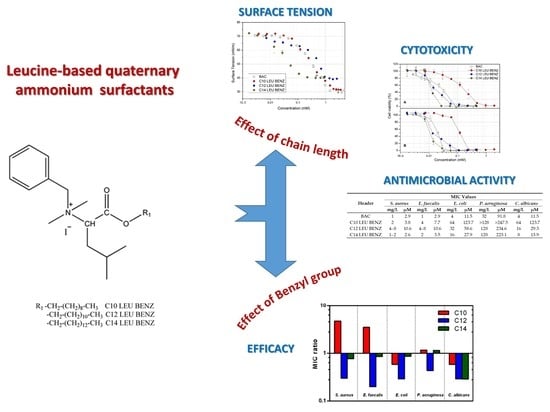
Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
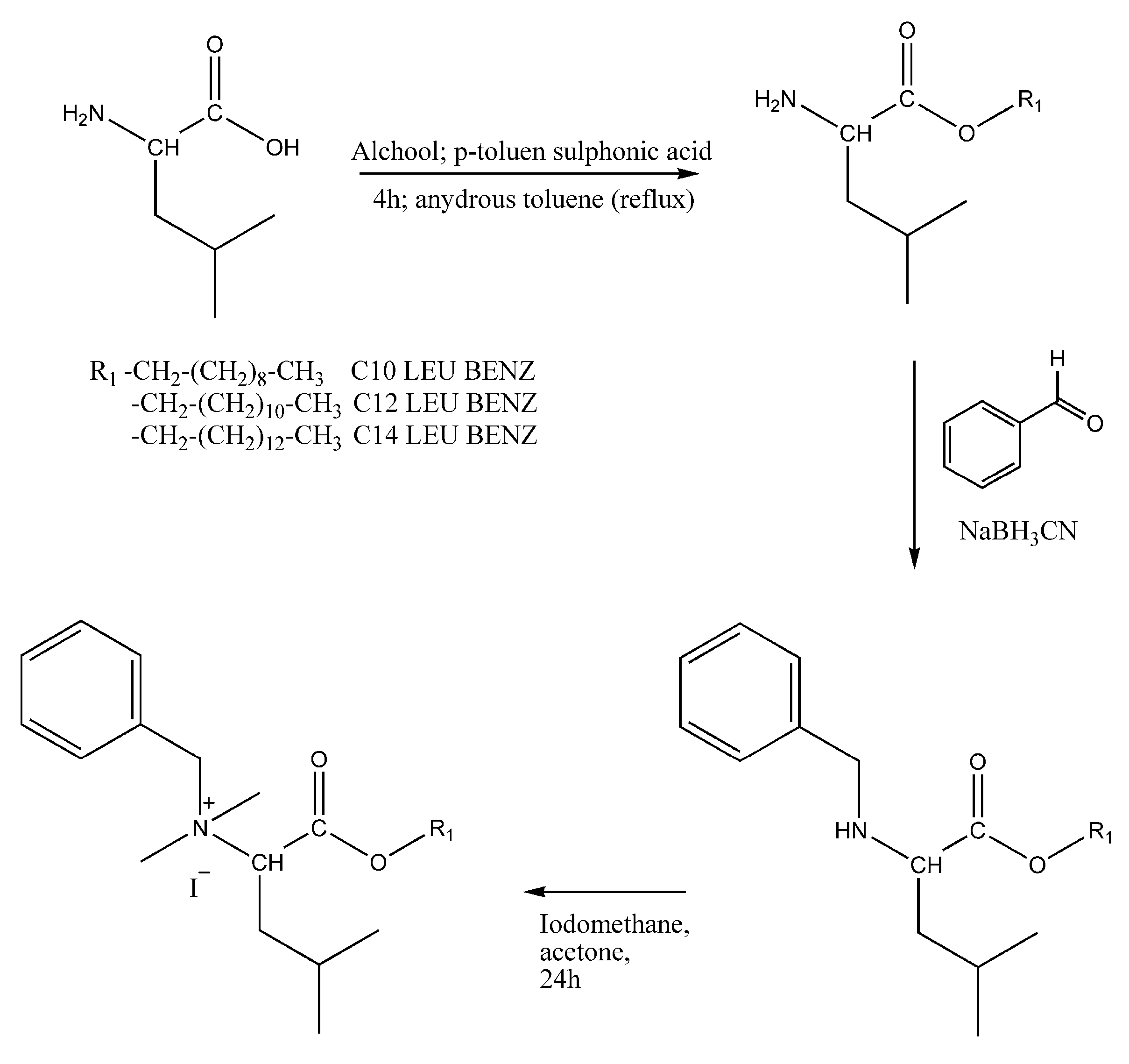
2.2.1. Synthesis and Chemical Characterisation of Benzyl Quaternary Ammonium Leucine-Based Surfactants
Synthesis of O-Acyl Leucine Derivatives
Synthesis of N-Benzyl-O-Acyl Leucine Derivatives
Synthesis of N,N,N-Benzyldimethyl-O-Acyl Leucine Derivatives
2.2.2. Surface Tension Measurements
2.2.3. Cytotoxicity Studies (MTS and LDH Assays) and Haemolytic Assay
2.2.4. Antimicrobial Assay and MIC Determination
3. Results
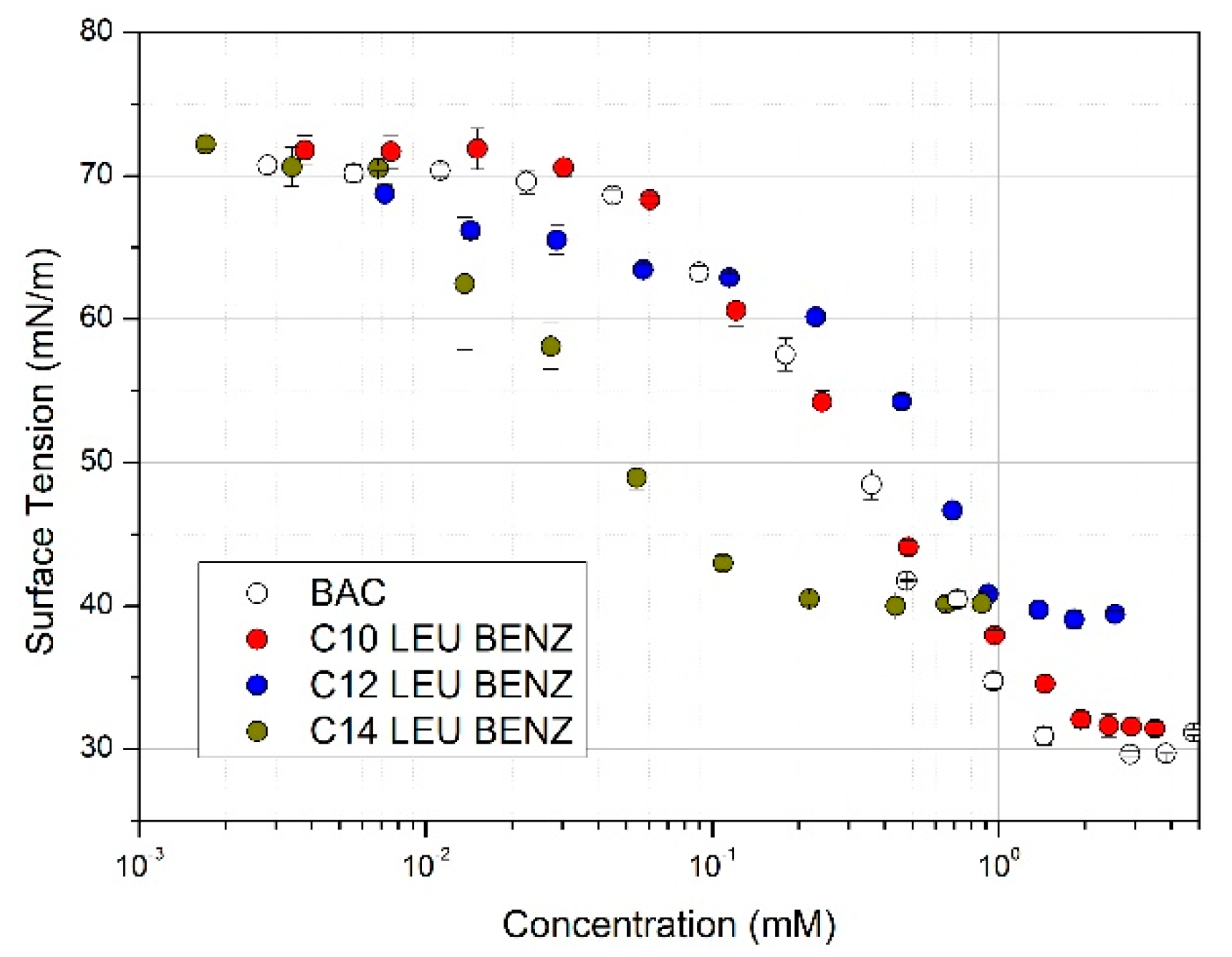
3.1. Tensiometric Analysis
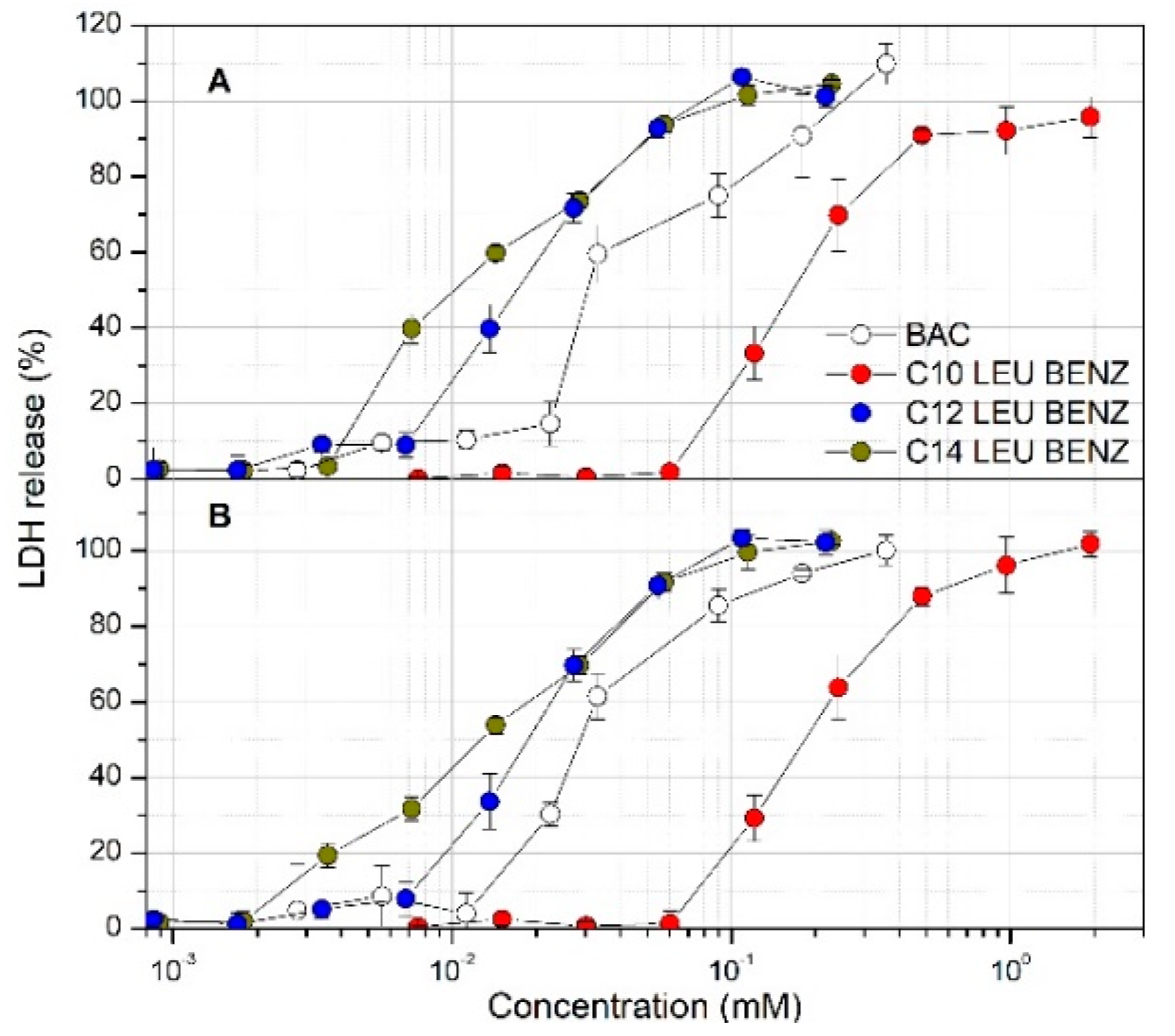
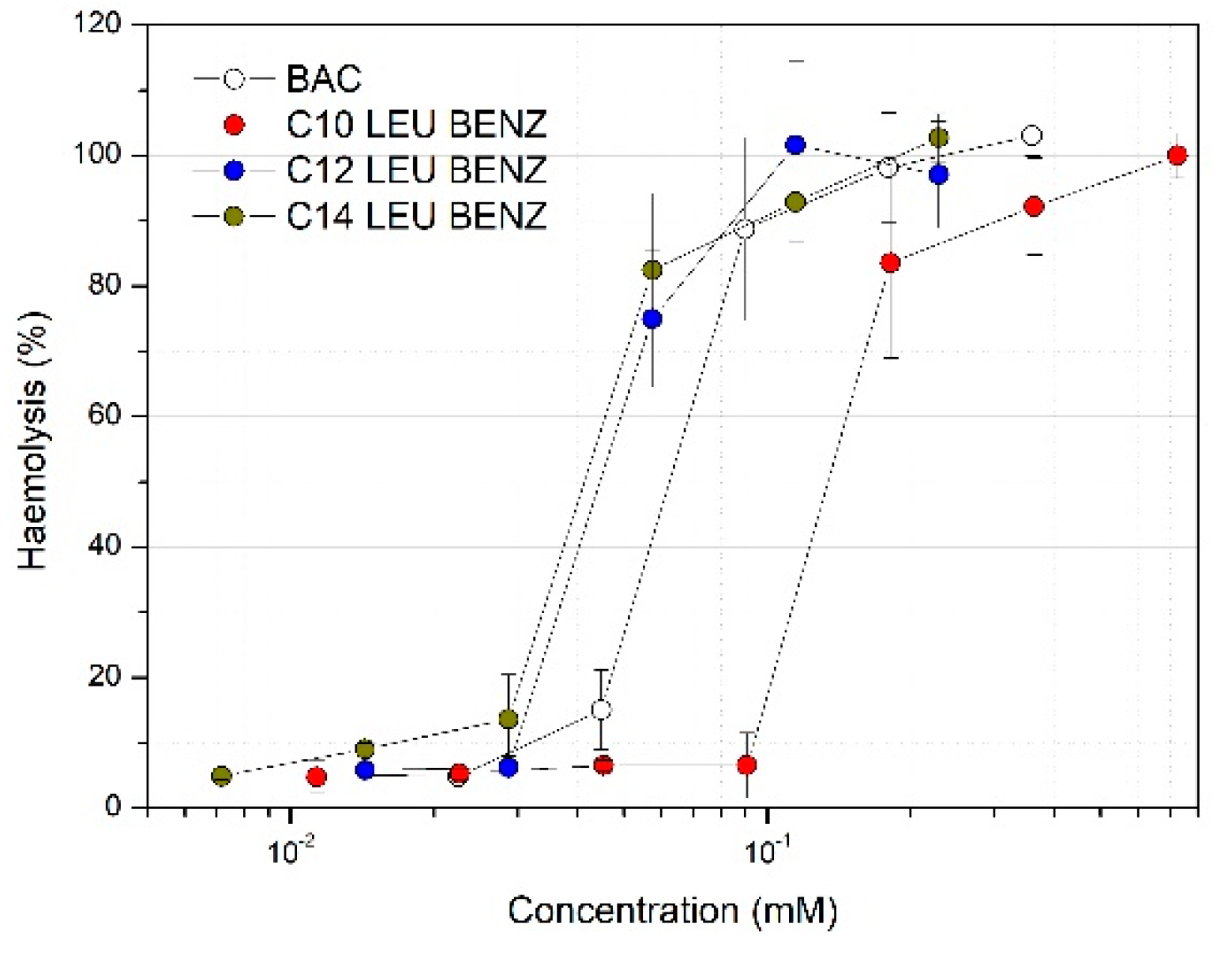
3.2. Cytotoxicity Studies and the Haemolitic Assay
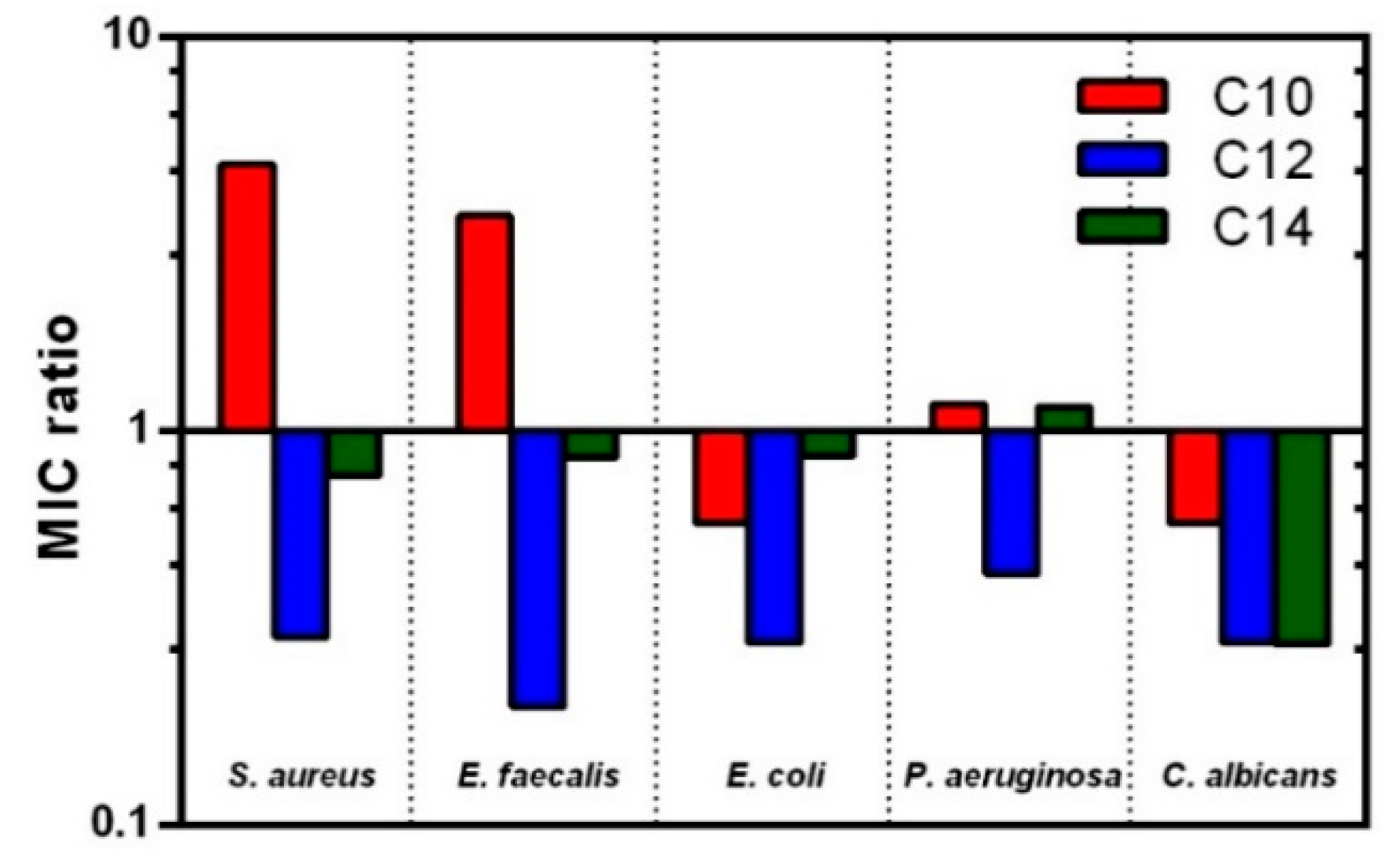
3.3. Antimicrobial Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bordes, R.; Holmberg, K. Amino acid-based surfactants–do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.C.; Pinazo, A.; Perez, L.; Clapes, P.; Angelet, M.; Garcia, M.T.; Vinardell, M.P.; Infante, M.R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. C. R. Chim. 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Zhao, H.; He, C.; Zhou, Y.; Yang, J.; Luo, C.; Xu, B. Study on foaming properties of N-acyl amino acid surfactants: Sodium N-acyl glycinate and sodium N-acyl phenylalaninate. Colloids Surf. Physicochem. Eng. Asp. 2019, 567, 240–248. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Casettari, L.; Cespi, M.; Fini, F.; Man, D.K.W.; Giorgioni, G.; Canala, S.; Lam, J.K.W.; Bonacucina, G.; Palmieri, G.F. Chemical–physical properties and cytotoxicity of N-decanoyl amino acid-based surfactants: Effect of polar heads. Colloids Surf. Physicochem. Eng. Asp. 2016, 492, 38–46. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Casettari, L.; Vllasaliu, D.; Cangiotti, M.; Ottaviani, M.F.; Giorgioni, G.; Bonacucina, G.; Palmieri, G.F. Correlation among chemical structure, surface properties and cytotoxicity of N-acyl alanine and serine surfactants. Eur. J. Pharm. Biopharm. 2016, 109, 93–102. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Comparative sensitivity of tumor and non-tumor cell lines as a reliable approach for in vitro cytotoxicity screening of lysine-based surfactants with potential pharmaceutical applications. Int. J. Pharm. 2011, 420, 51–58. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino acid–based surfactants: New antimicrobial agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef]
- Morán, C.; Clapés, P.; Comelles, F.; García, T.; Pérez, L.; Vinardell, P.; Mitjans, M.; Infante, M.R. Chemical Structure/Property Relationship in Single-Chain Arginine Surfactants. Langmuir 2001, 17, 5071–5075. [Google Scholar] [CrossRef]
- Pérez, L.; Garcia, M.T.; Ribosa, I.; Vinardell, M.P.; Manresa, A.; Infante, M.R. Biological properties of arginine-based gemini cationic surfactants. Environ. Toxicol. Chem. 2002, 21, 1279–1285. [Google Scholar] [CrossRef]
- Mezei, A.; Pérez, L.; Pinazo, A.; Comelles, F.; Infante, M.R.; Pons, R. Self Assembly of pH-Sensitive Cationic Lysine Based Surfactants. Langmuir 2012, 28, 16761–16771. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tyagi, V.K. Arginine Based Novel Cationic Surfactants: A Review. Tenside Surfactants Deterg. 2014, 51, 202–214. [Google Scholar] [CrossRef]
- Delbeke, E.I.P.; Roman, B.I.; Marin, G.B.; Van Geem, K.M.; Stevens, C.V. A new class of antimicrobial biosurfactants: Quaternary ammonium sophorolipids. Green Chem. 2015, 17, 3373–3377. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Petrelli, D.; Vitali, L.A.; Vllasaliu, D.; Cespi, M.; Giorgioni, G.; Elmowafy, E.; Bonacucina, G.; Palmieri, G.F. Quaternary ammonium surfactants derived from leucine and methionine: Novel challenging surface active molecules with antimicrobial activity. J. Mol. Liq. 2019, 283, 249–256. [Google Scholar] [CrossRef]
- de Loos, M.; van Esch, J.H.; Kellogg, R.M.; Feringa, B.L. C3-Symmetric, amino acid based organogelators and thickeners: A systematic study of structure–property relations. Tetrahedron 2007, 63, 7285–7301. [Google Scholar] [CrossRef]
- Nie, B.; Zhan, T.-G.; Zhou, T.-Y.; Xiao, Z.-Y.; Jiang, G.-F.; Zhao, X. Self-Assembly of Chiral Propeller-like Supermolecules with Unusual “Sergeants-and-Soldiers” and “Majority-Rules” Effects. Chem. Asian J. 2014, 9, 754–758. [Google Scholar] [CrossRef]
- Laschat, S.; Fröhlich, R.; Wibbeling, B. Preparation of 2, 3, 4-Trisubstituted Piperidines by a Formal Hetero-Ene Reaction of Amino Acid Derivatives. J. Org. Chem. 1996, 61, 2829–2838. [Google Scholar] [CrossRef]
- Naresh Chary, V.; Dinesh Kumar, C.; Vairamani, M.; Prabhakar, S. Characterization of amino acid-derived betaines by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2012, 47, 79–88. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Zakharova, L.Y.; Pashirova, T.N.; Fernandes, A.R.; Doktorovova, S.; Martins-Gomes, C.; Silva, A.M.; Souto, E.B. Self-assembled quaternary ammonium surfactants for pharmaceuticals and biotechnology. Org. Mater. Smart Nanocarriers Drug Deliv. 2018, 601–618. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Wu, J.; Yang, J.; Ma, W.; Bai, L.; Zhao, B.; Song, B. The Effects of Amide Bonds and Aromatic Rings on the Surface Properties and Antimicrobial Activity of Cationic Surfactants. J. Surfactants Deterg. 2019, 22, 315–325. [Google Scholar] [CrossRef]
- Liu, W.-S.; Wang, C.-H.; Sun, J.-F.; Hou, G.-G.; Wang, Y.-P.; Qu, R.-J. Synthesis, Characterization and Antibacterial Properties of Dihydroxy Quaternary Ammonium Salts with Long Chain Alkyl Bromides. Chem. Biol. Drug Des. 2015, 85, 91–97. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- El-Faham, A.; Hozzein, W.N.; Wadaan, M.A.M.; Khattab, S.N.; Ghabbour, H.A.; Fun, H.-K.; Siddiqui, M.R. Microwave Synthesis, Characterization, and Antimicrobial Activity of Some Novel Isatin Derivatives. J. Chem. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Gore, R.G.; Myles, L.; Spulak, M.; Beadham, I.; Garcia, T.M.; Connon, S.J.; Gathergood, N. A new generation of aprotic yet Brønsted acidic imidazolium salts: Effect of ester/amide groups in the C-2, C-4 and C-5 on antimicrobial toxicity and biodegradation. Green Chem. 2013, 15, 2747. [Google Scholar] [CrossRef]

| Species | Strain | Note |
|---|---|---|
| Staphylococcus aureus | ATCC 25923 | Gram-positive |
| Enterococcus faecalis | ATCC 29212 | Gram-positive |
| Escherichia coli | ATCC 25922 | Gram-negative |
| Pseudomonas aeruginosa | ATCC 27853 | Gram-negative |
| Candida albicans | ATCC 24433 | Fungus, Yeast |
| Surfactant | Tensiometry | |||
|---|---|---|---|---|
| CMC (mM) | γCMC (mN/m) | 106 Γmax (mol/m2) | Amin (A2) | |
| BAC | 1.02 ± 0.06 | 31.16 ± 0.05 | 5.35 ± 0.27 | 31.08 ± 1.60 |
| C10 LEU BENZ | 1.88 ± 0.16 | 31.75 ± 0.09 | 6.76 ± 0.22 | 24.09 ± 1.45 |
| C12 LEU BENZ | 0.92 ± 0.11 | 40.38 ± 0.13 | 6.10 ± 0.24 | 27.27 ± 1.34 |
| C14 LEU BENZ | 0.12 ± 0.08 | 40.35 ± 0.11 | 7.39 ± 0.31 | 22.46 ± 1.11 |
| Surfactant | MTS Assay (EC50 mM) | LDH Assay (EC50 mM) | Haemolysis (HC50 mM) | ||
|---|---|---|---|---|---|
| Caco-2 | Calu-3 | Caco-2 | Calu-3 | ||
| BAC | 0.033 ± 0.001 | 0.022 ± 0.005 | 0.048 ± 0.004 | 0.029 ± 0.005 | 0.064 ± 0.004 |
| C10 LEU BENZ | 0.160 ± 0.007 | 0.117 ± 0.005 | 0.158 ± 0.015 | 0.187 ± 0.024 | 0.148 ± 0.018 |
| C12 LEU BENZ | 0.016 ± 0.003 | 0.011 ± 0.002 | 0.019 ± 0.002 | 0.019 ± 0.003 | 0.054 ± 0.004 |
| C14 LEU BENZ | 0.013 ± 0.002 | 0.014 ± 0.002 | 0.012 ± 0.002 | 0.014 ± 0.002 | 0.027 ± 0.006 |
| Surfactant | MIC Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | E. coli | P. aeruginosa | C. albicans | ||||||
| mg/L | µM | mg/L | µM | mg/L | µM | mg/L | µM | mg/L | µM | |
| BAC | 1 | 2.9 | 1 | 2.9 | 4 | 11.5 | 32 | 91.8 | 4 | 11.5 |
| C10 LEU BENZ | 2 | 3.8 | 4 | 7.7 | 64 | 123.7 | >128 | >247.5 | 64 | 123.7 |
| C12 LEU BENZ | 4–8 | 10.6 | 4–8 | 10.6 | 32 | 58.6 | 128 | 234.6 | 16 | 29.3 |
| C14 LEU BENZ | 1–2 | 2.6 | 2 | 3.5 | 16 | 27.9 | 128 | 223.1 | 8 | 13.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perinelli, D.R.; Petrelli, D.; Vitali, L.A.; Bonacucina, G.; Cespi, M.; Vllasaliu, D.; Giorgioni, G.; Palmieri, G.F. Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics 2019, 11, 287. https://doi.org/10.3390/pharmaceutics11060287
Perinelli DR, Petrelli D, Vitali LA, Bonacucina G, Cespi M, Vllasaliu D, Giorgioni G, Palmieri GF. Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics. 2019; 11(6):287. https://doi.org/10.3390/pharmaceutics11060287
Chicago/Turabian StylePerinelli, Diego Romano, Dezemona Petrelli, Luca Agostino Vitali, Giulia Bonacucina, Marco Cespi, Driton Vllasaliu, Gianfabio Giorgioni, and Giovanni Filippo Palmieri. 2019. "Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity" Pharmaceutics 11, no. 6: 287. https://doi.org/10.3390/pharmaceutics11060287
APA StylePerinelli, D. R., Petrelli, D., Vitali, L. A., Bonacucina, G., Cespi, M., Vllasaliu, D., Giorgioni, G., & Palmieri, G. F. (2019). Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics, 11(6), 287. https://doi.org/10.3390/pharmaceutics11060287