Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment
Abstract
:1. Introduction
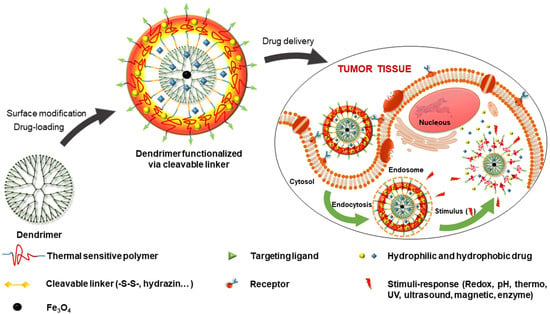
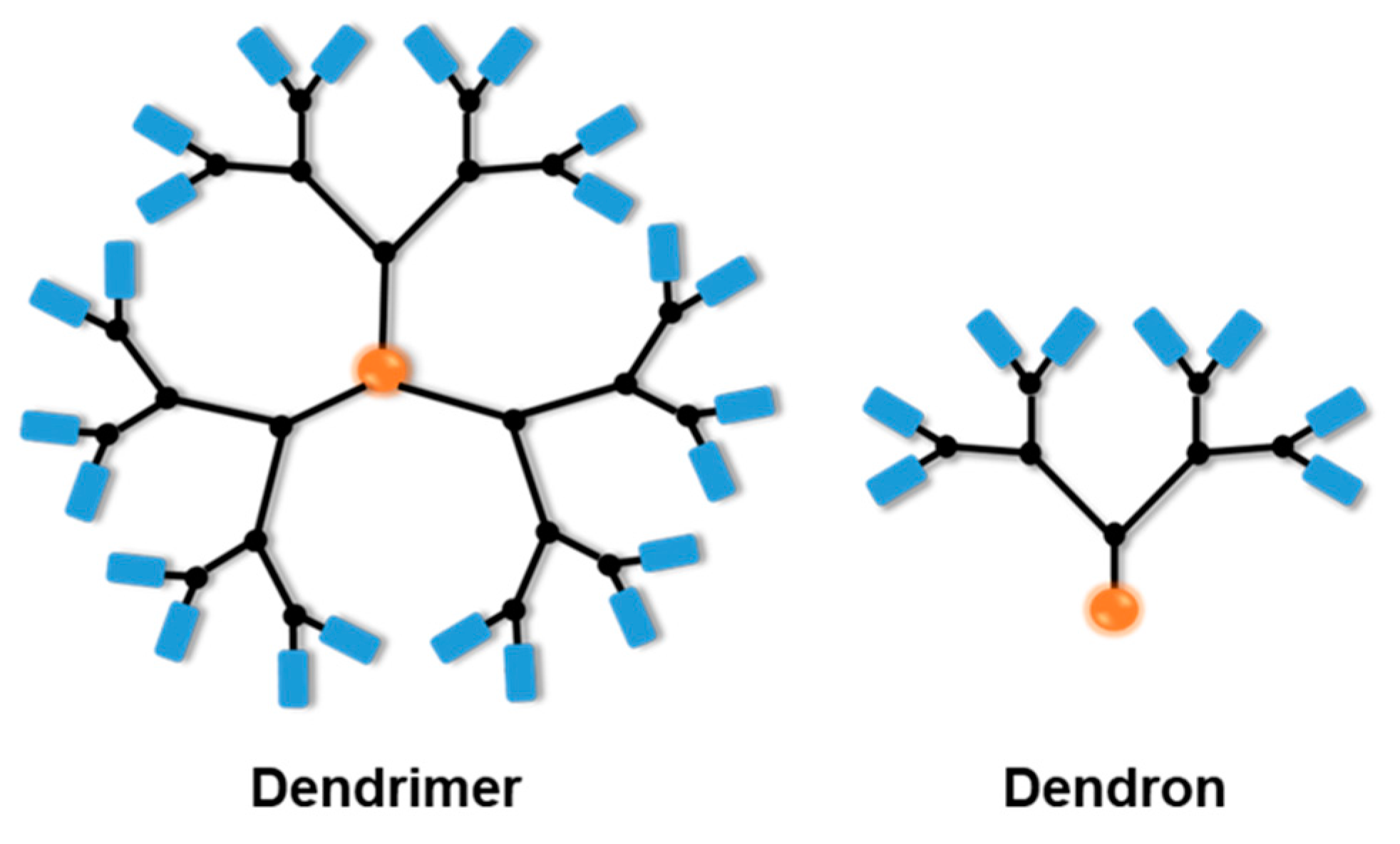
2. Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment
3. Recent Trends of Stimuli-Responsive Dendrimers in Controlling the Release of Drug at Target Sites
3.1. Functionalized Dendrimer with Polymers and Targeting Agents
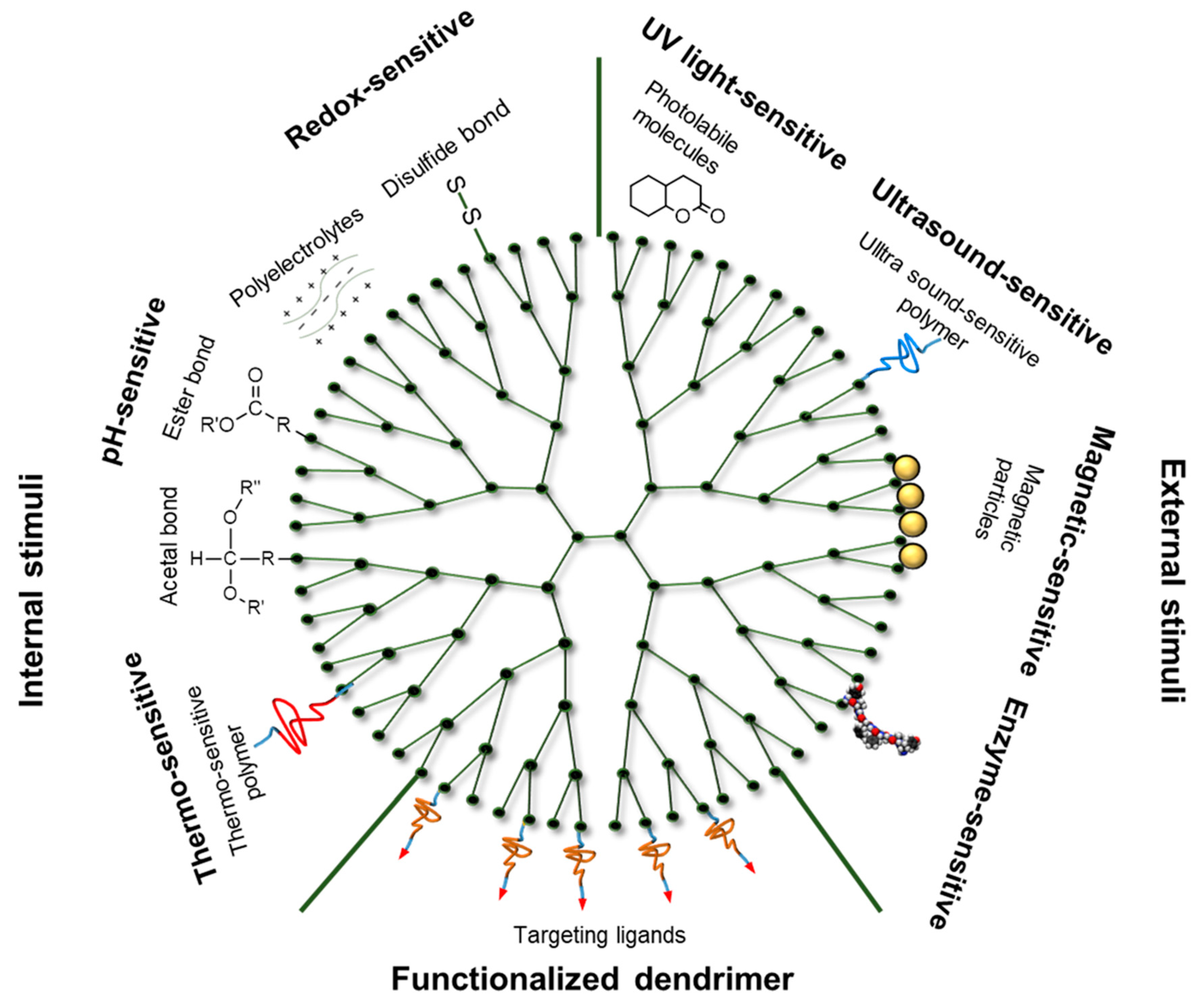
3.2. Internal-Stimuli-Responsive Dendrimers
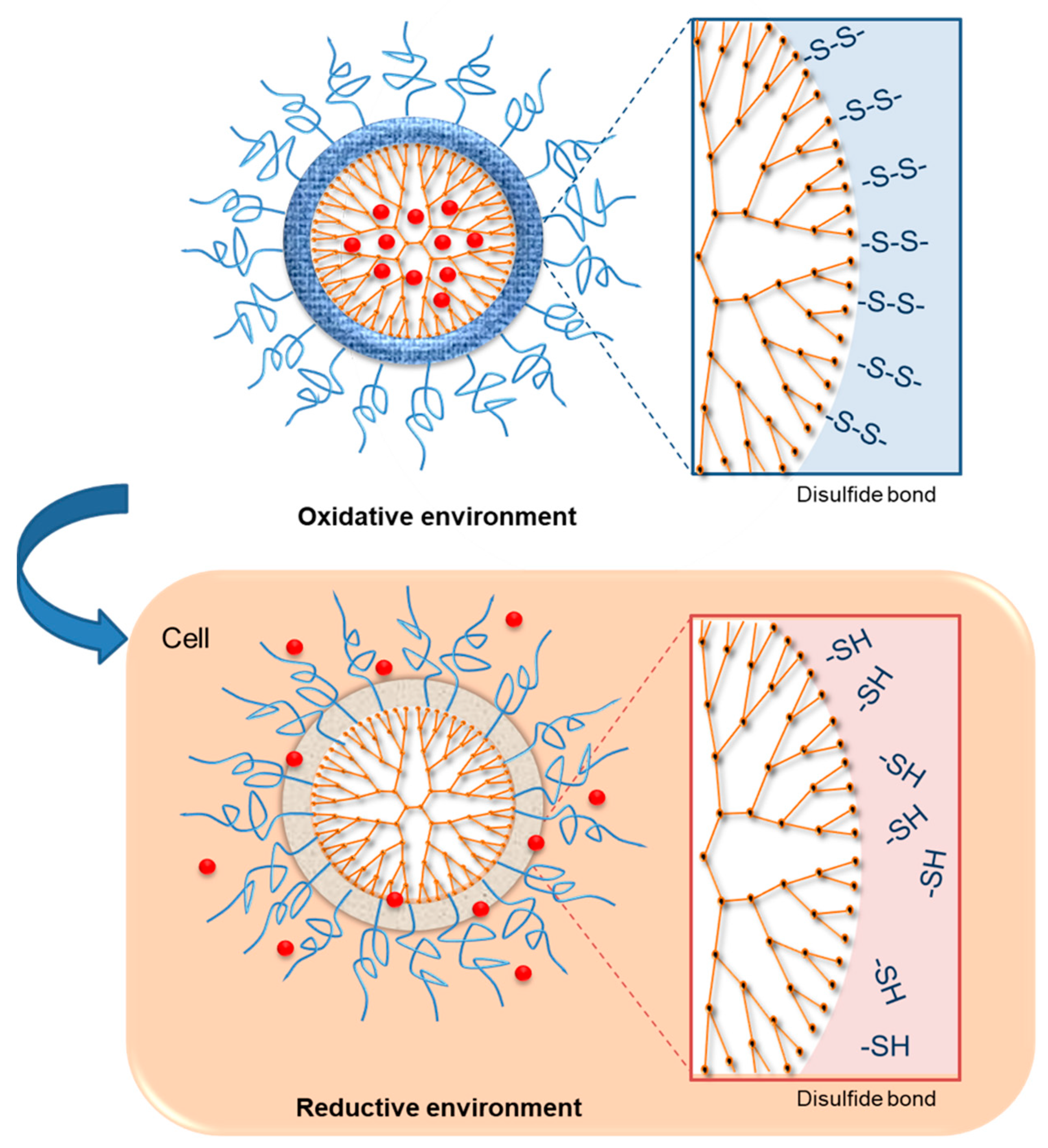
3.2.1. Redox-Sensitive Dendrimers
3.2.2. pH-Sensitive Dendrimers
3.2.3. Thermo-Sensitive Dendrimers
3.3. External-Stimuli-Responsive Dendrimers
3.3.1. UV Light-Sensitive Dendrimers
3.3.2. Ultrasound-Sensitive Dendrimers
3.3.3. Magnetic-Sensitive Dendrimers
3.3.4. Enzyme-Sensitive Dendrimers
4. Current Advances in General Biomedical Applications of Multi-Stimuli-Responsive Dendrimers
4.1. Drug Delivery
4.2. Diagnostics
4.3. Tissue Regeneration
5. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mohapatra, S.; Ranjan, S.; Dasgupta, N.; Kumar, R.; Thomas, S. Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development and in vitro evaluation of liposomes using soy lecithin to encapsulate paclitaxel. Int. J. Biomater. 2017, 2017, 8234712. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.A.; do Val, L.D.; Doherty, A.; de Magalhães, J.P. From humans to hydra: Patterns of cancer across the tree of life. Biol. Rev. 2018, 93, 1715–1734. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Phuong, T.H.D.; Tran, N.Q.; Nguyen, C.K.; Nguyen, D.H. Polymeric chitosan based nanogels as a potential platform for dual targeted drug delivery in cancer therapy. Int. J. Nanotechnol. 2018, 15, 188–198. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Liu, M.; Wang, K.; Tao, L.; Wan, Q.; Wei, Y. Recent progress and advances in redox-responsive polymers as controlled delivery nanoplatforms. Mater. Chem. Front. 2017, 1, 807–822. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Q.; Yang, C.; Zhang, Q.; Chen, W.; Shen, Y.; Sui, M. A multi-stimuli responsive nanoparticulate SN38 prodrug for cancer chemotherapy. J. Mater. Chem. B 2017, 5, 661–670. [Google Scholar] [CrossRef]
- Nguyen, D.H. Design and decoration of heparin on porous nanosilica via reversible disulfide linkages for controlled drug release. J. IKEEE 2017, 21, 320–330. [Google Scholar]
- Liu, F.-H.; Hou, C.-Y.; Zhang, D.; Zhao, W.-J.; Cong, Y.; Duan, Z.-Y.; Qiao, Z.-Y.; Wang, H. Enzyme-sensitive cytotoxic peptide–dendrimer conjugates enhance cell apoptosis and deep tumor penetration. Biomater. Sci. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Le, N.T.T.; Pham, L.P.T.; Nguyen, D.H.T.; Le, N.H.; Tran, T.V.; Nguyen, C.K.; Nguyen, D.H. Liposome-based nanocarrier system for phytoconstituents. Nov. Drug Deliv. Syst. Phytoconst. 2019, 45–68. [Google Scholar]
- Le, N.T.T.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Hoang Thi, T.T. Soy lecithin-derived liposomal delivery systems: Surface modification and current applications. Int. J. Mol. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Bach, L.G.; Tran, D.-H.N.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Thi, T.T.H. Partial surface modification of low generation polyamidoamine dendrimers: Gaining insight into their potential for improved carboplatin delivery. Biomolecules 2019, 9, 214. [Google Scholar] [CrossRef]
- Gonzaga, R.V.; da Silva Santos, S.; da Silva, J.V.; Prieto, D.C.; Savino, D.F.; Giarolla, J.; Ferreira, E.I. Targeting groups employed in selective dendrons and dendrimers. Pharmaceutics 2018, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 12, 908–931. [Google Scholar] [CrossRef]
- Nguyen, D.T.D.; Bach, L.G.; Nguyen, T.H.; Ho, M.H.; Ho, M.N.; Nguyen, D.H.; Nguyen, C.K.; Thi, T.T.H. Preparation and characterization of oxaliplatin drug delivery vehicle based on PEGylated half-generation PAMAM dendrimer. J. Polym. Res. 2019, 26, 116. [Google Scholar] [CrossRef]
- Araújo, R.; Santos, S.; Ferreira, E.I.; Giarolla, J. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.D.; Jemal, A. Cancer statistics. Cancer J. Clin 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Le, P.N.; Nguyen, D.H.; Nguyen, C.K.; Tran, N.Q. Dendrimers for controlled release drug delivery. In Dendrimers for Drug Delivery; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 207–224. [Google Scholar]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for drug delivery to the central nervous system. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Thi, H.; Thanh, T. Modified carboxyl-terminated PAMAM dendrimers as great cytocompatible nano-based drug delivery system. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef]
- Le, N.T.T.; Thi, Y.N.N.; Thi, B.L.P.; Hoang, N.L.; Nguyen, C.K.; Nguyen, D.H. Nanoliposomes as an efficient drug carrier system for paclitaxel delivery. In Proceedings of the International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh, Vietnam, 27–29 June 2018; pp. 193–196. [Google Scholar]
- Mendes, L.P.; Pan, J.; Torchilin, V. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; de la Mata, F.; Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-responsive supramolecular hydrogels and their applications in regenerative medicine. Macromol. Biosci. 2019, 19, 1800259. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.-S.; Xu, W.; Huang, Q.-Y.; Zhang, Y.-R.; Shi, X.-X.; Huang, H.; Li, H.-J.; Du, J.-Z.; Wang, J. Multi-stimuli responsive poly(amidoamine) dendrimers with peripheral N-dialkylaminoethyl carbamate moieties. Polym. Chem. 2019, 10, 656–662. [Google Scholar] [CrossRef]
- Marson, D.; Laurini, E.; Aulic, S.; Fermeglia, M.; Pricl, S. Evolution from covalent to self-assembled PAMAM-based dendrimers as nanovectors for siRNA delivery in cancer by coupled in silico-experimental studies. Part I: Covalent siRNA nanocarriers. Pharmaceutics 2019, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Laurini, E.; Marson, D.; Aulic, S.; Fermeglia, M.; Pricl, S. Evolution from covalent to self-assembled PAMAM-based dendrimers as nanovectors for siRNA delivery in cancer by coupled in silico-experimental studies. Part II: Self-assembled siRNA nanocarriers. Pharmaceutics 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H. 5-fluorouracil encapsulated CS-mPEG nanogels for controlling drug release. Vietnam J. Chem. 2017, 55, 446. [Google Scholar]
- Nguyen, D.H. Potential 5-fluorouracil encapsulated mPEG-Chitosan nanogels for controlling drug release. J. Adv. Med Pharm. Sci. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Pethe, A.M.; Yadav, K.S. Polymers, responsiveness and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 395–405. [Google Scholar] [CrossRef]
- Hu, W.; Qiu, L.; Cheng, L.; Hu, Q.; Liu, Y.; Hu, Z.; Cheng, L. Redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for intracellular delivery of doxorubicin. Acta Biomater. 2016, 36, 241–253. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, C.K.; Nguyen, D.H. Redox and pH responsive poly(amidoamine) dendrimer-heparin conjugates via disulfide linkages for letrozole delivery. BioMed Res. Int. 2017, 2017, 8589212. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, L.; Cheng, L.; Zheng, M.; Lei, Q.; Hu, Z.; Chen, D. Redox and pH-responsive poly(amidoamine) dendrimer–poly(ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug release. Colloids Surf. B Biointerfaces 2014, 123, 254–263. [Google Scholar] [CrossRef]
- Shen, Y.; Ma, X.; Zhang, B.; Zhou, Z.; Sun, Q.; Jin, E.; Fan, M. Degradable dual pH-and temperature-responsive photoluminescent dendrimers. Chem. A Eur. J. 2011, 17, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H. Superelastic and pH-responsive degradable dendrimer cryogels prepared by cryo-aza-michael addition reaction. Sci. Rep. 2018, 8, 7155. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Patil, N.G.; Augustine, R.; Zhang, Y.; Hong, S.C.; Kim, I. Synthesis of stimuli-responsive heterofunctional dendrimer by Passerini multicomponent reaction. ACS Omega 2019, 4, 6660–6668. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, W.-W.; Xu, D.-G. Stimuli-responsive nanoscale drug delivery systems for cancer therapy. J. Drug Target. 2019, 27, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Kong, H.; Zeng, Y.; Liu, G. Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment. Sci. Technol. Adv. Mater. 2018, 19, 771–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Yu, B.; Gao, L.; Cong, H.; Song, N.; Lu, C. Stimuli responsive nanoparticles for controlled anti-cancer drug release. Curr. Med. Chem. 2018, 25, 1837–1866. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Which dendrimer to attain the desired properties? Focus on phosphorhydrazone dendrimers. Molecules 2018, 23, 622. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, B.S.P.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surface. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Le, N.T.T.; Bach, L.G.; Nguyen, D.C.; Le, T.H.X.; Pham, K.H.; Nguyen, D.H.; Thi, H.; Thanh, T. Evaluation of factors affecting antimicrobial activity of bacteriocin from Lactobacillus plantarum microencapsulated in alginate-gelatin capsules and its application on pork meat as a bio-preservative. Int. J. Environ. Res. Public Health 2019, 16, 1017. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.R.; Nain, A.; Jain, S.; Punjabi, N.; Mukherji, S.; Satija, J. Dendrimer as a multifunctional capping agent for metal nanoparticles for use in bioimaging, drug delivery and sensor applications. J. Mater. Chem. B 2018, 16, 2368–2384. [Google Scholar] [CrossRef]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.; Thanh, T.; Tran, D.-H.N.; Bach, L.G.; Vu-Quang, H.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional magnetic core-shell system-based iron oxide nanoparticle coated with biocompatible copolymer for anticancer drug delivery. Pharmaceutics 2019, 11, 120. [Google Scholar]
- Otto, D.P.; de Villiers, M.M. Poly(amidoamine) dendrimers as a pharmaceutical excipient. Are we there yet? J. Pharm. Sci. 2018, 107, 75–83. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer enhanced antibacterial effect of vancomycin hydrochloride against gram-negative bacteria. J. Pharm. Pharm. Sci. 2018, 22, 10–21. [Google Scholar] [CrossRef]
- Yao, J.; Feng, J.; Chen, J. External-stimuli responsive systems for cancer theranostic. Asian J. Pharm. Sci. 2016, 11, 585–595. [Google Scholar] [CrossRef] [Green Version]
- WooáBae, J. Dendron-mediated self-assembly of highly PEGylated block copolymers: A modular nanocarrier platform. Chem. Commun. 2011, 47, 10302–10304. [Google Scholar]
- Ho, M.N.; Bach, L.G.; Nguyen, T.H.; Ho, M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, C.H.; Nguyen, N.V.; Thi, T.T.H. PEGylated poly(amidoamine) dendrimers-based drug loading vehicles for delivering carboplatin in treatment of various cancerous cells. J. Nanopart. Res. 2019, 21, 43. [Google Scholar] [CrossRef]
- Tran, D.H.N.; Nguyen, T.H.; Vo, T.N.N.; Pham, L.P.T.; Vo, D.M.H.; Nguyen, C.K.; Bach, L.G.; Nguyen, D.H. Self-assembled poly(ethylene glycol) methyl ether-grafted gelatin nanogels for efficient delivery of curcumin in cancer treatment. J. Appl. Polym. Sci. 2019, 136, 47544. [Google Scholar] [CrossRef]
- Pham, D.C.; Nguyen, T.H.; Ngoc, U.T.P.; Le, N.T.T.; Tran, T.V.; Nguyen, D.H. Preparation, characterization and antifungal properties of chitosan-silver nanoparticles synergize fungicide against Pyricularia oryzae. J. Nanosci. Nanotechnol. 2018, 18, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Martin-Serrano, Á.; Gómez, R.; Ortega, P.; de la Mata, F.J. Nanosystems as vehicles for the delivery of antimicrobial peptides (AMPs). Pharmaceutics 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological aspects of the design of nanocarriers for therapeutic peptides and proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Misra, R.; Parveen, S. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. In Nanomedicine in Cancer; Pan Stanford: Singapore, 2017; pp. 73–124. [Google Scholar]
- Arrigo, R.; Teresi, R.; Gambarotti, C.; Parisi, F.; Lazzara, G.; Dintcheva, N. Sonication-induced modification of carbon nanotubes: Effect on the rheological and thermo-oxidative behaviour of polymer-based nanocomposites. Materials 2018, 11, 383. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S. Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. Selective surface PEGylation of UiO-66 nanoparticles for enhanced stability, cell uptake, and pH-responsive drug delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef]
- Saliev, T. The advances in biomedical applications of carbon nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Vigneswari, S.; Chai, J.; Shantini, K.; Bhubalan, K.; Amirul, A. Designing novel interfaces via surface functionalization of short-chain-length polyhydroxyalkanoates. Adv. Polym. Technol. 2019, 2019, 3831251. [Google Scholar] [CrossRef]
- Bao, B.Q.; Le, N.H.; Nguyen, D.H.T.; Tran, T.V.; Pham, L.P.T.; Bach, L.G.; Ho, H.M.; Nguyen, T.H.; Nguyen, D.H. Evolution and present scenario of multifunctionalized mesoporous nanosilica platform: A mini review. Mater. Sci. Eng. C 2018, 91, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, N.; Chen, L.; Meng, X.; Liu, Y.; Li, Y.; Wang, J. Synthesis and characterization of DOX-conjugated dendrimer-modified magnetic iron oxide conjugates for magnetic resonance imaging, targeting, and drug delivery. J. Mater. Chem. 2012, 22, 9594–9601. [Google Scholar] [CrossRef]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Chen, D. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan–PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Pan, D.; Luo, K.; He, B.; Cheng, G.; Zhang, C.; Gu, Z. PEGylated dendrimer-doxorubicin cojugates as pH-sensitive drug delivery systems: Synthesis and in vitro characterization. J. Biomed. Nanotechnol. 2015, 11, 964–978. [Google Scholar] [CrossRef]
- Thi, N.T.N.; Le, N.H.; Vo, U.V.; Nguyen, C.K.; Nguyen, D.H. Engineering of hollow mesoporous silica nanoparticles enhancing drug-loading capacity. In Proceedings of the International Conference on the Development of Biomedical Engineering in Vietnam, Ho Chi Minh, Vietnam, 27–29 June 2018; pp. 197–201. [Google Scholar]
- Narmani, A.; Kamali, M.; Amini, B.; Salimi, A.; Panahi, Y. Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process Biochem. 2018, 69, 178–187. [Google Scholar] [CrossRef]
- Huang, A.H.; Han, S.P.; Lu, Y.P.; Ma, R.; Zheng, H.S.; Li, F.Z. Preparation and in vitro evaluation of arsenic trioxide glioma targeting drug delivery system loaded by PAMAM dendrimers co-modified with RGDyC and PEG. China J. Chin. Mater. Med. 2018, 43, 1618–1625. [Google Scholar]
- Wang, X.; Wu, X.; Fan, W.; Ding, B.; Wang, X.; Zhang, W.; Cai, Z. Surface modification with pluronic P123 enhances transfection efficiency of PAMAM dendrimer. Macromol. Res. 2012, 20, 162–167. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; HuangFu, M.; Xiao, Y.; Zhang, T.; Han, M.; Gao, J. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine 2016, 11, 2917–2934. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Cheng, Y. Glutathione-triggered “off–on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Pang, Y.; Huang, P.; Zhu, X.; Zhou, Y.; Yan, D. Molecular self-assembly of a homopolymer: An alternative to fabricate drug-delivery platforms for cancer therapy. Angew. Chem. Int. Ed. 2011, 50, 9162–9166. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Q.; Zhu, W.; Zhao, M.; Ping, Y.; Tang, G. Structure-invertible nanoparticles for triggered co-delivery of nucleic acids and hydrophobic drugs for combination cancer therapy. Adv. Funct. Mater. 2015, 25, 3380–3392. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Lv, X.; Liu, C.; Qi, L.; Song, X.; Yu, A. Enhancement of all-trans retinoic acid-induced differentiation by pH-sensitive nanoparticles for solid tumor cells. Macromol. Biosci. 2014, 14, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Hosseini, S.H.; Alizadeh, M.; Bennett, C. Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids Surf. B Biointerfaces 2014, 116, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zheng, S.; Pan, Z.; Liu, Z. Phase transition effects on mechanical properties of NIPA hydrogel. Polymers 2018, 10, 358. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Singh, A.K. Dendrimers: A novel carrier system for drug delivery. J. Drug Deliv. Ther. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Parham, N.; Panahi, H.A.; Feizbakhsh, A.; Moniri, E. Synthesis of high generation thermo-sensitive dendrimers for extraction of rivaroxaban from human fluid and pharmaceutic samples. J. Chromatogr. A 2018, 1545, 12–21. [Google Scholar] [CrossRef]
- Kojima, C.; Tsumura, S.; Harada, A.; Kono, K. A collagen-mimic dendrimer capable of controlled release. J. Am. Chem. Soc. 2009, 131, 6052–6053. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, L.; Wan, Y.; Zhu, X.; Wang, Z. Thermosensitivity of low generation poly (amidoamine) dendrimers with enriching peripheral functional groups. Colloids Surf. A Physicochem. Eng. Asp. 2012, 403, 164–168. [Google Scholar] [CrossRef]
- Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules 2016, 21, 1715. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Gao, M.; He, H.; Kuang, G.; Wei, Y. Photoresponsive nanocarriers based on PAMAM dendrimers with ao-nitrobenzyl shell. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 551–557. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Ramireddy, R.; Thayumanavan, S. Photoregulated release of noncovalent guests from dendritic amphiphilic nanocontainers. Angew. Chem. Int. Ed. 2011, 50, 3038–3042. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Djordjevic, I.; Pokholenko, O.; Zhang, H.; Zhang, J.; Steele, T.W. On-demand bioadhesive dendrimers with reduced cytotoxicity. Molecules 2018, 23, 796. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, J.; Yun, M.; Kim, C. Photoinduced release of guest molecules by supramolecular transformation of self-assembled aggregates derived from dendrons. Angew. Chem. Int. Ed. 2008, 47, 2959–2963. [Google Scholar] [CrossRef] [PubMed]
- Zardad, A.-Z.; Choonara, Y.; du Toit, L.; Kumar, P.; Mabrouk, M.; Kondiah, P.; Pillay, V. A review of thermo-and ultrasound-responsive polymeric systems for delivery of chemotherapeutic agents. Polymers 2016, 8, 359. [Google Scholar] [CrossRef]
- Huang, B.; Dong, W.J.; Yang, G.Y.; Wang, W.; Ji, C.H.; Zhou, F.N. Dendrimer-coupled sonophoresis-mediated transdermal drug-delivery system for diclofenac. Drug Des. Dev. Ther. 2015, 9, 3867–3876. [Google Scholar]
- Manikkath, J.; Manikkath, A.; Shavi, G.V.; Bhat, K.; Mutalik, S. Low frequency ultrasound and PAMAM dendrimer facilitated transdermal delivery of ketoprofen. J. Drug Deliv. Sci. Technol. 2017, 41, 334–343. [Google Scholar] [CrossRef]
- Martinho, N.; Florindo, H.; Silva, L.; Brocchini, S.; Zloh, M.; Barata, T. Molecular modeling to study dendrimers for biomedical applications. Molecules 2014, 19, 20424–20467. [Google Scholar] [CrossRef]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7. [Google Scholar] [CrossRef]
- Li, X.; Tian, L.; Ali, Z.; Wang, W.; Zhang, Q. Design of flexible dendrimer-grafted flower-like magnetic microcarriers for penicillin G acylase immobilization. J. Mater. Sci. 2018, 53, 937–947. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Luo, K.; She, W.; Guo, C.; Yang, Y.; Gu, Z. Peptide dendrimer–doxorubicin conjugate-based nanoparticles as an enzyme-responsive drug delivery system for cancer therapy. Adv. Healthc. Mater. 2014, 3, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Gu, Z. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Joung, Y.K.; Choi, J.H.; Moon, H.T.; Park, K.D. Targeting ligand-functionalized and redox-sensitive heparin-pluronic nanogels for intracellular protein delivery. Biomed. Mater. 2011, 6, 055004. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Choi, J.H.; Joung, Y.K.; Park, K.D. Disulfide-crosslinked heparin-pluronic nanogels as a redox-sensitive nanocarrier for intracellular protein delivery. J. Bioact. Compat. Polym. 2011, 26, 287–300. [Google Scholar] [CrossRef]
- Yan, L.; Li, X. Biodegradable stimuli-responsive polymeric micelles for treatment of malignancy. Curr. Pharm. Biotechnol. 2016, 17, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of amino-functional polyester dendrimers based on Bis-MPA as nonviral vectors for siRNA delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef]
- Aped, I.; Mazuz, Y.; Sukenik, C.N. Variations in the structure and reactivity of thioester functionalized self-assembled monolayers and their use for controlled surface modification. Beilstein J. Nanotechnol. 2012, 3, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Vo, U.V.; Nguyen, C.K.; Nguyen, V.C.; Tran, T.V.; Thi, B.Y.T.; Nguyen, D.H. Gelatin-poly(ethylene glycol) methyl ether-functionalized porous Nanosilica for efficient doxorubicin delivery. J. Polym. Res. 2019, 26, 6. [Google Scholar] [CrossRef]
- Kojima, C. Design of stimuli-responsive dendrimers. Expert Opin. Drug Deliv. 2010, 7, 307–319. [Google Scholar] [CrossRef]
- Du, X.J.; Wang, Z.Y.; Wang, Y.C. Redox-sensitive dendrimersome assembled from amphiphilic Janus dendrimer for siRNA delivery. Biomater. Sci. 2018, 6, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Kocak, G.; Solmaz, G.; Tuncer, C.; Bütün, V. Modification of glycidyl methacrylate based block copolymers and their aqueous solution behaviours. Eur. Polym. J. 2019, 110, 364–377. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.; Rossignol, J. Use of polyamidoamine dendrimers in brain diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.N.; Bach, L.G.; Nguyen, D.H.; Nguyen, C.H.; Nguyen, C.K.; Tran, N.Q.; Nguyen, N.V.; Thi, T.T.H. PEGylated PAMAM dendrimers loading oxaliplatin with prolonged release and high payload without burst effect. Biopolymers 2019, 110, e23272. [Google Scholar] [CrossRef]
- Moura, L.I.; Malfanti, A.; Peres, C.; Matos, A.I.; Guegain, E.; Sainz, V.; Zloh, M.; Vicent, M.; Florindo, H.F. Functionalized branched polymers: Promising immunomodulatory tools for the treatment of cancer and immune disorders. Mater. Horiz. 2019. [Google Scholar] [CrossRef]
- Ray, S.; Li, Z.; Hsu, C.-H.; Hwang, L.-P.; Lin, Y.-C.; Chou, P.-T.; Lin, Y.-Y. Dendrimer-and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics 2018, 8, 6322–6349. [Google Scholar] [CrossRef]
- Qi, X.; Qin, J.; Fan, Y.; Qin, X.; Jiang, Y.; Wu, Z. Carboxymethyl chitosan-modified polyamidoamine dendrimer enables progressive drug targeting of tumors via pH-sensitive charge inversion. J. Biomed. Nanotechnol. 2016, 12, 667–678. [Google Scholar] [CrossRef]
- Liu, K.C.; Yeo, Y. Zwitterionic chitosan–polyamidoamine dendrimer complex nanoparticles as a pH-sensitive drug carrier. Mol. Pharm. 2013, 10, 1695–1704. [Google Scholar] [CrossRef]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-based drug delivery nanosystems for the treatment of brain tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhu, M.; Gong, Y.; Tang, H.; Li, J.; Cao, Y. Thermoresponsive polymers with lower critical solution temperature-or upper critical solution temperature-type phase behaviour do not induce toxicity to human endothelial cells. Basic Clin. Pharmacol. Toxicol. 2017, 120, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pehkonen, S.O.; Yuan, S. Tailored Thin Coatings for Corrosion Inhibition Using a Molecular Approach; Academic Press: Cambridge, MA, USA, 2018; Volume 23. [Google Scholar]
- Sánchez-Moreno, P.; de Vicente, J.; Nardecchia, S.; Marchal, J.; Boulaiz, H. Thermo-sensitive nanomaterials: Recent advance in synthesis and biomedical applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, X. Temperature responsive nanoparticles based on PEGylated polyaspartamide derivatives for drug delivery. Polymers 2019, 11, 316. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Li, W.; Zhang, A.; Chen, Y.; Feldman, K.; Wu, H.; Schlüter, A.D. Low toxic, thermoresponsive dendrimers based on oligoethylene glycols with sharp and fully reversible phase transitions. Chem. Commun. 2008, 45, 5948–5950. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef]
- Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; García-Calvo, E.; Rosal, R.; Fernández-Piñas, F. First evidences of PAMAM dendrimer internalization in microorganisms of environmental relevance: A linkage with toxicity and oxidative stress. Nanotoxicology 2015, 9, 706–718. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.; Godinho, M.; Borges, J.; Soares, P. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Moghaddam, Z.S.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Noriega-Luna, B.; Godínez, L.A.; Rodríguez, F.J.; Rodríguez, A.; Larrea, G.; Sosa-Ferreyra, C.; Mercado-Curiel, R.; Manríquez, J.; Bustos, E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 2014, 39. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Zhang, H.; Zou, M.; Yan, D.; Chen, D.; Zhao, Y. A responsive porous hydrogel particle-based delivery system for oncotherapy. Nanoscale 2019, 11, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Nguyen, T.N.Q.; Hoang, D.T.; Nguyen, D.H. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetics, biodistribution, and pharmacodynamics of drug delivery systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Edwards, C.; de Noni, I.; Patel, H.; El, S.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Bach, L.G.; Pham, T.T.; Le, N.T.T.; Ngoc, U.T.P.; Tran, D.-H.N.; Nguyen, D.H. Synthesis and antifungal activity of chitosan-silver nanocomposite synergize fungicide against Phytophthora capsici. J. Macromol. Sci. Part A 2019, 56, 522–528. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Maurya, S.D.; Das, M.K.; Tilak, V.K.; Verma, K.K.; Dhakar, R.C. Dendrimers in drug delivery, diagnosis and therapy: Basics and potential applications. J. Drug Deliv. Ther. 2016, 6, 67–92. [Google Scholar] [CrossRef]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Mignani, S.; Tripathi, R.P.; Chen, L.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. New ways to treat tuberculosis using dendrimers as nanocarriers. Pharmaceutics 2018, 10, 105. [Google Scholar] [CrossRef]
- Nagpal, K.; Mohan, A.; Thakur, S.; Kumar, P. Dendritic platforms for biomimicry and biotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 861–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Tekade, M.; Kesharwani, P.; Iyer, A.K.; Kalia, K.; Tekade, R.K. The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today 2017, 22, 652–664. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; de Silva, C.E.R.; Barthès, J.G.D.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Shukla, S.; Shukla, S.K.; Govender, P.P.; Giri, N. Biodegradable polymeric nanostructures in therapeutic applications: Opportunities and challenges. RSC Adv. 2016, 6, 94325–94351. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Seo, S.J.; Lee, S.Y.; Choi, S.J.; Kim, H.W. Tumor-targeting co-delivery of drug and gene from temperature-triggered micelles. Macromol. Biosci. 2015, 15, 1198–1204. [Google Scholar] [CrossRef]
- Baker, J.R., Jr. Dendrimer-based nanoparticles for cancer therapy. ASH Educ. Program Book 2009, 2009, 708–719. [Google Scholar] [CrossRef] [Green Version]
- Mhlwatika, Z.; Aderibigbe, B. Application of dendrimers for the treatment of infectious diseases. Molecules 2018, 23, 2205. [Google Scholar] [CrossRef] [PubMed]
- Madaan, U.; Yzeiraj, E.; Meade, M.; Clark, J.F.; Rushlow, C.A.; Savage-Dunn, C. BMP signaling determines body size via transcriptional regulation of collagen genes in caenorhabditis elegans. Genetics 2018, 210, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
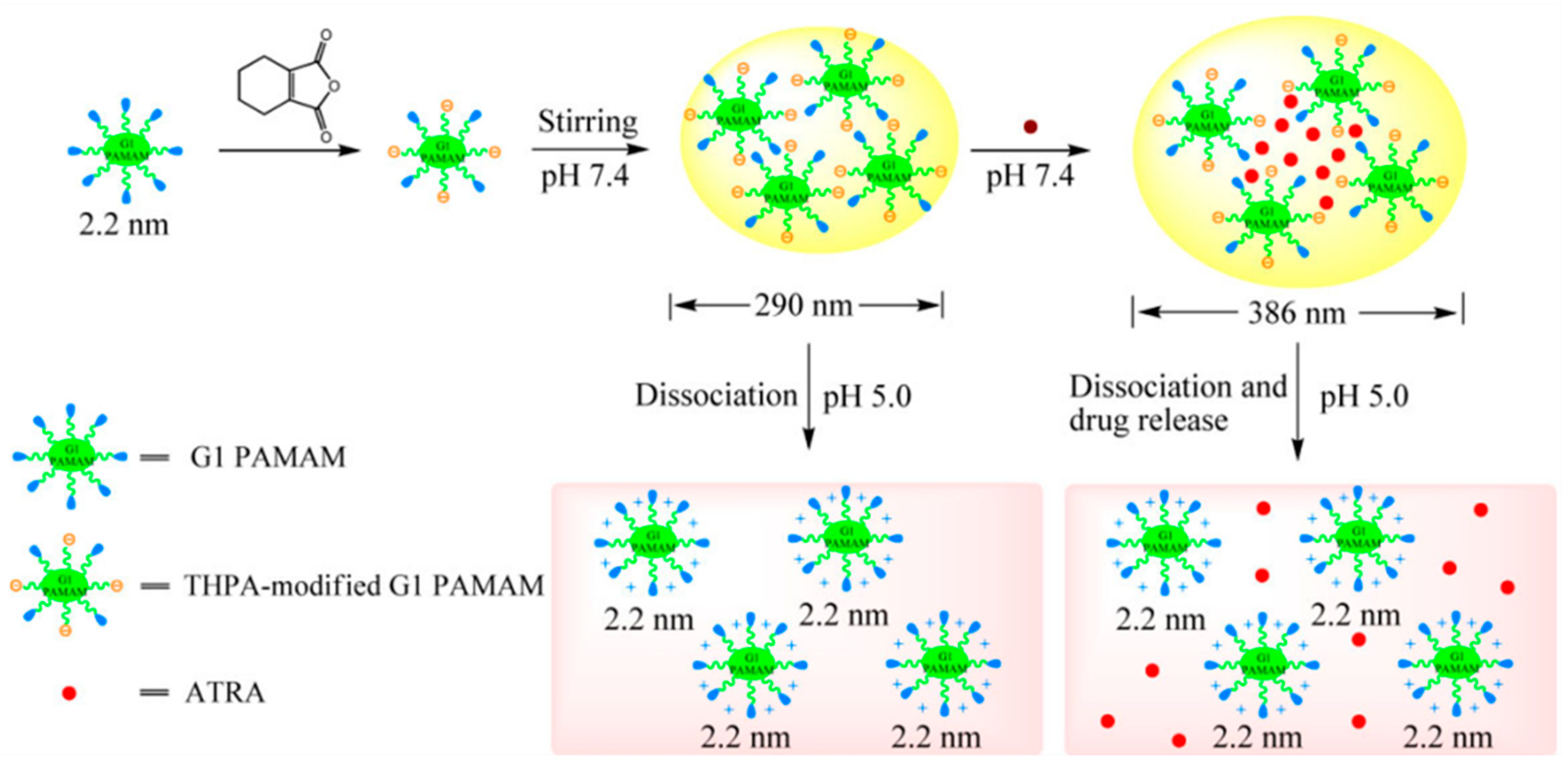

| Polymer | Generation | Modification | Responsiveness | Payload | Ref. |
|---|---|---|---|---|---|
| PAMAM | G4 | PEG | Redox and pH | Doxorubicin | [31] |
| PAMAM | G3.5 | Heparin | Redox and pH | Letrozole | [32] |
| PAMAM | G4 | PEG | Redox | Doxorubicin | [33] |
| PBAE | G4 | 2-(N,N-dimethylamino)ethyl acrylate | pH and temperature | Doxorubicin | [34] |
| PAMAM | G4 | PEG-chitosan-folic acid | pH | pDNA | [35] |
| Stimuli | Responsive moiety | Ref. | |
|---|---|---|---|
| Internal stimuli | Reduction | Au−S | [74] |
| Disulfide | [75,76] | ||
| pH | 3,4,5,6-tetrahydrophthalic anhydride | [77] | |
| Hydrazine | [34,68,78] | ||
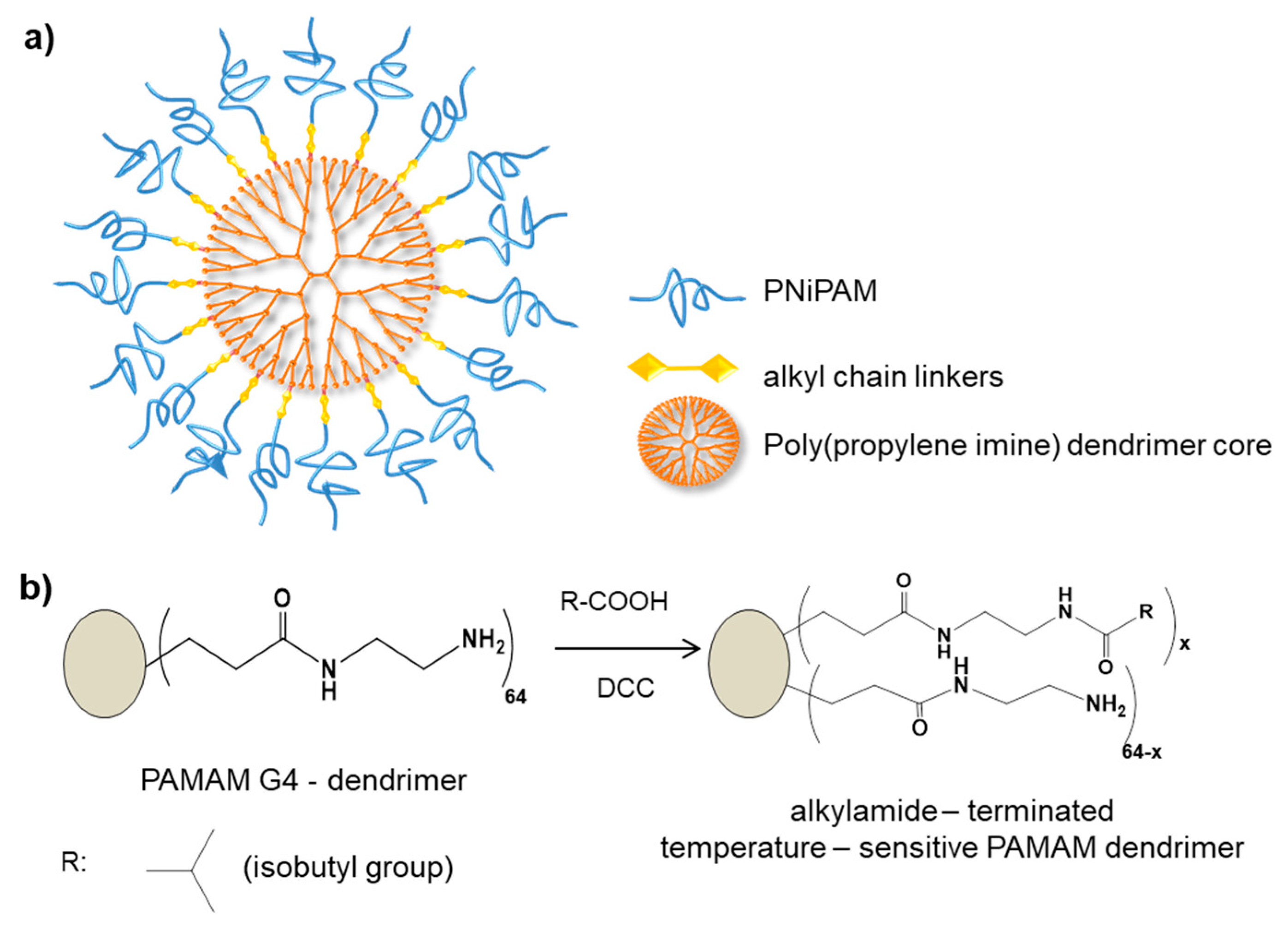
| Temperature | Poly (NiPAM) | [79,80,81] | |
| Isobutyramide (IBAM) | [82] | ||
| 4-(isopropylamino)-4-oxobutanoic acid (IPAOBA) and 4-(diethylamino)-4-oxobutanoic acid (DEAOBA) | [83] | ||
| External stimuli | Light | Ortho-nitrobenzyl (ONB) | [51,84,85,86] |
| 4-(3-(trifluoromethyl)-3H-diazirin-3-yl) benzyl bromide | [87] | ||
| Aso-nitrobenzyl and diazobenzene | [88] | ||
| Ultrasound | Sonophoresis | [89,90,91,92,93,94] | |
| Magnetic field | Fe3O4@SiO2-NH2 | [95] | |
| Enzyme | Gly-Phe-Leu-Gly (GFLG) | [96] | |
| Lysine | [97] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics 2019, 11, 591. https://doi.org/10.3390/pharmaceutics11110591
Le NTT, Nguyen TNQ, Cao VD, Hoang DT, Ngo VC, Hoang Thi TT. Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics. 2019; 11(11):591. https://doi.org/10.3390/pharmaceutics11110591
Chicago/Turabian StyleLe, Ngoc Thuy Trang, Thi Nhu Quynh Nguyen, Van Du Cao, Duc Thuan Hoang, Van Cuong Ngo, and Thai Thanh Hoang Thi. 2019. "Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment" Pharmaceutics 11, no. 11: 591. https://doi.org/10.3390/pharmaceutics11110591
APA StyleLe, N. T. T., Nguyen, T. N. Q., Cao, V. D., Hoang, D. T., Ngo, V. C., & Hoang Thi, T. T. (2019). Recent Progress and Advances of Multi-Stimuli-Responsive Dendrimers in Drug Delivery for Cancer Treatment. Pharmaceutics, 11(11), 591. https://doi.org/10.3390/pharmaceutics11110591