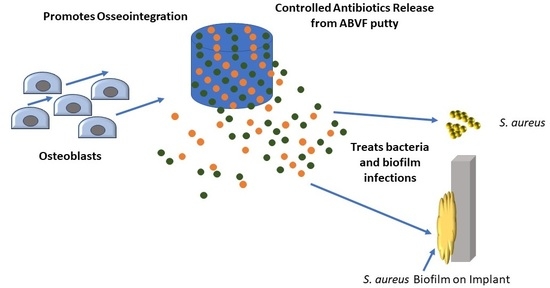
Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vancomycin Free-Base (V-fb)
2.3. Fabrication and In Vitro Characterization
2.3.1. Preparation of ABVF
2.3.2. In Vitro Drug Release Kinetics
2.3.3. In Vitro Antibacterial Activity
2.3.4. In Vitro Antibiofilm Assay
2.3.5. In Vitro Cytocompatibility
2.4. In Vivo Assessment
2.4.1. Rat Osteomyelitis Model
2.4.2. X-ray and Microcomputed Tomography (μ-CT)
2.4.3. Bone Volume
2.4.4. Histology
2.4.5. Bacterial Colony Count
2.5. Statistical Analysis
3. Results
3.1. In Vitro Drug Release Kinetics
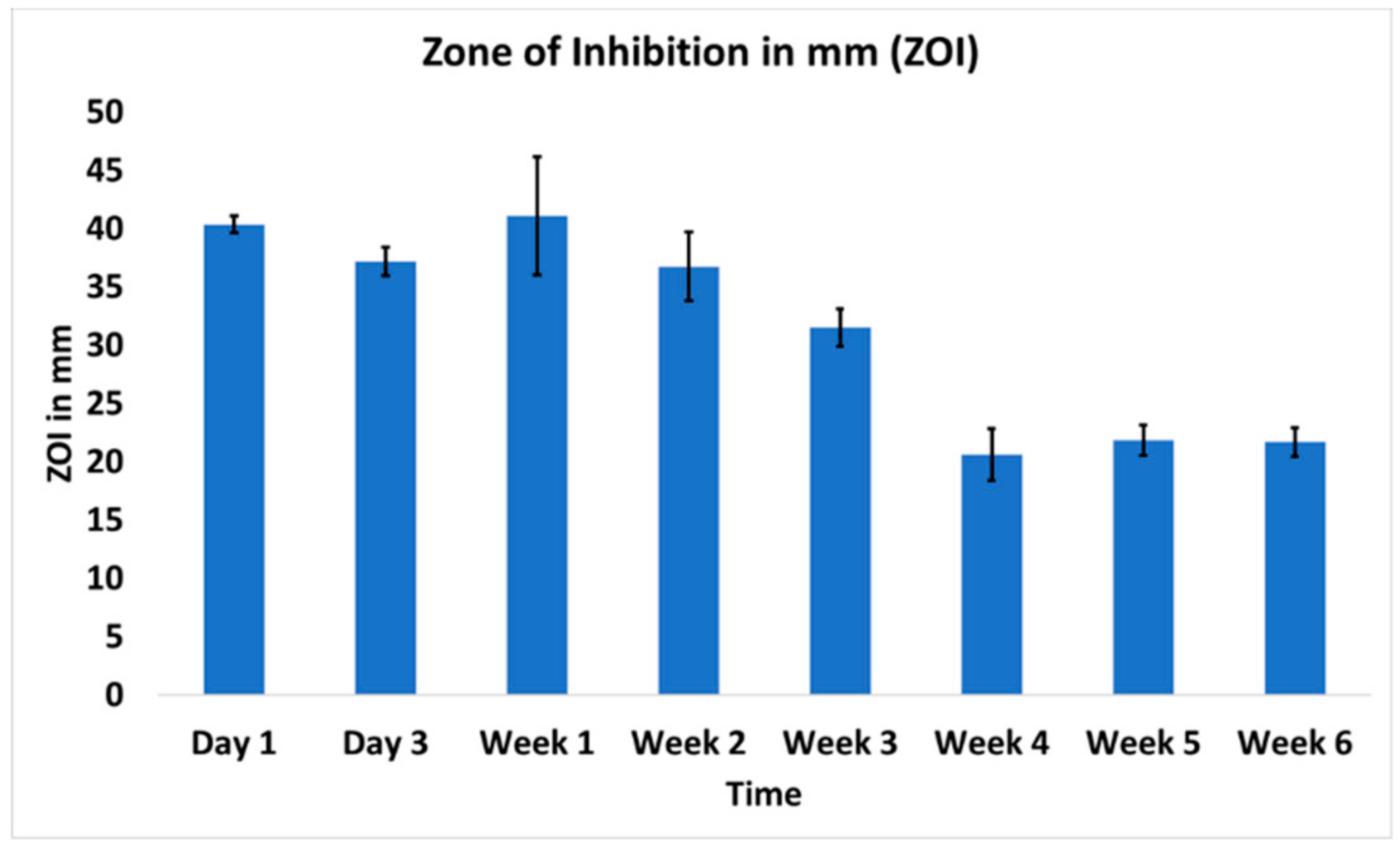
3.2. In Vitro Antibacterial Activity
3.3. In Vitro Antibiofilm Assay
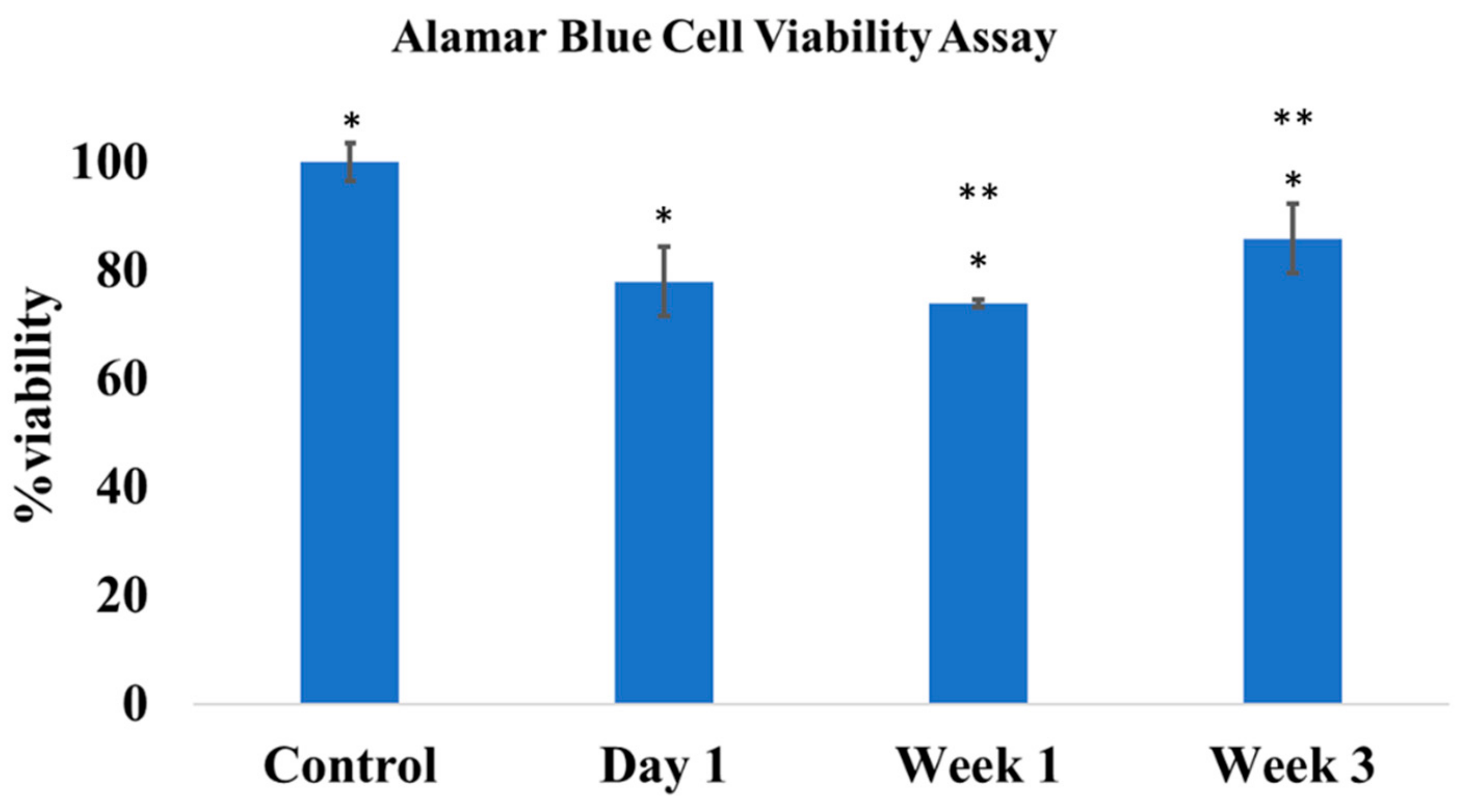
3.4. In Vitro Cytocompatibility
3.5. Rat Osteomyelitis Models
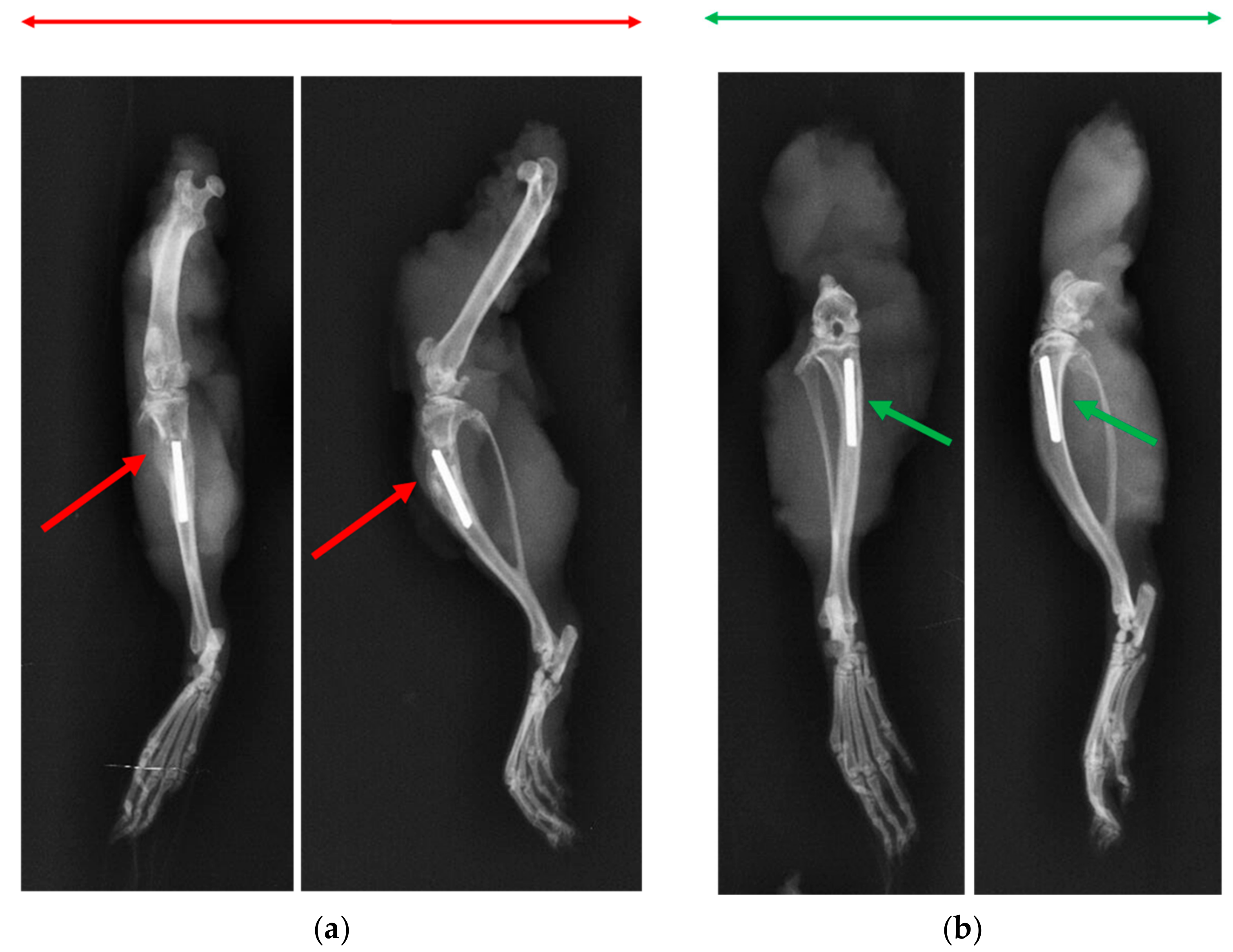
3.6. X-ray and Microcomputed Tomography (μ-CT)
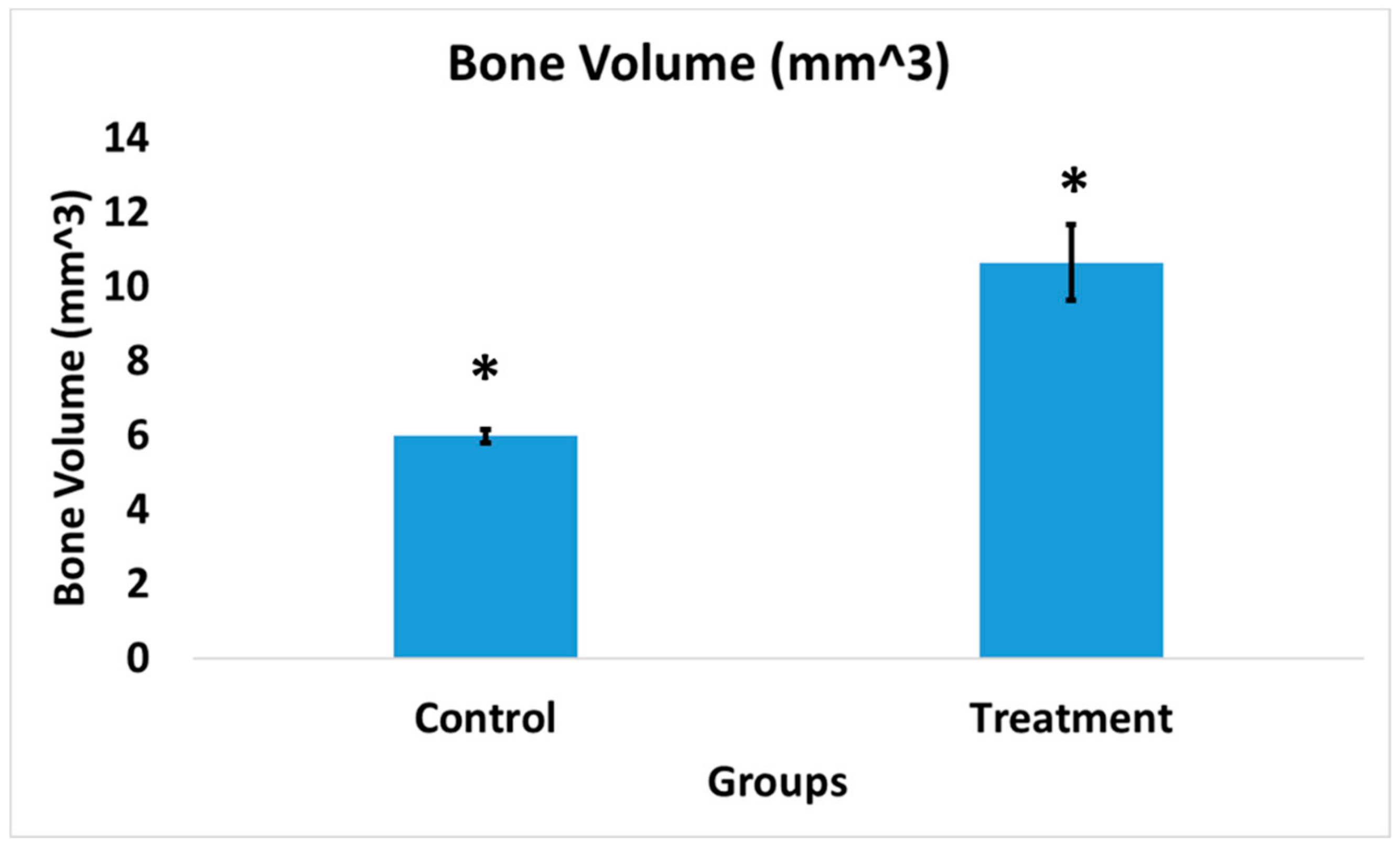
3.7. Bone Volume of Newly Formed Bone
3.8. Histology
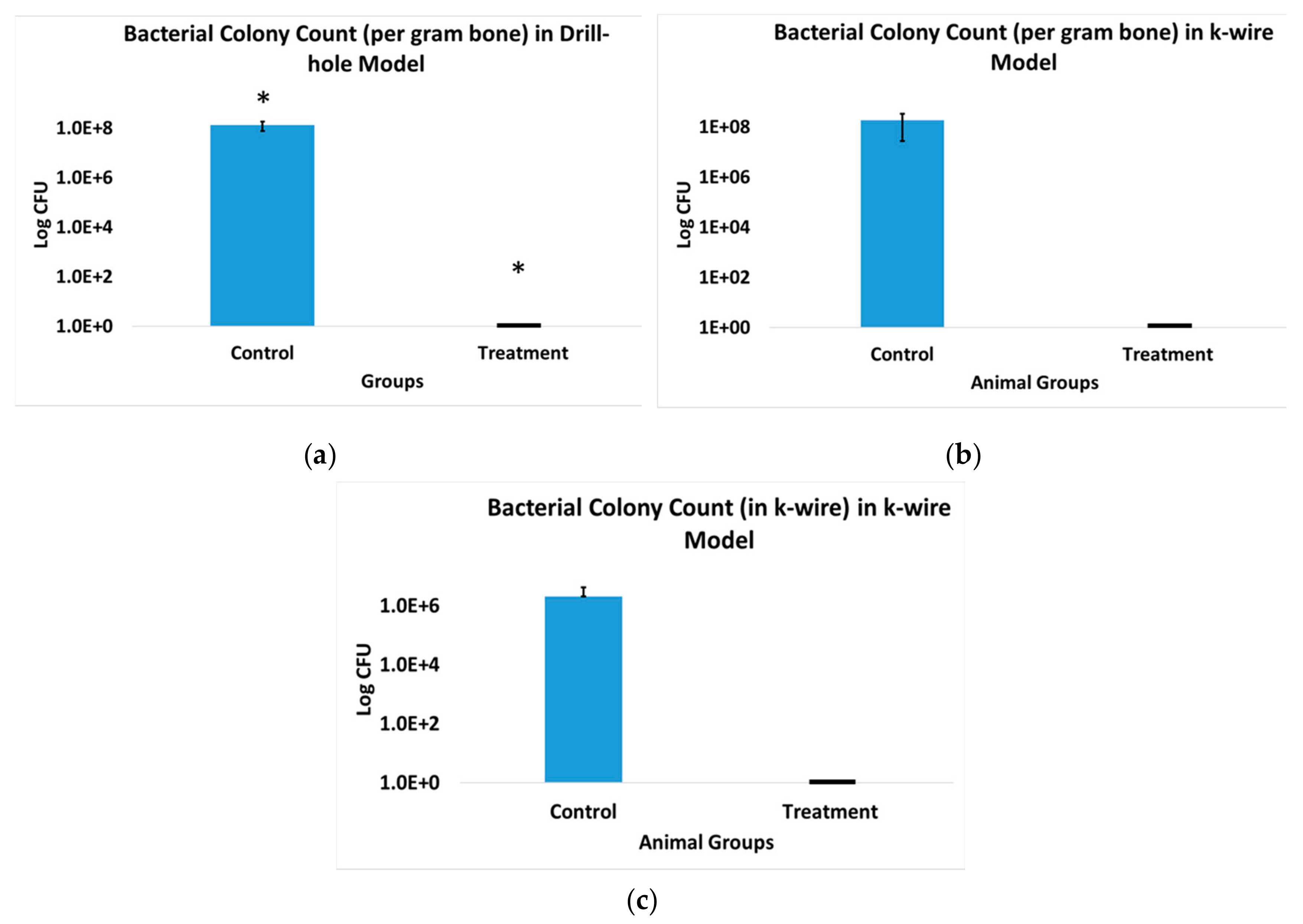
3.9. Bacterial Colony Count
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.M.; Lau, E.; Schmier, J.; Ong, K.L.; Zhao, K.; Parvizi, J. Infection Burden for Hip and Knee Arthroplasty in the United States. J. Arthroplast. 2008, 23, 984–991. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. JBJS 2007, 89, 780–785. [Google Scholar]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jämsen, E.; Varonen, M.; Huhtala, H.; Lehto, M.U.K.; Lumio, J.; Konttinen, Y.T.; Moilanen, T. Incidence of Prosthetic Joint Infections After Primary Knee Arthroplasty. J. Arthroplast. 2010, 25, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sloan, M.; Sheth, N. Changing Demographics in Primary and Revision Total Joint Arthroplasty, 2000–2014. In Proceedings of the American Academy of Orthopaedic Surgeons 2018 Annual Meeting, New Orleans, LA, USA, 6–10 March 2018. [Google Scholar]
- Ter Boo, G.-J.A.; Grijpma, D.W.; Moriarty, T.F.; Richards, R.G.; Eglin, D. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 2015, 52, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i5–i10. [Google Scholar] [CrossRef] [Green Version]
- Conterno, L.O.; da Silva Filho, C.R. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst. Rev. 2009, 3, CD004439. [Google Scholar]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of Antibacterials into Bone. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Schwartzenberger, J.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Revision Total Knee Arthroplasty Infection: Incidence and Predictors. Clin. Orthop. 2010, 468, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-M.; Zhang, Y.; Chen, F.; Khutsishvili, I.; Fehringer, E.V.; Marky, L.A.; Bayles, K.W.; Wang, D. Prevention of Orthopedic Device-Associated Osteomyelitis Using Oxacillin-Containing Biomineral-Binding Liposomes. Pharm. Res. 2012, 29, 3169–3179. [Google Scholar] [CrossRef] [Green Version]
- Choong, P.F.M.; Dowsey, M.M.; Carr, D.; Daffy, J.; Stanley, P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin based regimen. Acta Orthop. 2007, 78, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Williams, D.; Cooper, T.; Randle, J. (Eds.) Biofilms in Infection Prevention and Control; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-397043-5. [Google Scholar]
- Stoodley, P.; Nistico, L.; Johnson, S.; Lasko, L.-A.; Baratz, M.; Gahlot, V.; Ehrlich, G.D.; Kathju, S. Direct Demonstration of Viable Staphylococcus aureus Biofilms in an Infected Total Joint Arthroplasty. J. Bone Jt. Surg. Am. 2008, 90, 1751–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chohfi, M.; Langlais, F.; Fourastier, J.; Minet, J.; Thomazeau, H.; Cormier, M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int. Orthop. 1998, 22, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidar, R.; Boghossian, A.D.; Atiyeh, B. Duration of post-surgical antibiotics in chronic osteomyelitis: Empiric or evidence-based? Int. J. Infect. Dis. 2010, 14, e752–e758. [Google Scholar] [CrossRef]
- Fraimow, H.S. Systemic Antimicrobial Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Bernice, F. Vancomycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Graziani, A.L.; Lawson, L.A.; Gibson, G.A.; Steinberg, M.A.; MacGregor, R.R. Vancomycin concentrations in infected and noninfected human bone. Antimicrob. Agents Chemother. 1988, 32, 1320–1322. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; Gunay, N.; Ertugrul, B.M.; Sakarya, S. Effects of vancomycin, daptomycin, and tigecycline on coagulase-negative staphylococcus biofilm and bacterial viability within biofilm: An in vitro biofilm model. Can. J. Microbiol. 2016, 62, 735–743. [Google Scholar] [CrossRef]
- Lima, A.L.L.; Oliveira, P.R.; Carvalho, V.C.; Cimerman, S.; Savio, E. Recommendations for the treatment of osteomyelitis. Braz. J. Infect. Dis. 2014, 18, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R.; Ochsner, P.E. Role of Rifampin for Treatment of Orthopedic Implant–Related Staphylococcal Infections: A Randomized Controlled Trial. JAMA 1998, 279, 1537–1541. [Google Scholar] [CrossRef]
- Sridhar, A.; Sandeep, Y.; Krishnakishore, C.; Sriramnaveen, P.; Manjusha, Y.; Sivakumar, V. Fatal poisoning by isoniazid and rifampicin. Indian J. Nephrol. 2012, 22, 385–387. [Google Scholar]
- McPherson, E.; Facs, M. Deactivation of Palacos R Bone Cement with the Addition of Rifampin Antibiotic Powder An In-Vivo Experience-Case Report. Reconstr. Rev. 2011, 1. [Google Scholar] [CrossRef]
- Puga, A.M.; Rey-Rico, A.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A. Hot melt poly-ε-caprolactone/poloxamine implantable matrices for sustained delivery of ciprofloxacin. Acta Biomater. 2012, 8, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.; Hasan, M.R.; Larson, J.; Brooks, B.D.; Liu, Q.; Jain, T.; Joy, A.; Brooks, A.E. An Osteoconductive Antibiotic Bone Eluting Putty with a Custom Polymer Matrix. Polymers 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; de Mesy Bentley, K.L.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium Phosphate Spacers for the Local Delivery of Sitafloxacin and Rifampin to Treat Orthopedic Infections: Efficacy and Proof of Concept in a Mouse Model of Single-Stage Revision of Device-Associated Osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- Nelson, C.L. The Current Status of Material Used for Depot Delivery of Drugs. Clin. Orthop. Relat. Res. 1976–2007 2004, 427, 72–78. [Google Scholar] [CrossRef]
- Tennent, D.J.; Shiels, S.M.; Sanchez, C.J.; Niece, K.L.; Akers, K.S.; Stinner, D.J.; Wenke, J.C. Time-Dependent Effectiveness of Locally Applied Vancomycin Powder in a Contaminated Traumatic Orthopaedic Wound Model. J. Orthop. Trauma 2016, 30, 531–537. [Google Scholar] [CrossRef]
- Cao, B.; Christophersen, L.; Kolpen, M.; Jensen, P.Ø.; Sneppen, K.; Høiby, N.; Moser, C.; Sams, T. Diffusion Retardation by Binding of Tobramycin in an Alginate Biofilm Model. PLoS ONE 2016, 11, e0153616. [Google Scholar] [CrossRef]
- Henry-Stanley, M.J.; Hess, D.J.; Wells, C.L. Aminoglycoside inhibition of Staphylococcus aureus biofilm formation is nutrient dependent. J. Med. Microbiol. 2014, 63, 861–869. [Google Scholar] [CrossRef]
- Jensen, L.K.; Bjarnsholt, T.; Kragh, K.N.; Aalbæk, B.; Henriksen, N.L.; Blirup, S.A.; Pankoke, K.; Petersen, A.; Jensen, H.E. In Vivo Gentamicin Susceptibility Test for Prevention of Bacterial Biofilms in Bone Tissue and on Implants. Antimicrob. Agents Chemother. 2019, 63, e01889-18. [Google Scholar]
- Cardile, A.P.; Sanchez, C.J.; Samberg, M.E.; Romano, D.R.; Hardy, S.K.; Wenke, J.C.; Murray, C.K.; Akers, K.S. Human plasma enhances the expression of Staphylococcal microbial surface components recognizing adhesive matrix molecules promoting biofilm formation and increases antimicrobial tolerance In Vitro. BMC Res. Notes 2014, 7, 457. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Tennent, D.J.; Akers, K.S.; Wenke, J.C. Determining potential of PMMA as a depot for rifampin to treat recalcitrant orthopaedic infections. Injury 2017, 48, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Wenke, J.C. Early wound irrigation improves the ability to remove bacteria. J. Bone Jt. Surg. Am. 2007, 89, 1723–1726. [Google Scholar] [CrossRef]
- Shiels, S.M.; Tennent, D.J.; Lofgren, A.L.; Wenke, J.C. Topical rifampin powder for orthopaedic trauma part II: Topical rifampin allows for spontaneous bone healing in sterile and contaminated wounds. J. Orthop. Res. 2018, 36, 3142–3150. [Google Scholar] [CrossRef] [Green Version]
- Shiels, S.M.; Tennent, D.J.; Wenke, J.C. Topical rifampin powder for orthopedic trauma part I: Rifampin powder reduces recalcitrant infection in a delayed treatment musculoskeletal trauma model. J. Orthop. Res. 2018, 36, 3136–3141. [Google Scholar] [CrossRef] [Green Version]
- Patel, R. Biofilms and Antimicrobial Resistance. Clin. Orthop. Relat. Res. 2005, 437, 41. [Google Scholar] [CrossRef]
- Gagnon, R.F.; Richards, G.K.; Kostiner, G.B. Time-kill efficacy of antibiotics in combination with rifampin against Staphylococcus epidermidis biofilms. Adv. Perit. Dial. Conf. Perit. Dial. 1994, 10, 189–192. [Google Scholar]
- Sanchez, C.J.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef]
- Pascual, A.; Ramirez de Arellano, E.; Perea, E.J. Activity of glycopeptides in combination with amikacin or rifampin against Staphylococcus epidermidis biofilms on plastic catheters. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 1994, 13, 515–517. [Google Scholar] [CrossRef]
- Peck, K.R.; Kim, S.W.; Jung, S.-I.; Kim, Y.-S.; Oh, W.S.; Lee, J.Y.; Jin, J.H.; Kim, S.; Song, J.-H.; Kobayashi, H. Antimicrobials as potential adjunctive agents in the treatment of biofilm infection with Staphylococcus epidermidis. Chemotherapy 2003, 49, 189–193. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.A.; Kohlhepp, S.J.; Kohnen, P.W.; Houghton, D.C.; Gilbert, D.N. Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob. Agents Chemother. 1986, 30, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosthetic-Joint Infections|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMra040181?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed (accessed on 28 July 2019).
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cell. Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Kluin, O.S.; Busscher, H.J.; Neut, D.; van der Mei, H.C. Poly(trimethylene carbonate) as a carrier for rifampicin and vancomycin to target therapy-recalcitrant staphylococcal biofilms. J. Orthop. Res. 2016, 34, 1828–1837. [Google Scholar] [CrossRef] [Green Version]


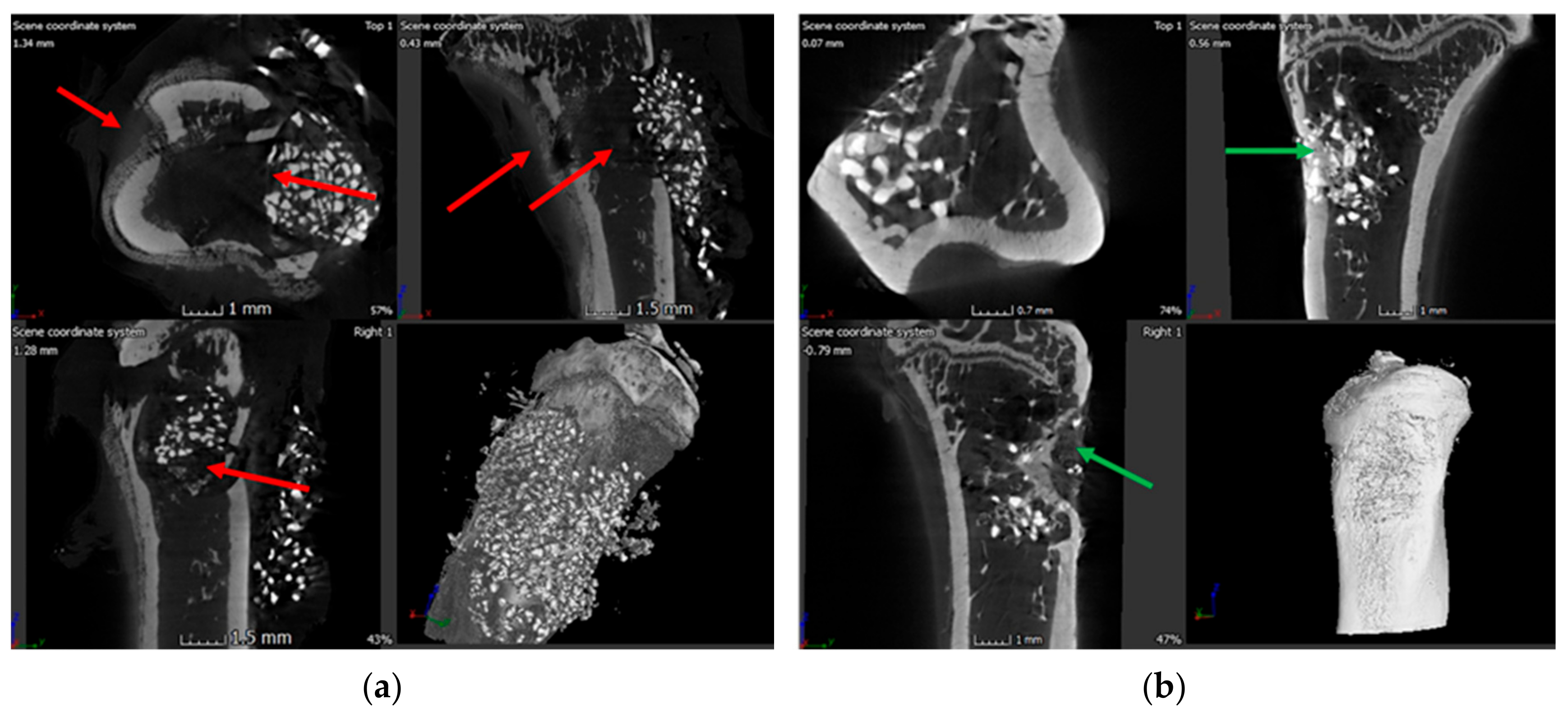
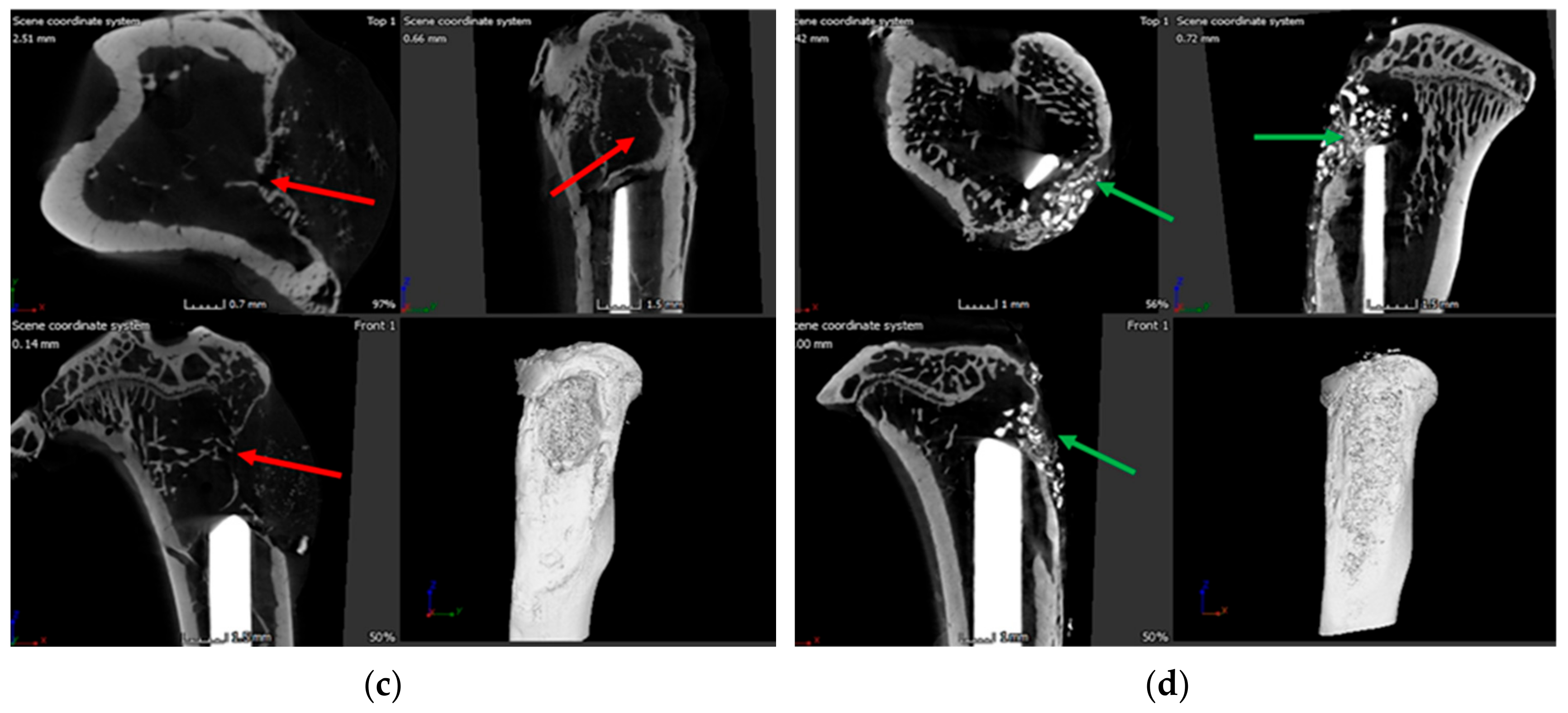


| Cohort | Implant | Surgery Model | Infectious Dose | Time to Sacrifice (Weeks) | Cohort Purpose | Morbidity/Infection | Cohort Size |
|---|---|---|---|---|---|---|---|
| 1 | BVF putty without antibiotics | Drill-hole Osteomyelitis | 108 CFU | 10 | Positive Infection control | Local infection | 12 |
| 2 | ABVF with vancomycin and rifampicin | Drill-hole Osteomyelitis | 108 CFU | 10 | Infection treatment | No infection | 5 |
| 3 | BVF putty without antibiotics | K-wire biofilm osteomyelitis model | Biofilm on k-wire | 6 | Positive Infection control | Local infection | 3 |
| 4 | ABVF with vancomycin and rifampicin | K-wire biofilm osteomyelitis model | Biofilm on k-wire | 6 | Infection treatment | No infection | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, R.; Schaner, K.; Schroeder, M.; Wohlers, A.; Shreffler, J.; Schaper, C.; Subramanian, H.; Brooks, A. Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis. Pharmaceutics 2019, 11, 592. https://doi.org/10.3390/pharmaceutics11110592
Hasan R, Schaner K, Schroeder M, Wohlers A, Shreffler J, Schaper C, Subramanian H, Brooks A. Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis. Pharmaceutics. 2019; 11(11):592. https://doi.org/10.3390/pharmaceutics11110592
Chicago/Turabian StyleHasan, Raquib, Kambri Schaner, Meredith Schroeder, Abbey Wohlers, Jacob Shreffler, Codi Schaper, Hariharaputhiran Subramanian, and Amanda Brooks. 2019. "Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis" Pharmaceutics 11, no. 11: 592. https://doi.org/10.3390/pharmaceutics11110592
APA StyleHasan, R., Schaner, K., Schroeder, M., Wohlers, A., Shreffler, J., Schaper, C., Subramanian, H., & Brooks, A. (2019). Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis. Pharmaceutics, 11(11), 592. https://doi.org/10.3390/pharmaceutics11110592