Development of an Acid-Protective Polymer Encapsulation Formulation for Oral Delivery of Salmonella Phages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacterial Strains and Culture Conditions
2.3. Bacteriophage Isolation and Characterization
2.3.1. Phage Enrichment and Spot Assay
2.3.2. Bulk Propagation of Bacteriophages
2.3.3. Assessment of Thermal and pH Sensitivity of Bacteriophages
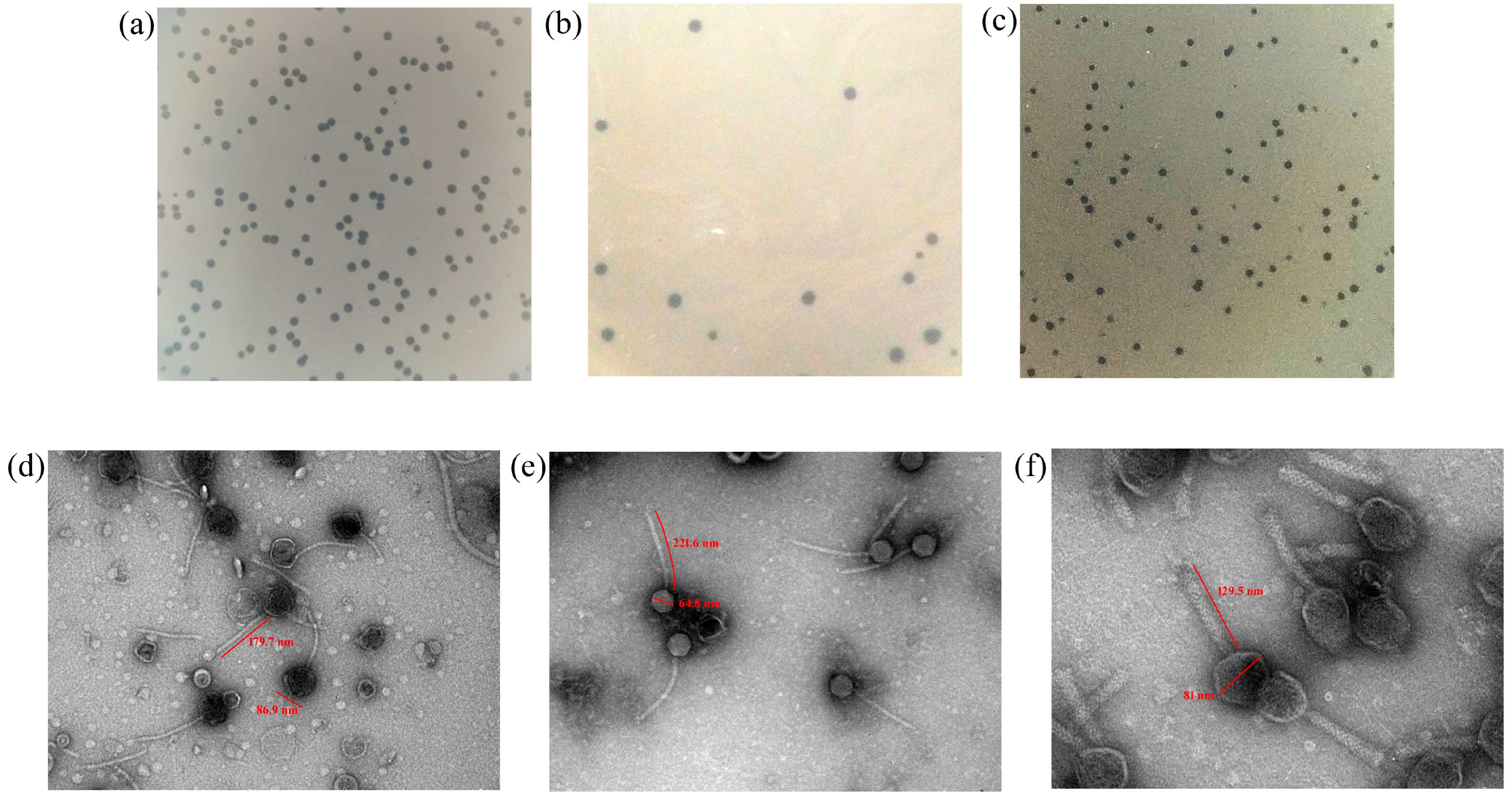
2.3.4. Transmission Electron Microscopy (TEM)
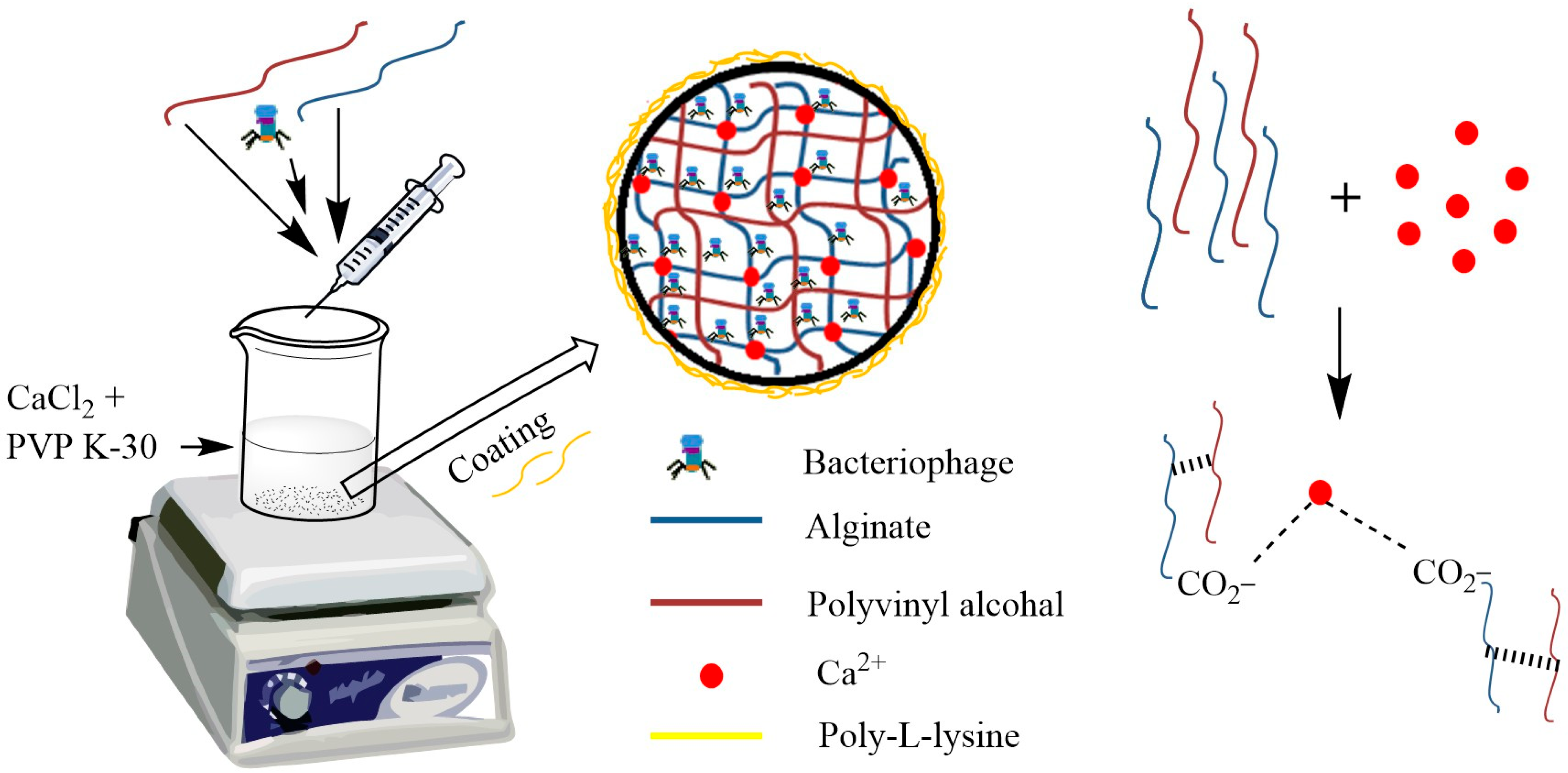
2.4. Preparation of Alginate Beads
2.5. Characterization of Alginate Beads
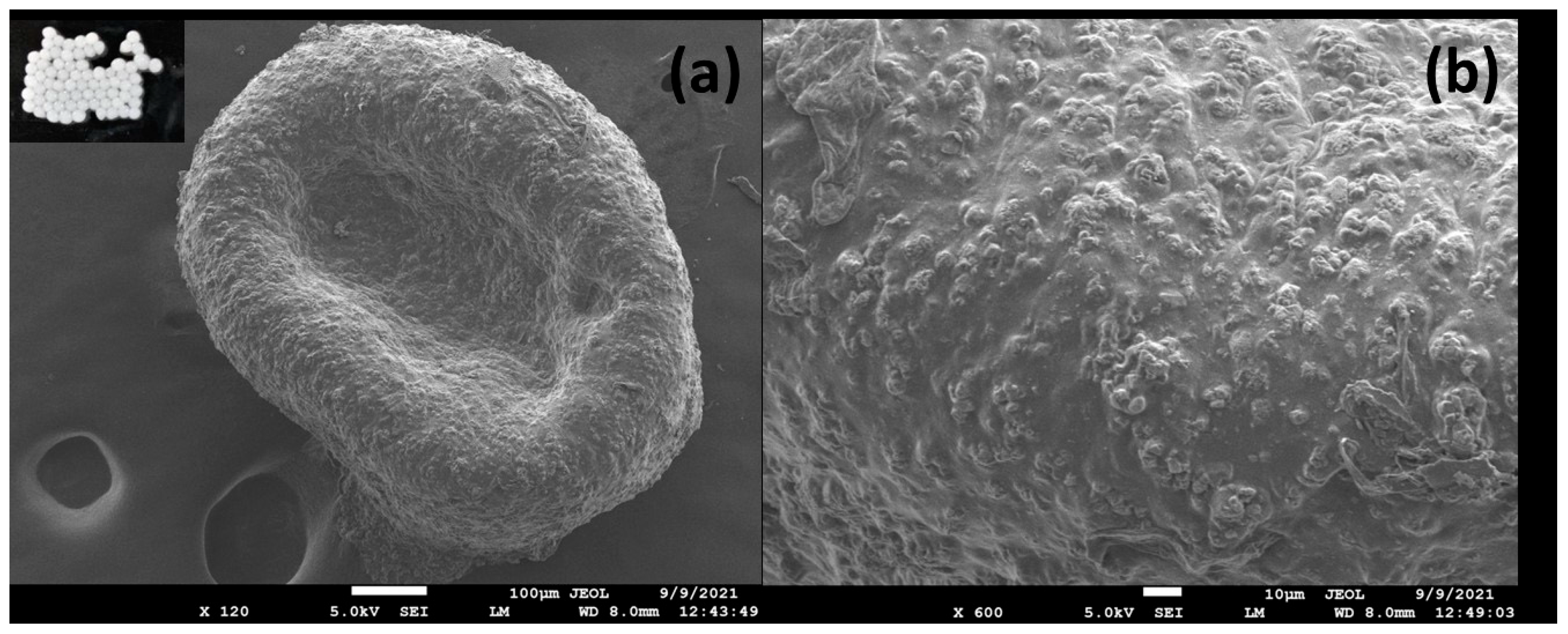
2.5.1. Bead Size and Morphology Assessment
2.5.2. Phage-Loading Efficiency
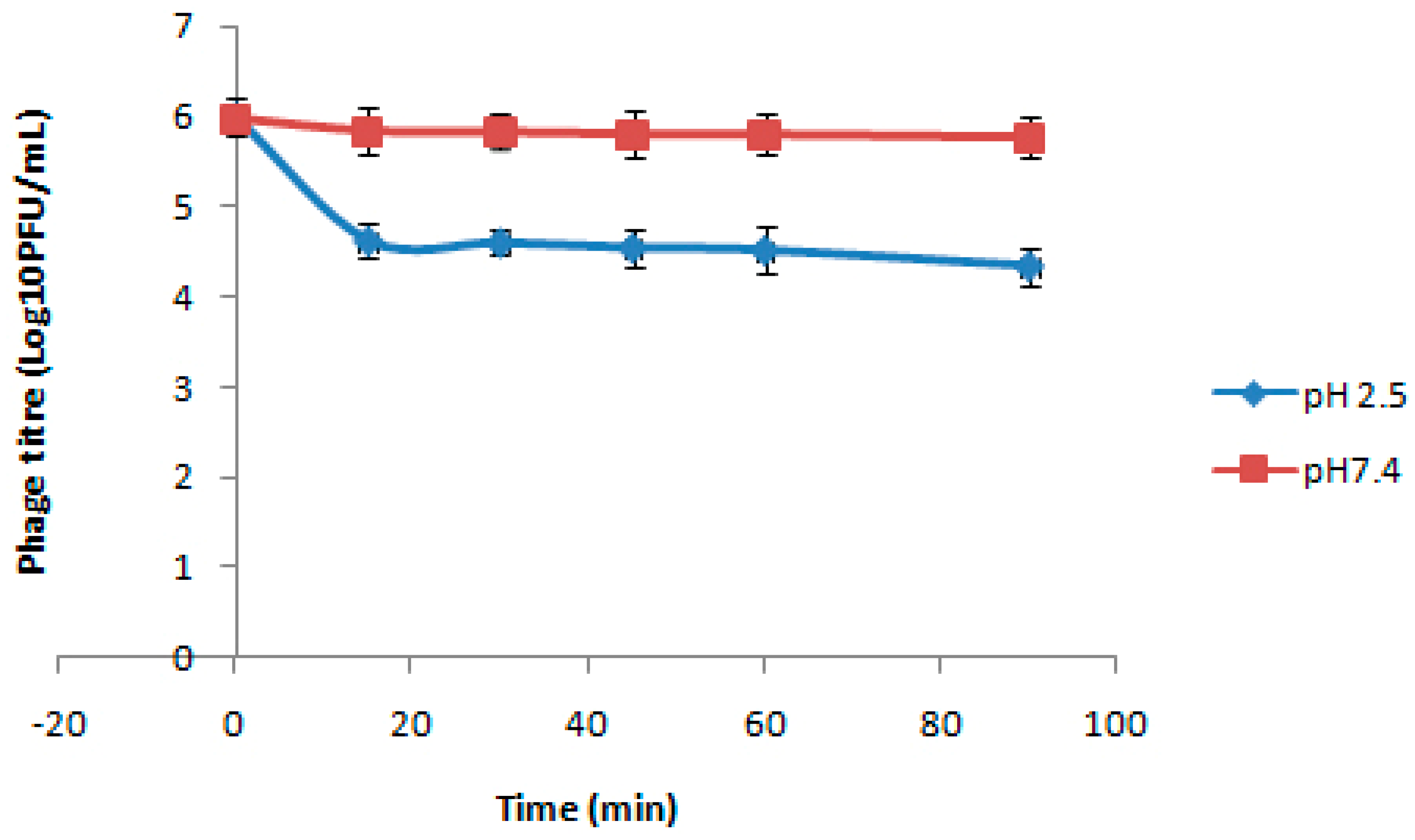
2.6. Stability of Beads at Different pH
2.7. Stability of Free and Encapsulated Phage in Bile Salts
2.8. Release at pH 7.4
2.9. Storage Stability
2.10. In Vivo Evaluation of Polymer-Encapsulated Formulation in Broiler Chickens
- Group A: Healthy birds with no infection or treatment (negative control).
- Group B: Birds infected with S. typhimurium and treated with phage-loaded beads.
- Group C: Birds infected with S. paratyphi and treated with phage-loaded beads.
- Group D: Birds infected with S. paratyphi only (positive control).
- Group E: Birds infected with S. typhimurium only (positive control).
2.11. Histopathological Study
2.12. Statistical Analysis
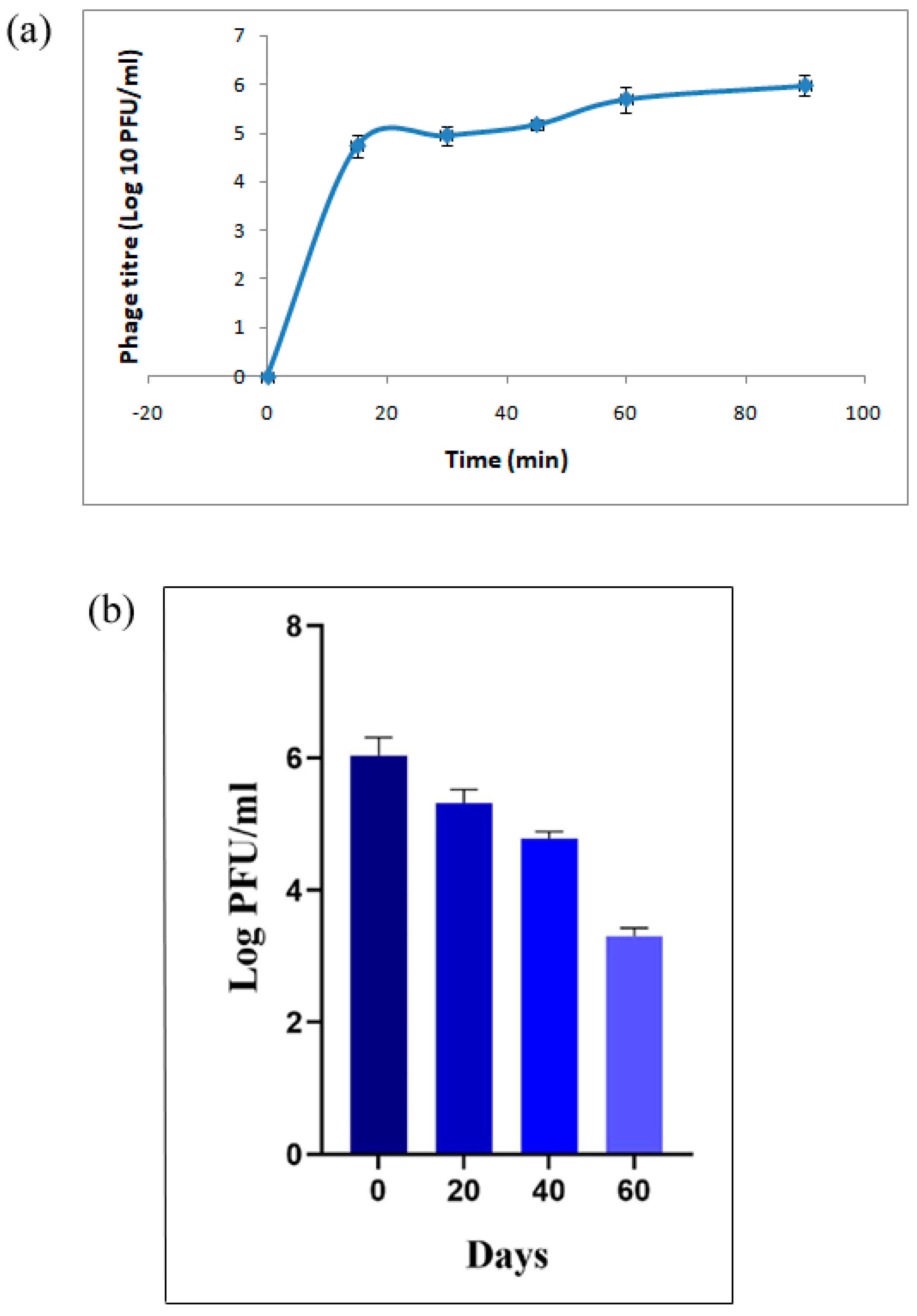
3. Results and Discussions
3.1. Bacteriophage Isolation
3.2. Preparation of Beads
3.3. Characterization
3.3.1. Bead Size and Morphology
3.3.2. Phage-Loading Efficiency
3.4. Stability of Beads at Different pH
3.5. Stability of Free and Encapsulated Phage in Bile Salts
3.6. Release at pH 7.4
3.7. Storage Stability
3.8. In Vivo Evaluation in Broiler Chickens
3.8.1. Assessment of Clinical Signs in Infected Birds
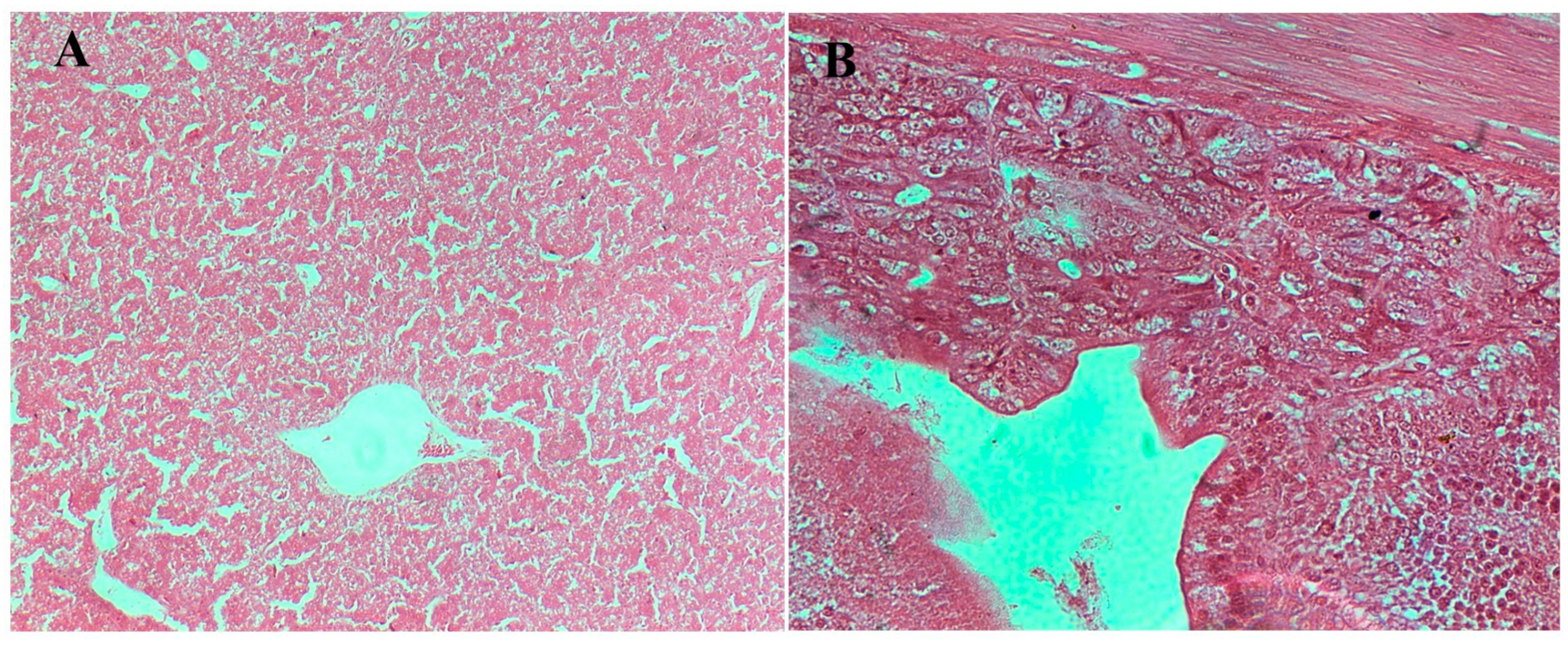
3.8.2. Gross Pathological Evaluation
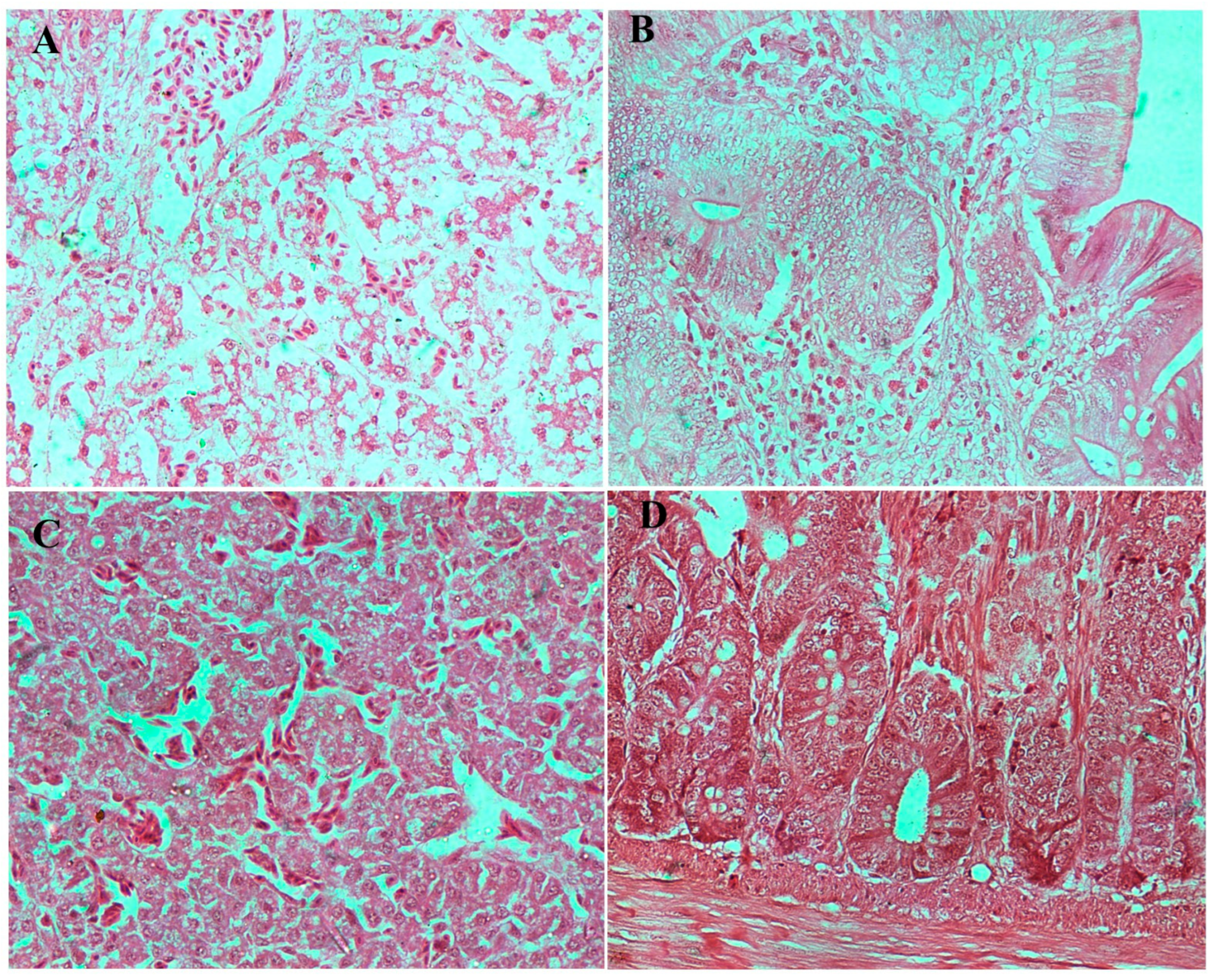
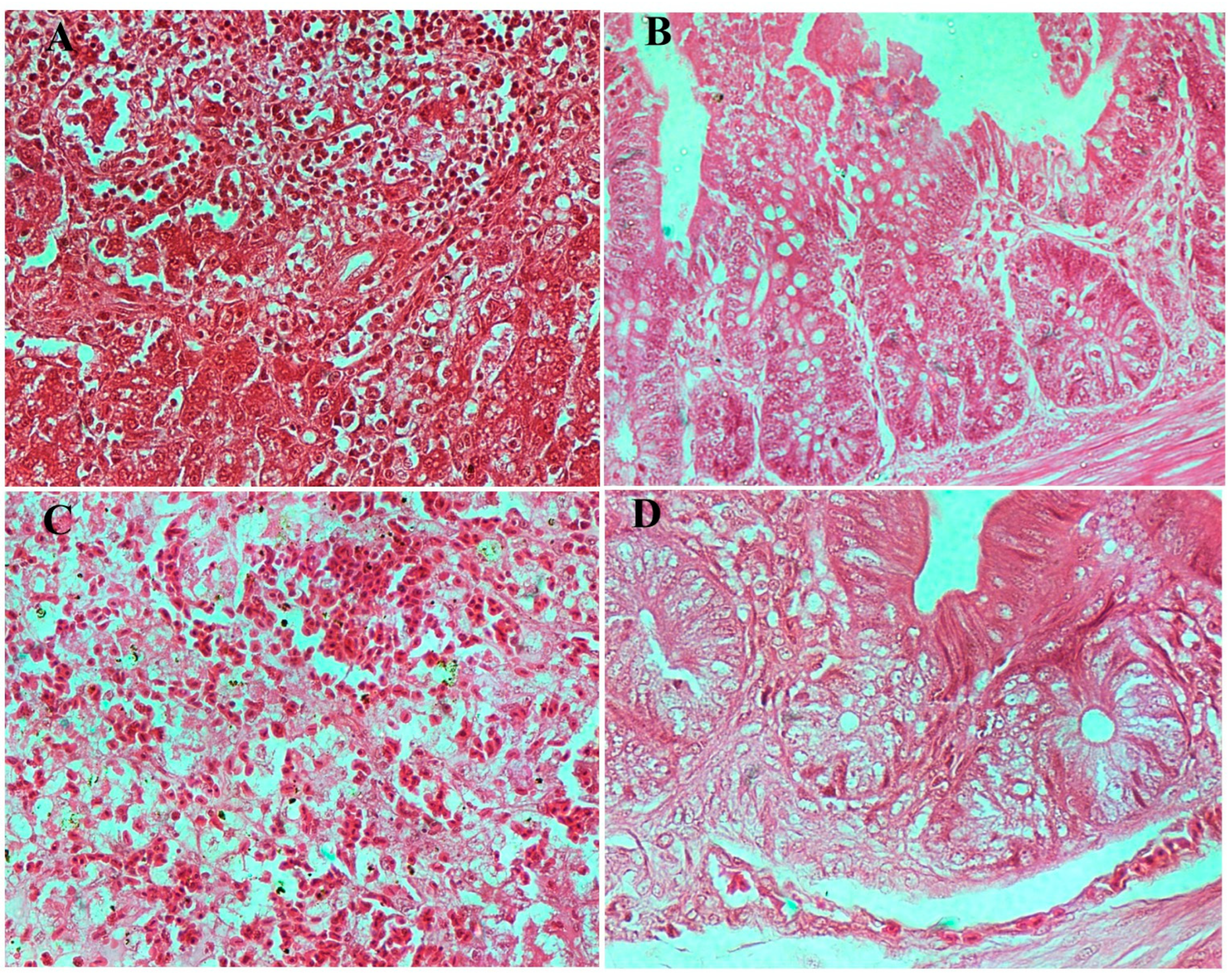
3.8.3. Histopathological Examination
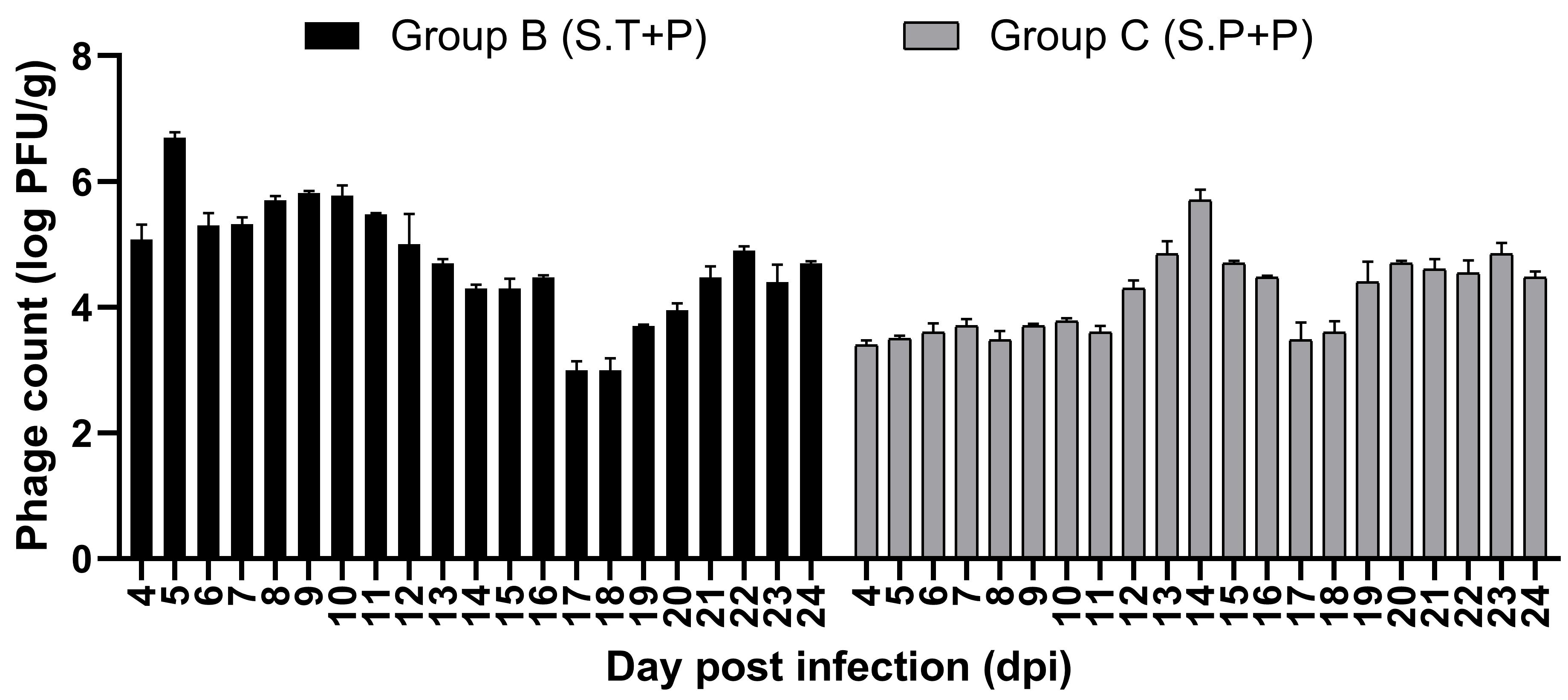
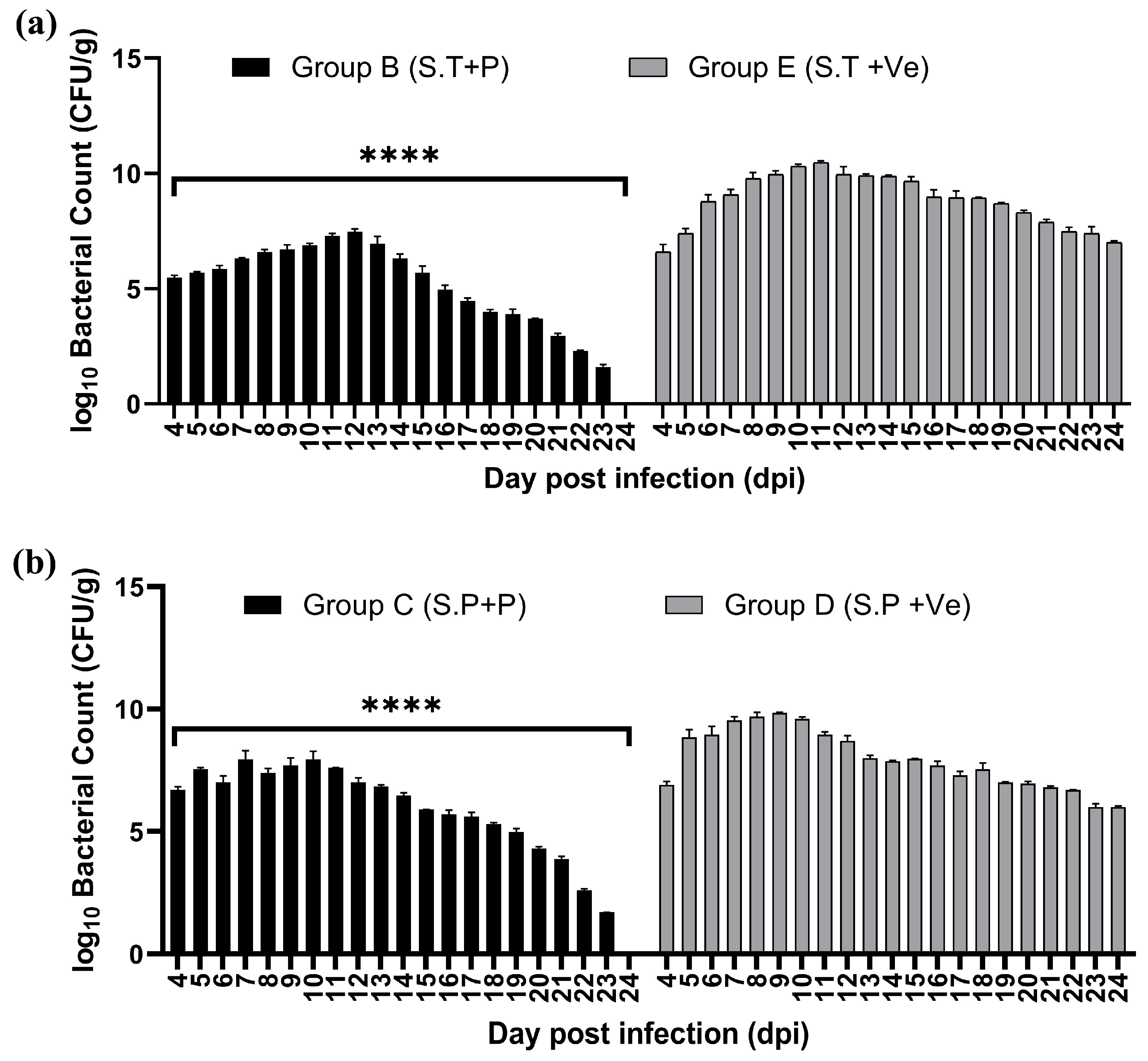
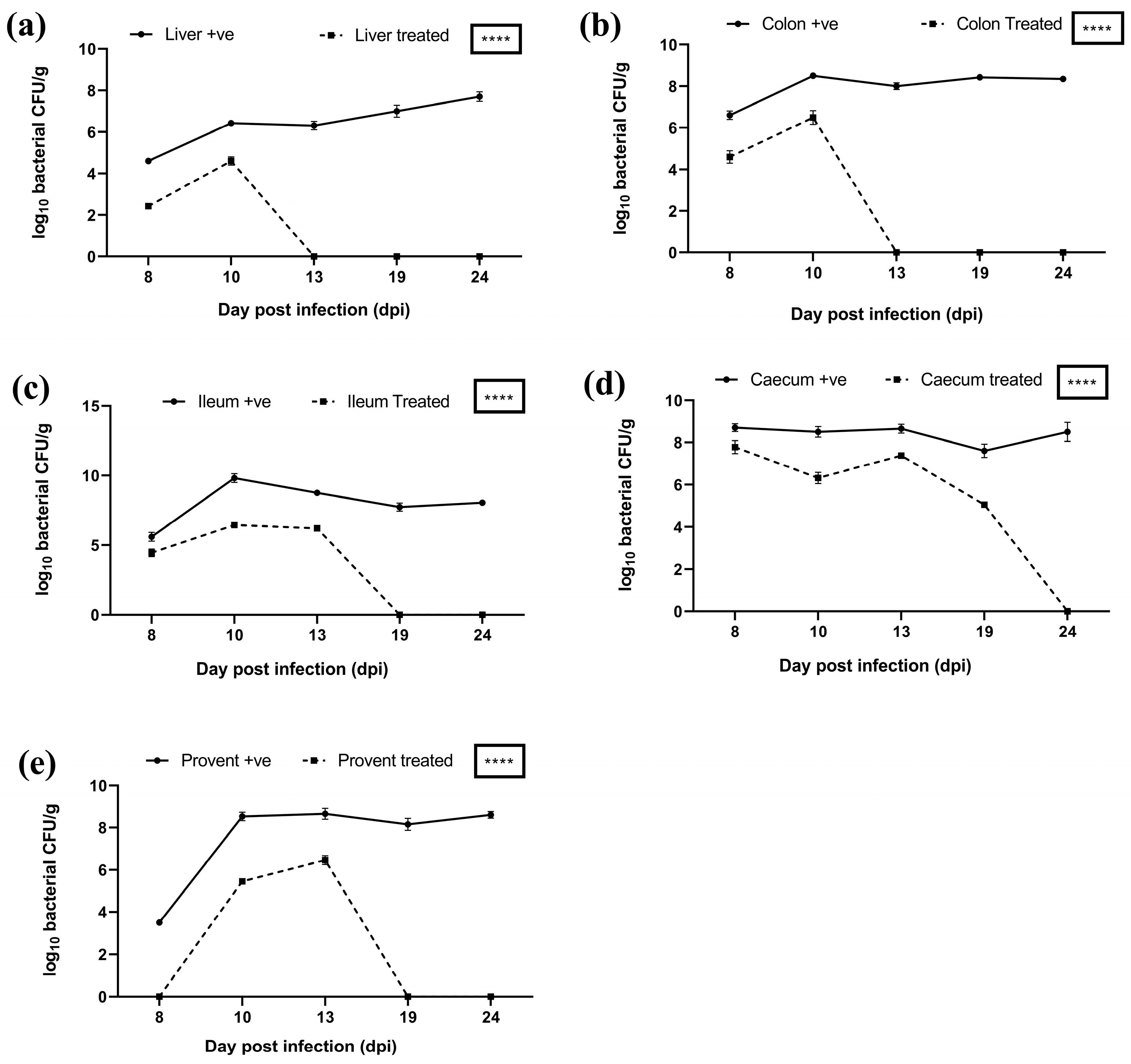
3.8.4. Estimation of Bacterial Load and Phage Presence in Feces and Different Organs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Integrated Surveillance of Antimicrobial Resistance: Guidance from a WHO Advisory Group; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Pires, S.M.; Vieira, A.R.; Hald, T.; Cole, D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014, 11, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Bopp, C.A.; Brenner, F.W.; Wells, J.G. Escherichia, Shigella, and Salmonella. In Manual of Clinical Microbiology; Murray, P., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press: Washington DC, USA, 1999; p. 437. [Google Scholar]
- Duan, B.; Shao, L.; Liu, R.; Msuthwana, P.; Hu, J.; Wang, C. Lactobacillus rhamnosus GG defense against Salmonella enterica serovar Typhimurium infection through modulation of M1 macrophage polarization. Microb. Pathog. 2021, 156, 104939. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.S.; Garrido, V.; Grillo, M.J. Non-typhoidal Salmonellosis. Mol. Vaccines 2013, 1, 329–342. [Google Scholar] [CrossRef]
- Elizabeth, L.H. Nontyphoidal salmonellosis. Food Saf. 2001, 32, 263–269. [Google Scholar]
- Crump, J.A.; Heyderman, R.S. A perspective on invasive Salmonella disease in Africa. Clin. Infect. Dis. 2015, 61 (Suppl. 4), S235–S240. [Google Scholar] [CrossRef] [PubMed]
- Uche, I.V.; MacLennan, C.A.; Saul, A.; Baker, S. A systematic review of the incidence, risk factors and case fatality rates of Invasive Nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl. Trop. Dis. 2017, 11, e0005118. [Google Scholar] [CrossRef] [PubMed]
- Ceyssens, P.J.; Mattheus, W.; Vanhoof, S.; Bertrand, S. Trends in serotype distribution and antimicrobial susceptibility in Salmonella enterica isolates from humans in Belgium, 2009 to 2013. Antimicrob. Agents Chemother. 2015, 59, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.M.; Hill-Cawthorne, G.A.; Ward, M.P.; Mor, S.M. Diversity of Salmonella serotypes from humans, food, domestic animals and wildlife in New South Wales, Australia. BMC Infect. Dis. 2018, 18, 623. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H.; et al. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Michael, G.B.; Schwarz, S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: An alarming trend? Clin. Microbiol. Infect. 2016, 22, 968–974. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Zhao, S.; Tate, H. Antimicrobial resistance in nontyphoidal Salmonella. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Alt, K.; Simon, S.; Helmeke, C.; Kohlstock, C.; Prager, R.; Tietze, E.; Rabsch, W.; Karagiannis, I.; Werber, D.; Frank, C.; et al. Outbreak of uncommon O4 non-agglutinating Salmonella typhimurium linked to minced pork, Saxony-Anhalt, Germany, January to April 2013. PLoS ONE 2015, 10, e0128349. [Google Scholar] [CrossRef] [PubMed]
- Folster, J.P.; Grass, J.E.; Bicknese, A.; Taylor, J.; Friedman, C.R.; Whichard, J.M. Characterization of resistance genes and plasmids from outbreaks and illness clusters caused by Salmonella resistant to ceftriaxone in the United States, 2011–2012. Microb. Drug Resist. 2017, 23, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.; Retamal, P.; Rojas-Aedo, J.F.; Fernández, J.; Fernández, A.; Lapierre, L. Multidrug-resistant outbreak-associated salmonella strains in irrigation water from the metropolitan region, Chile. Zoonoses Public Health 2017, 64, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wan, Y.; Du, P.; Bai, L. The epidemiology of monophasic Salmonella typhimurium. Foodborne Pathog. Dis. 2020, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ćwiek, K.; Korzekwa, K.; Tabiś, A.; Bania, J.; Bugla-Płoskońska, G.; Wieliczko, A. Antimicrobial resistance and biofilm formation capacity of Salmonella enterica serovar Enteritidis strains isolated from poultry and humans in Poland. Pathogens 2020, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Azim, S.; Ali, A.; Andleeb, S.; Ahsan, A.; Imran, M.; Rahman, A. Antimicrobial resistance profiling of biofilm-forming non-typhoidal Salmonella enterica isolates from poultry and its associated food products from Pakistan. Antibiotics 2021, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Lack of New Antibiotics Threatens Global Efforts to Contain Drug-Resistant Infections. 2020. Available online: https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections (accessed on 12 March 2025).
- Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D.; Participants of the 3rd World Healthcare-Associated Infections Forum. Ready for a world without antibiotics? The Pensières Antibiotic Resistance Call to Action. Antimicrob. Resist. Infect. Control 2012, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Almeida, G.M.; Sundberg, L.R. The forgotten tale of Brazilian phage therapy. Lancet Infect. Dis. 2020, 20, e90–e101. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.aphage.com/blog/ (accessed on 25 April 2020).
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.; Garton, N.J.; Stapley, A.G.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Moghtader, F.; Eğri, S.; Piskin, E. Phages in modified alginate beads. Artif. Cells Nanomed. Biotechnol. 2017, 45, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Wang, Q.; Lim, L.T.; Balamurugan, S. Encapsulation of Listeria phage A511 by alginate to improve its thermal stability. In Bacteriophages; Humana Press: New York, NY, USA, 2018; pp. 89–95. [Google Scholar]
- Śliwka, P.; Mituła, P.; Mituła, A.; Skaradziński, G.; Choińska-Pulit, A.; Niezgoda, N.; Weber-Dąbrowska, B.; Żaczek, M.; Skaradzińska, A. Encapsulation of bacteriophage T4 in mannitol-alginate dry macrospheres and survival in simulated gastrointestinal conditions. LWT 2019, 99, 238–243. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. AMB Express 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H.C. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy. J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Narra, K.; Dhanalekshmi, U.; Rangaraj, G.; Raja, D.; Kumar, C.S.; Reddy, P.N.; Mandal, A.B. Effect of formulation variables on rifampicin loaded alginate beads. Iran. J. Pharm. Sci. 2012, 11, 715. [Google Scholar] [PubMed]
- Abdalla, K.F.; Kamoun, E.A.; El Maghraby, G.M. Optimization of the entrapment efficiency and release of ambroxol hydrochloride alginate beads. J. Appl. Pharm. Sci. 2015, 5, 13–19. [Google Scholar] [CrossRef]
- Swanstrom, M.; Adams, M.H. Agar layer method for production of high titer phage stocks. Proc. Soc. Exp. Biol. Med. 1951, 78, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, X.; Sabour, P.M.; Chambers, J.R.; Wang, Q. Preparation and characterization of dry powder bacteriophage K for intestinal delivery through oral administration. LWT—Food Sci. Technol. 2015, 60, 263–270. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology; McGraw-Hill: New York, NY, USA, 1968; p. xii-258. [Google Scholar]
- Ma, Y.-H.; Islam, G.S.; Wu, Y.; Sabour, P.M.; Chambers, J.R.; Wang, Q.; Wu, S.-X.; Griffiths, M.W. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poult. Sci. 2016, 92, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Şanlı, O.; Ay, N.; Işıklan, N. Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur. J. Pharm. Biopharm. 2007, 65, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Guowei, D.; Adriane, K.; Chen, X.; Jie, C.; Yinfeng, L. PVP magnetic nanospheres: Biocompatibility, in vitro and in vivo bleomycin release. Int. J. Pharm. 2007, 328, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Khatua, S.; Hasnain, M.S.; Sen, K.K. Development of diclofenac sodium-loaded alginate-PVP K 30 microbeads using central composite design. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 356. [Google Scholar]
- Koo, J.; DePaola, A.; Marshall, D.L. Effect of simulated gastric fluid and bile on survival of Vibrio vulnificus and Vibrio vulnificus phage. J. Food Prot. 2000, 63, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Kawabuchi, M.; Watanabe, A.; Sugihara, M.; Sakurai, Y.; Okano, T. Effect of Ca2+-alginate gel dissolution on release of dextran with different molecular weights. J. Control Release 1999, 58, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Giannella, R.A. Salmonella. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 21. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8435/ (accessed on 18 March 2025).
- Khan, M.A.S.; Rahman, S.R. Use of phages to treat antimicrobial-resistant Salmonella infections in poultry. Vet. Sci. 2022, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B.; Piątek, R. Bacteriophages as potential tools for use in antimicrobial therapy and vaccine development. Pharmaceuticals 2021, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef] [PubMed]
- Pelyuntha, W.; Yafa, A.; Ngasaman, R.; Yingkajorn, M.; Chukiatsiri, K.; Champoochana, N.; Vongkamjan, K. Oral administration of a phage cocktail to reduce salmonella colonization in broiler gastrointestinal tract-a pilot study. Animals 2022, 12, 3087. [Google Scholar] [CrossRef] [PubMed]


| S. No. | Host | Phage | Plaque Characteristics |
|---|---|---|---|
| 1 | Salmonella typhimurium | Salmonella phage ΦST143 | 1.5 mm in diameter, clear, circular |
| 2 | Salmonella enteritidis | Salmonella phage ΦST187 | 2 mm in diameter, clear, circular |
| 3 | Salmonella enteritidis | Salmonella phage ΦST188 | 1.5 mm in diameter, clear, circular |
| Preparation Type | Initial Titre (log10 PFU/mL) | Titre After 3 h (log10 PFU/mL) |
|---|---|---|
| Free phage | 9.04 | 5.60 |
| Encapsulated phage | 6.04 | 5.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernela, M.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Vashisth, M.; Anand, T. Development of an Acid-Protective Polymer Encapsulation Formulation for Oral Delivery of Salmonella Phages. Viruses 2025, 17, 1205. https://doi.org/10.3390/v17091205
Bernela M, Virmani N, Bera BC, Vaid RK, Vashisth M, Anand T. Development of an Acid-Protective Polymer Encapsulation Formulation for Oral Delivery of Salmonella Phages. Viruses. 2025; 17(9):1205. https://doi.org/10.3390/v17091205
Chicago/Turabian StyleBernela, Manju, Nitin Virmani, Bidhan Chand Bera, Rajesh Kumar Vaid, Medhavi Vashisth, and Taruna Anand. 2025. "Development of an Acid-Protective Polymer Encapsulation Formulation for Oral Delivery of Salmonella Phages" Viruses 17, no. 9: 1205. https://doi.org/10.3390/v17091205
APA StyleBernela, M., Virmani, N., Bera, B. C., Vaid, R. K., Vashisth, M., & Anand, T. (2025). Development of an Acid-Protective Polymer Encapsulation Formulation for Oral Delivery of Salmonella Phages. Viruses, 17(9), 1205. https://doi.org/10.3390/v17091205







