Iontophoretic Drug Delivery in the Oral Cavity
Abstract
1. Introduction
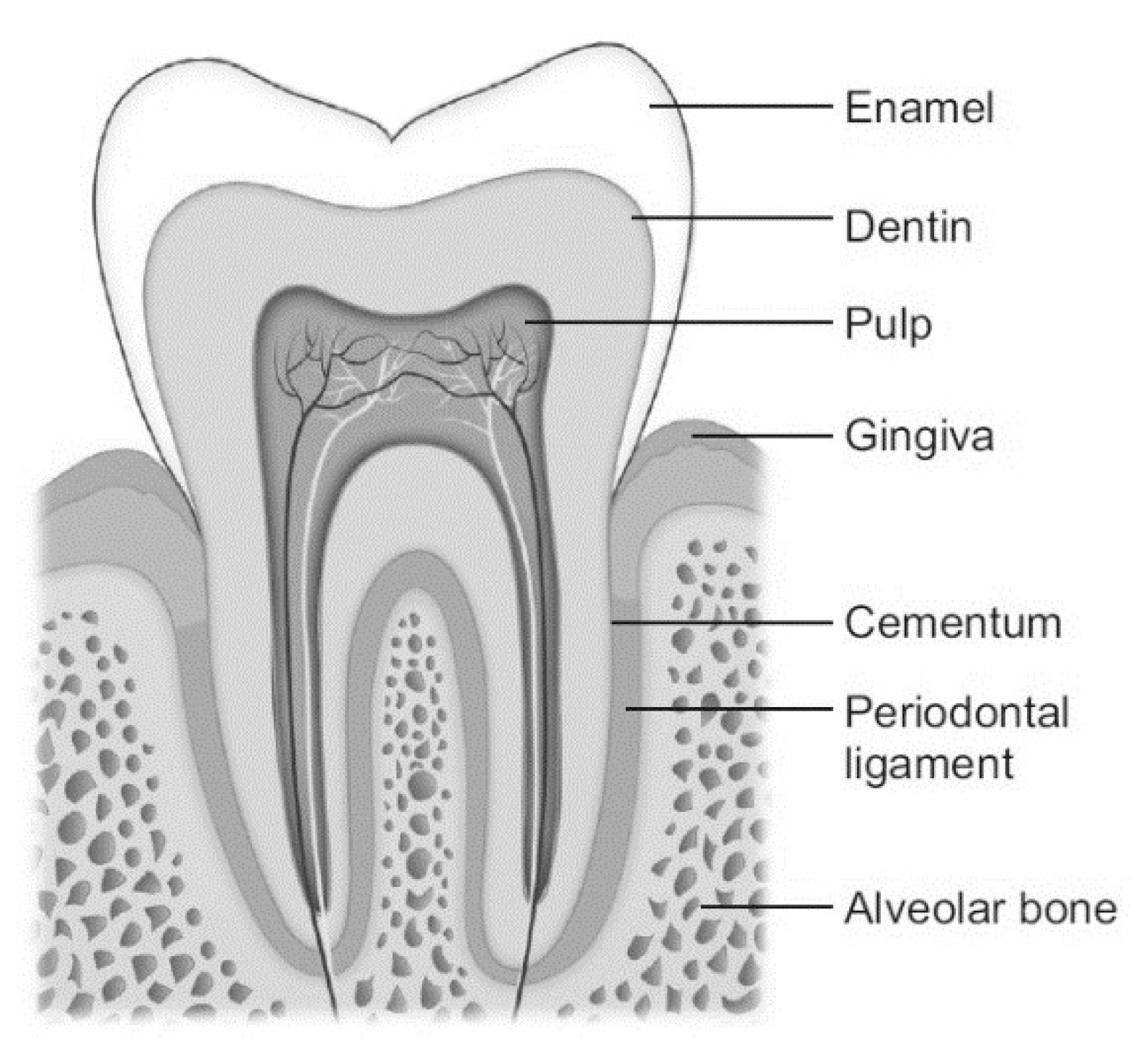
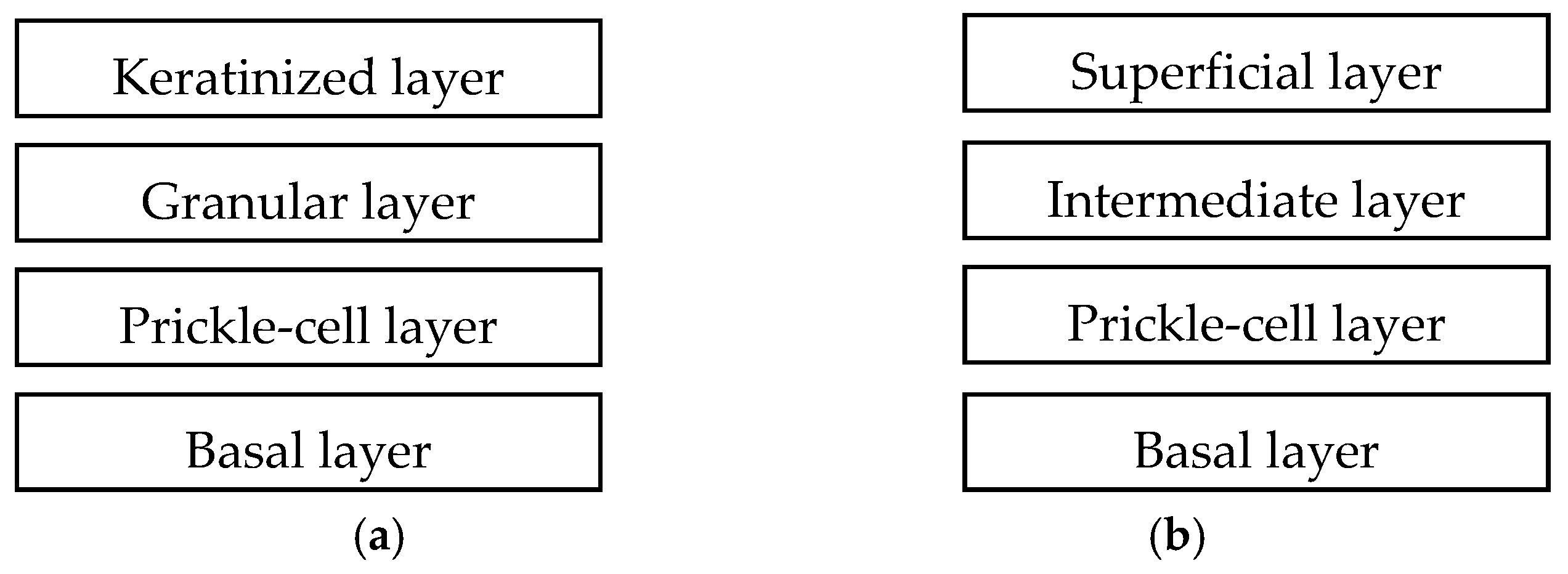
2. Anatomy
3. Oral Diseases
4. Systemic Drug Delivery in the Oral Cavity
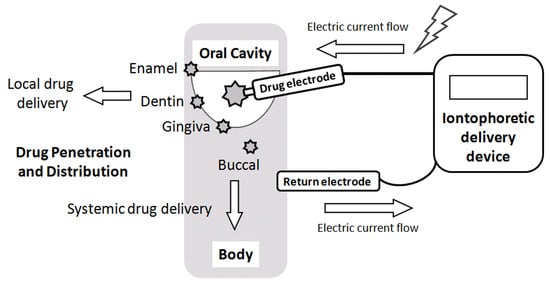
5. Local Drug Delivery in the Oral Cavity
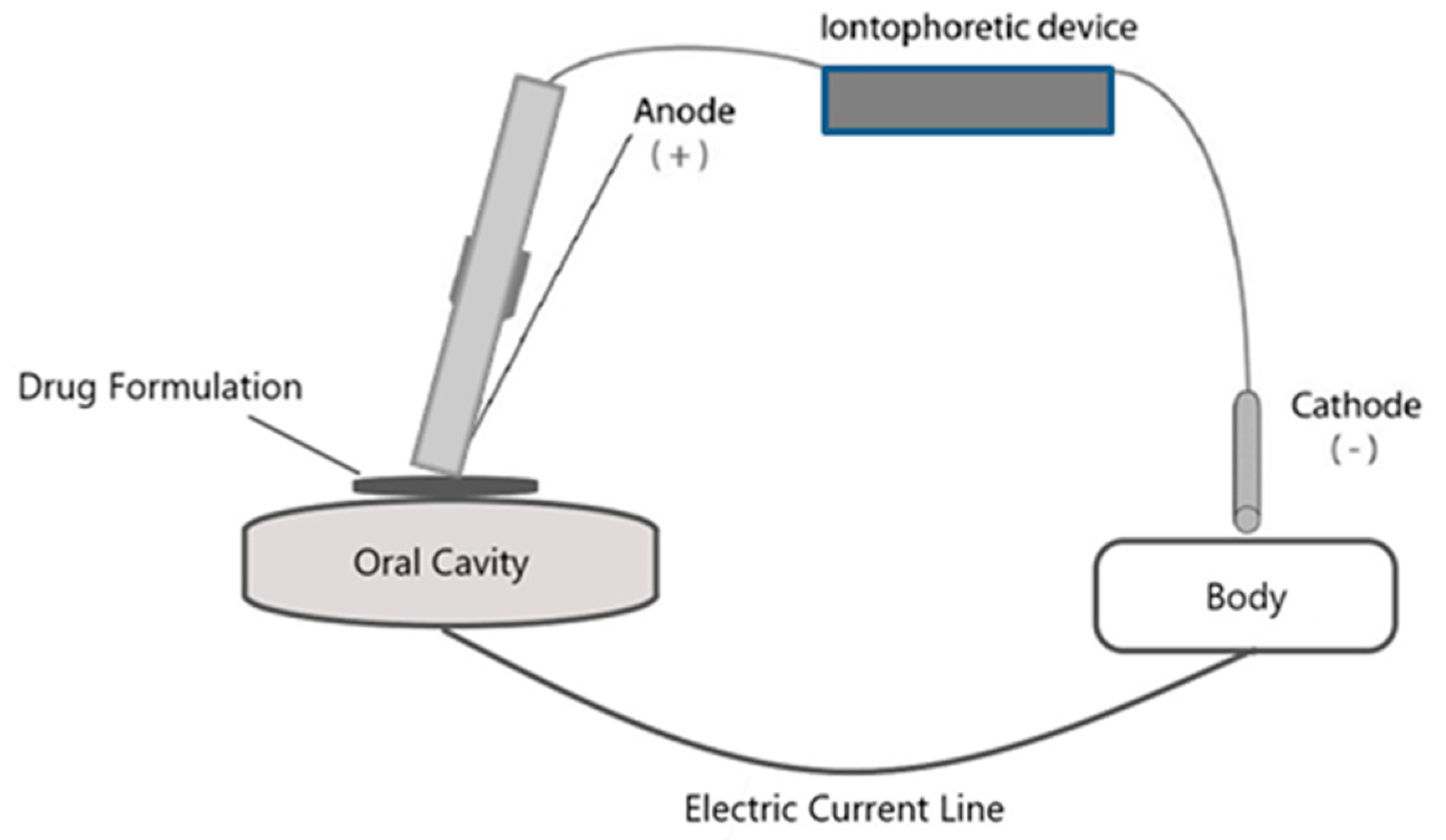
6. Iontophoretic Drug Delivery
7. Iontophoresis on Oral Mucosa
7.1. Buccal Iontophoresis
7.2. Palate Iontophoresis
8. Iontophoresis on Enamel and Dentin
9. Conclusions: Challenges and Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Laine, F.J.; Smoker, W.R.K. Oral cavity: Anatomy and pathology. Semin Ultrasound CT MR 1995, 16, 527–545. [Google Scholar] [CrossRef]
- Dawes, C. What is the critical pH and why does a tooth dissolve in acid? J. Can. Dent. Assoc. 2003, 69, 722–724. [Google Scholar] [PubMed]
- Vongsavan, N.; Matthews, B. Fluid flow through cat dentine in vivo. Arch. Oral Biol. 1992, 37, 175–185. [Google Scholar] [CrossRef]
- Husain, M.A. Dental anatomy and nomenclature for the radiologist. Radiol. Clin. N. Am. 2018, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.; Berardi, T.; Stoopler, E.T. Anatomic and examination considerations of the oral cavity. Med. Clin. N. Am. 2014, 98, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Banthitkhunanon, P.; Chintakanan, S.; Wanachantararak, S.; Vongsavan, N.; Matthews, B. Effects of enamel and dentine thickness on laser doppler blood-flow signals recorded from the underlying pulp cavity in human teeth in vitro. Arch. Oral Biol. 2013, 58, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.B.; Robinson, J.R. Oral cavity as a site for bioadhesive drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 43–74. [Google Scholar] [CrossRef]
- Sattar, M.; Sayed, O.M.; Lane, M.E. Oral transmucosal drug delivery—Current status and future prospects. Int. J. Pharm. 2014, 471, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Kremer, M.J. Biology of oral mucosa and esophagus. JNCI Monogr. 2001, 29, 7–15. [Google Scholar] [CrossRef]
- Squier, C.A. The permeability of oral mucosa. Crit. Rev. Oral Biol. Med. 1991, 2, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.A.; Rooney, L. The permeability of keratinized and nonkeratinized oral epithelium to lanthanum in vivo. J. Ultrastruct Res. 1976, 54, 286–295. [Google Scholar] [CrossRef]
- Squier, C.; Brogden, K.A. Regional differences in the oral mucosa. In Human Oral Mucosa; John Wiley & Sons: West Sussex, UK, 2013; pp. 77–98. [Google Scholar]
- Claffey, N.; Shanley, D. Relationship of gingival thickness and bleeding to loss of probing attachment in shallow sites following nonsurgical periodontal therapy. J. Clin. Periodontol. 1986, 13, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.L.; Savitha, B. Thickness of gingiva in association with age, gender and dental arch location. J. Clin. Periodontol. 2005, 32, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Cox, P.S.; Squier, C.A.; Downing, D.T. Lipids of epidermis and keratinized and non-keratinized oral epithelia. Comp. Biochem. Physiol. B 1986, 83, 529–531. [Google Scholar] [CrossRef]
- Cate, J.M.; Arends, J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977, 11, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.V.; Drake, D.R.; Hill, J.R.; Brogden, K.A.; Fischer, C.L.; Wertz, P.W. Organization, barrier function and antimicrobial lipids of the oral mucosa. Int. J. Cosmet. Sci. 2013, 35, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.F.H. Biophysical kinetic modeling of buccal absorption. Adv. Drug Deliv. Rev. 1993, 12, 61–97. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Modeling the oral cavity: In vitro and in vivo evaluations of buccal drug delivery systems. J. Control. Release 2012, 161, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Churchman, S.T.; Cruchley, A.T.; Braden, M.; Williams, D.M. Electrically induced transport of macromolecules through oral buccal mucosa. Dent. Mater. 2013, 29, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Ikinci, G.; Kas, S.; Yousefi-Rad, A.; Sargon, M.F.; Hincal, A.A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm 2000, 193, 197–203. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Reed, B.L.; Finnin, B.C. Buccal penetration enhancers—How do they really work? J. Control. Release 2005, 105, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Franco Neto, C.A.; Parolo, C.C.; Rosing, C.K.; Maltz, M. Comparative analysis of the effect of two chlorhexidine mouthrinses on plaque accumulation and gingival bleeding. Braz. Oral. Res. 2008, 22, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Demke, R. Plaque inhibition: The science and application of oral rinses. Dent. Today 2012, 31, 96–101. [Google Scholar] [PubMed]
- Franz-Montan, M.; Ribeiro, L.N.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; de Araujo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, R.; Vandana, K.L.; Prakash, S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J. Indian Soc. Periodontol. 2011, 15, 304–309. [Google Scholar] [PubMed]
- Matesanz-Perez, P.; Garcia-Gargallo, M.; Figuero, E.; Bascones-Martinez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Renugalakshmi, A.; Vinothkumar, T.S.; Kandaswamy, D. Nanodrug delivery systems in dentistry: A review on current status and future perspectives. Curr. Drug Deliv. 2011, 8, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Kasting, G.B. Theoretical models for iontophoretic delivery. Adv. Drug Deliv. Rev. 1992, 9, 177–199. [Google Scholar] [CrossRef]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Costello, C.T.; Jeske, A.H. Iontophoresis: Applications in transdermal medication delivery. Phys. Ther. 1995, 75, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Hao, J.; Liddell, M.R. Electrotransport across membranes in biological media: Electrokinetic theories and applications in drug delivery. In Transport in Biological Media, 1st ed.; Becker, S., Kuznetsov, A., Eds.; Elsevier: Philadelphia, PA, USA, 2013; Chapter 11. [Google Scholar]
- Oliashirazi, A.; Wilson-Byrne, T.; Shuler, F.D.; Parvizi, J. Patient-controlled fentanyl iontophoretic transdermal system improved postoperative mobility compared to intravenous patient-controlled analgesia morphine: A pooled analysis of randomized, controlled trials. Pain Pract. 2017, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, E.R.; Ding, L.; Itri, L.M. The efficacy and safety of the fentanyl iontophoretic transdermal system (IONSYS®) in the geriatric population: Results of a meta-analysis of phase III and IIIb trials. Drugs Aging 2016, 33, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J. Sumatriptan iontophoretic transdermal system: A novel approach to migraine-specific therapy. Neurol. Clin. Pract. 2014, 4, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Tepper, D.E. Sumatriptan iontophoretic patch for migraine. Headache 2014, 54, 1563–1564. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Ammann, R.A.; Metlagel, A.; Schoeni, M.H.; Swiss Paediatric Respiratory Research Group. Conductivity determined by a new sweat analyzer compared with chloride concentrations for the diagnosis of cystic fibrosis. J. Pediatr. 2005, 146, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.B.; Turcios, N.L.; Gibson, L.E. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J. Pediatr. 1994, 124, 255–260. [Google Scholar] [CrossRef]
- Dixit, N.; Bali, V.; Baboota, S.; Ahuja, A.; Ali, J. Iontophoresis—An approach for controlled drug delivery: A review. Curr. Drug Deliv. 2007, 4, 1–10. [Google Scholar] [PubMed]
- Fischer, G.A. Iontophoretic drug delivery using the iomed phoresor system. Expert Opin. Drug Deliv. 2005, 2, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Syms, C.A., 3rd; Grantham, M.L. Otologic iontophoresis: A no-papoose technique. Ann. Otol. Rhinol. Laryngol. 2013, 122, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Comeau, M.; Brummett, R. Anesthesia of the human tympanic membrane by iontophoresis of a local anesthetic. Laryngoscope 1978, 88, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.J.; Tamada, J.A.; Potts, R.O.; Jovanovic, L.; Garg, S.; Cygnus Research Team. Clinical evaluation of the glucowatch biographer: A continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001, 16, 621–629. [Google Scholar] [CrossRef]
- Cohen, A.E.; Assang, C.; Patane, M.A.; From, S.; Korenfeld, M.; Avion Study, I. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology 2012, 119, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Halhal, M.; Renard, G.; Courtois, Y.; BenEzra, D.; Behar-Cohen, F. Iontophoresis: From the lab to the bed side. Exp. Eye Res. 2004, 78, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.D.; Lauber, C.A.; Cappaert, T. The effect of dexamethasone iontophoresis on decreasing pain and improving function in patients with musculoskeletal conditions. J. Sport Rehabil. 2015, 24, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Ballard, A. Iontophoresis for palmar and plantar hyperhidrosis. Dermatol. Clin. 2014, 32, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, T.M.; Ferguson, E.; Febbraro, S.; Bakhtyari, A.; King, M.; Mundasad, M. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 2003, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Patane, M.A.; Cohen, A.; From, S.; Torkildsen, G.; Welch, D.; Ousler, G.W., 3rd. Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: Results of a randomized clinical trial. Clin. Ophthalmol. 2011, 5, 633–643. [Google Scholar] [PubMed]
- Siah, T.W.; Hampton, P.J. The effectiveness of tap water iontophoresis for palmoplantar hyperhidrosis using a monday, wednesday, and friday treatment regime. Dermatol. Online J. 2013, 19, 14. [Google Scholar] [PubMed]
- Dagash, H.; McCaffrey, S.; Mellor, K.; Roycroft, A.; Helbling, I. Tap water iontophoresis in the treatment of pediatric hyperhidrosis. J. Pediatr. Surg. 2017, 52, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Brown, J.; Vafaie, J.; Schwartz, R.A. Palmoplantar hyperhidrosis: A therapeutic challenge. Am. Fam. Physician 2004, 69, 1117–1120. [Google Scholar]
- Gratieri, T.; Kalia, Y.N. Mathematical models to describe iontophoretic transport in vitro and in vivo and the effect of current application on the skin barrier. Adv. Drug Deliv. Rev. 2013, 65, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.K.; Zhang, Y.; Zhu, H.; Higuchi, W.I.; White, H.S. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J. Pharm. Sci. 2005, 94, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Mudry, B.; Carrupt, P.A.; Guy, R.H.; Delgado-Charro, M.B. Quantitative structure-permeation relationship for iontophoretic transport across the skin. J. Control. Release 2007, 122, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Smitayothin, T.L.; Vongsavan, K.; Rirattanapong, P.; Kraivaphan, P.; Vongsavan, N.; Matthews, B. The iontophoresis of lignocaine with epinephrine into carious dentine for pain control during cavity preparation in human molars. Arch. Oral Biol. 2015, 60, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Moscicka-Studzinska, A.; Kijenska, E.; Ciach, T. Electroosmotic flow as a result of buccal iontophoresis—Buccal mucosa properties. Eur. J. Pharm. Biopharm. 2009, 72, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Giannola, L.I.; Florena, A.M.; De Caro, V.; Schumacher, A.; Gottsche, T.; Paderni, C.; Wolff, A. Bioavailability in vivo of naltrexone following transbuccal administration by an electronically-controlled intraoral device: A trial on pigs. J. Control. Release 2010, 145, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Paderni, C.; Campisi, G.; Schumacher, A.; Gottsche, T.; Giannola, L.I.; De Caro, V.; Wolff, A. Controlled delivery of naltrexone by an intraoral device: In vivo study on human subjects. Int. J. Pharm. 2013, 452, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cubayachi, C.; Couto, R.O.; de Gaitani, C.M.; Pedrazzi, V.; Freitas, O.; Lopez, R.F. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Telo, I.; Tratta, E.; Guasconi, B.; Nicoli, S.; Pescina, S.; Govoni, P.; Santi, P.; Padula, C. In-vitro characterization of buccal iontophoresis: The case of sumatriptan succinate. Int. J. Pharm. 2016, 506, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J. Buccal iontophoretic delivery of atenolol.HCl employing a new in vitro three-chamber permeation cell. J. Control. Release 2001, 70, 83–95. [Google Scholar] [CrossRef]
- Moscicka-Studzinska, A.; Czarnecka, K.; Ciach, T. Electrically enhanced and controlled drug delivery through buccal mucosa. Acta Pol. Pharm. 2008, 65, 767–769. [Google Scholar] [PubMed]
- Gratieri, T.; Kalia, Y.N. Targeted local simultaneous iontophoresis of chemotherapeutics for topical therapy of head and neck cancers. Int. J. Pharm. 2014, 460, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Churchman, S.T.; Cruchley, A.T.; Braden, M.; Williams, D.M. Delivery of macromolecules across oral mucosa from polymeric hydrogels is enhanced by electrophoresis (iontophoresis). Dent. Mater. 2013, 29, e299–e307. [Google Scholar] [CrossRef] [PubMed]
- Ciach, T.; Moscicka-Studzinska, A. Buccal iontophoresis: An opportunity for drug delivery and metabolite monitoring. Drug Discov. Today 2011, 16, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Baig, A.; White, D.J.; Li, S.K. Characterization of cornified oral mucosa for iontophoretically enhanced delivery of chlorhexidine. Eur. J. Pharm. Biopharm. 2016, 99, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Silva, S.M.; Damaj, B.B.; Martin, R.; Michniak-Kohn, B.B. Transdermal and transbuccal drug delivery systems: Enhancement using iontophoretic and chemical approaches. Int. J. Pharm. 2011, 421, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Chinnapareddy, S. Novel Biomedical Approaches in Health Monitoring and Oral Care. Ph.D. Thesis, Clarkson Univeristy, Potsdam, NY, USA, 2012. [Google Scholar]
- Ikeda, H.; Suda, H. Facilitatory effect of ac-iontophoresis of lidocaine hydrochloride on the permeability of human enamel and dentine in extracted teeth. Arch. Oral Biol. 2013, 58, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Simon, L.; Hu, L.; Michniak-Kohn, B. Effects of iontophoresis and chemical enhancers on the transport of lidocaine and nicotine across the oral mucosa. Pharm. Res. 2012, 29, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Thongkukiatkun, W.; Vongsavan, K.; Kraivaphan, P.; Rirattanapong, P.; Vongsavan, N.; Matthews, B. Effects of the iontophoresis of lignocaine with epinephrine into exposed dentine on the sensitivity of the dentine in man. Arch. Oral Biol. 2015, 60, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Puapichartdumrong, P.; Ikeda, H.; Suda, H. Facilitation of iontophoretic drug delivery through intact and caries-affected dentine. Int. Endod. J. 2003, 36, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Damaj, B.B.; Martin, R.; Michniak-Kohn, B.B. Enhanced in vitro transbuccal drug delivery of ondansetron HCL. Int. J. Pharm. 2011, 404, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Chun, K.H.; Jeon, S.O.; Kang, J.W.; Lee, S. Enhanced transbuccal salmon calcitonin (sCT) delivery: Effect of chemical enhancers and electrical assistance on in vitro sCT buccal permeation. Eur. J. Pharm. Biopharm. 2011, 79, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Kim, M.J.; Jeon, S.O.; Seo, J.E.; Jeong, S.H.; Kang, J.W.; Choi, Y.W.; Lee, S. Strategic approaches for enhancement of in vivo transbuccal peptide drug delivery in rabbits using iontophoresis and chemical enhancers. Pharm. Res. 2015, 32, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Kwon, H.K.; Kim, B.I. Application of fluoride iontophoresis to improve remineralization. J. Oral Rehabil. 2009, 36, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.E.; Kim, B.I. Can the application of fluoride iontophoresis improve remineralisation of early caries lesions? Oral Health Prev. Dent. 2016, 14, 177–182. [Google Scholar] [PubMed]
- Lee, Y.E.; Baek, H.J.; Choi, Y.H.; Jeong, S.H.; Park, Y.D.; Song, K.B. Comparison of remineralization effect of three topical fluoride regimens on enamel initial carious lesions. J. Dent. 2010, 38, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Saito, A.; Watanabe, K.; Saeki, K.; Nakashima, H.; Maki, K. Preventive effects of iontophoresis on bovine enamel decalcification through enhancing uptake and transportation of fluoride—In vitro study. Pediatr. Dent. J. 2018, 28, 103–109. [Google Scholar] [CrossRef]
- Ren, W.; Baig, A.; Li, S.K. Passive and iontophoretic transport of fluorides across enamel in vitro. J. Pharm. Sci. 2014, 103, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Weil, T.M. Reduction of enamel acid solubility with electrophoretic fluoride applications. J. Dent. Res. 1966, 45, 1563. [Google Scholar] [CrossRef] [PubMed]
- Barbakow, F.H.; van der Merwe, E.H.M.; Scanes, S.G.; Retief, D.H.; Fatti, L.P. An in vivo evaluation of an iontophoretic technique for increasing the surface fluoride content in enamel. J. Dent. Assoc. S. Afr. 1973, 28, 624–629. [Google Scholar]
- Patil, A.R.; Varma, S.; Suragimath, G.; Abbayya, K.; Zope, S.A.; Kale, V. Comparative evaluation of efficacy of iontophoresis with 0.33% sodium fluoride gel and diode laser alone on occlusion of dentinal tubules. J. Clin. Diagn. Res. 2017, 11, ZC123–ZC126. [Google Scholar] [CrossRef] [PubMed]
- Stowell, E.C.; Taylor, J.B. Influence of electrical potential on ion migration in teeth. 2. Quantitative measurements of I-131 penetration by an acid-leaching technique. J. Dent. Res. 1964, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Fait, D.; Gedalia, I.; Hermel, J.; Weinman, J. The fluoride uptake by the enamel of human teeth “in vitro” after immersion in 2 per cent NaF solution with iontophoresis. Refuat Hapeh Vehashinayim 1970, 19, 7–8. [Google Scholar] [PubMed]
- Call-Smith, K.; Gangarosa, L.P., Sr. Iontophoresis: A new approach to some old problems. New Dent. 1979, 10, 20–22. [Google Scholar] [PubMed]
- Bechtold, A.W. Iontophoretic Toothbrush. U.S. Patent 3,520,297, 14 July 1970. [Google Scholar]
- Kanai, M. Device for Penetrating Teeth with Fluoride. U.S. Patent 2,834,344, 13 May 1958. [Google Scholar]
- Gangarosa, L.P. Iontophoresis in Dental Practice, 1st ed.; Quintessence: Chicago, IL, USA, 1983. [Google Scholar]
- Aparna, S.; Setty, S.; Thakur, S. Comparative efficacy of two treatment modalities for dentinal hypersensitivity: A clinical trial. Indian J. Dent. Res. 2010, 21, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pandit, I.K.; Srivastava, N.; Gugnani, N. Comparative evaluation of 2% sodium fluoride iontophoresis and other cavity liners beneath silver amalgam restorations. J. Indian Soc. Pedod. Prev. Dent. 2010, 28, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Gangarosa, L.P.; Park, N.H. Practical considerations in iontophoresis of fluoride for desensitizing dentin. J. Prosthet. Dent. 1978, 39, 173–178. [Google Scholar] [CrossRef]
- Carlo, G.T.; Ciancio, S.G.; Seyrek, S.K. An evaluation of iontophoretic application of fluoride for tooth desensitization. J. Am. Dent. Assoc. 1982, 105, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Gangarosa, L.P.S. Fluoride iontophoresis for tooth desensitization. J. Am. Dent. Assoc. 1986, 112, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Gangarosa, L.P. Current strategies for dentist-applied treatment in the management of hypersensitive dentine. Arch. Oral Biol. 1994, 39, S101–S106. [Google Scholar] [CrossRef]
- Gergova, R.T.; Gueorgieva, T.; Dencheva-Garova, M.S.; Krasteva-Panova, A.Z.; Kalchinov, V.; Mitov, I.; Kamenoff, J. Antimicrobial activity of different disinfection methods against biofilms in root canals. J. Investig. Clin. Dent. 2016, 7, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pucher, J.J.; Lamendola-Sitenga, K.; Ferguson, D.; Van Swoll, R. The effectiveness of an ionic toothbrush in the removal of dental plaque and reduction on gingivitis in orthodontic patients. J. West. Soc. Periodontol. Periodontal Abstr. 1999, 47, 101–107. [Google Scholar] [PubMed]
- Gaberšček, M.; Klemenc, F. Electrophoretic efficiency of an ionic toothbrush. Acta Chim. Slov. 2006, 53, 521–526. [Google Scholar]
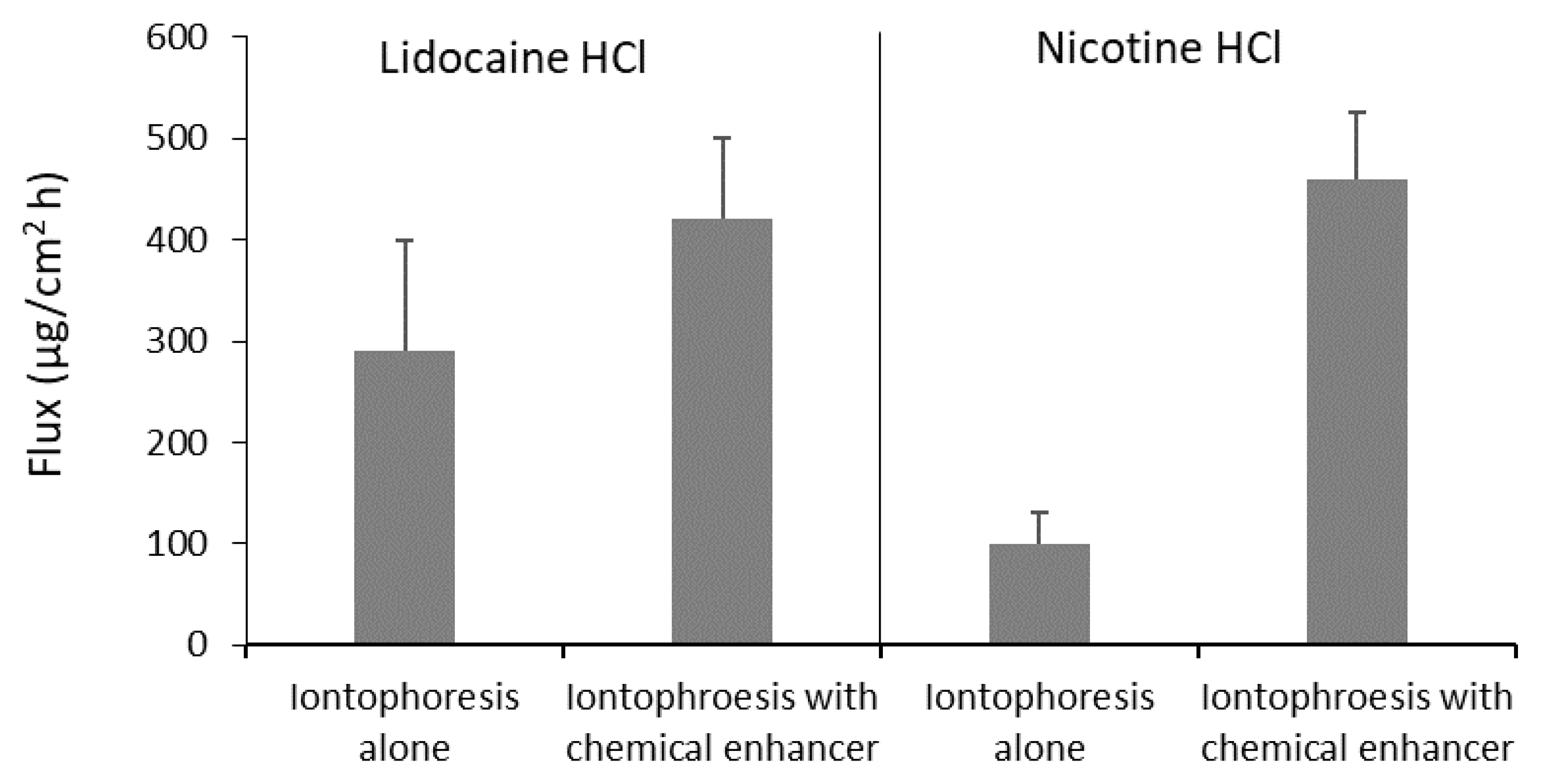
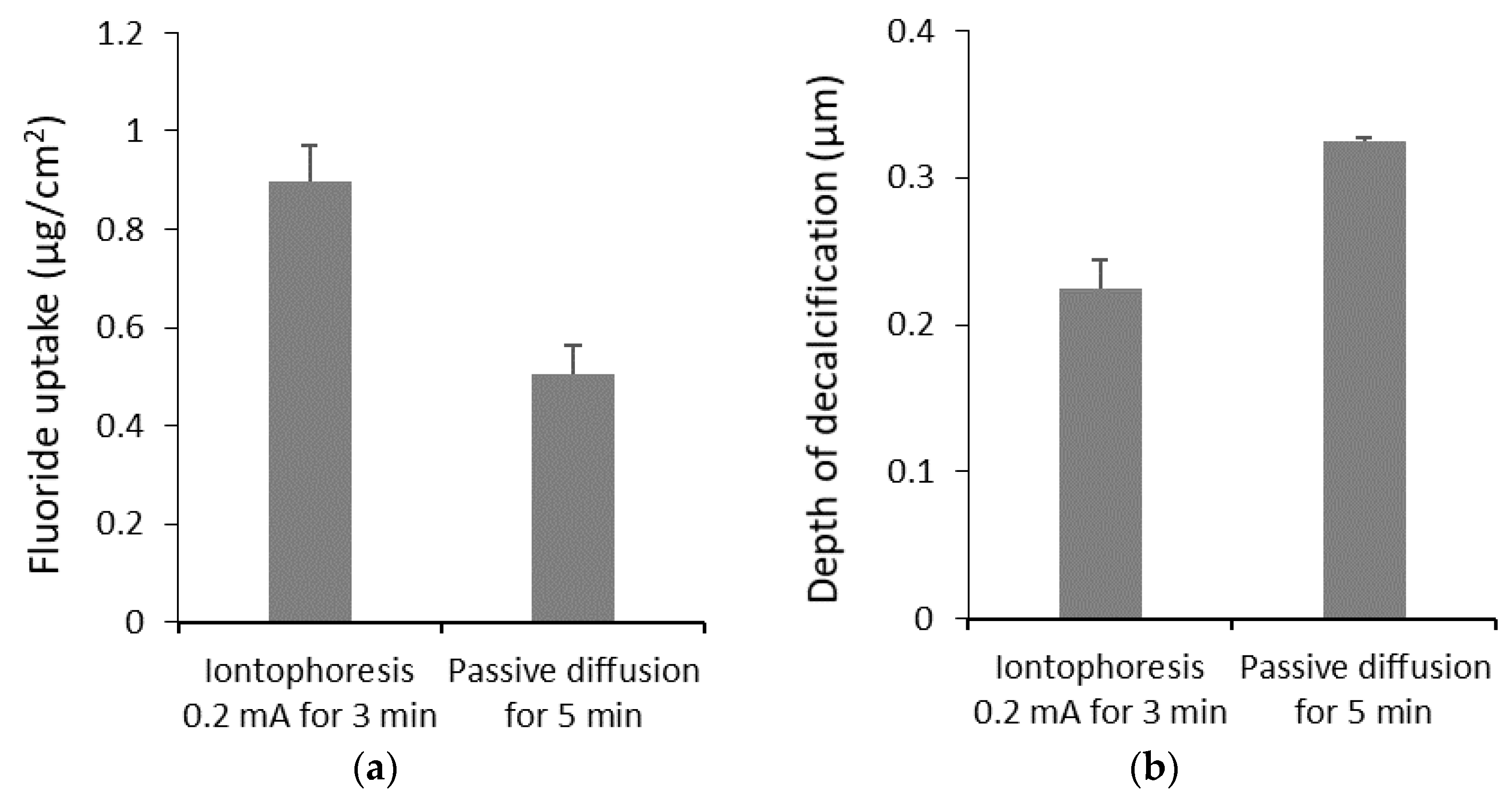
| Permeant/Drug | Formulation | Tissue | Iontophoresis Condition | Iontophoretic Enhancement or Effect | Reference |
|---|---|---|---|---|---|
| 3 kDa Dextran | Hydrogel (1 mg/mL) | Porcine buccal | 0.1 mA 8 h | 7.1 | Patel et al. [67] |
| 10 kDa Dextran | Hydrogel (1 mg/mL) | Porcine buccal | 0.1 mA 8 h | 32 | |
| 12 kDa Parvalbumin | Hydrogel (1 mg/mL) | Porcine buccal | 0.1 mA 8 h | 36 | |
| 5-Fluoruuracil | Solution (20 mM) Gel (10 mM) | Bovine buccal Bovine buccal | 1 mA/cm2 20 min 1 mA/cm2 10 min | 8 4 | Gratieri and Kalia [66] |
| Atenolol | Solution (0.027–0.10 M) | Porcine buccal | 0.1 mA/cm2 8 h 0.2 mA/cm2 8 h 0.3 mA/cm2 8 h 0.4 mA/cm2 8 h | 6 18 36 58 | Jacobsen [64] |
| Chlorhexidine | Solution (0.12%) | Bovine palate | 0.5 mA 2 h | 10 | Ren et al. [69] |
| Diltiazem | Gel (2%) | Porcine buccal | 0.3 mA 8 h | 3 b | Hu et al. [70] |
| Dexamethasone | Solution (10 µg/mL) | Bovine palate | 0.1 mA 8 h | 2.1 | Ren et al. [69] |
| Eosyn Y dye | Solution (7.3 mM) | Human enamel | 6 mA 60 min | Dye increase in the cracks | Chinnapareddy et al. [71] |
| Lidocaine | Solution (1, 2, 5, 10, 20%) | Human enamel/dentin | 3 V, 1 kHz, 10 min | 6 b | Ikeda et al. [72] |
| Lidocaine | Solution (10 mg/mL) | Porcine buccal | 0.5 mA/cm2 6 h | 4 | Telò et al. [63] |
| Lidocaine | Gel | Porcine buccal | 0.3 mA/cm2 8 h with chemical enhancers | 8 b | Wei et al. [73] |
| Lidocaine | Gel (2.5%) | Porcine buccal | 0.3 mA 8 h | 5.4 b | Hu et al. [70] |
| Lidocaine | Hydrogels (2.5% w/w) | Porcine buccal | 1 mA/cm2 1 h | 4 | Cubayachi et al. [62] |
| Leucovorin | Solution (20 mM) Gel (10 mM) | Bovine buccal Bovine buccal | 1 mA/cm2 20 min 1 mA/cm2 10 min | 3 3 | Gratieri and Kalia [66] |
| Lignocaine with epinephrine | Solution (20% lidocaine, 0.1% epinephrine) | Human dentin | 0.2 mA 2 min | Anesthetized 85% of participants | Smitayothin et al. [58] |
| Lignocaine with epinephrine | Solution (20% lidocaine, 0.1% epinephrine) | Human dentin | 0.12 mA 90 s | Fully anesthetized for 40 min | Thongkukiatkun et al. [74] |
| Mannitol | Solution (1 mg/mL) | Bovine palate | 0.1 mA 8 h | 6.2 | Ren et al. [69] |
| Metronidazole | Solution (0.05 M) | Human dentin | 0.05 mA 10 min | 2 b | Puapichartdumrong et al. [75] |
| Nicotine | Gel | Porcine buccal | 0.3 mA/cm2 8 h | 450 b | Wei et al. [73] |
| Nicotine | Gel (2%) | Porcine buccal | 0.3 mA 8 h | 90 b | Hu et al. [70] |
| Naproxen | Solution (0.05 M) | Human dentin | 0.05 mA 10 min | 2 b | Puapichartdumrong et al. [75] |
| Ondansetron | Gel (0.5%) | Porcine buccal | 0.1 mA 8 h 0.2 mA 8 h 0.3 mA 8 h | 3.3 5.2 7.1 | Hu et al. [76] |
| Prilocaine | Hydrogels (2.5%) | Porcine buccal | 1 mA/cm2 1 h | 8.5 | Cubayachi et al. [62] |
| Salmon calcitonin (sCT) | Solution (200 IU) | Porcine buccal | 0.5 mA/cm2 8 h | 66 | Oh et al. [77] |
| Salmon calcitonin (sCT) | Hydrogel (200 IU) | Rabbit buccal | 0.5 mA/cm2 | Hypocalcemic effect drop 20% | Oh et al. [78] |
| Sodium salicylate | Solution (0.15 M) | Bovine palate | 0.1 mA 8 h | 9.4 | Ren et al. [69] |
| Sodium salicylate | Solution (0.05 M) | Human dentin | 0.05 mA 10 min | 2 b | Puapichartdumrong et al. [75] |
| Sodium fluoride | Solution (0.05 M) | Bovine palate | 0.1 mA 8 h | 50 b | Ren et al. [69] |
| Sodium fluoride | Solution (2%) | Bovine enamel | 0.4 mA 4 min | No differences in remineralization | Kim et al. [79] |
| Sodium fluoride | Solution (2%) | Bovine enamel | 0.3 mA 4 min for 5 days | 16% increase in fluoride content, no difference in lesion depth | Kim et al. [80] |
| Sodium fluoride | Solution (2%) | Bovine enamel | 0.2 mA 4 min | No differences in remineralization | Lee et al. [81] |
| Sodium fluoride | Solution (2%) | Bovine enamel | 0.5 mA 3 min 0.5 mA 5 min 0.5 mA 10 min | 1.6 1.9 1.5 | Tanaka et al. [82] |
| Sodium fluoride | Solution (1000 ppm) as NaF, MFP, or SnF2 | Bovine enamel | 0.1 mA 8 h | 60–80 | Ren et al. [83] |
| Sodium fluoride | Solution (4%) | Rat enamel | 0.5–0.6 mA 30 s | Double resistivity to acid | Wagner et al. [84] |
| Sodium fluoride | Gel (1.1%) | Human enamel | 10 mA-min | Increase fluoride retention from 34 to 52% | Barbakow et al. [85] |
| Sodium fluoride | Gel (0.33%) | Human dentin | 1.5% mA 3 min | Close 50% open tubules | Patil et al. [86] |
| Sodium fluoride | Acidulated phosphate fluoride gel (1.23%) | Bovine enamel | 0.4 mA 4 min | No differences in remineralization | Kim et al. [79] |
| Sodium fluoride | Acidulated phosphate fluoride gel (1.23%) | Bovine enamel | 0.2 mA 4 min | No differences in remineralization | Lee et al. [81] |
| Sodium iodide | Solution (0.04 M) | Human enamel | 1–12.5 V 15 min–24 h | 3.2 | Stowell and Taylor [87] |
| Sumatriptan succinate | Solution (33 mM) | Porcine buccal | 0.75 mA/cm2 2 h | 16 | Telò et al. [63] |
| Tetraethylammonium | Solution (0.15 M) | Bovine palate | 0.1 mA 8 h | 42 | Ren et al. [69] |
| System | Membrane | Drug/Permeant | Enhancer | Reference |
|---|---|---|---|---|
| In vitro, porcine | Non-cornified, Buccal | Lidocaine, nicotine | 0.3 mA/cm2 for 8 h; and Pre-treatment with dodecyl-2-(N,N-dimethylamino) propionate hydrochloride, N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane, azone, and propylene glycol | Wei et al. [73] |
| In vitro, porcine | Non-cornified, Buccal | Lidocaine, prilocaine | 1 mA/cm2 for 1 h | Cubayachi et al. [62] |
| In vitro, porcine | Non-cornified, Buccal | Diltiazem, lidocaine, nicotine | 0.1 mA or 0.3 mA for 8 h; or Pre-treatment with dodecyl-2-(N,N-dimethylamino) propionate hydrochloride, N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane, and azone | Hu et al. [70] |
| In vitro, porcine | Non-cornified, Buccal | Ondansetron | 0.1, 0.2 and 0.3 mA for 8 h; or Pre-treatment with dodecyl 2-(N,N-dimethylamino) propionate, N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane, azone | Hu et al. [76] |
| In vitro, porcine | Non-cornified, Buccal | Atenolol | 0.1, 0.2, 0.3 and 0.4 mA/cm2 for 8 h | Jacobsen [64] |
| In vitro, porcine | Non-cornified, Buccal | Salmon calcitonin | 0.5 mA/cm2; and N-acetyl-l-cysteine, deoxyglycocholate, or ethanol for 8 h | Oh et al. [77] |
| In vitro, porcine | Non-cornified, Buccal | Galantamine, naltrexone | 0.4–1.5 mA/cm2 | Mościcka-Studzińska et al. [65] |
| In vitro, porcine | Non-cornified, Buccal | Sodium dodecyl sulfate, urea as water promoter | 0.25–1.0 mA/cm2 | Mościcka-Studzińska et al. [59] |
| In vitro, porcine | Non-cornified, Buccal | Dextrans (3 and 10 kDa), parvalbumin (12 kDa) | 0.1 mA for 8 h | Patel et al. [67] |
| In vitro, porcine | Non-cornified, Buccal | Dextrans (3 and 10 kDa), parvalbumin (12 kDa), bovine serum albumin (66 kDa) | 0.1 mA for 8 h | Patel et al. [20] |
| In vitro, porcine | Non-cornified, esophageal epithelium | Sumatriptan succinate, lidocaine | 0.38, 0.5, 0.75, 1.08, 1.5, 5.83 mA/cm2 for 2 h | Telò et al. [63] |
| In vitro, bovine | Non-cornified, Buccal | 5-Fluorouracil, leucovorin | 1 mA/cm2 for 10–20 min | Gratieri and Kalia [66] |
| In vitro, bovine | Cornified, Palate | Tetraethylammonium, salicylate, mannitol, dexamethasone, fluoride, chlorhexidine | 0.1 mA for 8 h | Ren et al. [69] |
| In vivo, porcine | Non-cornified, Buccal | Naltrexone | 2 mA/cm² for 10 min IntelliDrug device | Campisi et al. [60] |
| In vivo, rabbit | Non-cornified, Buccal | Salmon calcitonin and hypocalcemic effect was measured | 0.5 mA/cm2; with N-acetyl-l-cysteine, deoxyglycocholate, or ethanol for 8 h | Oh et al. [78] |
| System | Membrane | Drug/Permeant | Enhancer | Reference |
|---|---|---|---|---|
| In vitro, bovine | Enamel | Fluoride for remineralization | 0.4 mA, 12 V for 4 min | Kim et al. [79] |
| In vitro, bovine | Enamel | Fluoride for remineralization in early caries | 0.1, 0.2, 0.3, 0.4 mA, 12 V for 4 min/day, 5 days | Kim et al. [80] |
| In vitro, bovine | Enamel | Fluoride for remineralization in early caries | 0.2 mA for 4 min | Lee et al. [81] |
| In vitro, bovine | Enamel | Fluoride for dental caries prevention | 0.2, 0.4, 0.5 mA for 3, 5, 10 min | Tanaka et al. [82] |
| In vitro, bovine | Enamel | Sodium fluoride, sodium monofluorophosphate, Tin (II) fluoride | 0.1 mA for 8 h | Ren et al. [83] |
| In vitro, human | Enamel | Eosyn Y dye | 0.7 and 6 mA for 60 min | Chinnapareddy [71] |
| In vitro, human | Enamel | Radiolabeled sodium iodide | 1–12.5 V | Stowell and Taylor [87] |
| In vitro, human | Enamel | Lidocaine | AC-iontophoresis 3 V, 1 kHz | Ikeda and Suda [72] |
| In vitro, human | Dentin | Metronidazole, sodium salicylate, naproxen | 0.05 mA for 10 min | Puapichartdumrong et al. [75] |
| In vitro, human | Dentin | Fluoride for tooth desensitization | 1.5 mA for 3 min | Patil et al. [86] |
| In vivo, rat | Enamel | Fluoride for acid resistivity | 0.5–0.6 mA for 30 s | Wagner et al. [84] |
| In vivo, human | Enamel | Fluoride for tooth desensitization | Increasing current until the patient felt a sensation | Aparna et al. [94] |
| In vivo, human | Enamel | Fluoride for dental caries prevention | 3–5 mA for 2–3.3 min (10 mA min) | Barbakow et al. [85] |
| In vivo, human | Dentin | Lignocaine with epinephrine | 0.2 mA for 2 min | Smitayothin et al. [58] |
| In vivo, human | Dentin | Lignocaine with epinephrine | 0.12 mA for 90 s | Thongkukiatkun et al. [74] |
| In vivo, human | Dentin | Fluoride for post-operative desensitization | Variable current, current slightly below the level when the patient felt a sensation | Gupta et al. [95] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanasathop, A.; Li, S.K. Iontophoretic Drug Delivery in the Oral Cavity. Pharmaceutics 2018, 10, 121. https://doi.org/10.3390/pharmaceutics10030121
Wanasathop A, Li SK. Iontophoretic Drug Delivery in the Oral Cavity. Pharmaceutics. 2018; 10(3):121. https://doi.org/10.3390/pharmaceutics10030121
Chicago/Turabian StyleWanasathop, Apipa, and S. Kevin Li. 2018. "Iontophoretic Drug Delivery in the Oral Cavity" Pharmaceutics 10, no. 3: 121. https://doi.org/10.3390/pharmaceutics10030121
APA StyleWanasathop, A., & Li, S. K. (2018). Iontophoretic Drug Delivery in the Oral Cavity. Pharmaceutics, 10(3), 121. https://doi.org/10.3390/pharmaceutics10030121