Depletion of Caspase-12 Alleviates Retinal Degeneration in Aged BALB/c Mice Following Systemic Neonatal Infection by Murine Cytomegalovirus (MCMV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Reagents and Antibodies
2.3. Mice
2.4. Experimental Design
2.5. Western Blot
2.6. PCR, Real-Time PCR and RT-PCR
2.7. Statistical Analysis
3. Results
3.1. MCMV Latency in Eyes of Caspase-12−/− and Caspase-12+/+ Mice Following Newborn Peritoneal Infection
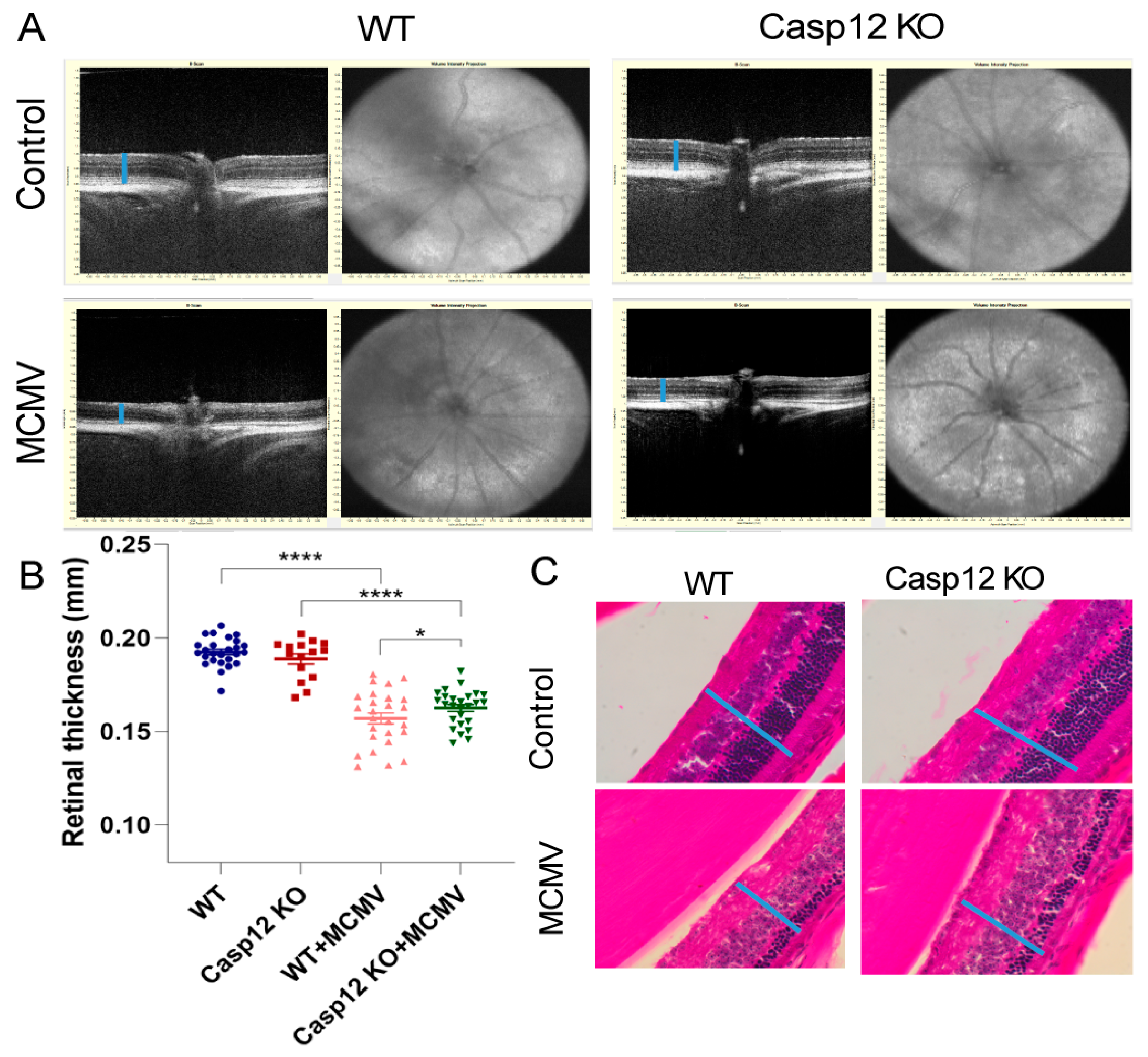
3.2. Caspase-12 Depletion Results in a Less Severe Retinal Degeneration in Aged MCMV-Infected Mice
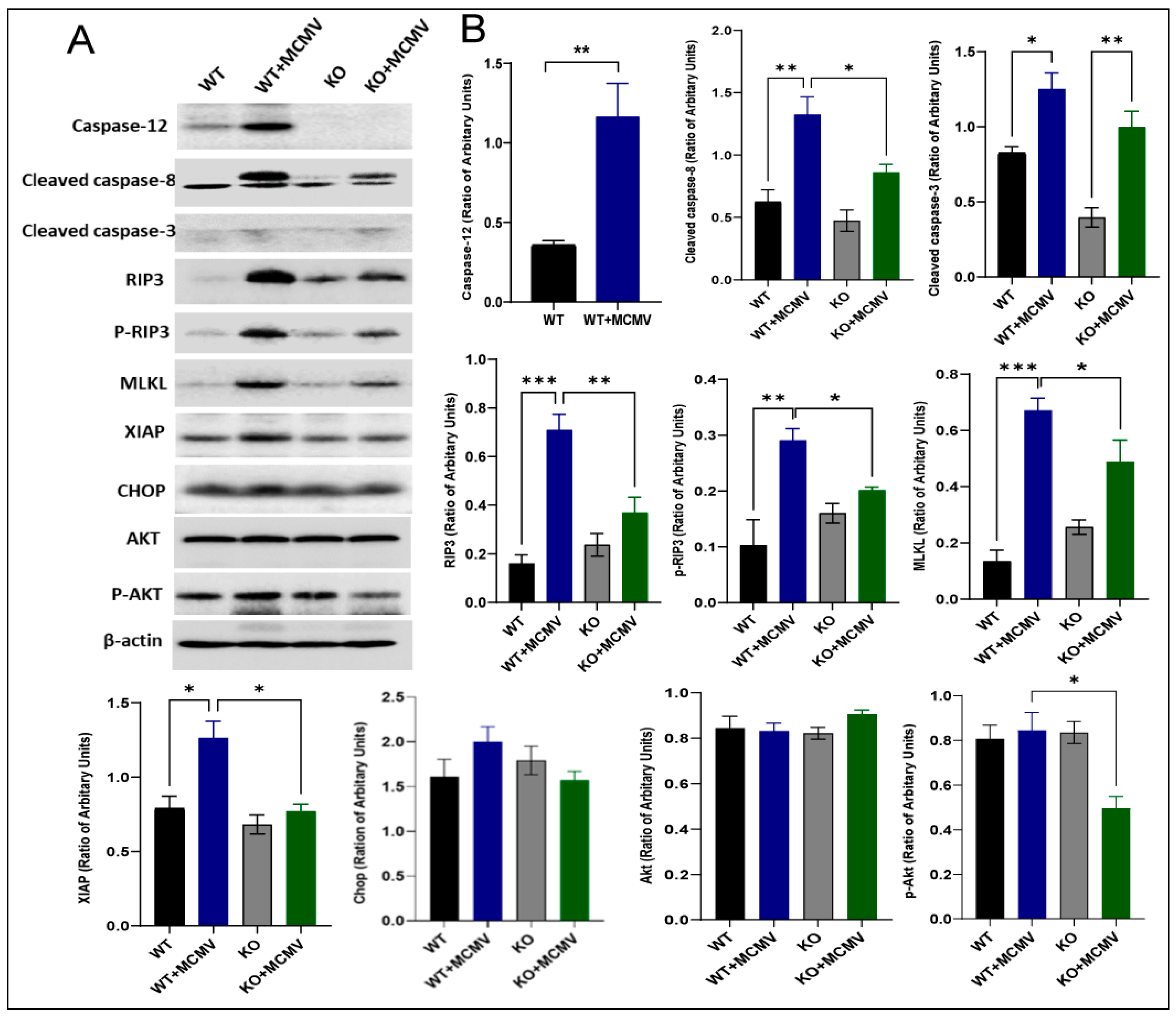
3.3. Analysis of Cell Death Pathways by Western Blot
3.4. p53 and NF-κB Activation
3.5. Analysis of Expression of Inflammatory Genes by Real-Time RT-PCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Demmler, G.J. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 1991, 13, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Wills, M.R.; Sinclair, J.H. Advances in the treatment of cytomegalovirus. Br. Med. Bull. 2019, 131, 5–17. [Google Scholar] [CrossRef]
- Presti, R.M.; Pollock, J.L.; Dal Canto, A.J.; O’Guin, A.K.; Virgin, H.W., IV. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 1998, 188, 577–588. [Google Scholar] [CrossRef]
- Dupont, L.; Reeves, M.B. Cytomegalovirus latency and reactivation: Recent insights into an age old problem. Rev. Med. Virol. 2016, 26, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Zhang, X.; Marshall, B.; Dong, Z.; Liu, Y.; Espinosa-Heidmann, D.G.; Zhang, M. Ocular cytomegalovirus latency exacerbates the development of choroidal neovascularization. J. Pathol. 2020, 251, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Nikitskaya, E.; Lebedeva, A.; Ivanova, O.; Maryukhnich, E.; Shpektor, A.; Grivel, J.C.; Margolis, L.; Vasilieva, E. Cytomegalovirus-Productive Infection Is Associated With Acute Coronary Syndrome. J. Am. Heart Assoc. 2016, 5, e003759. [Google Scholar] [CrossRef]
- Nerheim, P.L.; Meier, J.L.; Vasef, M.A.; Li, W.G.; Hu, L.; Rice, J.B.; Gavrila, D.; Richenbacher, W.E.; Weintraub, N.L. Enhanced cytomegalovirus infection in atherosclerotic human blood vessels. Am. J. Pathol. 2004, 164, 589–600. [Google Scholar] [CrossRef]
- Sorlie, P.D.; Nieto, F.J.; Adam, E.; Folsom, A.R.; Shahar, E.; Massing, M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: The atherosclerosis risk in communities (ARIC) study. Arch. Intern. Med. 2000, 160, 2027–2032. [Google Scholar] [CrossRef]
- Popovic, M.; Smiljanic, K.; Dobutovic, B.; Syrovets, T.; Simmet, T.; Isenovic, E.R. Human cytomegalovirus infection and atherothrombosis. J. Thromb. Thrombolysis 2012, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Skowasch, D.; Jabs, A.; Andrie, R.; Dinkelbach, S.; Schiele, T.M.; Wernert, N.; Luderitz, B.; Bauriedel, G. Pathogen burden, inflammation, proliferation and apoptosis in human in-stent restenosis. Tissue characteristics compared to primary atherosclerosis. J. Vasc. Res. 2004, 41, 525–534. [Google Scholar] [CrossRef]
- Firth, C.; Harrison, R.; Ritchie, S.; Wardlaw, J.; Ferro, C.J.; Starr, J.M.; Deary, I.J.; Moss, P. Cytomegalovirus infection is associated with an increase in systolic blood pressure in older individuals. QJM 2016, 109, 595–600. [Google Scholar] [CrossRef]
- Haarala, A.; Kahonen, M.; Lehtimaki, T.; Aittoniemi, J.; Jylhava, J.; Hutri-Kahonen, N.; Taittonen, L.; Laitinen, T.; Juonala, M.; Viikari, J.; et al. Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: The Cardiovascular Risk in Young Finns Study. Clin. Exp. Immunol. 2012, 167, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Espinosa-Heidmann, D.G.; Legra, J.; Dubovy, S.R.; Suner, I.J.; Sedmak, D.D.; Dix, R.D.; Cousins, S.W. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am. J. Ophthalmol. 2004, 138, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bidanset, D.J.; Beadle, J.R.; Wan, W.B.; Hostetler, K.Y.; Kern, E.R. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J. Infect. Dis. 2004, 190, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Bidanset, D.J.; Rybak, R.J.; Hartline, C.B.; Kern, E.R. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J. Infect. Dis. 2001, 184, 192–195. [Google Scholar] [CrossRef]
- Blalock, E.L.; Chien, H.; Dix, R.D. Systemic reduction of interleukin-4 or interleukin-10 fails to reduce the frequency or severity of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression. Ophthalmol. Eye Dis. 2012, 4, 79–90. [Google Scholar] [CrossRef]
- Blalock, E.L.; Chien, H.; Dix, R.D. Murine cytomegalovirus downregulates interleukin-17 in mice with retrovirus-induced immunosuppression that are susceptible to experimental cytomegalovirus retinitis. Cytokine 2013, 61, 862–875. [Google Scholar] [CrossRef][Green Version]
- Chien, H.; Dix, R.D. Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: Apoptosis, necroptosis, and pyroptosis. J. Virol. 2012, 86, 10961–10978. [Google Scholar] [CrossRef]
- Dix, R.D.; Cousins, S.W. Interleukin-2 immunotherapy of murine cytomegalovirus retinitis during MAIDS correlates with increased intraocular CD8+ T-cell infiltration. Ophthalmic Res. 2003, 35, 154–159. [Google Scholar] [CrossRef]
- Dix, R.D.; Cousins, S.W. Interleukin-2 immunotherapy and AIDS-related cytomegalovirus retinitis. Curr. HIV Res. 2004, 2, 333–342. [Google Scholar] [CrossRef]
- Dix, R.D.; Cousins, S.W. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: Correlation with intraocular levels of tumor necrosis factor-alpha and interferon-gamma. Curr. Eye Res. 2004, 29, 173–180. [Google Scholar] [CrossRef]
- Dix, R.D.; Cousins, S.W. Cell-mediated cytotoxicity of murine cytomegalovirus-infected target cells allows for release of residual infectious virus. Arch. Virol. 2005, 150, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Ekworomadu, C.O.; Hernandez, E.; Cousins, S.W. Perforin knockout mice, but not mice with MAIDS, show protection against experimental cytomegalovirus retinitis after adoptive transfer of immune cells with a functional perforin cytotoxic pathway. Arch. Virol. 2004, 149, 2235–2244. [Google Scholar] [CrossRef]
- Dix, R.D.; Podack, E.R.; Cousins, S.W. Loss of the perforin cytotoxic pathway predisposes mice to experimental cytomegalovirus retinitis. J. Virol. 2003, 77, 3402–3408. [Google Scholar] [CrossRef]
- Laycock, K.A.; Fenoglio, E.D.; Hook, K.K.; Pepose, J.S. An in vivo model of human cytomegalovirus retinal infection. Am. J. Ophthalmol. 1997, 124, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Laycock, K.A.; Kumano, Y.; Pepose, J.S. Reproduction of antiviral effect in an in vivo model of human cytomegalovirus retinal infection. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 527–530. [Google Scholar] [CrossRef]
- Prichard, M.N.; Quenelle, D.C.; Bidanset, D.J.; Komazin, G.; Chou, S.; Drach, J.C.; Kern, E.R. Human cytomegalovirus UL27 is not required for viral replication in human tissue implanted in SCID mice. Virol. J. 2006, 3, 18. [Google Scholar] [CrossRef][Green Version]
- Saleh, M.; Mathison, J.C.; Wolinski, M.K.; Bensinger, S.J.; Fitzgerald, P.; Droin, N.; Ulevitch, R.J.; Green, D.R.; Nicholson, D.W. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 2006, 440, 1064–1068. [Google Scholar] [CrossRef]
- Vande Walle, L.; Jimenez Fernandez, D.; Demon, D.; Van Laethem, N.; Van Hauwermeiren, F.; Van Gorp, H.; Van Opdenbosch, N.; Kayagaki, N.; Lamkanfi, M. Does caspase-12 suppress inflammasome activation? Nature 2016, 534, E1–E4. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Barik, S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 2001, 80, 441–454. [Google Scholar] [CrossRef]
- Jordan, R.; Wang, L.; Graczyk, T.M.; Block, T.M.; Romano, P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 2002, 76, 9588–9599. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Arjona, A.; Zhang, Y.; Sultana, H.; Dai, J.; Yang, L.; LeBlanc, P.M.; Doiron, K.; Saleh, M.; Fikrig, E. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat. Immunol. 2010, 11, 912–919. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, P.M.; Yeretssian, G.; Rutherford, N.; Doiron, K.; Nadiri, A.; Zhu, L.; Green, D.R.; Gruenheid, S.; Saleh, M. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 2008, 3, 146–157. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef]
- Obeng, E.A.; Boise, L.H. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2005, 280, 29578–29587. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, H.; Okamoto, H.; Yoshimura, A.; Yoshida, H. ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J. Cell Sci. 2006, 119, 3958–3966. [Google Scholar] [CrossRef]
- Garcia de la Cadena, S.; Massieu, L. Caspases and their role in inflammation and ischemic neuronal death. Focus on caspase-12. Apoptosis 2016, 21, 763–777. [Google Scholar] [CrossRef]
- Mo, J.; Marshall, B.; Covar, J.; Zhang, N.Y.; Smith, S.B.; Atherton, S.S.; Zhang, M. Role of Bax in death of uninfected retinal cells during murine cytomegalovirus retinitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7137–7146. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Covar, J.; Marshall, B.; Dong, Z.; Atherton, S.S. Lack of TNF-alpha promotes caspase-3-independent apoptosis during murine cytomegalovirus retinitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1800–1808. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Marshall, B.; Dong, Z.; Smith, S.B.; Zhang, M. The Role of Caspase-12 in Retinal Bystander Cell Death and Innate Immune Responses against MCMV Retinitis. Int. J. Mol. Sci. 2021, 22, 8135. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhang, X.; Marshall, B.; Dong, Z.; Smith, S.B.; Espinosa-Heidmann, D.G.; Zhang, M. Retinal and Choroidal Pathologies in Aged BALB/c Mice Following Systemic Neonatal Murine Cytomegalovirus Infection. Am. J. Pathol. 2021, 191, 1787–1804. [Google Scholar] [CrossRef]
- Atherton, S.S.; Newell, C.K.; Kanter, M.Y.; Cousins, S.W. T-Cell Depletion Increases Susceptibility to Murine Cytomegalovirus Retinitis. Investig. Ophth Vis. Sci. 1992, 33, 3353–3360. [Google Scholar]
- Zhang, X.; Xu, J.; Marshall, B.; Dong, Z.; Liu, Y.; Espinosa-Heidmann, D.G.; Zhang, M. Transcriptome Analysis of Retinal and Choroidal Pathologies in Aged BALB/c Mice Following Systemic Neonatal Murine Cytomegalovirus Infection. Int. J. Mol. Sci. 2023, 24, 4322. [Google Scholar] [CrossRef]
- Zhang, M.; Marshall, B.; Atherton, S.S. Murine cytomegalovirus infection and apoptosis in organotypic retinal cultures. Investig. Ophthalmol. Vis. Sci. 2008, 49, 295–303. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Mo, J.; Liu, X.; Marshall, B.; Atherton, S.S.; Dong, Z.; Smith, S.; Zhang, M. Depletion of the Receptor-Interacting Protein Kinase 3 (RIP3) Decreases Photoreceptor Cell Death During the Early Stages of Ocular Murine Cytomegalovirus Infection. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2445–2458. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Mo, J.; Marshall, B.; Perry, L.; Dong, Z.; Zhang, M. Inflammation and outer blood-retina barrier (BRB) compromise following choroidal murine cytomegalovirus (MCMV) infections. Mol. Vis. 2018, 24, 379–394. [Google Scholar]
- Fischer, H.; Koenig, U.; Eckhart, L.; Tschachler, E. Human caspase 12 has acquired deleterious mutations. Biochem. Biophys. Res. Commun. 2002, 293, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Vaillancourt, J.P.; Graham, R.K.; Huyck, M.; Srinivasula, S.M.; Alnemri, E.S.; Steinberg, M.H.; Nolan, V.; Baldwin, C.T.; Hotchkiss, R.S.; et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 2004, 429, 75–79. [Google Scholar] [CrossRef]
- Kalai, M.; Lamkanfi, M.; Denecker, G.; Boogmans, M.; Lippens, S.; Meeus, A.; Declercq, W.; Vandenabeele, P. Regulation of the expression and processing of caspase-12. J. Cell Biol. 2003, 162, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000, 150, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, D.H.; Boo, J.H.; Kim, Y.H.; Park, I.S.; Mook-Jung, I. ER stress-induced caspase-12 activation is inhibited by PKC in neuronal cells. Apoptosis Int. J. Program. Cell Death 2005, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, H.; Kam, T.I.; Tai, M.L.; Lee, J.Y.; Noh, J.Y.; Shim, S.M.; Seo, S.J.; Kong, Y.Y.; Nakagawa, T.; et al. E2-25K/Hip-2 regulates caspase-12 in ER stress-mediated Abeta neurotoxicity. J. Cell Biol. 2008, 182, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zulliger, R.; Lecaude, S.; Eigeldinger-Berthou, S.; Wolf-Schnurrbusch, U.E.; Enzmann, V. Caspase-3-independent photoreceptor degeneration by N-methyl-N-nitrosourea (MNU) induces morphological and functional changes in the mouse retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 859–869. [Google Scholar] [CrossRef][Green Version]
- Sanges, D.; Comitato, A.; Tammaro, R.; Marigo, V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 17366–17371. [Google Scholar] [CrossRef]
- Sanges, D.; Marigo, V. Cross-talk between two apoptotic pathways activated by endoplasmic reticulum stress: Differential contribution of caspase-12 and AIF. Apoptosis 2006, 11, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Taniguchi, M.; Honda, A.; Imaizumi, K.; Tohyama, M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci. Lett. 2004, 357, 127–130. [Google Scholar] [CrossRef]
- Hetz, C.; Russelakis-Carneiro, M.; Maundrell, K.; Castilla, J.; Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003, 22, 5435–5445. [Google Scholar] [CrossRef]
- Kim, H.T.; Waters, K.; Stoica, G.; Qiang, W.; Liu, N.; Scofield, V.L.; Wong, P.K. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spongiform encephalomyelopathy. Lab. Investig. 2004, 84, 816–827. [Google Scholar] [CrossRef]
- Shibata, M.; Hattori, H.; Sasaki, T.; Gotoh, J.; Hamada, J.; Fukuuchi, Y. Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience 2003, 118, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.W.; Andrabi, S.A.; Wang, H.; Kim, N.S.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 18314–18319. [Google Scholar] [CrossRef]
- Yu, S.W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef]
- Lu, M.; Sun, X.L.; Qiao, C.; Liu, Y.; Ding, J.H.; Hu, G. Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol. Aging 2014, 35, 421–430. [Google Scholar] [CrossRef]
- Pant, V.; Sun, C.; Lozano, G. Tissue specificity and spatio-temporal dynamics of the p53 transcriptional program. Cell Death Differ. 2023, 30, 897–905. [Google Scholar] [CrossRef]
- Makino, Y.; Hikita, H.; Fukumoto, K.; Sung, J.H.; Sakano, Y.; Murai, K.; Sakane, S.; Kodama, T.; Sakamori, R.; Kondo, J.; et al. Constitutive Activation of the Tumor Suppressor p53 in Hepatocytes Paradoxically Promotes Non-Cell Autonomous Liver Carcinogenesis. Cancer Res. 2022, 82, 2860–2873. [Google Scholar] [CrossRef]
- Castillo, J.P.; Frame, F.M.; Rogoff, H.A.; Pickering, M.T.; Yurochko, A.D.; Kowalik, T.F. Human cytomegalovirus IE1-72 activates ataxia telangiectasia mutated kinase and a p53/p21-mediated growth arrest response. J. Virol. 2005, 79, 11467–11475. [Google Scholar] [CrossRef] [PubMed]
- Lazo, P.A.; Santos, C.R. Interference with p53 functions in human viral infections, a target for novel antiviral strategies? Rev. Med. Virol. 2011, 21, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsurumi, T. Genome guardian p53 and viral infections. Rev. Med. Virol. 2013, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Taura, M.; Eguma, A.; Suico, M.A.; Shuto, T.; Koga, T.; Komatsu, K.; Komune, T.; Sato, T.; Saya, H.; Li, J.D.; et al. p53 regulates Toll-like receptor 3 expression and function in human epithelial cell lines. Mol. Cell. Biol. 2008, 28, 6557–6567. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Fontela, C.; Macip, S.; Martinez-Sobrido, L.; Brown, L.; Ashour, J.; Garcia-Sastre, A.; Lee, S.W.; Aaronson, S.A. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008, 205, 1929–1938. [Google Scholar] [CrossRef]
- Hummer, B.T.; Li, X.L.; Hassel, B.A. Role for p53 in gene induction by double-stranded RNA. J. Virol. 2001, 75, 7774–7777. [Google Scholar] [CrossRef]


| Gene | Functions | WT | KO |
|---|---|---|---|
| M36 | Anti-cell death | 5/6 | 5/6 |
| M37 | Anti-cell death | 5/6 | 5/6 |
| M38.5 | Anti-cell death | 4/6 | 4/6 |
| M41 | Anti-cell death | 4/6 | 3/6 |
| M45 | Anti-cell death | 2/6 | 3/6 |
| M04 | Immune modulation | 2/6 | 3/6 |
| M18 | Early | 0/6 | 1/6 |
| M80 | Protein assembly | 4/6 | 2/6 |
| Increased Expression | Decreased Expression | ||
|---|---|---|---|
| Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation |
| Atg12 | 2.47 | Atg5 | −2.07 |
| Casp1 | 3.53 | Azi2 | −4.25 |
| Chuk | 3.03 | Ccl5 | −2.87 |
| Cxcl9 | 7.35 | Ctsb | −4.4 |
| Fadd | 2.24 | Cxcl10 | −2.49 |
| Ifna2 | 3 | Cxcl11 | −2.05 |
| Irf5 | 4.51 | Cyld | −2.83 |
| Nod2 | 4.81 | Ddx3x | −3.31 |
| Spp1 | 3.65 | Ddx58 | −2.06 |
| Tlr7 | 5.91 | Fos | −3.11 |
| Tlr8 | 3.66 | Hsp90aa1 | −3.99 |
| Tradd | 2.34 | Irak1 | −2.61 |
| Irf7 | −4.4 | ||
| Isg15 | −3.77 | ||
| Jun | −2.16 | ||
| Map2k1 | −3.6 | ||
| Map2k3 | −10.97 | ||
| Map3k7 | −3.87 | ||
| Mapk14 | −2.01 | ||
| Mapk3 | −3.07 | ||
| Mx1 | −4.99 | ||
| Nfkb1 | −8.96 | ||
| Nfkbia | −2.68 | ||
| Pstpip1 | −2.27 | ||
| Ripk1 | −4.87 | ||
| Stat1 | −3.27 | ||
| Tlr3 | −3.02 | ||
| Traf6 | −2.01 | ||
| Actb | −9.03 | ||
| Gapdh | −3.36 | ||
| Gusb | −2.33 | ||
| Hsp90ab1 | −3.45 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhang, X.; Liao, Y.; Shi, T.; Marshall, B.; Zhang, M. Depletion of Caspase-12 Alleviates Retinal Degeneration in Aged BALB/c Mice Following Systemic Neonatal Infection by Murine Cytomegalovirus (MCMV). Viruses 2025, 17, 1206. https://doi.org/10.3390/v17091206
Xu J, Zhang X, Liao Y, Shi T, Marshall B, Zhang M. Depletion of Caspase-12 Alleviates Retinal Degeneration in Aged BALB/c Mice Following Systemic Neonatal Infection by Murine Cytomegalovirus (MCMV). Viruses. 2025; 17(9):1206. https://doi.org/10.3390/v17091206
Chicago/Turabian StyleXu, Jinxian, Xinyan Zhang, Yi Liao, Ting Shi, Brendan Marshall, and Ming Zhang. 2025. "Depletion of Caspase-12 Alleviates Retinal Degeneration in Aged BALB/c Mice Following Systemic Neonatal Infection by Murine Cytomegalovirus (MCMV)" Viruses 17, no. 9: 1206. https://doi.org/10.3390/v17091206
APA StyleXu, J., Zhang, X., Liao, Y., Shi, T., Marshall, B., & Zhang, M. (2025). Depletion of Caspase-12 Alleviates Retinal Degeneration in Aged BALB/c Mice Following Systemic Neonatal Infection by Murine Cytomegalovirus (MCMV). Viruses, 17(9), 1206. https://doi.org/10.3390/v17091206






