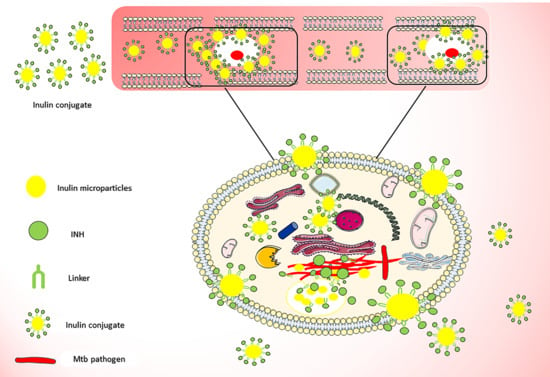
Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
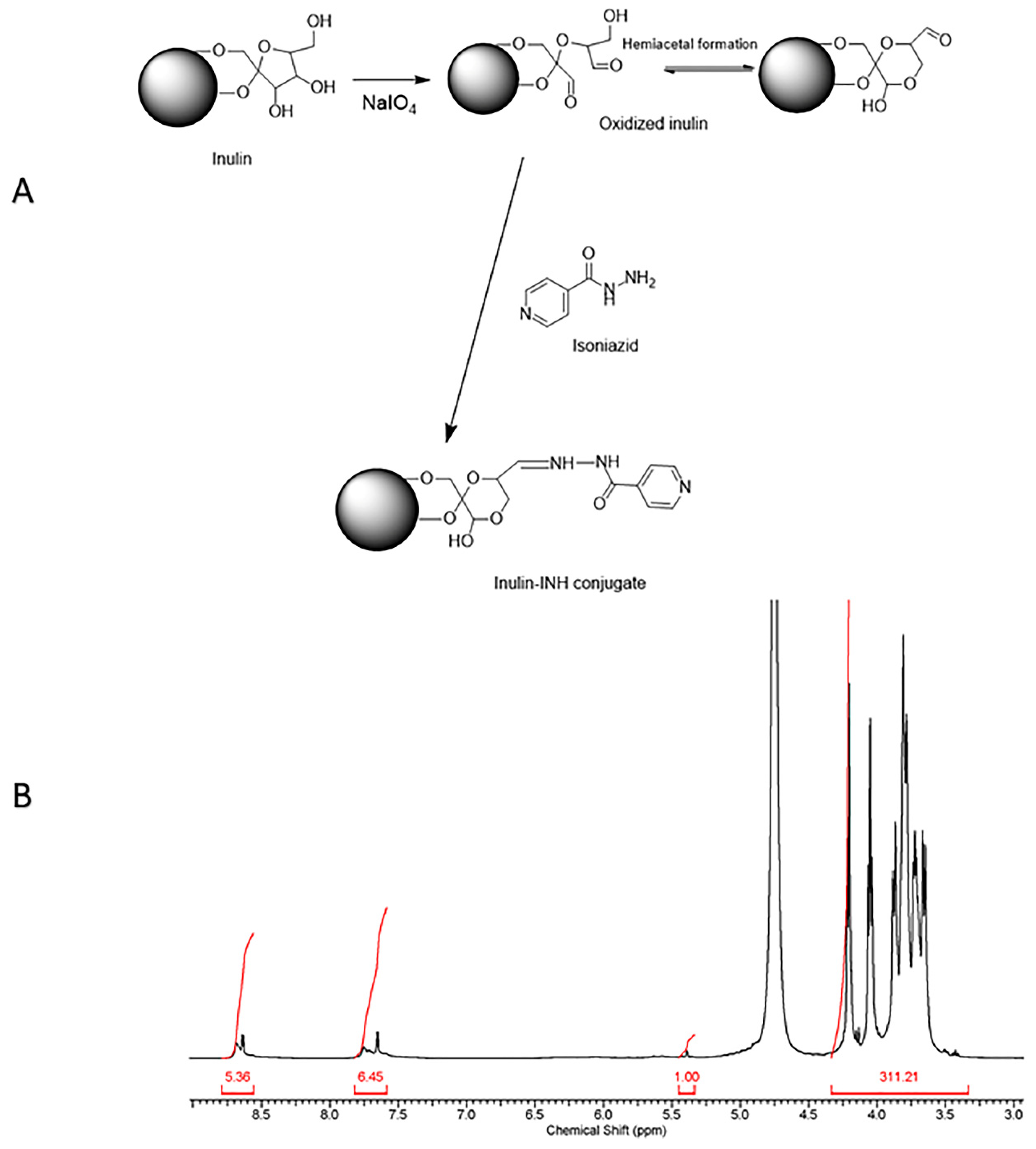
2.2. Oxidation of Inulin Particles
2.3. Isoniazid (INH) Coupling to the Oxidized Inulin Particles
2.4. Characterization of Inulin-INH
2.4.1. Nuclear Magnetic Resonance (NMR)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. X-Ray Powder Diffraction (XRD)
2.4.6. Scanning Electron Microscopy (SEM)
2.4.7. Zeta Potential
2.5. INH Loading Content Determination
2.6. Release of INH from the Synthesized Conjugate
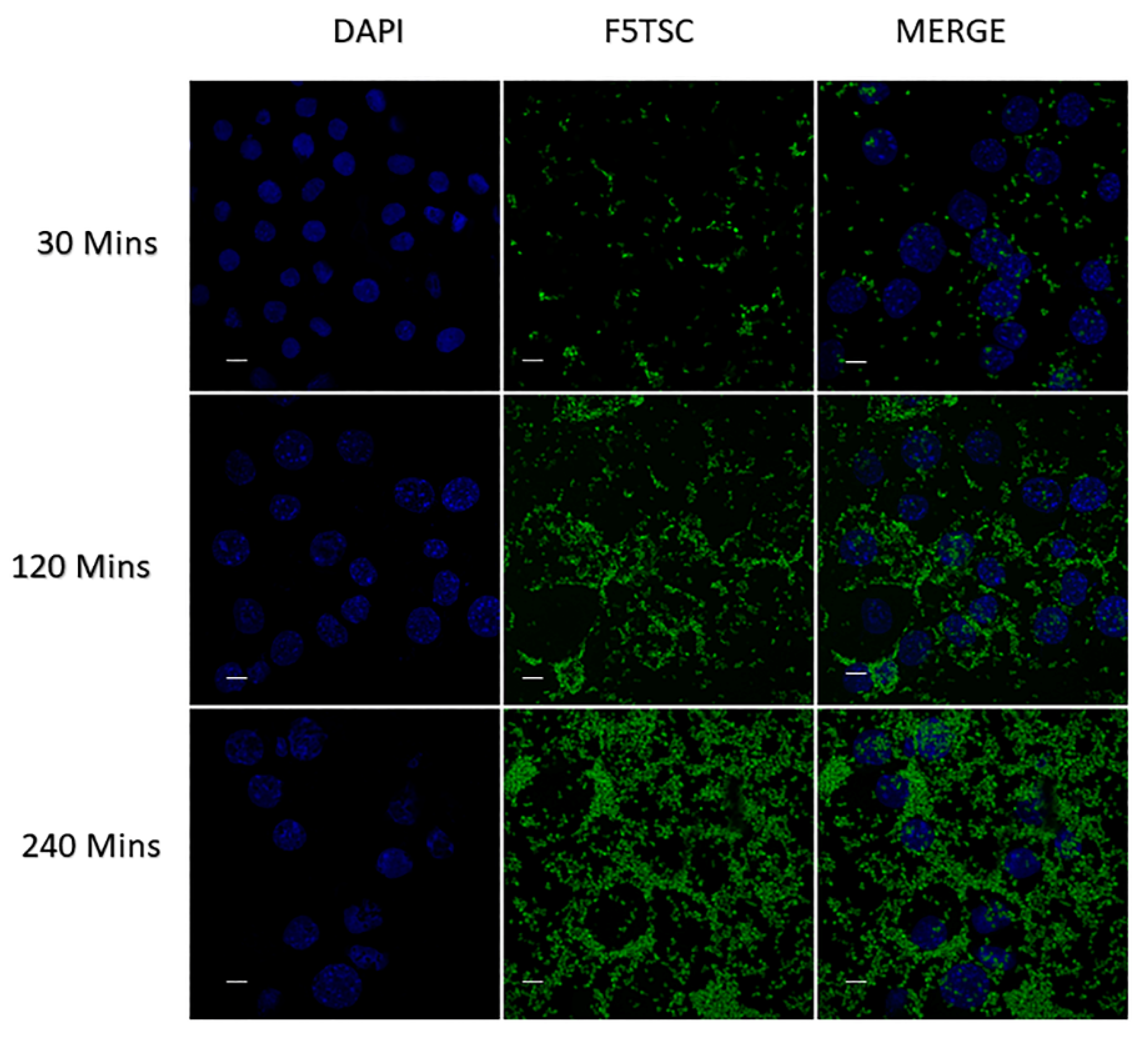
2.7. Efficient Uptake of Fluorescein-5-Thiosemicarbizide (F5TSC) Labelled Inulin Particles by RAW 264.7 Cells
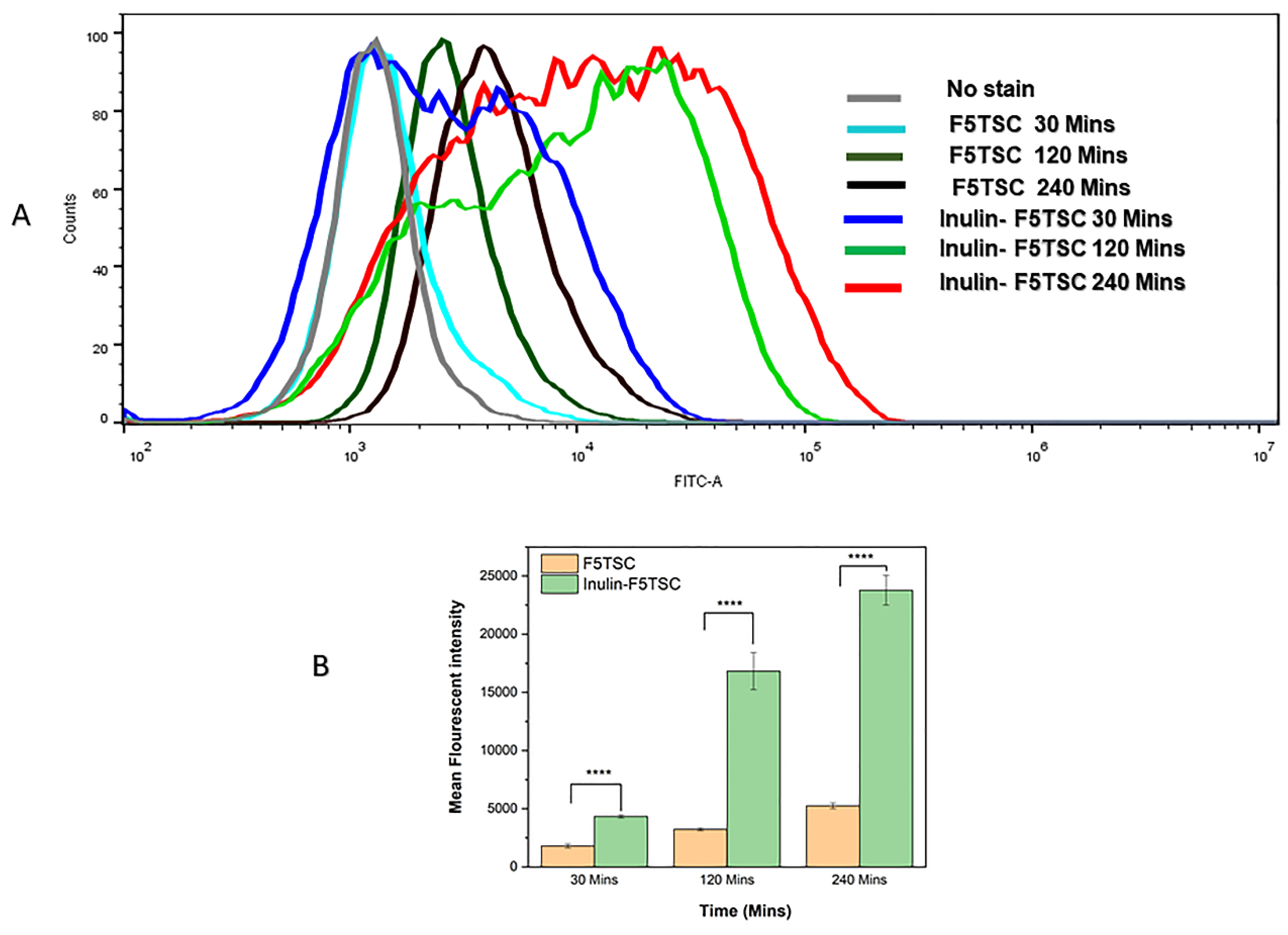
2.8. Flow Cytometry Measurements (FACS)
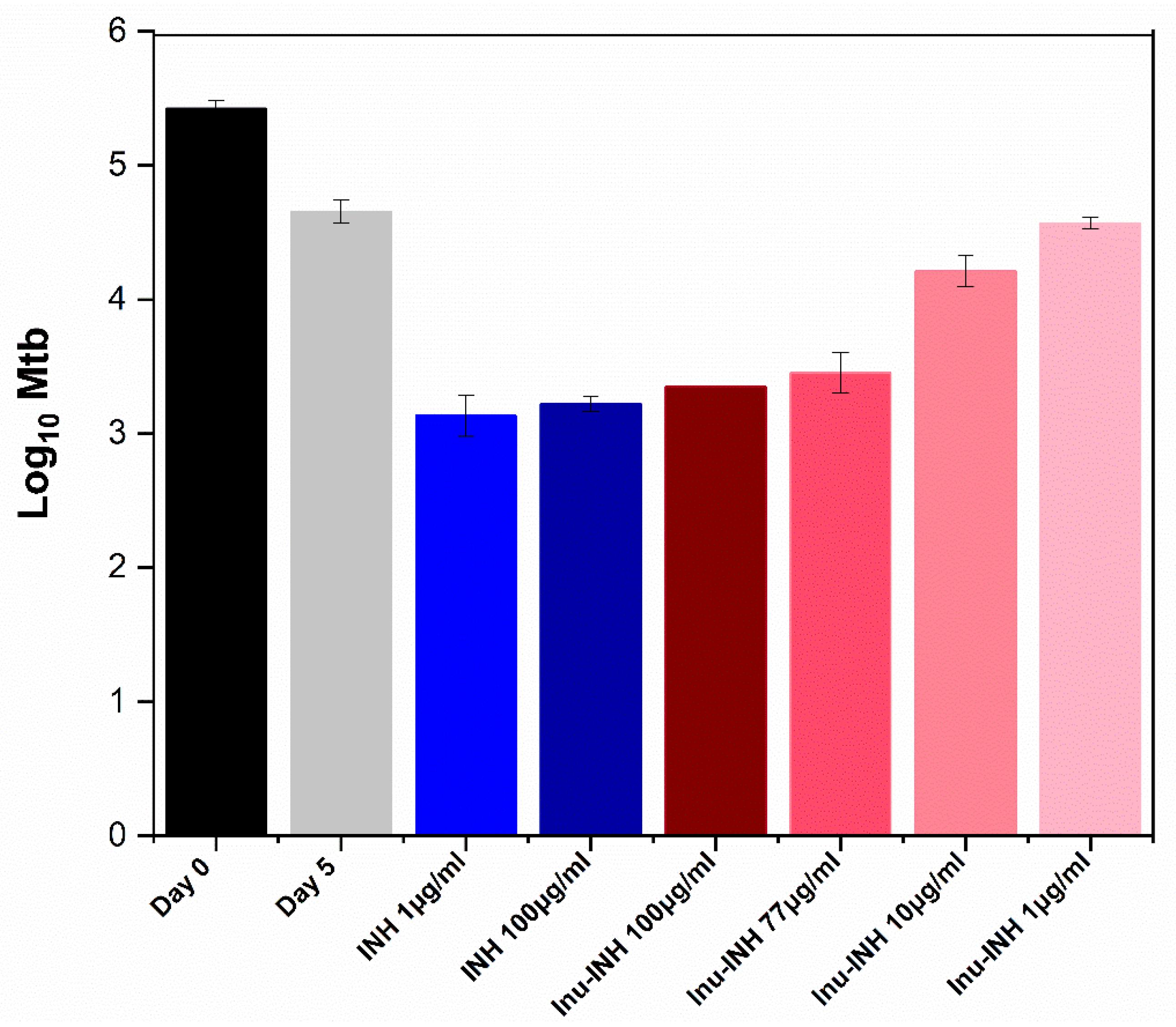
2.9. Intracellular Antibacterial Assay
3. Results and Discussion
3.1. Isoniazid Coupling to Delta Inulin Particles
3.2. Characterization of Inulin Isoniazid Conjugate Using Proton Nuclear Magnetic Resonance (1H NMR)
3.3. Effect of Oxidation Time and Reaction Parameter on Loading
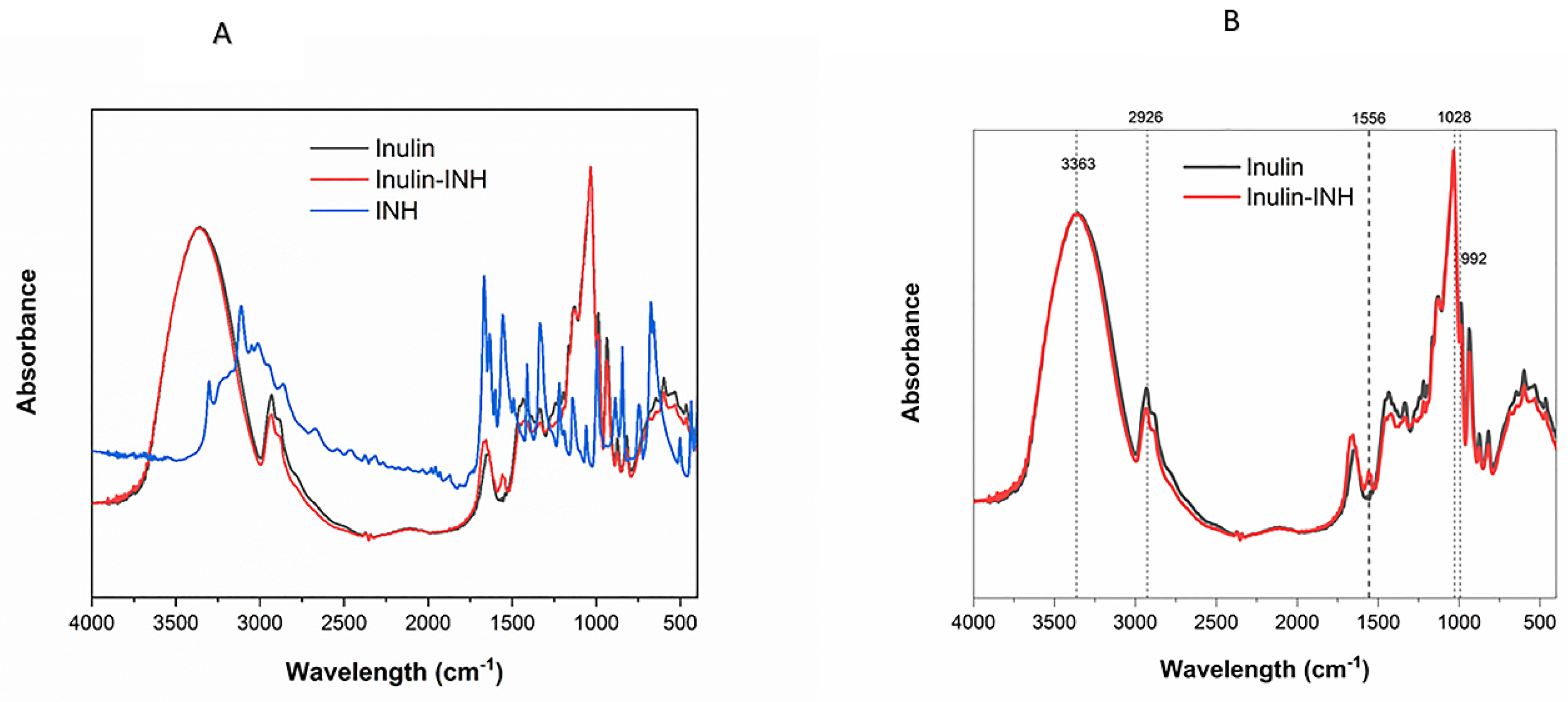
3.4. FTIR
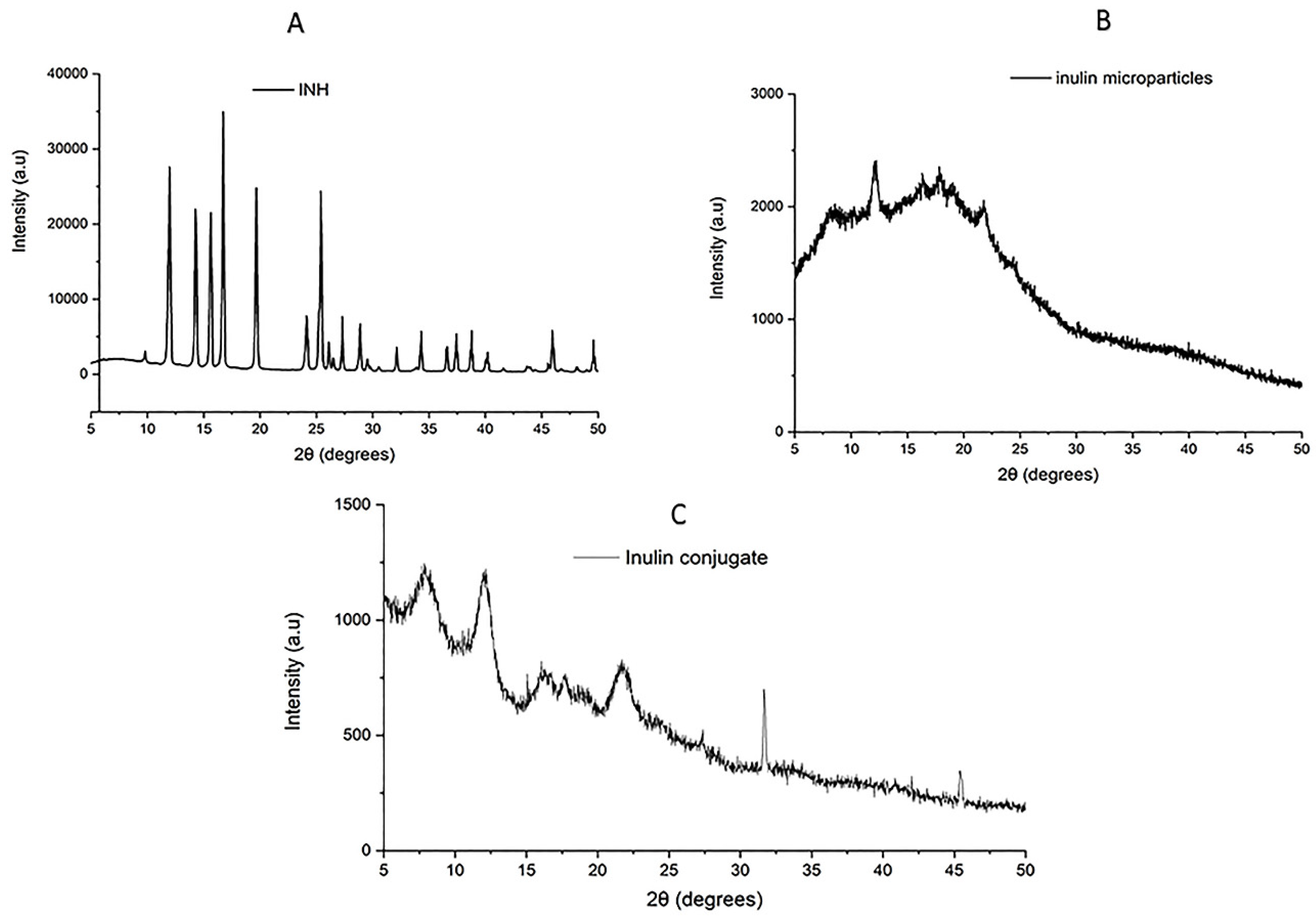
3.5. XRD of Inulin-INH Conjugate and Inulin Microparticles
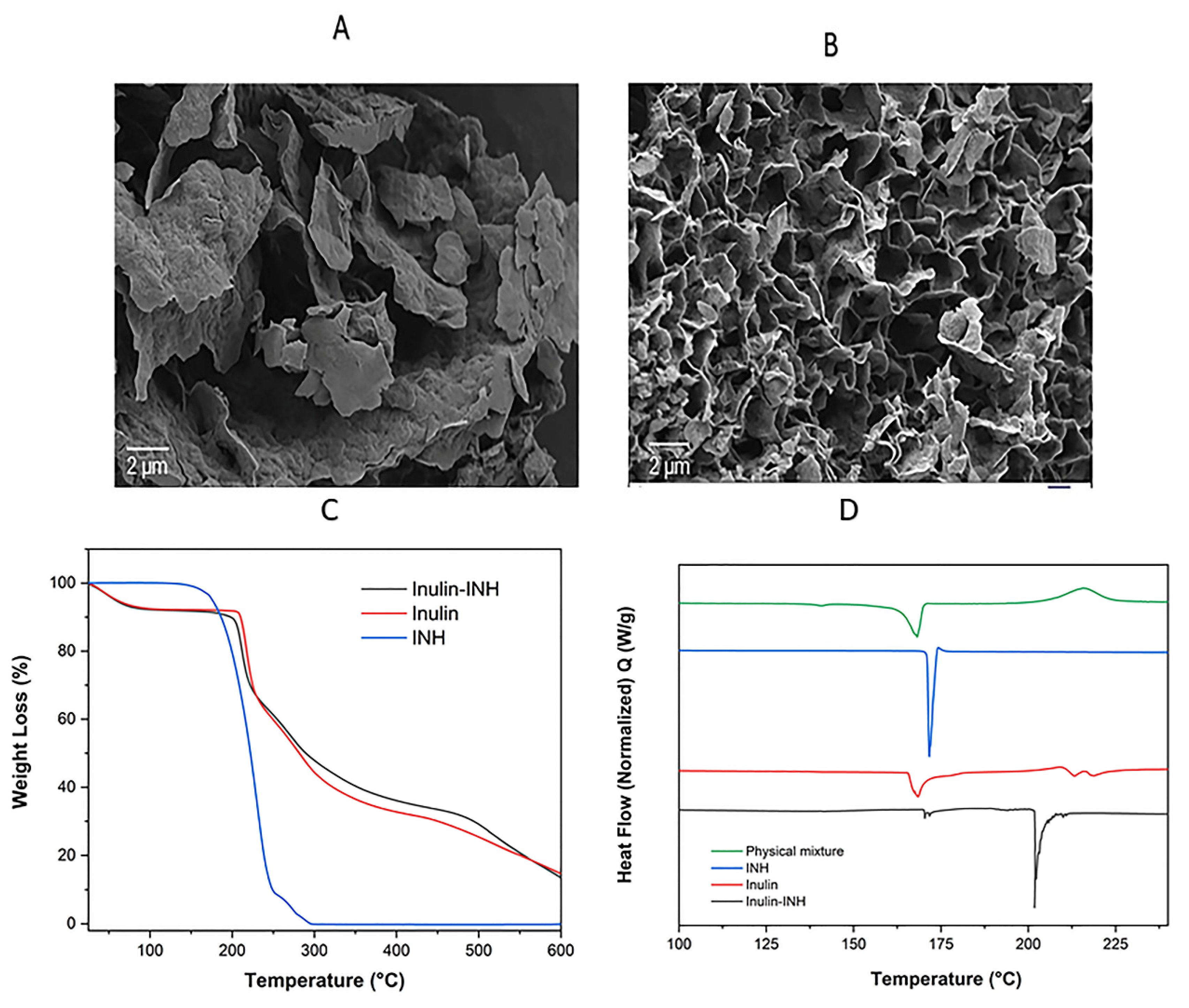
3.6. Scanning Electron Microscopy (SEM)
3.7. Zeta Potential
3.8. TGA
3.9. DSC
3.10. In Vitro pH-Controlled Drug Release
3.11. Cellular Uptake of INUF5TSC by RAW 264.7 Cells
3.12. Quantification of RAW 264.7 Cellular Uptake
3.13. Antimycobacterial Activity Against Infected Macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ndlovu, H.; Marakalala, M.J. Granulomas and Inflammation: Host-Directed Therapies for Tuberculosis. Front. Immunol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Global Tuberculosis Report 2018; World Health Organisation: Geneva, Switzerland, 2018. [Google Scholar]
- Palomino, J.C.; Martin, A. Drug Resistance Mechanisms in Mycobacterium tuberculosis. Antibiotics 2014, 3, 317–340. [Google Scholar] [CrossRef]
- Koch, A.; Cox, H.; Mizrahi, V. Drug-resistant tuberculosis: Challenges and opportunities for diagnosis and treatment. Curr. Opin. Pharmacol. 2018, 42, 7–15. [Google Scholar] [CrossRef]
- Singh, G.; Kesharwani, P.; Srivastava, A.K. Tuberculosis Treated by Multiple Drugs: An Overview. Curr. Drug Deliv. 2018, 15, 312–320. [Google Scholar] [CrossRef]
- Deribew, A.; Hailemichael, Y.; Tesfaye, M.; Desalegn, D.; Wogi, A.; Daba, S. The synergy between TB and HIV co-infection on perceived stigma in Ethiopia. BMC Res. Notes 2010, 3, 249. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.; Jansson, M.; Sköld, M.; Rottenberg, M.E.; Källenius, G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012, 8, e1002464. [Google Scholar] [CrossRef]
- Mayer, K.H.; Hamilton, C.D. Synergistic Pandemics: Confronting the Global HIV and Tuberculosis Epidemics. Clin. Infect. Dis. 2010, 50, S67–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arranz-Trullén, J.; Lu, L.; Pulido, D.; Bhakta, S.; Boix, E. Host Antimicrobial Peptides: The Promise of New Treatment Strategies against Tuberculosis. Front. Immunol. 2017, 8, 1499. [Google Scholar] [CrossRef] [PubMed]
- Peyron, P.; Vaubourgeix, J.; Poquet, Y.; Levillain, F.; Botanch, C.; Bardou, F.; Daffe, M.; Emile, J.F.; Marchou, B.; Cardona, P.J.; et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008, 4, e1000204. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMicking, J.D.; Taylor, G.A.; McKinney, J.D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 2003, 302, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell. Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Divangahi, M.; Remold, H.G. Evasion of innate immunity by Mycobacterium tuberculosis: Is death an exit strategy? Nat. Rev. Microbiol. 2010, 8, 668–674. [Google Scholar] [CrossRef] [PubMed]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74, ftw068. [Google Scholar] [CrossRef]
- Anisimova, Y.V.; Gelperina, S.I.; Peloquin, C.A.; Heifets, L.B. Nanoparticles as Antituberculosis Drugs Carriers: Effect on Activity Against Mycobacterium tuberculosis in Human Monocyte-Derived Macrophages. J. Nanopart. Res. 2000, 2, 165–171. [Google Scholar] [CrossRef]
- Kisich, K.O.; Gelperina, S.; Higgins, M.P.; Wilson, S.; Shipulo, E.; Oganesyan, E.; Heifets, L. Encapsulation of moxifloxacin within poly(butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. Int. J. Pharm. 2007, 345, 154–162. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Chaturvedi, A.P.; Tripathi, Y.B.; Mishra, B. Macrophage-specific targeting of isoniazid through mannosylated gelatin microspheres. Aaps Pharmscitech 2011, 12, 900–908. [Google Scholar] [CrossRef]
- Barrow, E.L.W.; Winchester, G.A.; Staas, J.K.; Quenelle, D.C.; Barrow, W.W. Use of microsphere technology for targeted delivery of rifampin to Mycobacterium tuberculosis-infected macrophages. Antimicrob. Agents Chemother. 1998, 42, 2682–2689. [Google Scholar] [CrossRef]
- Grossman, J.G.; Nywening, T.M.; Belt, B.; Ahlers, M.; Hawkins, W.G.; Strasberg, S.M.; Goedegebuure, P.S.; Linehan, D.; Fields, R.C. Targeting inflammatory monocytes in human metastatic colorectal cancer. J. Clin. Oncol. 2017, 35, 605. [Google Scholar] [CrossRef]
- Brown, J.M.; Recht, L.; Strober, S. The Promise of Targeting Macrophages in Cancer Therapy. Clin. Cancer Res. 2017, 23, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009, 6, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gegia, M.; Winters, N.; Benedetti, A.; van Soolingen, D.; Menzies, D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 223–234. [Google Scholar] [CrossRef]
- Quémard, A.; Lacave, C.; Lanéelle, G. Isoniazid inhibition of mycolic acid synthesis by cell extracts of sensitive and resistant strains of Mycobacterium aurum. Antimicrob. Agents Chemother. 1991, 35, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Nkanga, C.I.; Krause, R.W.M. Encapsulation of Isoniazid-conjugated Phthalocyanine-In-Cyclodextrin-In-Liposomes Using Heating Method. Sci. Rep. 2019, 9, 11485. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Krause, R.W.M. Conjugation of isoniazid to a zinc phthalocyanine via hydrazone linkage for pH-dependent liposomal controlled release. Appl. Nanosci. 2018, 8, 1313–1323. [Google Scholar] [CrossRef]
- Silva, M.; Lara, A.S.; Leite, C.Q.; Ferreira, E.I. Potential tuberculostatic agents: Micelle-forming copolymer poly(ethylene glycol)-poly(aspartic acid) prodrug with isoniazid. Arch. Der Pharm. 2001, 334, 189–193. [Google Scholar] [CrossRef]
- Tripodo, G.; Perteghella, S.; Grisoli, P.; Trapani, A.; Torre, M.L.; Mandracchia, D. Drug delivery of rifampicin by natural micelles based on inulin: Physicochemical properties, antibacterial activity and human macrophages uptake. Eur. J. Pharm. Biopharm. 2019, 136, 250–258. [Google Scholar] [CrossRef]
- Hwang, A.A.; Lee, B.Y.; Clemens, D.L.; Dillon, B.J.; Zink, J.I.; Horwitz, M.A. pH-Responsive Isoniazid-Loaded Nanoparticles Markedly Improve Tuberculosis Treatment in Mice. Small 2015, 11, 5066–5078. [Google Scholar] [CrossRef] [Green Version]
- Clemens, D.L.; Lee, B.Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef]
- Pandey, R.; Zahoor, A.; Sharma, S.; Khuller, G.K. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis 2003, 83, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kaur, I.P. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int. J. Pharm. 2013, 441, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Dutt, M.; Khuller, G.K. Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in Poly (dl-lactide-co-glycolide) microparticles. J. Antimicrob. Chemother. 2001, 47, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Singh, H. Nanostructured drug delivery for better management of tuberculosis. J. Control. Release 2014, 184, 36–50. [Google Scholar] [CrossRef]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Z.; Guo, W.; Wang, J.; Feng, Y.; Kong, X.; Hong, Z. Specifically targeted delivery of protein to phagocytic macrophages. Int. J. Nanomed. 2015, 10, 1743–1757. [Google Scholar]
- Jain, N.K.; Mishra, V.; Mehra, N.K. Targeted drug delivery to macrophages. Expert Opin. Drug Deliv. 2013, 10, 353–367. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Silva, D.G.; Cooper, P.D.; Petrovsky, N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol. Cell Biol. 2004, 82, 611–616. [Google Scholar] [CrossRef]
- Cooper, P.D.; Carter, M. Anti-complementary action of polymorphic “solubility forms” of particulate inulin. Mol. Immunol. 1986, 23, 895–901. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; McComb, C.; Steele, E.J. The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine 1991, 9, 408–415. [Google Scholar] [CrossRef]
- Cooper, P.D.; Carter, M. The anti-melanoma activity of inulin in mice. Mol. Immunol. 1986, 23, 903–908. [Google Scholar] [CrossRef]
- Murugappan, S.; Frijlink, H.W.; Petrovsky, N.; Hinrichs, W.L.J. Enhanced pulmonary immunization with aerosolized inactivated influenza vaccine containing delta inulin adjuvant. Eur. J. Pharm. Sci. 2015, 66, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Heddle, R.; Russo, P.; Petrovsky, N.; Hanna, R.; Smith, A. Immunotherapy–2076. A controlled study of delta inulin-adjuvanted honey bee venom immunotherapy. World Allergy Organ. J. 2013, 6, 158. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Song, Y.; Chung, R.; Wang, L.; Liu, L.; Hayball, J.D.; Petrovsky, N.; Garg, S. Design and Characterization of Inulin Conjugate for Improved Intracellular and Targeted Delivery of Pyrazinoic Acid to Monocytes. Pharmaceutics 2019, 11, 243. [Google Scholar] [CrossRef]
- Favrot, L.; Ronning, D.R. Targeting the mycobacterial envelope for tuberculosis drug development. Expert Rev. Anti-Infect. Ther. 2012, 10, 1023–1036. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Barclay, T.; Song, Y.; Joyce, P.; Sakala, I.G.; Petrovsky, N.; Garg, S. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine 2017, 35, 4382–4388. [Google Scholar] [CrossRef]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising β-d-[2 → 1] poly(fructo-furanosyl) α-d-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Barclay, T.G.; Ginic-Markovic, M.; Gerson, A.R.; Petrovsky, N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydr. Polym. 2014, 103, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Rajapaksha, H.; Thilagam, A.; Qian, G.; Ginic-Markovic, M.; Cooper, P.D.; Gerson, A.; Petrovsky, N. Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydr. Polym. 2016, 143, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkanga, C.; Walker, R.; Krause, R. pH-Dependent release of isoniazid from isonicotinic acid (4-hydroxy-benzylidene)-hydrazide loaded liposomes. J. Drug Deliv. Sci. Technol. 2018, 45, 264–271. [Google Scholar] [CrossRef]
- Marques, M.; Löbenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Schacht, E.; Vermeersch, J.; Vandoorne, F.; Vercauteren, R.; Remon, J.P. Synthesis and characterization of some modified polysaccharides containing drug moieties. J. Control. Release 1985, 2, 245–256. [Google Scholar] [CrossRef]
- Schacht, E.; Ruys, L.; Vermeersch, J.; Remon, J.P.; Duncan, R. Use of polysaccharides as drug carriers. Dextran and inulin derivatives of procainamide. Ann. N. Y. Acad. Sci. 1985, 446, 199–212. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In situ hydrogel constructed by starch-based nanoparticles via a Schiff base reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef]
- Keshk, S.M.; Ramadan, A.M.; Bondock, S. Physicochemical characterization of novel Schiff bases derived from developed bacterial cellulose 2,3-dialdehyde. Carbohydr. Polym. 2015, 127, 246–251. [Google Scholar] [CrossRef]
- Zuberbuhler, K.; Casi, G.; Bernardes, G.J.L.; Neri, D. Fucose-specific conjugation of hydrazide derivatives to a vascular-targeting monoclonal antibody in IgG format. Chem. Commun. 2012, 48, 7100–7102. [Google Scholar] [CrossRef]
- Berezin, A.S.; Skorik, Y.A. Chitosan-isoniazid conjugates: Synthesis, evaluation of tuberculostatic activity, biodegradability and toxicity. Carbohydr. Polym. 2015, 127, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.J.; Simões, P.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Maia, J.; Ferreira, L.; Carvalho, R.; Ramos, M.A.; Gil, M.H. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer 2005, 46, 9604–9614. [Google Scholar] [CrossRef] [Green Version]
- Cooper, P.D.; Barclay, T.G.; Ginic-Markovic, M.; Petrovsky, N. The polysaccharide inulin is characterized by an extensive series of periodic isoforms with varying biological actions. Glycobiology 2013, 23, 1164–1174. [Google Scholar] [CrossRef]
- Matthews, S.E.; Pouton, C.W.; Threadgill, M.D. Macromolecular systems for chemotherapy and magnetic resonance imaging. Adv. Drug Deliv. Rev. 1996, 18, 219–267. [Google Scholar] [CrossRef]
- Sodanapalli, R.; Nair, R.; Bachala, T. Preparation and Pharmaceutical Characterization of Supra molecular Complex of Isoniazid with L(+) Tartaric acid. J. Biomed. Sci. Res. 2011, 3, 397–402. [Google Scholar]
- Gunasekaran, S.; Sailatha, E.; Srinivasan, S.; Kumaresan, S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. IJPAP 2009, 47, 12–18. [Google Scholar]
- Zhao, C.; Liu, X.; Zhang, X.; Yan, H.; Qian, Z.; Li, X.; Ma, Z.; Han, Q.; Pei, C. A facile one-step method for preparation of Fe3O4/CS/INH nanoparticles as a targeted drug delivery for tuberculosis. Mater. Sci. Eng. C 2017, 77, 1182–1188. [Google Scholar] [CrossRef]
- Banik, N.; Iman, M.; Hussain, A.; Ramteke, A.; Boruah, R.; Maji, T.K. Soy flour nanoparticles for controlled drug delivery: Effect of crosslinker and montmorillonite (MMT). New J. Chem. 2013, 37, 3981–3990. [Google Scholar] [CrossRef]
- Fukuoka, E.; Makita, M.; Yamamura, S. Pattern Fitting Procedure for the Characterization of Crystals and/or Crystallities in Tablets. Chem. Pharm. Bull. 1993, 41, 2166–2171. [Google Scholar] [CrossRef]
- Zabot, G.L.; Silva, E.K.; Azevedo, V.M.; Meireles, M.A.A. Replacing modified starch by inulin as prebiotic encapsulant matrix of lipophilic bioactive compounds. Food Res. Int. 2016, 85, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.W.; Kim, M.S.; Kim, J.S.; Park, H.J.; Lee, S.; Woo, J.S.; Hwang, S.J. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.; Vemuri, K.; Venkateswara Swamy, K.; Khan, E.M.; Sritharan, M.; Padhye, S. Synthesis, characterization, molecular docking and anti-tubercular activity of Plumbagin–Isoniazid Analog and its β-cyclodextrin conjugate. Bioorganic Med. Chem. Lett. 2014, 24, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Cassano, R.; Valenti, D.; Trombino, S.; Ferrarelli, T.; Picci, N.; Fadda, A.M.; Manconi, M. Isoniazid-gelatin conjugate microparticles containing rifampicin for the treatment of tuberculosis. J. Pharm. Pharmacol. 2013, 65, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, M.; Sadighian, S.; Rostamizadeh, K.; Khoeini, F.; Naghibi, M.; Bayat, N.; Habibizadeh, M.; Hamidi, M. Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. Bioimpacts 2015, 5, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and characterization of isoniazid-loaded crude soybean lecithin liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [CrossRef]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemical studies on the biopolymer inulin: A critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009, 91, 687–699. [Google Scholar] [CrossRef]
- Swapna, B.; Maddileti, D.; Nangia, A. Cocrystals of the Tuberculosis Drug Isoniazid: Polymorphism, Isostructurality, and Stability. Cryst. Growth Des. 2014, 14, 5991–6005. [Google Scholar] [CrossRef]
- Aryal, S.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Doxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriers. J. Mater. Chem. 2009, 19, 7879–7884. [Google Scholar] [CrossRef]
- Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenço, J.P.; Rosa da Costa, A.M.; Grenha, A. Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules 2016, 21, 702. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Biggs, D.L.; Manning, M.C.; Randolph, T.W.; Christians, U.; Hybertson, B.M.; Ng, K.-Y. Microparticle-based lung delivery of INH decreases INH metabolism and targets alveolar macrophages. J. Control. Release 2005, 107, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Ankur, B.; Anne, G.; Goutam, R.; Amit Kumar, G.; Amit Kumar, J.; Abhinav, M. Pulmonary Delivery of Anti-Tubercular Drugs Using Ligand Anchored pH Sensitive Liposomes for the Treatment of Pulmonary Tuberculosis. Curr. Drug Deliv. 2016, 13, 909–922. [Google Scholar]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.M.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable Fucoidan Microparticles Combining Two Antitubercular Drugs with Potential Application in Pulmonary Tuberculosis Therapy. Polymers 2018, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Edagwa, B.J.; Guo, D.; Puligujja, P.; Chen, H.; McMillan, J.; Liu, X.; Gendelman, H.E.; Narayanasamy, P. Long-acting antituberculous therapeutic nanoparticles target macrophage endosomes. FASEB J 2014, 28, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef]
- Jayaram, R.; Shandil, R.K.; Gaonkar, S.; Kaur, P.; Suresh, B.L.; Mahesh, B.N.; Jayashree, R.; Nandi, V.; Bharath, S.; Kantharaj, E.; et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 2004, 48, 2951–2957. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Chung, R.; Song, Y.; Nagalingam, G.; Triccas, J.; Wang, L.; Liu, L.; Hayball, J.D.; et al. Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery. Pharmaceutics 2019, 11, 555. https://doi.org/10.3390/pharmaceutics11110555
Afinjuomo F, Barclay TG, Parikh A, Chung R, Song Y, Nagalingam G, Triccas J, Wang L, Liu L, Hayball JD, et al. Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery. Pharmaceutics. 2019; 11(11):555. https://doi.org/10.3390/pharmaceutics11110555
Chicago/Turabian StyleAfinjuomo, Franklin, Thomas G. Barclay, Ankit Parikh, Rosa Chung, Yunmei Song, Gayathri Nagalingam, Jamie Triccas, Lixin Wang, Liang Liu, John D. Hayball, and et al. 2019. "Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery" Pharmaceutics 11, no. 11: 555. https://doi.org/10.3390/pharmaceutics11110555
APA StyleAfinjuomo, F., Barclay, T. G., Parikh, A., Chung, R., Song, Y., Nagalingam, G., Triccas, J., Wang, L., Liu, L., Hayball, J. D., Petrovsky, N., & Garg, S. (2019). Synthesis and Characterization of pH-Sensitive Inulin Conjugate of Isoniazid for Monocyte-Targeted Delivery. Pharmaceutics, 11(11), 555. https://doi.org/10.3390/pharmaceutics11110555