinolone metabolite formation and clearance in patients with cystic fibrosis compared to healthy volunteers. Eur. J. Pharm. Sci. 2018, 123, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Allen, S.E.; Amsden, G.W. Altered steady state pharmacokinetics of levofloxacin in adult cystic fibrosis patients receiving calcium carbonate. J. Cyst. Fibros. 2006, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Sorgel, F.; Kinzig, M. Pharmacokinetics of gyrase inhibitors, part 2: Renal and hepatic elimination pathways and drug interactions. Am. J. Med. 1993, 94, 56S–69S. [Google Scholar] [PubMed]
- Sorgel, F.; Kinzig, M. Pharmacokinetics of gyrase inhibitors, part 1: Basic chemistry and gastrointestinal disposition. Am. J. Med. 1993, 94, 44S–55S. [Google Scholar] [PubMed]
- Zlotos, G.; Bucker, A.; Kinzig-Schippers, M.; Sorgel, F.; Holzgrabe, U. Plasma protein binding of gyrase inhibitors. J. Pharm. Sci. 1998, 87, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Sorgel, F.; Bulitta, J.; Kinzig-Schippers, M. How well do gyrase inhibitors work? The pharmacokinetics of quinolones. Pharm. Unserer Zeit 2001, 30, 418–427. [Google Scholar]
- Sorgel, F. Metabolism of gyrase inhibitors. Rev. Infect. Dis. 1989, 11, S1119–S1129. [Google Scholar] [CrossRef]
- Lode, H.; Hoffken, G.; Boeckk, M.; Deppermann, N.; Borner, K.; Koeppe, P. Quinolone pharmacokinetics and metabolism. J. Antimicrob. Chemother. 1990, 26, 41–49. [Google Scholar] [CrossRef]
- Fitton, A. The quinolones. An overview of their pharmacology. Clin. Pharmacokinet. 1992, 22, 1–11. [Google Scholar] [CrossRef]
- Bressolle, F.; Goncalves, F.; Gouby, A.; Galtier, M. Pefloxacin clinical pharmacokinetics. Clin. Pharmacokinet. 1994, 27, 418–446. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Henwood, J.M. Pefloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1989, 37, 628–668. [Google Scholar] [CrossRef] [PubMed]
- Olcay, E.; Beytemur, O.; Kaleagasioglu, F.; Gulmez, T.; Mutlu, Z.; Olgac, V. Oral toxicity of pefloxacin, norfloxacin, ofloxacin and ciprofloxacin: Comparison of biomechanical and histopathological effects on achilles tendon in rats. J. Toxicol. Sci. 2011, 36, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Pouzaud, F.; Bernard-Beaubois, K.; Thevenin, M.; Warnet, J.M.; Hayem, G.; Rat, P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: Involvement of oxidative stress. J. Pharmacol. Exp. Ther. 2004, 308, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Casparian, J.M.; Luchi, M.; Moffat, R.E.; Hinthorn, D. Quinolones and tendon ruptures. South. Med. J. 2000, 93, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Holford, N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of lean bodyweight. Clin. Pharmacokinet. 2005, 44, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Vinks, A.A.; Van Rossem, R.N.; Mathot, R.A.; Heijerman, H.G.; Mouton, J.W. Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation. Antimicrob. Agents Chemother. 2007, 51, 3049–3055. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Duffull, S.B.; Kinzig-Schippers, M.; Holzgrabe, U.; Stephan, U.; Drusano, G.L.; Sorgel, F. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2007, 51, 2497–2507. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Duffull, S.B.; Landersdorfer, C.B.; Kinzig, M.; Holzgrabe, U.; Stephan, U.; Drusano, G.L.; Sorgel, F. Comparison of the pharmacokinetics and pharmacodynamic profile of carumonam in cystic fibrosis patients and healthy volunteers. Diagn. Microbiol. Infect. Dis. 2009, 65, 130–141. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Kinzig, M.; Landersdorfer, C.B.; Holzgrabe, U.; Stephan, U.; Sorgel, F. Comparable population pharmacokinetics and pharmacodynamic breakpoints of cefpirome in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2011, 55, 2927–2936. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Landersdorfer, C.B.; Huttner, S.J.; Drusano, G.L.; Kinzig, M.; Holzgrabe, U.; Stephan, U.; Sorgel, F. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 2010, 54, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, M.; Jaehde, U.; Koch, H.U.; Malter, U.; Naber, K.G.; Sorgel, F. High-performance liquid chromatographic assays for gyrase inhibitors in plasma, urine, several body fluids, and tissues. Rev. Infect. Dis. 1988, 10, S98–S100. [Google Scholar]
- Rubinstein, E.; Dautrey, S.; Farinoti, R.; St Julien, L.; Ramon, J.; Carbon, C. Intestinal elimination of sparfloxacin, fleroxacin, and ciprofloxacin in rats. Antimicrob. Agents Chemother. 1995, 39, 99–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinzig, M.; Seelmann, R.; Mahr, G.; Sorgel, F.; Naber, K.G.; Weidekamm, E.; Stockel, K. Significant gastrointestinal secretion of fleroxacin in man (abstr. 388). In Proceedings of the 17th International Congress on Chemotherapy, Berlin, Germany, 26 June 1991; Futuramed Publishers: Munich, Germany, 1991. [Google Scholar]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Okusanya, O.O.; Forrest, A.; Bhavnani, S.M.; Clark, K.; Still, J.G.; Fernandes, P.; Ambrose, P.G. Population pharmacokinetics of fusidic acid: Rationale for front-loaded dosing regimens due to autoinhibition of clearance. Antimicrob. Agents Chemother. 2013, 57, 498–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentre, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Paik, S.H.; Chi, Y.H.; Kim, T.H.; Shin, S.; Landersdorfer, C.B.; Jiao, Y.; Yadav, R.; Shin, B.S. Characterizing the time-course of antihypertensive activity and optimal dose range of fimasartan via mechanism-based population modeling. Eur. J. Pharm. Sci. 2017, 107, 32–44. [Google Scholar] [CrossRef]
- Bauer, R.J.; Guzy, S.; Ng, C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J. 2007, 9, E60–E83. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Bingolbali, A.; Shin, B.S.; Landersdorfer, C.B. Development of a new pre- and post-processing tool (sadapt-tran) for nonlinear mixed-effects modeling in s-adapt. AAPS J. 2011, 13, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Landersdorfer, C.B. Performance and robustness of the monte carlo importance sampling algorithm using parallelized s-adapt for basic and complex mechanistic models. AAPS J. 2011, 13, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- James, W. Research on Obesity; Her Majesty’s Stationery Office: London, UK, 1976. [Google Scholar]
- Barraclough, M.; Taylor, C.J. Twenty-four hour ambulatory gastric and duodenal ph profiles in cystic fibrosis: Effect of duodenal hyperacidity on pancreatic enzyme function and fat absorption. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Isenberg, J.N.; Ament, M.E. Gastric acid hypersecretion in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Fondacaro, J.D.; Heubi, J.E.; Kellogg, F.W. Intestinal bile acid malabsorption in cystic fibrosis: A primary mucosal cell defect. Pediatr. Res. 1982, 16, 494–498. [Google Scholar] [CrossRef]
- Yahiaoui, Y.; Jablonski, M.; Hubert, D.; Mosnier-Pudar, H.; Noel, L.H.; Stern, M.; Grenet, D.; Grunfeld, J.P.; Chauveau, D.; Fakhouri, F. Renal involvement in cystic fibrosis: Diseases spectrum and clinical relevance. Clin. J. Am. Soc. Nephrol. 2009, 4, 921–928. [Google Scholar] [CrossRef]
- Jouret, F.; Bernard, A.; Hermans, C.; Dom, G.; Terryn, S.; Leal, T.; Lebecque, P.; Cassiman, J.J.; Scholte, B.J.; De Jonge, H.R.; et al. Cystic fibrosis is associated with a defect in apical receptor-mediated endocytosis in mouse and human kidney. J. Am. Soc. Nephrol. 2007, 18, 707–718. [Google Scholar] [CrossRef]
- Katz, S.M.; Krueger, L.J.; Falkner, B. Microscopic nephrocalcinosis in cystic fibrosis. N. Engl. J. Med. 1988, 319, 263–266. [Google Scholar] [CrossRef]
- Jaehde, U.; Sorgel, F.; Stephan, U.; Schunack, W. Effect of an antacid containing magnesium and aluminum on absorption, metabolism, and mechanism of renal elimination of pefloxacin in humans. Antimicrob. Agents Chemother. 1994, 38, 1129–1133. [Google Scholar] [CrossRef]
- Petitjean, O.; Pangon, B.; Brion, N.; Tod, M.; Chaplain, C.; Le Gros, V.; Louchahi, K.; Allouch, P. Pharmacokinetics and bactericidal activities of one 800-milligram dose versus two 400-milligram doses of intravenously administered pefloxacin in healthy volunteers. Antimicrob. Agents Chemother. 1993, 37, 737–740. [Google Scholar] [CrossRef][Green Version]
- Frydman, A.M.; Le Roux, Y.; Lefebvre, M.A.; Djebbar, F.; Fourtillan, J.B.; Gaillot, J. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400 mg bid) in young healthy volunteers. J. Antimicrob. Chemother. 1986, 17, 65–79. [Google Scholar] [CrossRef]
- Wise, R.; Griggs, D.; Andrews, J.M. Pharmacokinetics of the quinolones in volunteers: A proposed dosing schedule. Rev. Infect. Dis. 1988, 10, S70–S77. [Google Scholar] [CrossRef]
- Sorgel, F.; Jaehde, U.; Naber, K.; Stephan, U. Pharmacokinetic disposition of quinolones in human body fluids and tissues. Clin. Pharmacokinet. 1989, 16, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Stass, H.; Kubitza, D.; Moller, J.G.; Delesen, H. Influence of activated charcoal on the pharmacokinetics of moxifloxacin following intravenous and oral administration of a 400 mg single dose to healthy males. Br. J. Clin. Pharmacol. 2005, 59, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Mulgaonkar, A.; Venitz, J.; Grundemann, D.; Sweet, D.H. Human organic cation transporters 1 (slc22a1), 2 (slc22a2), and 3 (slc22a3) as disposition pathways for fluoroquinolone antimicrobials. Antimicrob. Agents Chemother. 2013, 57, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Dautrey, S.; Felice, K.; Petiet, A.; Lacour, B.; Carbon, C.; Farinotti, R. Active intestinal elimination of ciprofloxacin in rats: Modulation by different substrates. Br. J. Pharmacol. 1999, 127, 1728–1734. [Google Scholar] [CrossRef]
- Lowes, S.; Simmons, N.L. Multiple pathways for fluoroquinolone secretion by human intestinal epithelial (caco-2) cells. Br. J. Pharmacol. 2002, 135, 1263–1275. [Google Scholar] [CrossRef]
- Naruhashi, K.; Tamai, I.; Inoue, N.; Muraoka, H.; Sai, Y.; Suzuki, N.; Tsuji, A. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob. Agents Chemother. 2002, 46, 344–349. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yano, I.; Saito, H.; Inui, K. Pharmacokinetic role of p-glycoprotein in oral bioavailability and intestinal secretion of grepafloxacin in vivo. J. Pharmacol. Exp. Ther. 2002, 300, 1063–1069. [Google Scholar] [CrossRef]
- Cormet-Boyaka, E.; Huneau, J.F.; Mordrelle, A.; Boyaka, P.N.; Carbon, C.; Rubinstein, E.; Tome, D. Secretion of sparfloxacin from the human intestinal caco-2 cell line is altered by p-glycoprotein inhibitors. Antimicrob. Agents Chemother. 1998, 42, 2607–2611. [Google Scholar] [CrossRef]
- Haslam, I.S.; Wright, J.A.; O’Reilly, D.A.; Sherlock, D.J.; Coleman, T.; Simmons, N.L. Intestinal ciprofloxacin efflux: The role of breast cancer resistance protein (abcg2). Drug Metab. Dispos. 2011, 39, 2321–2328. [Google Scholar] [CrossRef]
- Zakelj, S.; Sturm, K.; Kristl, A. Ciprofloxacin permeability and its active secretion through rat small intestine in vitro. Int. J. Pharm. 2006, 313, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Van De Leur, J.J.; Vollaard, E.J.; Janssen, A.J.; Dofferhoff, A.S. Concentration of pefloxacin in feces during infection prophylaxis in neutropenic patients. Antimicrob. Agents Chemother. 1995, 39, 1182–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janin, N.; Meugnier, H.; Desnottes, J.F.; Woehrle, R.; Fleurette, J. Recovery of pefloxacin in saliva and feces and its action on oral and fecal floras of healthy volunteers. Antimicrob. Agents Chemother. 1987, 31, 1665–1668. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Demographic Variable | Patients with CF | Healthy Volunteers |
|---|---|---|
| Number of subjects (males/females) | 8 (2/6) c | 10 (5/5) |
| Age (year) | 19 [17–24] d | 24 [18–27] |
| Height (cm) | 166 [158–175] d | 174 [168–191] |
| Total body weight (WT) (kg) | 46.3 [35.5–63.5] d | 77.5 [55.0–82.0] |
| Fat-free mass (FFM) a (kg) | 33.3 [27.3–46.4] d | 52.1 [37.7–64.0] |
| Lean body mass (LBM) b (kg) | 38.0 [31.0–47.1] d | 55.6 [43.0–64.5] |
| Body mass index (kg m−2) | 17.6 [13.4–22.2] d | 21.6 [19.5–27.3] |
| Pharmacokinetic Parameter | Patients with CF | Healthy Volunteers | ||
|---|---|---|---|---|
| Oral | Intravenous | Oral | Intravenous | |
| Total clearance (L/h) | 6.47 [3.50–10.4] | 6.47 [3.81–12.6] | 8.81 [4.76–12.6] | 8.35 [5.56–16.0] |
| Renal clearance (L/h) | 0.650 [0.442–0.890] | 0.796 [0.457–1.08] | 0.564 [0.426–0.870] | 0.654 [0.395–1.01] |
| Nonrenal clearance (L/h) | 5.65 [3.06–9.97] | 5.74 [3.23–11.8] | 8.32 [4.24–12.0] | 7.60 [5.17–15.0] |
| Volume of distribution at steady-state (L) | 105 [73.0–142] | 98.1 [82.2–166] | 105 [91.1–200] | 98.2 [80.5–202] |
| Time to peak concentration (h) | 1.13 [0.50–3.00] | 0.50 [0.50–0.67] | 1.50 [0.50–4.00] | 0.54 [0.50–1.50] |
| Peak concentration (mg/L) | 4.49 [3.45–8.94] | 7.40 [3.68–11.0] | 4.19 [2.68–5.32] | 4.76 [2.35–7.56] |
| Terminal half-life (h) | 11.7 [9.58–19.2] | 12.3 [10.6–20.1] | 11.7 [7.95–15.9] | 9.40 [6.18–12.4] |
| Mean residence time (h) | 15.4 [13.3–25.5] | 15.4 [13.0–25.1] | 14.1 [10.4–20.1] | 13.2 [9.85–16.2] |
| Oral bioavailability (%) | 107 [58–178] | 99 [80–141] | ||
| Area under the plasma concentration time curve (mg·h/L) | 61.8 [38.4–114] | 61.8 [31.7–105] | 45.4 [31.6–84.0] | 48.0 [25.0–71.9] |
| Fraction of dose excreted as pefloxacin in urine (%) | 11.1 [4.24–15.6] | 13.2 [6.83–17.5] | 6.54 [4.73–10.9] | 7.44 [6.30–10.2] |
| Fraction of dose excreted as norfloxacin in urine (%) | 15.9 [9.10–20.0] | 14.8 [8.77–27.1] | 15.3 [10.7–17.7] | 14.0 [11.6–22.4] |
| Fraction of dose excreted as pefloxacin N-oxide in urine (%) | 18.3 [16.3–20.5] | 18.6 [15.4–21.7] | 17.2 [11.8–18.3] | 14.8 [10.3–17.2] |
| PK Parameters | Symbol | Unit | Patients with CF | Healthy Volunteers | ||
|---|---|---|---|---|---|---|
| Pop. Mean (SE%) | BSV a (SE%) | Pop. Mean (SE%) | BSV a (SE%) | |||
| Pefloxacin | ||||||
| Oral bioavailability | FBIO | - | 1.00 (5.60%) | 0.146 (109%) | 1.03 (4.70%) | 0.127 (48.2%) |
| Absorption lag-time | Tlag | min | 13.3 (4.00%) | 0.0712 (121%) | 13.3 (4.00%) | 0.0712 (121%) |
| Absorption half-life | Tabs | min | 19.8 (36.9%) | 1.10 (54.1%) | 11.2 (31.0%) | 0.983 (114%) |
| Reabsorption half-life from intestine | Treabs | min | 65.4 (15.3%) | 0.334 (83.1%) | 20.8 (19.3%) | 0.804 (107%) |
| Volume of distribution for central compartment | V1 b | L | 37.4 (19.6%) | 0.435 (40.9%) | 40.8 (12.9%) | 0.435 (40.9%) |
| Volume of distribution for peripheral compartment | V2 b | L | 59.9 (15.5%) | 0.131 (194%) | 65.4 (5%) | 0.131 (194%) |
| Total clearance | CLTOT b | L/h | 8.44 (12.8%) c | - | 9.26 (7.10%) c | - |
| Non-renal clearance | CLNR b | L/h | 7.37 (14.5%) | 0.238 (35.0%) | 8.56 (7.70%) | 0.238 (35.0%) |
| Renal clearance | CLR b | L/h | 1.07 (14.1%) | 0.168 (44.6%) | 0.705 (6.50%) | 0.168 (44.6%) |
| Distribution clearance | CLD b | L/h | 406 (33.7%) | 1.19 (51.8%) | 406 (33.7%) | 1.19 (51.8%) |
| Gut clearance for enterohepatic circulation | CLGUT b | L/h | 66 (fixed) | 0 (fixed) | 66 (fixed) | 0 (fixed) |
| Plasma concentration associated with half-maximal CLGUT | KmEX | mg/L | 1.44 (12.0%) | 0.1 (fixed) | 1.44 (12.0%) | 0.1 (fixed) |
| Norfloxacin | ||||||
| Formation fraction | fmNOR | - | 0.201 d | [0.152 to 0.313] d | 0.174 d | [0.147 to 0.195] d |
| Formation clearance | CLfNOR b | L/h | 1.48 e | 1.49 e | ||
| Pefloxacin N-oxide | ||||||
| Formation fraction | fmNOX | - | 0.244 d | [0.171 to 0.272] d | 0.178 d | [0.151 to 0.205] d |
| Formation clearance | CLfNOX b | L/h | 1.80 e | 1.52 e | ||
| Body Size Model a | FCYF, CLNR | FCYF, CLR | FCYF, CLT | FCYF, VSS |
|---|---|---|---|---|
| No body size model | 0.649 (34.8%) | 1.17 (28.5%) | 0.688 b | 0.568 (22.6%) |
| WT linear scaling | 0.983 (9.5%) | 1.75 (10.0%) | 1.04 | 0.948 (10.2%) |
| WT allometric | 0.891 (6.7%) | 1.59 (8.2%) | 0.944 | 0.926 (6.9%) |
| FFM linear scaling | 0.956 (7.1%) | 1.67 (8.3%) | 1.01 | 0.976 (6.6%) |
| FFM allometric | 0.861 (12.3%) | 1.53 (12.5%) c | 0.912 | 0.916 (14.7%) |
| Body Size Model | Relative between Subject Variance (%) | ||||
|---|---|---|---|---|---|
| CLNR | CLR | CLTOT | V1 | V2 | |
| WT linear scaling | 100 a | 100 | 100 | 100 | 100 |
| WT allometric | 108 b | 87 | 107 | 103 | 94 |
| FFM linear scaling | 64 b,c | 207 d | 67 d | 60 | 108 |
| FFM allometric | 79 b,c | 155 d | 80 d | 74 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulitta, J.B.; Jiao, Y.; Landersdorfer, C.B.; Sutaria, D.S.; Tao, X.; Shin, E.; Höhl, R.; Holzgrabe, U.; Stephan, U.; Sörgel, F. Comparable Bioavailability and Disposition of Pefloxacin in Patients with Cystic Fibrosis and Healthy Volunteers Assessed via Population Pharmacokinetics. Pharmaceutics 2019, 11, 323. https://doi.org/10.3390/pharmaceutics11070323
Bulitta JB, Jiao Y, Landersdorfer CB, Sutaria DS, Tao X, Shin E, Höhl R, Holzgrabe U, Stephan U, Sörgel F. Comparable Bioavailability and Disposition of Pefloxacin in Patients with Cystic Fibrosis and Healthy Volunteers Assessed via Population Pharmacokinetics. Pharmaceutics. 2019; 11(7):323. https://doi.org/10.3390/pharmaceutics11070323
Chicago/Turabian StyleBulitta, Jürgen B., Yuanyuan Jiao, Cornelia B. Landersdorfer, Dhruvitkumar S. Sutaria, Xun Tao, Eunjeong Shin, Rainer Höhl, Ulrike Holzgrabe, Ulrich Stephan, and Fritz Sörgel. 2019. "Comparable Bioavailability and Disposition of Pefloxacin in Patients with Cystic Fibrosis and Healthy Volunteers Assessed via Population Pharmacokinetics" Pharmaceutics 11, no. 7: 323. https://doi.org/10.3390/pharmaceutics11070323
APA StyleBulitta, J. B., Jiao, Y., Landersdorfer, C. B., Sutaria, D. S., Tao, X., Shin, E., Höhl, R., Holzgrabe, U., Stephan, U., & Sörgel, F. (2019). Comparable Bioavailability and Disposition of Pefloxacin in Patients with Cystic Fibrosis and Healthy Volunteers Assessed via Population Pharmacokinetics. Pharmaceutics, 11(7), 323. https://doi.org/10.3390/pharmaceutics11070323