Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds
Abstract
:1. Introduction
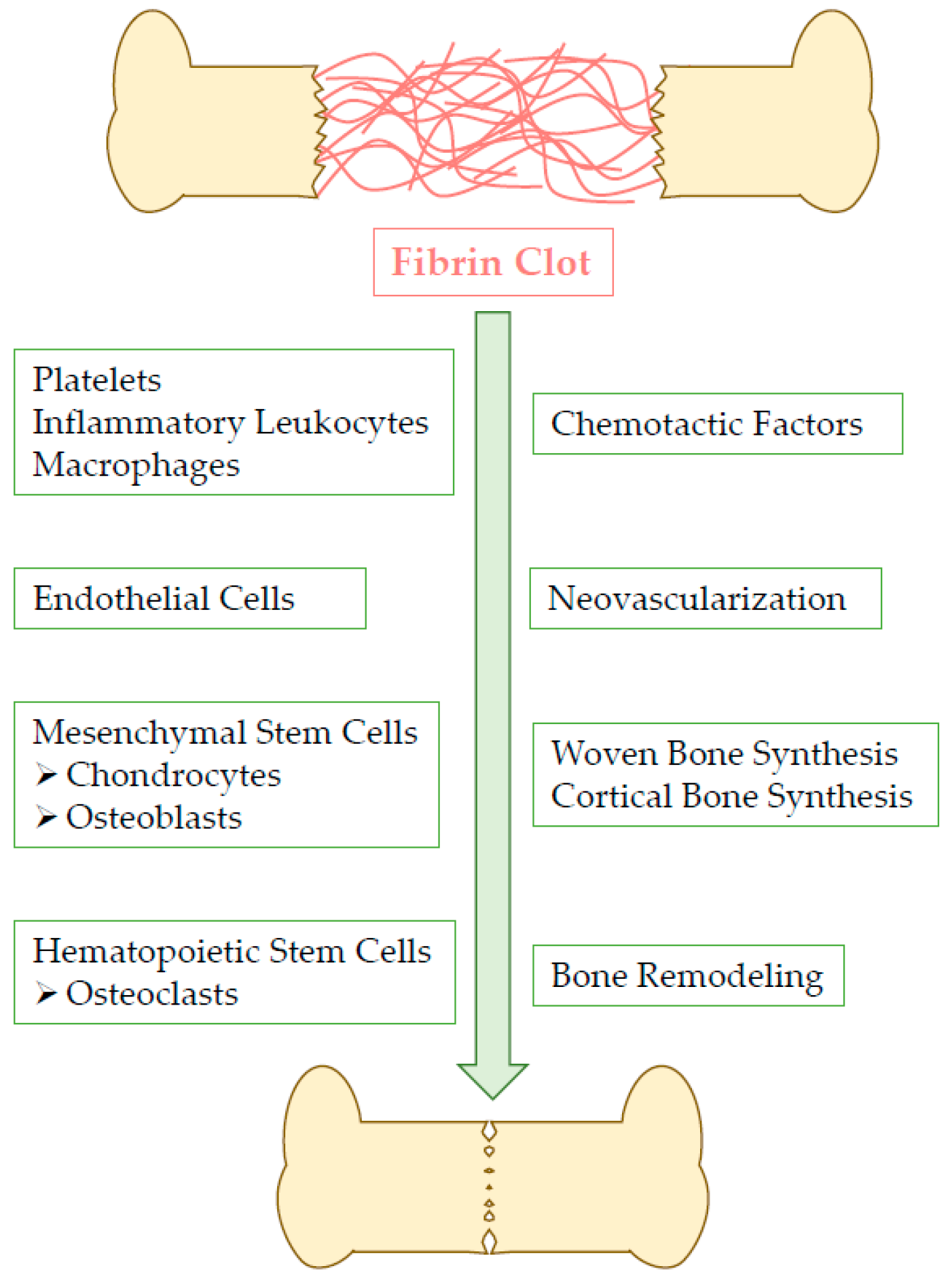
2. Fibrin Clot Action within Bone Healing Process
3. Fibrin Physiological Properties
3.1. Composition, Structure, and Mechanical Properties
3.2. Biological Properties
4. Preparing Fibrin for Bone Repair
5. Fibrin Handling for Bioactive Compounds Delivery
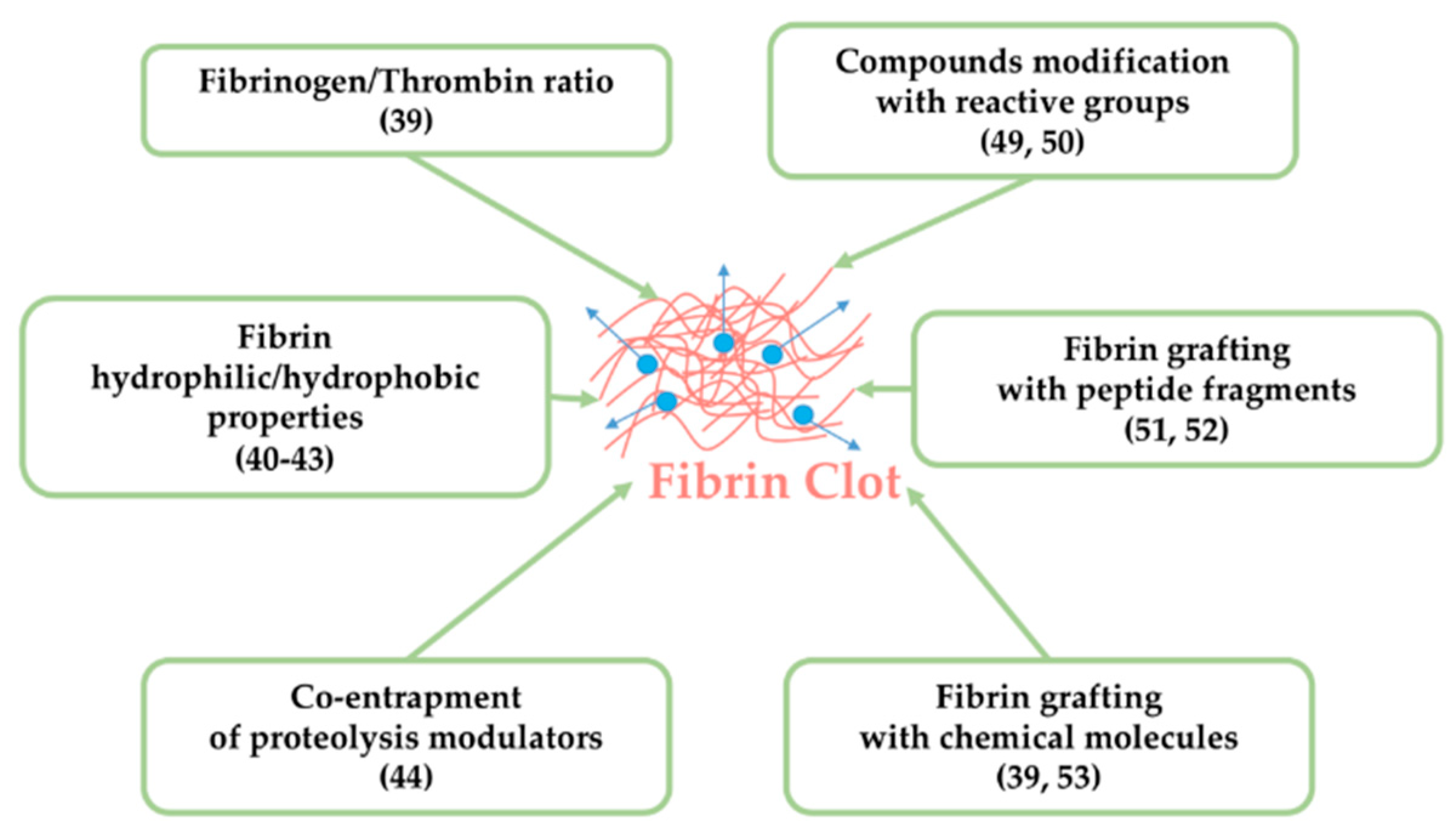
5.1. Tuning Fibrin Intrinsic Characteristics
5.2. Modifying Fibrin and/or Active Agents for Efficient Combination and Delivery
6. Active Agents Used in Fibrin-Based Delivery Strategies
6.1. Active Agents for Bone Infections
6.2. Active Agents for Pain Relief and Cancer Treatment
6.3. Growth Factors and Cytokines
6.4. Genes
6.5. miRNAs/siRNA
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verron, E.; Bouler, J.M.; Guicheux, J. Controlling the biological function of calcium phosphate bone substitutes with drugs. Acta Biomater. 2012, 8, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Fracture healing: Fracture healing understood as the result of a fascinating cascade of physical and biological interactions. Part I. An Attempt to Integrate Observations from 30 Years AO Research. Acta Chir. Orthop. Traumatol. Cechoslov. 2014, 81, 355–364. [Google Scholar]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [PubMed] [Green Version]
- Hoppe, B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thromb. Haemost. 2014, 112, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Sproul, E.P.; Hannan, R.T.; Brown, A.C. Controlling Fibrin Network Morphology, Polymerization, and Degradation Dynamics in Fibrin Gels for Promoting Tissue Repair. Methods Mol. Biol. 2018, 1758, 85–99. [Google Scholar] [PubMed]
- Welsch, N.; Brown, A.C.; Barker, T.H.; Lyon, L.A. Enhancing clot properties through fibrin-specific self-cross-linked PEG side-chain microgels. Colloids Surf. B Biointerfaces 2018, 166, 89–97. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Z.; Kim, O.V.; Litvinov, R.I.; Weisel, J.W.; Alber, M. Model predictions of deformation, embolization and permeability of partially obstructive blood clots under variable shear flow. J. R. Soc. Interface 2017, 14, 20170441. [Google Scholar] [CrossRef] [PubMed]
- Bannish, B.E.; Chernysh, I.N.; Keener, J.P.; Fogelson, A.L.; Weisel, J.W. Molecular and Physical Mechanisms of Fibrinolysis and Thrombolysis from Mathematical Modeling and Experiments. Sci. Rep. 2017, 7, 6914. [Google Scholar] [CrossRef]
- Kim, O.V.; Litvinov, R.I.; Weisel, J.W.; Alber, M.S. Structural basis for the nonlinear mechanics of fibrin networks under compression. Biomaterials 2014, 35, 6739–6749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, O.V.; Litvinov, R.I.; Chen, J.; Chen, D.Z.; Weisel, J.W.; Alber, M.S. Compression-induced structural and mechanical changes of fibrin-collagen composites. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 60–61, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Ateshian, G.A. Grading of osteoarthritic cartilage: Correlations between histology and biomechanics. J. Orthop. Res. 2016, 34, 8–9. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biol. J. Int. Soc. Matrix Biol. 2017, 60–61, 110–123. [Google Scholar] [CrossRef]
- Kim, O.V.; Liang, X.; Litvinov, R.I.; Weisel, J.W.; Alber, M.S.; Purohit, P.K. Foam-like compression behavior of fibrin networks. Biomech. Model. Mechanobiol. 2016, 15, 213–228. [Google Scholar] [CrossRef]
- Yakovlev, S.; Medved, L. Effect of fibrinogen, fibrin, and fibrin degradation products on transendothelial migration of leukocytes. Thromb. Res. 2018, 162, 93–100. [Google Scholar] [CrossRef]
- Yakovlev, S.; Cao, C.; Galisteo, R.; Zhang, L.; Strickland, D.K.; Medved, L. Fibrin-VLDL Receptor-Dependent Pathway Promotes Leukocyte Transmigration by Inhibiting Src Kinase Fyn and is a Target for Fibrin β15-42 Peptide. Thromb. Haemost. 2019. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Bensaıd, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar] [CrossRef]
- de la Puente, P.; Ludeña, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Sarraf, F.; Shoichet, M.S.; Davies, J.E. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J. Biomed. Mater. Res. A 2004, 71, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Catelas, I.; Sese, N.; Wu, B.M.; Dunn, J.C.Y.; Helgerson, S.; Tawil, B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006, 12, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Tawil, B.; Dunn, J.C.Y.; Wu, B.M. The behavior of human mesenchymal stem cells in 3D fibrin clots: Dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006, 12, 1587–1595. [Google Scholar] [CrossRef]
- Davis, H.E.; Miller, S.L.; Case, E.M.; Leach, J.K. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011, 7, 691–699. [Google Scholar] [CrossRef]
- Linsley, C.; Wu, B.; Tawil, B. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng. Part A 2013, 19, 1416–1423. [Google Scholar] [CrossRef]
- Colley, H.; McArthur, S.L.; Stolzing, A.; Scutt, A. Culture on fibrin matrices maintains the colony-forming capacity and osteoblastic differentiation of mesenchymal stem cells. Biomed. Mater. 2012, 7, 045015. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, H.-J.; Kim, T.-I.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. The effects of the modulation of the fibronectin-binding capacity of fibrin by thrombin on osteoblast differentiation. Biomaterials 2012, 33, 4089–4099. [Google Scholar] [CrossRef]
- Law, J.X.; Chowdhury, S.R.; Aminuddin, B.S.; Ruszymah, B.H.I. Role of plasma-derived fibrin on keratinocyte and fibroblast wound healing. Cell Tissue Bank. 2017, 18, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Albala, D.M. Fibrin sealants in clinical practice. Cardiovasc. Surg. 2003, 11 (Suppl. 1), 5–11. [Google Scholar] [CrossRef]
- Morikawa, T. Tissue sealing. Am. J. Surg. 2001, 182, 29S–35S. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Kruskall, M.S.; Uhl, L. Cryoprecipitate. Patterns of use. Am. J. Clin. Pathol. 2003, 119, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, P.; Winge, S.; Karlsson, G. Large-scale preparation of thrombin from human plasma. Thromb. Res. 2008, 122, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, W.L.; Nur, I.; Meidler, R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2011, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Madsen, T.; Zhu, H.; Semple, E. Stability of human thrombin produced from 11 mL of plasma using the thrombin processing device. J. Extra Corpor. Technol. 2005, 37, 390–395. [Google Scholar] [PubMed]
- Xuan, F.; Lee, C.-U.; Son, J.-S.; Jeong, S.-M.; Choi, B.-H. A comparative study of the regenerative effect of sinus bone grafting with platelet-rich fibrin-mixed Bio-Oss® and commercial fibrin-mixed Bio-Oss®: An experimental study. J. Cranio-Maxillofac. Surg. 2014, 42, e47–e50. [Google Scholar] [CrossRef]
- Jeon, O.; Ryu, S.H.; Chung, J.H.; Kim, B.-S. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J. Control. Release 2005, 105, 249–259. [Google Scholar] [CrossRef]
- Greco, F.; de Palma, L.; Spagnolo, N.; Rossi, A.; Specchia, N.; Gigante, A. Fibrin–antibiotic mixtures: An in vitro study assessing the possibility of using a biologic carrier for local drug delivery. J. Biomed. Mater. Res. 1991, 25, 39–51. [Google Scholar] [CrossRef]
- Tredwell, S.; Jackson, J.K.; Hamilton, D.; Lee, V.; Burt, H.M. Use of fibrin sealants for the localized, controlled release of cefazolin. Can. J. Surg. J. Can. Chir. 2006, 49, 347–352. [Google Scholar]
- Woolverton, C.J.; Huebert, K.; Burkhart, B.; MacPhee, M. Subverting bacterial resistance using high dose, low solubility antibiotics in fibrin. Infection 1999, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yamaoka, Y.; Shinoyama, M.; Kamiya, A. Novel drug delivery system using autologous fibrin glue--release properties of anti-cancer drugs. Biol. Pharm. Bull. 2000, 23, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.E.; Griffith, M.; Hincke, M. Characterization and Inhibition of Fibrin Hydrogel–Degrading Enzymes During Development of Tissue Engineering Scaffolds. Tissue Eng. 2007, 13, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Bacon-Baguley, T.; Ogilvie, M.L.; Gartner, T.K.; Walz, D.A. Thrombospondin binding to specific sequences within the A alpha- and B beta-chains of fibrinogen. J. Biol. Chem. 1990, 265, 2317–2323. [Google Scholar] [PubMed]
- Voland, C.; Serre, C.M.; Delmas, P.; Clézardin, P. Platelet-osteosarcoma cell interaction is mediated through a specific fibrinogen-binding sequence located within the N-terminal domain of thrombospondin 1. J. Bone Miner. Res. 2000, 15, 361–368. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, B.; Li, S.; Duan, C. Fibronectin binds insulin-like growth factor-binding protein 5 and abolishes Its ligand-dependent action on cell migration. J. Biol. Chem. 2004, 279, 4269–4277. [Google Scholar] [CrossRef]
- Campbell, P.G.; Durham, S.K.; Hayes, J.D.; Suwanichkul, A.; Powell, D.R. Insulin-like growth factor-binding protein-3 binds fibrinogen and fibrin. J. Biol. Chem. 1999, 274, 30215–30221. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.D.; Durrieu, M.C.; Laroche, G. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater. 2016, 36, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Schmoekel, H.G.; Weber, F.E.; Schense, J.C.; Grätz, K.W.; Schawalder, P.; Hubbell, J.A. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol. Bioeng. 2005, 89, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.-G.; Bhang, S.H.; Kim, H.-J.; Im, G.-I.; Lee, H.; Park, J.-H.; Kim, B.-S. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng. Part A 2011, 17, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Yang, M.-C.; Chung, T.-W. Liposomes/chitosan scaffold/human fibrin gel composite systems for delivering hydrophilic drugs--release behaviors of tirofiban in vitro. Drug Deliv. 2008, 15, 149–157. [Google Scholar] [CrossRef]
- Fu, J.; Ni, M.; Li, H.; Li, X.; Chai, W.; Zhou, Y.; Hao, L.; Chen, J. The proper timing of second-stage revision in treating periprosthetic knee infection: Reliable indicators and risk factors. J. Orthop. Surg. 2018, 13, 214. [Google Scholar] [CrossRef]
- Redl, H.; Schlag, G.; Stanek, G.; Hirschl, A.; Seelich, T. In vitro properties of mixtures of fibrin seal and antibiotics. Biomaterials 1983, 4, 29–32. [Google Scholar] [CrossRef]
- Osada, T.; Yamamura, K.; Yano, K.; Fujimoto, K.; Mizuno, K.; Sakurai, T.; Nabeshima, T. Distribution and serum concentration of sisomicin released from fibrin glue-sealed dacron graft in the rat and human. J. Biomed. Mater. Res. 2000, 52, 53–57. [Google Scholar] [CrossRef]
- Fujimoto, K.; Yamamura, K.; Osada, T.; Hayashi, T.; Nabeshima, T.; Matsushita, M.; Nishikimi, N.; Sakurai, T.; Nimura, Y. Subcutaneous tissue distribution of vancomycin from a fibrin glue/Dacron graft carrier. J. Biomed. Mater. Res. 1997, 36, 564–567. [Google Scholar] [CrossRef]
- Ozaki, S.; Saito, A.; Nakaminami, H.; Ono, M.; Noguchi, N.; Motomura, N. Comprehensive evaluation of fibrin glue as a local drug-delivery system-efficacy and safety of sustained release of vancomycin by fibrin glue against local methicillin-resistant Staphylococcus aureus infection. J. Artif. Organs 2014, 17, 42–49. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008, 9, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Dupleichs, M.; Gao, Q.; Badran, Z.; Janvier, P.; Bouler, J.-M.; Gauthier, O.; Tamimi, F.; Verron, E. Delivery systems of local anesthetics in bone surgery: Are they efficient and safe? Drug Discov. Today 2018, 23, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Dupleichs, M.; Masson, M.; Gauthier, O.; Dutilleul, M.; Bouler, J.-M.; Verron, E.; Janvier, P. Pain Management After Bone Reconstruction Surgery Using an Analgesic Bone Cement: A Functional Noninvasive In Vivo Study Using Gait Analysis. J. Pain 2018, 19, 1169–1180. [Google Scholar] [CrossRef]
- Verron, E.; Gauthier, O.; Janvier, P.; Le Guen, H.; Holopherne, D.; Cavagna, R.; Bouler, J.-M. Analgesic properties of calcium phosphate apatite loaded with bupivacaine on postoperative pain. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 89–96. [Google Scholar] [CrossRef]
- Zhibo, X.; Miaobo, Z. Effect of sustained-release lidocaine on reduction of pain after subpectoral breast augmentation. Aesthet. Surg. J. 2009, 29, 32–34. [Google Scholar] [CrossRef]
- Verron, E.; Schmid-Antomarchi, H.; Pascal-Mousselard, H.; Schmid-Alliana, A.; Scimeca, J.-C.; Bouler, J.-M. Therapeutic strategies for treating osteolytic bone metastases. Drug Discov. Today 2014, 19, 1419–1426. [Google Scholar] [CrossRef]
- Kitazawa, H.; Sato, H.; Adachi, I.; Masuko, Y.; Horikoshi, I. Microdialysis assessment of fibrin glue containing sodium alginate for local delivery of doxorubicin in tumor-bearing rats. Biol. Pharm. Bull. 1997, 20, 278–281. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Tsiridis, E.; Upadhyay, N.; Giannoudis, P. Molecular aspects of fracture healing: Which are the important molecules? Injury 2007, 38 (Suppl. 1), S11–S25. [Google Scholar] [CrossRef]
- Kwong, F.N.K.; Harris, M.B. Recent developments in the biology of fracture repair. J. Am. Acad. Orthop. Surg. 2008, 16, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Schützenberger, S.; Schultz, A.; Hausner, T.; Hopf, R.; Zanoni, G.; Morton, T.; Kropik, K.; van Griensven, M.; Redl, H. The optimal carrier for BMP-2: A comparison of collagen versus fibrin matrix. Arch. Orthop. Trauma Surg. 2012, 132, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Kim, C.-S.; Han, D.-K.; Cho, I.-H.; Jung, U.-W.; Choi, S.-H.; Kim, C.-K.; Cho, K.-S. The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials 2006, 27, 3810–3816. [Google Scholar] [CrossRef]
- Patel, V.V.; Zhao, L.; Wong, P.; Pradhan, B.B.; Bae, H.W.; Kanim, L.; Delamarter, R.B. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J. 2006, 6, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-Y.; Kang, S.-W.; Kim, J.-W.; Suh, S.-W.; Ko, Y.-J.; Park, J.-H. Optimal condition of heparin-conjugated fibrin with bone morphogenetic protein-2 for spinal fusion in a rabbit model. Cytotherapy 2014, 16, 1441–1448. [Google Scholar] [CrossRef]
- Vila, O.F.; Martino, M.M.; Nebuloni, L.; Kuhn, G.; Pérez-Amodio, S.; Müller, R.; Hubbell, J.A.; Rubio, N.; Blanco, J. Bioluminescent and micro-computed tomography imaging of bone repair induced by fibrin-binding growth factors. Acta Biomater. 2014, 10, 4377–4389. [Google Scholar] [CrossRef]
- Burkhardt, M.A.; Gerber, I.; Moshfegh, C.; Lucas, M.S.; Waser, J.; Emmert, M.Y.; Hoerstrup, S.P.; Schlottig, F.; Vogel, V. Clot-entrapped blood cells in synergy with human mesenchymal stem cells create a pro-angiogenic healing response. Biomater. Sci. 2017, 5, 2009–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Lan, X.; Xu, M.; Mao, Z.; Lv, H.; Yao, Q.; Tang, P. Improvement in angiogenesis and osteogenesis with modified cannulated screws combined with VEGF/PLGA/fibrin glue in femoral neck fractures. J. Mater. Sci. Mater. Med. 2014, 25, 1165–1172. [Google Scholar] [CrossRef]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesfin, A.; Buchowski, J.M.; Zebala, L.P.; Bakhsh, W.R.; Aronson, A.B.; Fogelson, J.L.; Hershman, S.; Kim, H.J.; Ahmad, A.; Bridwell, K.H. High-dose rhBMP-2 for adults: Major and minor complications: A study of 502 spine cases. J. Bone Joint Surg. Am. 2013, 95, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Morton, T.J.; Fürst, W.; van Griensven, M.; Redl, H. Controlled release of substances bound to fibrin-anchors or of DNA. Drug Deliv. 2009, 16, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Dockery, P.; O’Brien, T.; Pandit, A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J. Biomed. Mater. Res. A 2009, 89, 876–884. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Shikanov, A.; Shea, L.D. Fibrin hydrogels for non-viral vector delivery in vitro. J. Control. Release 2009, 136, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Bara, J.J.; Dresing, I.; Zeiter, S.; Anton, M.; Daculsi, G.; Eglin, D.; Nehrbass, D.; Stadelmann, V.A.; Betts, D.C.; Müller, R.; et al. A doxycycline inducible, adenoviral bone morphogenetic protein-2 gene delivery system to bone. J. Tissue Eng. Regen. Med. 2018, 12, e106–e118. [Google Scholar] [CrossRef]
- Arriaga, M.A.; Ding, M.-H.; Gutierrez, A.S.; Chew, S.A. The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering. Biotechnol. J. 2019, 14, e1900084. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumarán, C.C.; Parra, M.V.; Olate, S.A.; Fernández, E.G.; Muñoz, F.T.; Haidar, Z.S. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials 2018, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Verra, W.C.; van Hilten, J.A.; Honohan, Á.; van Zwet, E.W.; van der Bom, J.G.; Nelissen, R.G.H.H. FIRST-research group the effect of a fibrin sealant on knee function after total knee replacement surgery. Results from the FIRST trial. A multicenter randomized controlled trial. PLoS ONE 2018, 13, e0200804. [Google Scholar] [CrossRef] [PubMed]
- Canellas, J.V.D.S.; Medeiros, P.J.D.; Figueredo, C.M.D.S.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M.; Tawil, B. Mesenchymal stem cell growth on and mechanical properties of fibrin-based biomimetic bone scaffolds. J. Biomed. Mater. Res. A 2016, 104, 2945–2953. [Google Scholar] [CrossRef]
- Sese, N.; Cole, M.; Tawil, B. Proliferation of human keratinocytes and cocultured human keratinocytes and fibroblasts in three-dimensional fibrin constructs. Tissue Eng. Part A 2011, 17, 429–437. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef]
- Kolehmainen, K.; Willerth, S.M. Preparation of 3D fibrin scaffolds for stem cell culture applications. J. Vis. Exp. JoVE 2012, 61, e3641. [Google Scholar] [CrossRef]
- Montgomery, A.; Wong, A.; Gabers, N.; Willerth, S.M. Engineering personalized neural tissue by combining induced pluripotent stem cells with fibrin scaffolds. Biomater. Sci. 2015, 3, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Praveen, G.; Sreerekha, P.R.; Menon, D.; Nair, S.V.; Chennazhi, K.P. Fibrin nanoconstructs: A novel processing method and their use as controlled delivery agents. Nanotechnology 2012, 23, 095102. [Google Scholar] [CrossRef]
- Arun Kumar, R.; Sivashanmugam, A.; Deepthi, S.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Nano-fibrin stabilized CaSO4 crystals incorporated injectable chitin composite hydrogel for enhanced angiogenesis & osteogenesis. Carbohydr. Polym. 2016, 140, 144–153. [Google Scholar] [PubMed]
- Vishnu Priya, M.; Sivshanmugam, A.; Boccaccini, A.R.; Goudouri, O.M.; Sun, W.; Hwang, N.; Deepthi, S.; Nair, S.V.; Jayakumar, R. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed. Mater. 2016, 11, 035017. [Google Scholar] [CrossRef] [PubMed]


| Growth Factor | Functions for Bone Regeneration |
|---|---|
| BMP (Bone matrix protein) | Recruiting MSCs to the injury site Triggering osteogenic differentiation of osteoprogenitors |
| FGF (Fibroblast growth factor) | Stimulating proliferation of endothelial cells and osteoblasts Promoting angiogenesis from preexisting vasculature Regulating MSC, endothelial cells, fibroblasts and osteoblasts |
| PDGF (Platelet-derived growth factor) | Promoting chemotaxis of MSC, macrophages, neutrophils, fibroblasts and osteoblasts Modulating collagenase synthesis and collagen secretion |
| VEGF (Vascular endothelial growth factor) | Stimulating proliferation and migration of endothelial cells to regulate angiogenesis during bone formation Promoting recruitment and survival of bone forming cells |
| TGF-β (Transforming growth factor β) | Stimulating proliferation of MSC, endothelial cells, fibroblasts and osteoblasts Inhibiting macrophage and lymphocyte proliferation Regulating collagenase synthesis and collagen secretion |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujoli, B.; Scimeca, J.-C.; Verron, E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics 2019, 11, 556. https://doi.org/10.3390/pharmaceutics11110556
Bujoli B, Scimeca J-C, Verron E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics. 2019; 11(11):556. https://doi.org/10.3390/pharmaceutics11110556
Chicago/Turabian StyleBujoli, Bruno, Jean-Claude Scimeca, and Elise Verron. 2019. "Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds" Pharmaceutics 11, no. 11: 556. https://doi.org/10.3390/pharmaceutics11110556
APA StyleBujoli, B., Scimeca, J.-C., & Verron, E. (2019). Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics, 11(11), 556. https://doi.org/10.3390/pharmaceutics11110556





