Mechanisms of Thromboinflammation in Viral Infections—A Narrative Review
Abstract
1. From Homeostasis to Thromboinflammation: Mechanisms and Implications
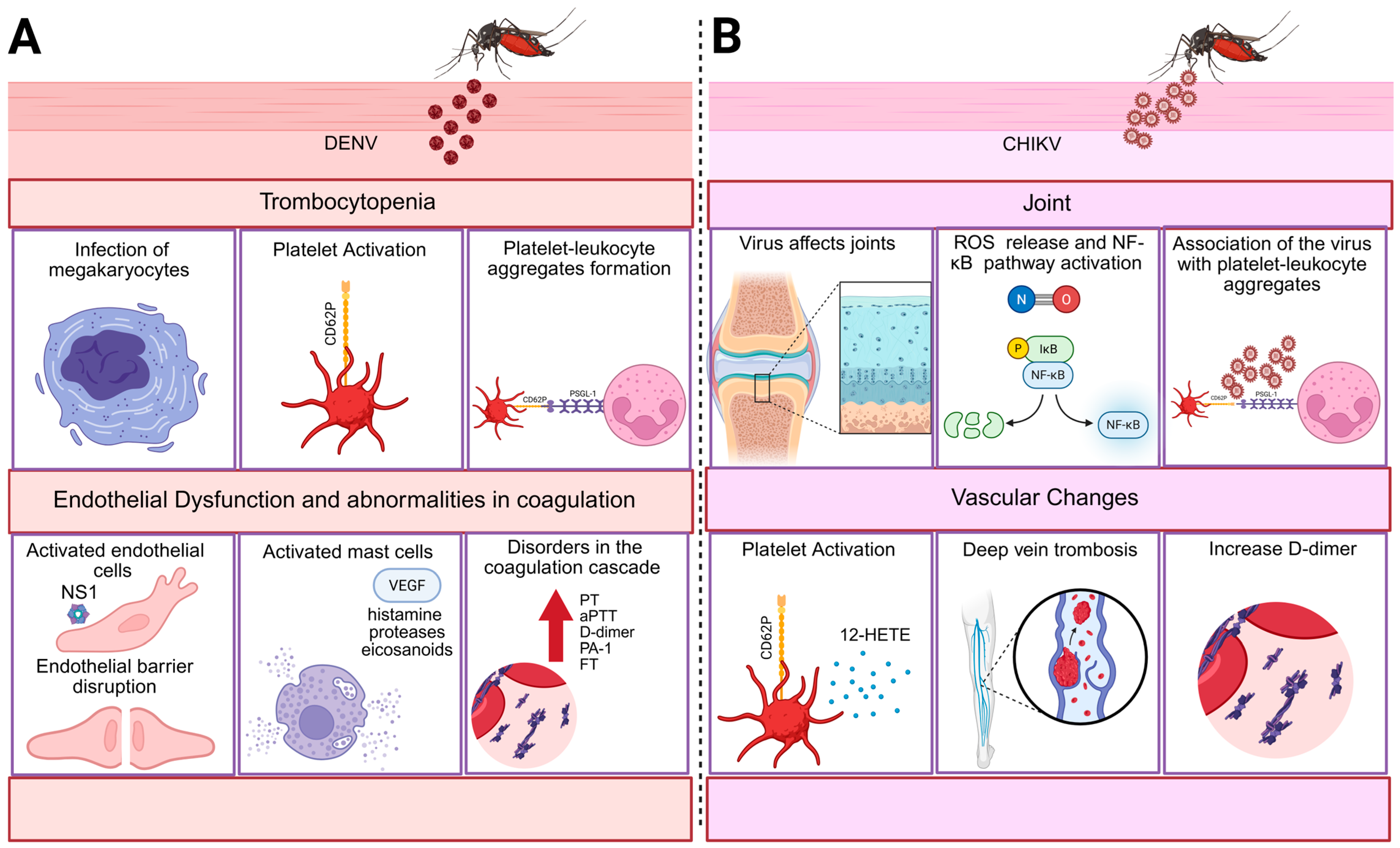
2. Thromboinflammatory Mechanisms Induced by Arboviruses
2.1. Dengue
2.1.1. Dengue-Induced Thrombocytopenia
2.1.2. Platelet–Leukocyte Aggregate Formation Mediated by P-Selectin
2.1.3. Endothelial Dysfunction
2.1.4. Abnormalities in Coagulation
2.1.5. Thromboinflammation in Dengue: Pregnancy Context
3. Chikungunya
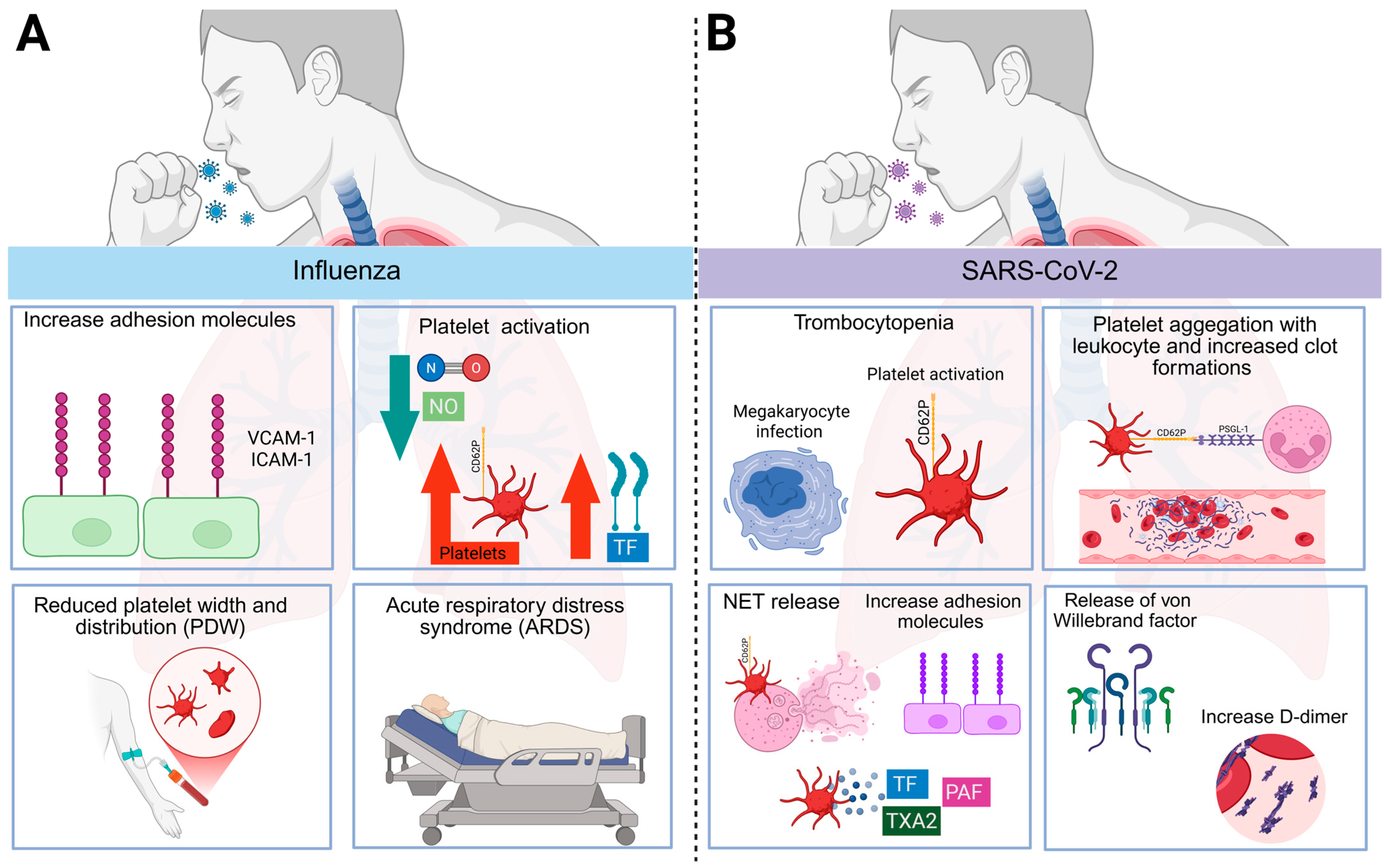
4. Influenza
5. SARS-CoV-2
6. The Evidence of Thromboinflammation and Post-Acute Viral Sequelae
7. Antithrombotic Strategies in Viral Infections: Anticoagulation and Antiplatelet Therapy
8. Conclusions
9. Methodology
| Thromboinflammatory Pathways | Dengue | Chikungunya | Influenza | SARS-CoV-2 |
|---|---|---|---|---|
| Platelet activation |
|
| ||
| Endothelial dysfunction |
|
|
| |
| Abnormalities in coagulation |
|
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aPTT | Activated partial thromboplastin time |
| ACE2 | Angiotensin-converting enzyme 2 |
| ADP | Adenosine diphosphate |
| ARDS | Respiratory distress syndrome |
| CHIKV | Chikungunya virus |
| DENV | Dengue virus |
| DHF | Dengue hemorrhagic fever |
| DIC | Disseminated intravascular coagulation |
| EGL | Endothelial glycocalyx-like layer |
| GP | Glycoprotein |
| GPIbα | Glycoprotein Ib alpha chain |
| HA | Hemagglutinin |
| HSCs | Hematopoietic stem cells |
| IAV | Influenza A virus |
| ICAM-1 | Intercellular adhesion molecule-1 |
| ISGs | Interferon-stimulated genes |
| MIF | Migration inhibitory factors |
| MCP-1 | Monocyte chemoattractant protein –1 |
| MKs | Megakaryocytes |
| MLR | Monocyte-to-lymphocyte ratio |
| MMPs | Matrix metalloproteinases |
| MRP | Myeloid-related protein |
| NA | Neuraminidase |
| NAP-2 | Neutrophil-activating peptide 2 |
| NETs | Neutrophil extracellular traps |
| NLR | Neutrophil-to-lymphocyte ratio |
| NO | Nitric oxide |
| NS1 | Non-structural protein 1 |
| PAF | Platelet-activating factor |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAR | Protease-activated receptor |
| PDW | Platelet distribution width |
| PF-4 | Platelet factor 4 |
| PLR | Platelet-to-lymphocyte ratio |
| PMAs | Platelet–monocyte aggregates |
| PSGL-1 | P-selectin glycoprotein ligand-1 |
| PT | Prothrombin time |
| ROS | Reactive oxygen species |
| SARS | Severe acute respiratory syndrome |
| sCD40L | Soluble CD40 ligand |
| SD | Severe dengue |
| TF | Tissue factor |
| TLR | Toll-like receptor |
| TXA2 | Thromboxane A2 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VLA-4 | Very late antigen-4 |
References
- Loof, T.G.; Mörgelin, M.; Johansson, L.; Oehmcke, S.; Olin, A.I.; Dickneite, G.; Norrby-Teglund, A.; Theopold, U.; Herwald, H. Coagulation, an Ancestral Serine Protease Cascade, Exerts a Novel Function in Early Immune Defense. Blood 2011, 118, 2589–2598. [Google Scholar] [CrossRef]
- Loof, T.G.; Schmidt, O.; Herwald, H.; Theopold, U. Coagulation Systems of Invertebrates and Vertebrates and Their Roles in Innate Immunity: The Same Side of Two Coins? J. Innate Immun. 2011, 3, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Thon, J.N.; Italiano, J.E. Platelets: Production, Morphology and Ultrastructure. In Antiplatelet Agents; Springer: Berlin, Heidelberg, 2012; pp. 3–22. [Google Scholar]
- Asquith, N.L.; Carminita, E.; Camacho, V.; Rodriguez-Romera, A.; Stegner, D.; Freire, D.; Becker, I.C.; Machlus, K.R.; Khan, A.O.; Italiano, J.E. The Bone Marrow Is the Primary Site of Thrombopoiesis. Blood 2024, 143, 272–278. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The Lung Is a Site of Platelet Biogenesis and a Reservoir for Haematopoietic Progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef]
- Massberg, S.; Gawaz, M.; Grüner, S.; Schulte, V.; Konrad, I.; Zohlnhöfer, D.; Heinzmann, U.; Nieswandt, B. A Crucial Role of Glycoprotein VI for Platelet Recruitment to the Injured Arterial Wall In Vivo. J. Exp. Med. 2003, 197, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M. Mechanisms Initiating Platelet Thrombus Formation. Thromb. Haemost. 1997, 78, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Almskog, L.M.; Ågren, A. Thromboinflammation vs. Immunothrombosis: Strategies for Overcoming Anticoagulant Resistance in COVID-19 and Other Hyperinflammatory Diseases. Is ROTEM Helpful or Not? Front. Immunol. 2025, 16, 1599639. [Google Scholar] [CrossRef] [PubMed]
- Schrottmaier, W.C.; Assinger, A. The Concept of Thromboinflammation. Hamostaseologie 2024, 44, 021–030. [Google Scholar] [CrossRef] [PubMed]
- Dhanesha, N.; Patel, R.B.; Doddapattar, P.; Ghatge, M.; Flora, G.D.; Jain, M.; Thedens, D.; Olalde, H.; Kumskova, M.; Leira, E.C.; et al. PKM2 Promotes Neutrophil Activation and Cerebral Thromboinflammation: Therapeutic Implications for Ischemic Stroke. Blood 2022, 139, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Polzin, A.; Benkhoff, M.; Thienel, M.; Barcik, M.; Mourikis, P.; Shchurovska, K.; Helten, C.; Ehreiser, V.; Zhe, Z.; von Wulffen, F.; et al. Long-Term FXa Inhibition Attenuates Thromboinflammation after Acute Myocardial Infarction and Stroke by Platelet Proteome Alteration. J. Thromb. Haemost. 2025, 23, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Gupta, A.; Forstner, D.; Guettler, J.; Ahrens, M.S.; Prakasan Sheeja, A.; Fatima, S.; Shamkeeva, S.; Lia, M.; Dathan-Stumpf, A.; et al. LMWH Prevents Thromboinflammation in the Placenta via HBEGF-AKT Signaling. Blood Adv. 2024, 8, 4756–4766. [Google Scholar] [CrossRef] [PubMed]
- Leal de Azeredo, E.; Solórzano, V.E.F.; de Oliveira, D.B.; Marinho, C.F.; de Souza, L.J.; da Cunha, R.V.; Damasco, P.V.; Kubelka, C.F.; De-Oliveira-Pinto, L.M. Increased Circulating Procoagulant and Anticoagulant Factors as TF and TFPI According to Severity or Infecting Serotypes in Human Dengue Infection. Microbes Infect. 2017, 19, 62–68. [Google Scholar] [CrossRef]
- Oliveira-Neto, J.T.d.; Souza, J.d.P.; Rodrigues, D.; Machado, M.R.; Alves, J.V.; Barros, P.R.; Bressan, A.F.; Silva, J.F.; Costa, T.J.; Costa, R.M.; et al. Acute Chikungunya Infection Induces Vascular Dysfunction by Directly Disrupting Redox Signaling in Endothelial Cells. Cells 2024, 13, 1770. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Agati, L.B.; Aguiar, V.C.R.; Wolosker, N.; Guerra, J.C.; de Almeida, R.P.; Alves, J.C.; Lopes, R.D.; Wakefield, T.W.; Comerota, A.J.; et al. Zika and Chikungunya Virus and Risk for Venous Thromboembolism. Clin. Appl. Thromb. 2019, 25, 1076029618821184. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.L.; Kang, L.-I.; de Assis Barros D’Elia Zanella, L.G.F.; Silveira, C.G.T.; Ho, Y.-L.; Foquet, L.; Bial, G.; McCune, B.T.; Duarte-Neto, A.N.; Thomas, A.; et al. Consumptive Coagulopathy of Severe Yellow Fever Occurs Independently of Hepatocellular Tropism and Massive Hepatic Injury. Proc. Natl. Acad. Sci. USA 2020, 117, 32648–32656. [Google Scholar] [CrossRef]
- Mackman, N.; Grover, S.P.; Antoniak, S. Tissue Factor Expression, Extracellular Vesicles, and Thrombosis after Infection with the Respiratory Viruses Influenza A Virus and Coronavirus. J. Thromb. Haemost. 2021, 19, 2652–2658. [Google Scholar] [CrossRef]
- Ryu, J.K.; Yan, Z.; Montano, M.; Sozmen, E.G.; Dixit, K.; Suryawanshi, R.K.; Matsui, Y.; Helmy, E.; Kaushal, P.; Makanani, S.K.; et al. Fibrin Drives Thromboinflammation and Neuropathology in COVID-19. Nature 2024, 633, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and Re-Emergence of Mosquito-Borne Arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Prim. 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- OMS. Recommendations for Treatment. Psychiatr. News 2009, 41, 29. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet Activation Determines the Severity of Thrombocytopenia in Dengue Infection. Sci. Rep. 2017, 7, 41697. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Lima, G.; Hottz, E.D.; de Assis, E.F.; Liechocki, S.; Souza, T.M.L.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T. Dengue Virus-Activated Platelets Modulate Monocyte Immunometabolic Response through Lipid Droplet Biogenesis and Cytokine Signaling. J. Leukoc. Biol. 2020, 108, 1293–1306. [Google Scholar] [CrossRef]
- Syenina, A.; Saron, W.A.A.; Jagaraj, C.J.; Bibi, S.; Arock, M.; Gubler, D.J.; Rathore, A.P.S.; Abraham, S.N.; St John, A.L. Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability. Viruses 2020, 12, 1379. [Google Scholar] [CrossRef]
- Adane, T.; Getawa, S. Coagulation Abnormalities in Dengue Fever Infection: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009666. [Google Scholar] [CrossRef]
- Rocha, F. Created in BioRender. Figure 1: Thromboinflammatory Mechanisms Are Induced by Arboviruses. BioRender. 2025. Available online: https://BioRender.com/daeu735 (accessed on 10 June 2025).
- Quirino-Teixeira, A.C.; Andrade, F.B.; Pinheiro, M.B.M.; Rozini, S.V.; Hottz, E.D. Platelets in Dengue Infection: More than a Numbers Game. Platelets 2022, 33, 176–183. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, D.W.; Li, K.K.H.; Li, C.K.; Chik, K.W.; Fok, T.F. Lung Damage May Induce Thrombocytopenia. Blood 2004, 104, 3026. [Google Scholar] [CrossRef]
- Moragas, L.J.; Arruda, L.V.; Oliveira, L.d.L.S.; Alves, F.d.A.V.; Salomão, N.G.; da Silva, J.F.R.; Basílio-de-Oliveira, C.A.; Basílio-de-Oliveira, R.P.; Mohana-Borges, R.; Azevedo, C.G.; et al. Detection of Viral Antigen and Inflammatory Mediators in Fatal Pediatric Dengue: A Study on Lung Immunopathogenesis. Front. Immunol. 2025, 16, 1487284. [Google Scholar] [CrossRef]
- Michels, M.; Alisjahbana, B.; de Groot, P.G.; Indrati, A.R.; Fijnheer, R.; Puspita, M.; Dewi, I.M.W.; van de Wijer, L.; de Boer, E.M.S.; Roest, M.; et al. Platelet Function Alterations in Dengue Are Associated with Plasma Leakage. Thromb. Haemost. 2014, 112, 352–362. [Google Scholar] [CrossRef]
- Hottz, E.D.; Oliveira, M.F.; Nunes, P.C.G.; Nogueira, R.M.R.; Valls-de-Souza, R.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, P.T.; Bozza, F.A. Dengue Induces Platelet Activation, Mitochondrial Dysfunction and Cell Death through Mechanisms That Involve DC-SIGN and Caspases. J. Thromb. Haemost. 2013, 11, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.A.; Oragui, E.E.; Stephens, A.C.; Daramola, O.A.; Dung, N.M.; Loan, H.T.; Chau, N.V.; Chambers, M.; Stepniewska, K.; Farrar, J.J.; et al. Coagulation Abnormalities in Dengue Hemorrhagic Fever: Serial Investigations in 167 Vietnamese Children with Dengue Shock Syndrome. Clin. Infect. Dis. 2002, 35, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Tripathi, A.; Duggal, S.; Banerjee, A.; Vrati, S. Dengue Virus Infection Impedes Megakaryopoiesis in MEG-01 Cells Where the Virus Envelope Protein Interacts with the Transcription Factor TAL-1. Sci. Rep. 2020, 10, 19587. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Spencer Clinton, J.L.; Rico-Hesse, R. Dengue Viruses Infect Human Megakaryocytes, with Probable Clinical Consequences. PLoS Negl. Trop. Dis. 2019, 13, e0007837. [Google Scholar] [CrossRef]
- Sridharan, A.; Chen, Q.; Tang, K.F.; Ooi, E.E.; Hibberd, M.L.; Chen, J. Inhibition of Megakaryocyte Development in the Bone Marrow Underlies Dengue Virus-Induced Thrombocytopenia in Humanized Mice. J. Virol. 2013, 87, 11648–11658. [Google Scholar] [CrossRef]
- Kaur, J.; Rawat, Y.; Sood, V.; Periwal, N.; Rathore, D.K.; Kumar, S.; Kumar, N.; Bhattacharyya, S. Replication of Dengue Virus in K562-Megakaryocytes Induces Suppression in the Accumulation of Reactive Oxygen Species. Front. Microbiol. 2022, 12, 784070. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Noisakran, S.; Onlamoon, N.; Hsiao, H.-M.; Roback, J.; Villinger, F.; Ansari, A.A.; Perng, G.C. Multiploid CD61+ Cells Are the Pre-Dominant Cell Lineage Infected during Acute Dengue Virus Infection in Bone Marrow. PLoS ONE 2012, 7, e52902. [Google Scholar] [CrossRef]
- La Russa, V.F.; Innis, B.L. 11 Mechanisms of Dengue Virus-Induced Bone Marrow Suppression. Baillieres. Clin. Haematol. 1995, 8, 249–270. [Google Scholar] [CrossRef]
- Poirault-Chassac, S.; Six, E.; Catelain, C.; Lavergne, M.; Villeval, J.-L.; Vainchenker, W.; Lauret, E. Notch/Delta4 Signaling Inhibits Human Megakaryocytic Terminal Differentiation. Blood 2010, 116, 5670–5678. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Schwertz, H.; Hottz, E.D.; Rowley, J.W.; Manne, B.K.; Washington, A.V.; Hunter-Mellado, R.; Tolley, N.D.; Christensen, M.; Eustes, A.S.; et al. Human Megakaryocytes Possess Intrinsic Antiviral Immunity through Regulated Induction of IFITM3. Blood 2019, 133, 2013–2026. [Google Scholar] [CrossRef]
- Morodomi, Y.; Kanaji, S.; Sullivan, B.M.; Zarpellon, A.; Orje, J.N.; Won, E.; Shapiro, R.; Yang, X.-L.; Ruf, W.; Schimmel, P.; et al. Inflammatory Platelet Production Stimulated by Tyrosyl-TRNA Synthetase Mimicking Viral Infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2212659119. [Google Scholar] [CrossRef]
- Pozner, R.G.; Ure, A.E.; Jaquenod de Giusti, C.; D’Atri, L.P.; Italiano, J.E.; Torres, O.; Romanowski, V.; Schattner, M.; Gómez, R.M. Junín Virus Infection of Human Hematopoietic Progenitors Impairs In Vitro Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling. PLoS Pathog. 2010, 6, e1000847. [Google Scholar] [CrossRef] [PubMed]
- Ravid, K.; Lu, J.; Zimmet, J.M.; Jones, M.R. Roads to Polyploidy: The Megakaryocyte Example. J. Cell. Physiol. 2002, 190, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Trugilho, M.R.d.O.; Hottz, E.D.; Brunoro, G.V.F.; Teixeira-Ferreira, A.; Carvalho, P.C.; Salazar, G.A.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T.; Perales, J. Platelet Proteome Reveals Novel Pathways of Platelet Activation and Platelet-Mediated Immunoregulation in Dengue. PLOS Pathog. 2017, 13, e1006385. [Google Scholar] [CrossRef]
- Wan, S.-W.; Yang, Y.-W.; Chu, Y.-T.; Lin, C.-F.; Chang, C.-P.; Yeh, T.-M.; Anderson, R.; Lin, Y.-S. Anti-Dengue Virus Nonstructural Protein 1 Antibodies Contribute to Platelet Phagocytosis by Macrophages. Thromb. Haemost. 2016, 115, 646–656. [Google Scholar] [CrossRef]
- Alonzo, M.T.G.; Lacuesta, T.L.V.; Dimaano, E.M.; Kurosu, T.; Suarez, L.C.; Mapua, C.A.; Akeda, Y.; Matias, R.R.; Kuter, D.J.; Nagata, S.; et al. Platelet Apoptosis and Apoptotic Platelet Clearance by Macrophages in Secondary Dengue Virus Infections. J. Infect. Dis. 2012, 205, 1321–1329. [Google Scholar] [CrossRef]
- Hottz, E.D.; Medeiros-de-Moraes, I.M.; Vieira-de-Abreu, A.; de Assis, E.F.; Vals-de-Souza, R.; Castro-Faria-Neto, H.C.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T. Platelet Activation and Apoptosis Modulate Monocyte Inflammatory Responses in Dengue. J. Immunol. 2014, 193, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Masri, M.F.B.; Mantri, C.K.; Rathore, A.P.S.; St. John, A.L. Peripheral Serotonin Causes Dengue Virus–Induced Thrombocytopenia through 5HT2 Receptors. Blood 2019, 133, 2325–2337. [Google Scholar] [CrossRef] [PubMed]
- Khazali, A.S.; Hadrawi, W.H.; Ibrahim, F.; Othman, S.; Nor Rashid, N. Thrombocytopenia in Dengue Infection: Mechanisms and a Potential Application. Expert Rev. Mol. Med. 2024, 26, e26. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, A.D.; Saliakhutdinova, S.M.; Sounbuli, K.; Selivanova, Y.A.; Andrianova, I.A.; Khabirova, A.I.; Litvinov, R.I.; Weisel, J.W. The Differential Formation and Composition of Leukocyte-Platelet Aggregates Induced by Various Cellular Stimulants. Thromb. Res. 2024, 241, 109092. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Role of Platelet P-Selectin and Microparticle PSGL-1 in Thrombus Formation. Trends Mol. Med. 2004, 10, 171–178. [Google Scholar] [CrossRef]
- Moore, K.L.; Stults, N.L.; Diaz, S.; Smith, D.F.; Cummings, R.D.; Varki, A.; McEver, R.P. Identification of a Specific Glycoprotein Ligand for P-Selectin (CD62) on Myeloid Cells. J. Cell Biol. 1992, 118, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, L.; Geng, Z.H.; Wang, H.-B.; Wang, J.-T.; Chen, M.; Geng, J.-G. P-Selectin Cross-Links PSGL-1 and Enhances Neutrophil Adhesion to Fibrinogen and ICAM-1 in a Src Kinase-Dependent, but GPCR-Independent Mechanism. Cell Adh. Migr. 2007, 1, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-J.; Jen, Y.-H.; Chang, J.-S.; Hsiao, H.-M.; Noisakran, S.; Perng, G.C. Frequency Alterations in Key Innate Immune Cell Components in the Peripheral Blood of Dengue Patients Detected by FACS Analysis. J. Innate Immun. 2011, 3, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.I.; Benoit, S.E.; Barnard, M.R.; Valeri, C.R.; Borbone, M.L.; Becker, R.C.; Hechtman, H.B.; Michelson, A.D. Increased Platelet Reactivity and Circulating Monocyte-Platelet Aggregates in Patients with Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 1998, 31, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.; Barrett, T.J.; Guo, Y.; Nardi, M.; Ramkhelawon, B.; Rockman, C.B.; Hochman, J.S.; Berger, J.S. Circulating Monocyte-Platelet Aggregates Are a Robust Marker of Platelet Activity in Cardiovascular Disease. Atherosclerosis 2019, 282, 11–18. [Google Scholar] [CrossRef]
- Passacquale, G.; Vamadevan, P.; Pereira, L.; Hamid, C.; Corrigall, V.; Ferro, A. Monocyte-Platelet Interaction Induces a Pro-Inflammatory Phenotype in Circulating Monocytes. PLoS ONE 2011, 6, e25595. [Google Scholar] [CrossRef]
- Lien, T.-S.; Chan, H.; Sun, D.-S.; Wu, J.-C.; Lin, Y.-Y.; Lin, G.-L.; Chang, H.-H. Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice. Front. Immunol. 2021, 12, 616394. [Google Scholar] [CrossRef]
- Sung, P.-S.; Huang, T.-F.; Hsieh, S.-L. Extracellular Vesicles from CLEC2-Activated Platelets Enhance Dengue Virus-Induced Lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef]
- Garishah, F.M.; Rother, N.; Riswari, S.F.; Alisjahbana, B.; Overheul, G.J.; van Rij, R.P.; van der Ven, A.; van der Vlag, J.; de Mast, Q. Neutrophil Extracellular Traps in Dengue Are Mainly Generated NOX-Independently. Front. Immunol. 2021, 12, 629167. [Google Scholar] [CrossRef]
- Böer, L.M.; Junqueira, I.C.; Nascimento, T.C.d.; Guilarde, A.O.; Féres, V.C.d.R.; Alcântara, K.C.d. Monocyte-Lymphocyte, Neutrophil-Lymphocyte, and Platelet-Lymphocyte Ratios as Inflammatory Biomarkers of Clinical Dengue Severity. Biosci. J. 2024, 40, e40038. [Google Scholar] [CrossRef]
- Li, N.; Ji, Q.; Hjemdahl, P. Platelet–Lymphocyte Conjugation Differs between Lymphocyte Subpopulations. J. Thromb. Haemost. 2006, 4, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Nikdoust, F.; Khorram, S.; Marashi, S.M.; Ghanavati, P.; Ameri, F.; Akbarzadeh, A.; Hasanvand, A.; Khodakarim, N. Dengue Virus Infection: How Platelet-Leukocyte Crosstalk Shapes Thrombotic Events and Inflammation. Mol. Biol. Rep. 2025, 52, 119. [Google Scholar] [CrossRef]
- Vats, R.; Brzoska, T.; Bennewitz, M.F.; Jimenez, M.A.; Pradhan-Sundd, T.; Tutuncuoglu, E.; Jonassaint, J.; Gutierrez, E.; Watkins, S.C.; Shiva, S.; et al. Platelet Extracellular Vesicles Drive Inflammasome–IL-1β–Dependent Lung Injury in Sickle Cell Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, V.; Hawley, A.; Farris, D.; Knipp, B.; Varga, A.; Wrobleski, S.; Thanapron, P.; Eagleton, M.; Myers, D.; Fowlkes, J.; et al. Decrease in Fibrin Content of Venous Thrombi in Selectin-Deficient Mice. J. Surg. Res. 2003, 109, 1–7. [Google Scholar] [CrossRef]
- Yokoyama, S.; Ikeda, H.; Haramaki, N.; Yasukawa, H.; Murohara, T.; Imaizumi, T. Platelet P-Selectin Plays an Important Role in Arterial Thrombogenesis by Forming Large Stable Platelet-Leukocyte Aggregates. J. Am. Coll. Cardiol. 2005, 45, 1280–1286. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, L.K.; Lye, D.C.; Leo, Y.S. Current Management of Severe Dengue Infection. Expert Rev. Anti. Infect. Ther. 2017, 15, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, S.; Wertheim, H.; Simmons, C.P.; Screaton, G.; Wills, B. Microvascular and Endothelial Function for Risk Prediction in Dengue: An Observational Study. Lancet 2015, 385, S102. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLOS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue Virus NS1 Protein Activates Cells via Toll-like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Chao, C.-H.; Yeh, T.-M. Macrophage Migration Inhibitory Factor Induces Vascular Leakage via Autophagy. Biol. Open 2015, 4, 244–252. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Liu, C.-C.; Perng, G.-C.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl. Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Quirino-Teixeira, A.C.; Rozini, S.V.; Barbosa-Lima, G.; Coelho, D.R.; Carneiro, P.H.; Mohana-Borges, R.; Bozza, P.T.; Hottz, E.D. Inflammatory Signaling in Dengue-Infected Platelets Requires Translation and Secretion of Nonstructural Protein 1. Blood Adv. 2020, 4, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-H.; Wu, W.-C.; Lai, Y.-C.; Tsai, P.-J.; Perng, G.-C.; Lin, Y.-S.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1 Activates Platelets via Toll-like Receptor 4, Leading to Thrombocytopenia and Hemorrhage. PLOS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef] [PubMed]
- Assunção-Miranda, I.; Amaral, F.A.; Bozza, F.A.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of Macrophage Migration Inhibitory Factor to the Pathogenesis of Dengue Virus Infection. FASEB J. 2010, 24, 218–228. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lei, H.-Y.; Liu, H.-S.; Lin, Y.-S.; Fu, T.-F.; Yeh, T.-M. Macrophage Migration Inhibitory Factor Induced by Dengue Virus Infection Increases Vascular Permeability. Cytokine 2011, 54, 222–231. [Google Scholar] [CrossRef]
- Anderson, R.; Wang, S.; Osiowy, C.; Issekutz, A.C. Activation of Endothelial Cells via Antibody-Enhanced Dengue Virus Infection of Peripheral Blood Monocytes. J. Virol. 1997, 71, 4226–4232. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.N.; Pastorino, B.; Grau, G.E.; Lou, J.; Tolou, H.; Couissinier-Paris, P. Dengue Virus Infection of Human Microvascular Endothelial Cells from Different Vascular Beds Promotes Both Common and Specific Functional Changes. J. Med. Virol. 2006, 78, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Murao, L.A.; Lan, N.T.P.; Huy, N.T.; Huong, V.T.Q.; Thuy, T.T.; Tham, V.D.; Nga, C.T.P.; Ha, T.T.N.; Ohmoto, Y.; et al. Association of Mast Cell-Derived VEGF and Proteases in Dengue Shock Syndrome. PLoS Negl. Trop. Dis. 2012, 6, e1505. [Google Scholar] [CrossRef]
- Brown, M.G.; King, C.A.; Sherren, C.; Marshall, J.S.; Anderson, R. A Dominant Role for FcγRII in Antibody-Enhanced Dengue Virus Infection of Human Mast Cells and Associated CCL5 Release. J. Leukoc. Biol. 2006, 80, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of Vasoactive Cytokines by Antibody-Enhanced Dengue Virus Infection of a Human Mast Cell/Basophil Line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef]
- Phan, D.T.; Ha, N.T.; Thuc, L.T.; Diet, N.H.; Phu, L.V.; Ninh, L.Y.; An, V.T. Some Changes in Immunity and Blood in Relation to Clinical States of Dengue Hemorrhagic Fever Patients in Vietnam. Haematologia 1991, 24, 13–21. [Google Scholar] [PubMed]
- Tuchinda, M.; Dhorranintra, B.; Tuchinda, P. Histamine Content in 24-Hour Urine in Patients with Dengue Haemorrhagic Fever. Southeast Asian J. Trop. Med. Public Health 1977, 8, 80–83. [Google Scholar]
- St John, A.L. Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology. PLoS Pathog. 2013, 9, e1003783. [Google Scholar] [CrossRef]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue Virus Selectively Induces Human Mast Cell Chemokine Production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; Hermann, L.L.; Issekutz, A.C.; Marshall, J.S.; Rowter, D.; Al-Afif, A.; Anderson, R. Dengue Virus Infection of Mast Cells Triggers Endothelial Cell Activation. J. Virol. 2011, 85, 1145–1150. [Google Scholar] [CrossRef]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA Sensors Enable Human Mast Cell Anti-Viral Chemokine Production and IFN-Mediated Protection in Response to Antibody-Enhanced Dengue Virus Infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Narayanan, K.; Sasikumar, S.; Philipose, J.; Surendran, S. Coagulopathy in Dengue Fever Patients. Int. J. Res. Med. Sci. 2014, 2, 1070. [Google Scholar] [CrossRef]
- Wills, B.; Van Ngoc, T.; Van, N.T.H.; Thuy, T.T.T.; Thuy, T.T.N.; Dung, N.M.; Diet, T.V.; Van Vinh Chau, N.; Trung, D.T.; Farrar, J. Hemostatic Changes in Vietnamese Children with Mild Dengue Correlate with the Severity of Vascular Leakage Rather than Bleeding. Am. J. Trop. Med. Hyg. 2009, 81, 638–644. [Google Scholar] [CrossRef]
- Avila-Aguero, M.L.; Avila-Aguero, C.R.; Um, S.L.; Soriano-Fallas, A.; Cañas-Coto, A.; Yan, S.B. Systemic Host Inflammatory and Coagulation Response in the Dengue Virus Primo-Infection. Cytokine 2004, 27, 173–179. [Google Scholar] [CrossRef]
- Ho, T.-S.; Wang, S.-M.; Lin, Y.-S.; Liu, C.-C. Clinical and Laboratory Predictive Markers for Acute Dengue Infection. J. Biomed. Sci. 2013, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Liu, C.-C.; Wang, S.-T.; Lei, H.-Y.; Liu, H.-S.; Lin, Y.-S.; Wu, H.-L.; Yeh, T.-M. Activation of Coagulation and Fibrinolysis during Dengue Virus Infection. J. Med. Virol. 2001, 63, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, H.; Martino, N.; Garrett, J.P.; Adam, A.P. Interleukin-6 Promotes a Sustained Loss of Endothelial Barrier Function via Janus Kinase-Mediated STAT3 Phosphorylation and de Novo Protein Synthesis. Am. J. Physiol. Physiol. 2018, 314, C589–C602. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; ten Cate, H. Disseminated Intravascular Coagulation. N. Engl. J. Med. 1999, 341, 586–592. [Google Scholar] [CrossRef]
- Trotta, R.F.; Diehl, L.F.; Shorr, A.F.; Hanzel, G.S.; Alkins, S.A. D-Dimer Assay Predicts Mortality in Critically Ill Patients without Disseminated Intravascular Coagulation or Venous Thromboembolic Disease. Intensive Care Med. 1999, 25, 207–210. [Google Scholar] [CrossRef]
- Nurnaningsih; Sunbanu, S.E.; Rusmawatiningtyas, D.; Arguni, E.; Makrufardi, F.; Kumara, I.F. Disseminated Intravascular Coagulation Initial Score as a Predictor of Mortality in Children with Dengue Shock Syndrome: A Retrospective Cohort Study. Ann. Med. Surg. 2022, 79, 103890. [Google Scholar] [CrossRef]
- Das, S.; Mallik, M.H.; Chattopadyay, P.; Mallick, S.; Karmakar, D.; Ghora, S.; Begum, F.; Chatterjee, B.; Thagriki, D.S.; Srivastava, A.K.; et al. Dengue Virus NS1 Leads to Downregulation of HNF4 Alpha in Liver Cells Resulting in a Decrease in Coagulation Factors I, V, X, and XIII, Contributing to Coagulopathy. J. Virol. 2024, 98, e01418-24. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Hensley, L.E.; Jahrling, P.B.; Larsen, T.; Geisbert, J.B.; Paragas, J.; Young, H.A.; Fredeking, T.M.; Rote, W.E.; Vlasuk, G.P. Treatment of Ebola Virus Infection with a Recombinant Inhibitor of Factor VIIa/Tissue Factor: A Study in Rhesus Monkeys. Lancet 2003, 362, 1953–1958. [Google Scholar] [CrossRef]
- Geisbert, T.W.; Young, H.A.; Jahrling, P.B.; Davis, K.J.; Kagan, E.; Hensley, L.E. Mechanisms Underlying Coagulation Abnormalities in Ebola Hemorrhagic Fever: Overexpression of Tissue Factor in Primate Monocytes/Macrophages Is a Key Event. J. Infect. Dis. 2003, 188, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Francischetti, I.M.B.; Seydel, K.B.; Monteiro, R.Q. Blood Coagulation, Inflammation, and Malaria. Microcirculation 2008, 15, 81–107. [Google Scholar] [CrossRef]
- de Azeredo, E.L.; Kubelka, C.F.; Alburquerque, L.M.; Barbosa, L.S.; Damasco, P.V.; Ávila, C.A.L.; Motta-Castro, A.R.C.; da Cunha, R.V.; Monteiro, R.Q. Tissue Factor Expression on Monocytes from Patients with Severe Dengue Fever. Blood Cells Mol. Dis. 2010, 45, 334–335. [Google Scholar] [CrossRef]
- Huerta-Zepeda, A.; Cabello-Gutiérrez, C.; Cime-Castillo, J.; Monroy-Martínez, V.; Manjarrez-Zavala, M.E.; Gutiérrez-Rodríguez, M.; Izaguirre, R.; Ruiz-Ordaz, B. Crosstalk between Coagulation and Inflammation during Dengue Virus Infection. Thromb. Haemost. 2008, 99, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, V.; Taskou, C.; Sarantaki, A.; Spandidos, D.; Gourounti, K.; Chaniotis, D.; Beloukas, A. Vector-borne Infectious Diseases in Pregnancy in the Era of Climate Change: A Focus on Mosquito- and Tick-borne Pathogens (Review). Exp. Ther. Med. 2025, 30, 174. [Google Scholar] [CrossRef] [PubMed]
- Steward, K.; Raja, A. Physiology, Ovulation and Basal Body Temperature; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Goicochea-Ríos, E.d.S.; Otiniano, N.M.; Rojas-Infantas, L.d.C.; Ocaña-Gutiérrez, V.R.; Gómez-Goicochea, N.I. Dengue Infection during Pregnancy and the Occurrence of Pathological Neonatal Outcome: A Systematic Review and Meta-Analysis. F1000Research 2025, 13, 1523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Wilson, M.E. Update on Non-Vector Transmission of Dengue: Relevant Studies with Zika and Other Flaviviruses. Trop. Dis. Travel Med. Vaccines 2016, 2, 15. [Google Scholar] [CrossRef]
- Kerdpanich, A.; Watanaveeradej, V.; Samakoses, R.; Chumnanvanakij, S.; Chulyamitporn, T.; Sumeksri, P.; Vuthiwong, C.; Kounruang, C.; Nisalak, A.; Endy, T. Perinatal Dengue Infection. Southeast Asian J. Trop. Med. Public Health 2001, 32, 488–493. [Google Scholar]
- Basurko, C.; Matheus, S.; Hildéral, H.; Everhard, S.; Restrepo, M.; Cuadro-Alvarez, E.; Lambert, V.; Boukhari, R.; Duvernois, J.-P.; Favre, A.; et al. Estimating the Risk of Vertical Transmission of Dengue: A Prospective Study. Am. J. Trop. Med. Hyg. 2018, 98, 1826–1832. [Google Scholar] [CrossRef]
- Machain-Williams, C.; Raga, E.; Baak-Baak, C.M.; Kiem, S.; Blitvich, B.J.; Ramos, C. Maternal, Fetal, and Neonatal Outcomes in Pregnant Dengue Patients in Mexico. BioMed Res. Int. 2018, 2018, 9643083. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Ahmed, K.S.; Mubashir, H.; Quddusi, A.; Farooq, A.; Ahmed, S.I.; Jamil, B.; Qureshi, R. Dengue and Malaria Infections in Pregnancy. Wien. Klin. Wochenschr. 2020, 132, 188–196. [Google Scholar] [CrossRef]
- Paixão, E.S.; Teixeira, M.G.; Costa, M.d.C.N.; Rodrigues, L.C. Dengue during Pregnancy and Adverse Fetal Outcomes: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016, 16, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Vouga, M.; Chiu, Y.-C.; Pomar, L.; de Meyer, S.V.; Masmejan, S.; Genton, B.; Musso, D.; Baud, D.; Stojanov, M. Dengue, Zika and Chikungunya during Pregnancy: Pre- and Post-Travel Advice and Clinical Management. J. Travel Med. 2019, 26, taz077. [Google Scholar] [CrossRef] [PubMed]
- Brar, R.; Sikka, P.; Suri, V.; Singh, M.P.; Suri, V.; Mohindra, R.; Biswal, M. Maternal and Fetal Outcomes of Dengue Fever in Pregnancy: A Large Prospective and Descriptive Observational Study. Arch. Gynecol. Obstet. 2021, 304, 91–100. [Google Scholar] [CrossRef]
- Nunes, P.C.G.; Daumas, R.P.; Sánchez-Arcila, J.C.; Nogueira, R.M.R.; Horta, M.A.P.; dos Santos, F.B. 30 Years of Fatal Dengue Cases in Brazil: A Review. BMC Public Health 2019, 19, 329. [Google Scholar] [CrossRef]
- Hariyanto, H.; Yahya, C.Q.; Wibowo, P.; Tampubolon, O.E. Management of Severe Dengue Hemorrhagic Fever and Bleeding Complications in a Primigravida Patient: A Case Report. J. Med. Case Rep. 2016, 10, 357. [Google Scholar] [CrossRef]
- Thaithumyanon, P.; Thisyakorn, U.; Deerojnawong, J.; Innis, B.L. Dengue Infection Complicated by Severe Hemorrhage and Vertical Transmission in a Parturient Woman. Clin. Infect. Dis. 1994, 18, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Kril, V.; Aïqui-Reboul-Paviet, O.; Briant, L.; Amara, A. New Insights into Chikungunya Virus Infection and Pathogenesis. Annu. Rev. Virol. 2021, 8, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.; Failloux, A.-B.; Weaver, S. Chikungunya Virus–Vector Interactions. Viruses 2014, 6, 4628–4663. [Google Scholar] [CrossRef] [PubMed]
- van Aalst, M.; Nelen, C.M.; Goorhuis, A.; Stijnis, C.; Grobusch, M.P. Long-Term Sequelae of Chikungunya Virus Disease: A Systematic Review. Travel Med. Infect. Dis. 2017, 15, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Roux, K.L.; Prevost, M.-C.; Fsihi, H.; et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A Mouse Model for Chikungunya: Young Age and Inefficient Type-I Interferon Signaling Are Risk Factors for Severe Disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Colavita, F.; Bordi, L.; Lalle, E.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Tropism of the Chikungunya Virus. Viruses 2019, 11, 175. [Google Scholar] [CrossRef]
- Ganu, M.A.; Ganu, A.S. Post-Chikungunya Chronic Arthritis--Our Experience with DMARDs over Two Year Follow Up. J. Assoc. Physicians India 2011, 59, 83–86. [Google Scholar]
- Lauridsen, C. From Oxidative Stress to Inflammation: Redox Balance and Immune System. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. ROS Signaling and Redox Biology in Endothelial Cells. Cell. Mol. Life Sci. 2015, 72, 3281–3303. [Google Scholar] [CrossRef] [PubMed]
- Bryce Larke, R.P.; Wheelock, E.F. Stabilization of Chikungunya Virus Infectivity by Human Blood Platelets. J. Infect. Dis. 1970, 122, 523–531. [Google Scholar] [CrossRef]
- Chernesky, M.A.; Larke, R.P.B. Contrasting Effects of Rabbit and Human Platelets on Chikungunya Virus Infectivity. Can. J. Microbiol. 1977, 23, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Azevedo-Quintanilha, I.; Campos, M.M.; Teixeira Monteiro, A.P.; Dantas do Nascimento, A.; Calheiros, A.S.; Oliveira, D.M.; Dias, S.S.G.; Soares, V.C.; Santos, J.d.C.; Tavares, I.; et al. Increased Platelet Activation and Platelet-Inflammasome Engagement during Chikungunya Infection. Front. Immunol. 2022, 13, 958820. [Google Scholar] [CrossRef] [PubMed]
- Duchez, A.-C.; Boudreau, L.H.; Naika, G.S.; Bollinger, J.; Belleannée, C.; Cloutier, N.; Laffont, B.; Mendoza-Villarroel, R.E.; Lévesque, T.; Rollet-Labelle, E.; et al. Platelet Microparticles Are Internalized in Neutrophils via the Concerted Activity of 12-Lipoxygenase and Secreted Phospholipase A 2-IIA. Proc. Natl. Acad. Sci. USA 2015, 112, E3564–E3573. [Google Scholar] [CrossRef]
- Alvarez, M.F.; Bolívar-Mejía, A.; Rodriguez-Morales, A.J.; Ramirez-Vallejo, E. Cardiovascular Involvement and Manifestations of Systemic Chikungunya Virus Infection: A Systematic Review. F1000Research 2017, 6, 390. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.A.; Adami de Sá, F.P.; Lupi, O.; Brasil, P.; von Ristow, A. Trombose Venosa Profunda e Vírus Chicungunha. J. Vasc. Bras. 2017, 16, 60–62. [Google Scholar] [CrossRef][Green Version]
- He, X.; Balati, A.; Wang, W.; Wang, H.; Zhang, B.; Li, C.; Yu, D.; Guo, S.; Zeng, H. Association of Thrombocytopenia and D-Dimer Elevation with in-Hospital Mortality in Acute Aortic Dissection. Ann. Med. 2025, 57, 2478477. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Tract Infections in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef]
- Hill, N.J.; Runstadler, J.A. A Bird’s Eye View of Influenza A Virus Transmission: Challenges with Characterizing Both Sides of a Co-Evolutionary Dynamic. Integr. Comp. Biol. 2016, 56, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.S.; Choi, J.Y.; Fieldhouse, J.K.; Borkenhagen, L.K.; Zemke, J.; Zhang, D.; Gray, G.C. The Continual Threat of Influenza Virus Infections at the Human–Animal Interface. Evol. Med. Public Health 2018, 2018, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cui, Q.; Rong, L. Competitive Cooperation of Hemagglutinin and Neuraminidase during Influenza A Virus Entry. Viruses 2019, 11, 458. [Google Scholar] [CrossRef]
- Rago, F.; Melo, E.M.; Miller, L.M.; Duray, A.M.; Batista Felix, F.; Vago, J.P.; de Faria Gonçalves, A.P.; Angelo, A.L.P.M.; Cassali, G.D.; de Gaetano, M.; et al. Treatment with Lipoxin A4 Improves Influenza A Infection Outcome, Induces Macrophage Reprogramming, Anti-Inflammatory and pro-Resolutive Responses. Inflamm. Res. 2024, 73, 1903–1918. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The Role of Platelets in Mediating a Response to Human Influenza Infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Dou, L.; Liu, Y.; Zhang, J. Relationship of Influenza Virus to Inflammatory Factors and Immune Function in Elderly Patients with COPD: A Retrospective Analysis. Technol. Health Care 2025, 33, 09287329251317307. [Google Scholar] [CrossRef]
- Fang, X.; Gao, J.; Zhang, Z.; Yang, X.; Wang, Q.; Wang, J. Platelet Distribution Width in Patients with Influenza A Virus-Induced Acute Respiratory Distress Syndrome: An Old Indicator with Promising Clinical Application. Diagn. Microbiol. Infect. Dis. 2025, 111, 116657. [Google Scholar] [CrossRef]
- Videm, V.; Albrigtsen, M. Soluble ICAM-1 and VCAM-1 as Markers of Endothelial Activation. Scand. J. Immunol. 2008, 67, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, G.; Berardi, E.; Messina, F.; Marcotullio, M.C.; Gresele, P. Effects of 3,5,4′-Tri-[4-(Nitrooxy)Butanoyl]Oxy Resveratrol, a New Nitric Oxide-Releasing Derivative of Resveratrol, on Platelet Activation. Pharmacol. Rep. 2025, 77, 729–738. [Google Scholar] [CrossRef]
- Antoniak, S.; Tatsumi, K.; Hisada, Y.; Milner, J.J.; Neidich, S.D.; Shaver, C.M.; Pawlinski, R.; Beck, M.A.; Bastarache, J.A.; Mackman, N. Tissue Factor Deficiency Increases Alveolar Hemorrhage and Death in Influenza A Virus-infected Mice. J. Thromb. Haemost. 2016, 14, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yu, H.; Oh, E.B.; Park, J.W.; Kim, S.; Kim, T.; Sohn, J.; Jin, B.-R.; Chang, T.-S. Targeting NADPH Oxidase with APX-115: Suppression of Platelet Activation and Thrombotic Response. Antioxid. Redox Signal. 2025, 43, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Mohtar, S.H.; Othman, R.; Hassan, R.M.; Liang, K.; Lei, D.; Xu, B. Platelet Distribution Width as an Useful Indicator of Influenza Severity in Children. BMC Infect. Dis. 2024, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.J.G.; Spaan, T.; Low, H.Z.; Di Iorio, D.; van den Brand, J.; Tieke, M.; Barendrecht, A.; Rohn, K.; van Amerongen, G.; Stittelaar, K.; et al. Influenza-Induced Thrombocytopenia Is Dependent on the Subtype and Sialoglycan Receptor and Increases with Virus Pathogenicity. Blood Adv. 2020, 4, 2967–2978. [Google Scholar] [CrossRef]
- Rommel, M.G.E.; Walz, L.; Fotopoulou, F.; Kohlscheen, S.; Schenk, F.; Miskey, C.; Botezatu, L.; Krebs, Y.; Voelker, I.M.; Wittwer, K.; et al. Influenza A Virus Infection Instructs Hematopoiesis to Megakaryocyte-Lineage Output. Cell Rep. 2022, 41, 111447. [Google Scholar] [CrossRef] [PubMed]
- Lê, V.B.; Schneider, J.G.; Boergeling, Y.; Berri, F.; Ducatez, M.; Guerin, J.-L.; Adrian, I.; Errazuriz-Cerda, E.; Frasquilho, S.; Antunes, L.; et al. Platelet Activation and Aggregation Promote Lung Inflammation and Influenza Virus Pathogenesis. Am. J. Respir. Crit. Care Med. 2015, 191, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Rondina, M.T.; Brewster, B.; Grissom, C.K.; Zimmerman, G.A.; Kastendieck, D.H.; Harris, E.S.; Weyrich, A.S. In Vivo Platelet Activation in Critically Ill Patients with Primary 2009 Influenza A(H1N1). Chest 2012, 141, 1490–1495. [Google Scholar] [CrossRef]
- Boilard, E.; Paré, G.; Rousseau, M.; Cloutier, N.; Dubuc, I.; Lévesque, T.; Borgeat, P.; Flamand, L. Influenza Virus H1N1 Activates Platelets through FcγRIIA Signaling and Thrombin Generation. Blood 2014, 123, 2854–2863. [Google Scholar] [CrossRef]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J.; Georas, S.N.; Veazey, J.; et al. Lung Megakaryocytes Are Immune Modulatory Cells. J. Clin. Investig. 2021, 131, e137377. [Google Scholar] [CrossRef]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Van Vinh Chau, N.; Khanh, T.H.; Dong, V.C.; et al. Fatal Outcome of Human Influenza A (H5N1) Is Associated with High Viral Load and Hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Avnon, L.S.; Munteanu, D.; Smoliakov, A.; Jotkowitz, A.; Barski, L. Thromboembolic Events in Patients with Severe Pandemic Influenza A/H1N1. Eur. J. Intern. Med. 2015, 26, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Obi, A.T.; Tignanelli, C.J.; Jacobs, B.N.; Arya, S.; Park, P.K.; Wakefield, T.W.; Henke, P.K.; Napolitano, L.M. Empirical Systemic Anticoagulation Is Associated with Decreased Venous Thromboembolism in Critically Ill Influenza A H1N1 Acute Respiratory Distress Syndrome Patients. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Liu, S.; Zhou, L.; Zhu, Y.; Wang, W.; Wei, M.; Xu, X.; Liu, Y.; Shuai, Q.; Yu, J.; et al. Immunogenicity and Safety of 1 versus 2 Doses of Quadrivalent-Inactivated Influenza Vaccine in Children Aged 3–8 Years with or without Previous Influenza Vaccination Histories. Hum. Vaccin. Immunother. 2025, 21, 2468074. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F. Created in BioRender. Figure 2: Respiratory Virus-Induced Thromboinflammation. BioRender. 2025. Available online: https://BioRender.com/flkjij2 (accessed on 10 June 2025).
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and Its Association with Mortality in Patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Gonçalves, J.J.; da Mata, C.P.S.M.; Lourenço, A.A.; Ribeiro, Á.L.; Ferreira, G.M.; Fraga-Silva, T.F.d.C.; de Souza, F.M.; Almeida, V.E.S.; Batista, I.A.; D‘Avila-Mesquita, C.; et al. Timeline Kinetics of Systemic and Airway Immune Mediator Storm for Comprehensive Analysis of Disease Outcome in Critically Ill COVID-19 Patients. Front. Immunol. 2022, 13, 903903. [Google Scholar] [CrossRef]
- Chae, D.-H.; Park, H.S.; Kim, K.-M.; Yu, A.; Park, J.H.; Oh, M.-K.; Choi, S.W.; Ryu, J.; Dunbar, C.E.; Yoo, H.M.; et al. SARS-CoV-2 Pseudovirus Dysregulates Hematopoiesis and Induces Inflammaging of Hematopoietic Stem and Progenitor Cells. Exp. Mol. Med. 2025, 57, 616–627. [Google Scholar] [CrossRef]
- de Maistre, E.; Savard, P.; Guinot, P.-G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute Respiratory Distress Syndrome: Causes, Pathophysiology, and Phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef]
- Mendes, S.; Guimarães, L.C.; Costa, P.A.C.; Fernandez, C.C.; Figueiredo, M.M.; Teixeira, M.M.; dos Santos, R.A.S.; Guimarães, P.P.G.; Frézard, F. Intranasal Liposomal Angiotensin-(1-7) Administration Reduces Inflammation and Viral Load in the Lungs during SARS-CoV-2 Infection in K18-HACE2 Transgenic Mice. Antimicrob. Agents Chemother. 2024, 68, e00835-24. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of Venous Thromboembolism in Hospitalized Patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Zhu, A.; Real, F.; Capron, C.; Rosenberg, A.R.; Silvin, A.; Dunsmore, G.; Zhu, J.; Cottoignies-Callamarte, A.; Massé, J.-M.; Moine, P.; et al. Infection of Lung Megakaryocytes and Platelets by SARS-CoV-2 Anticipate Fatal COVID-19. Cell. Mol. Life Sci. 2022, 79, 365. [Google Scholar] [CrossRef]
- Garcia, C.; Vardon-Bounes, F.; Compagnon, B.; Guilbeau-Frugier, C.; Voisin, S.; Rieu, J.-B.; De Mas, V.; Payrastre, B.; Ribes, A. Detection of SARS-CoV-2 in Bone Marrow Megakaryocytes and Elevated Emperipolesis in COVID-19 Patients with Thrombocytopenia. J. Thromb. Haemost. 2025, 23, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, S.; Petsini, F.; Detopoulou, M.; Theoharides, T.C.; Demopoulos, C.A. Is There an Interplay between the SARS-CoV-2 Spike Protein and Platelet-Activating Factor? BioFactors 2022, 48, 1271–1283. [Google Scholar] [CrossRef]
- Petry, J.; Shoykhet, M.; Weiser, T.; Griesbaum, L.; Bashiri Dezfouli, A.; Verschoor, A.; Wollenberg, B. SARS-CoV-2 S1 Protein Induces IgG-Mediated Platelet Activation and Is Prevented by 1.8-Cineole. Biomed. Pharmacother. 2025, 187, 118100. [Google Scholar] [CrossRef] [PubMed]
- Zuchtriegel, G.; Uhl, B.; Puhr-Westerheide, D.; Pörnbacher, M.; Lauber, K.; Krombach, F.; Reichel, C.A. Platelets Guide Leukocytes to Their Sites of Extravasation. PLOS Biol. 2016, 14, e1002459. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, T.; Weiss, R.; Huber, S.; Ebeyer-Masotta, M.; Mostageer, M.; Emprechtinger, R.; Knabl, L.; Knabl, L.; Würzner, R.; Weber, V. Expression of Tissue Factor and Platelet/Leukocyte Markers on Extracellular Vesicles Reflect Platelet–Leukocyte Interaction in Severe COVID-19. Int. J. Mol. Sci. 2023, 24, 16886. [Google Scholar] [CrossRef]
- Rapkiewicz, A.V.; Mai, X.; Carsons, S.E.; Pittaluga, S.; Kleiner, D.E.; Berger, J.S.; Thomas, S.; Adler, N.M.; Charytan, D.M.; Gasmi, B.; et al. Megakaryocytes and Platelet-Fibrin Thrombi Characterize Multi-Organ Thrombosis at Autopsy in COVID-19: A Case Series. eClinicalMedicine 2020, 24, 100434. [Google Scholar] [CrossRef]
- Andrade, A.C.; Lacasse, E.; Dubuc, I.; Gudimard, L.; Puhm, F.; Queiroz, C.; Allaeys, I.; Prunier, J.; Dumais, É.; Flamand, N.; et al. Loss of Platelet 12-Lipoxygenase Aggravates the Severity of Sars-Cov-2 Infection. Blood 2023, 142, 3938. [Google Scholar] [CrossRef]
- Chouchane, O.; Léopold, V.; Michels, E.H.A.; de Brabander, J.; van Linge, C.C.A.; Klarenbeek, A.M.; Wiersinga, W.J.; van ‘t Veer, C.; van der Poll, T. Differential Platelet Protein Release Profiles in Community-Acquired Pneumonia and COVID-19. ERJ Open Res. 2025, 11, 00863–02024. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Azevedo-Quintanilha, I.G.; Palhinha, L.; Teixeira, L.; Barreto, E.A.; Pão, C.R.R.; Righy, C.; Franco, S.; Souza, T.M.L.; Kurtz, P.; et al. Platelet Activation and Platelet-Monocyte Aggregate Formation Trigger Tissue Factor Expression in Patients with Severe COVID-19. Blood 2020, 136, 1330–1341. [Google Scholar] [CrossRef]
- Hottz, E.D.; Martins-Gonçalves, R.; Palhinha, L.; Azevedo-Quintanilha, I.G.; de Campos, M.M.; Sacramento, C.Q.; Temerozo, J.R.; Soares, V.C.; Dias, S.S.G.; Teixeira, L.; et al. Platelet-Monocyte Interaction Amplifies Thromboinflammation through Tissue Factor Signaling in COVID-19. Blood Adv. 2022, 6, 5085–5099. [Google Scholar] [CrossRef]
- Baycan, O.F. Plasminogen Activator Inhibitor-1 Levels as an Indicator of Severity and Mortality for COVID-19. North. Clin. Istanb. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 Patients in Intensive Care Unit: A Report of Thromboelastography Findings and Other Parameters of Hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and Cardiac Pathology in African American Patients with COVID-19: An Autopsy Series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Cornwell, M.; Myndzar, K.; Rolling, C.C.; Xia, Y.; Drenkova, K.; Biebuyck, A.; Fields, A.T.; Tawil, M.; Luttrell-Williams, E.; et al. Platelets Amplify Endotheliopathy in COVID-19. Sci. Adv. 2021, 7, eabh2434. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.O.; Yusuf, A.M.; Jayakumar, M.N.; Ansari, A.W.; Elhassan, M.; AbdulKarim, F.; Kannan, M.; Halwani, R.; Ahmad, F. SARS-CoV-2 Infection Induces Soluble Platelet Activation Markers and PAI-1 in the Early Moderate Stage of COVID-19. Int. J. Lab. Hematol. 2022, 44, 712–721. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Yin, Y.; Zhang, Y.; Cao, Y.; Lin, X.; Huang, L.; Hoffmann, D.; Lu, M.; Qiu, Y. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Arce, N.A.; Cao, W.; Brown, A.K.; Legan, E.R.; Wilson, M.S.; Xu, E.-R.; Berndt, M.C.; Emsley, J.; Zhang, X.F.; Li, R. Activation of von Willebrand Factor via Mechanical Unfolding of Its Discontinuous Autoinhibitory Module. Nat. Commun. 2021, 12, 2360. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Terra, P.O.C.; Donadel, C.D.; Oliveira, L.C.; Menegueti, M.G.; Auxiliadora-Martins, M.; Calado, R.T.; De Santis, G.C. Neutrophil-to-lymphocyte Ratio and D-dimer Are Biomarkers of Death Risk in Severe COVID-19: A Retrospective Observational Study. Health Sci. Rep. 2022, 5, e514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer Levels on Admission to Predict In-hospital Mortality in Patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Asseri, A.A.; Al-Qahtani, S.M.; Alzaydani, I.A.; Al-Jarie, A.; Alyazidi, N.S.; Alrmelawi, A.A.; Alqahtani, A.M.; Alsulayyim, R.S.; Alzailaie, A.K.; Abdullah, D.M.; et al. Clinical and Epidemiological Characteristics of Respiratory Syncytial Virus, SARS-CoV-2 and Influenza Paediatric Viral Respiratory Infections in Southwest Saudi Arabia. Ann. Med. 2025, 57, 2445791. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent Endotheliopathy in the Pathogenesis of Long COVID Syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent Clotting Protein Pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Is Accompanied by Increased Levels of Antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Taquet, M.; Skorniewska, Z.; Hampshire, A.; Chalmers, J.D.; Ho, L.-P.; Horsley, A.; Marks, M.; Poinasamy, K.; Raman, B.; Leavy, O.C.; et al. Acute Blood Biomarker Profiles Predict Cognitive Deficits 6 and 12 Months after COVID-19 Hospitalization. Nat. Med. 2023, 29, 2498–2508. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with Covid-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent Complement Dysregulation with Signs of Thromboinflammation in Active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence in the Human Body and Brain at Autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Garg, R.K.; Rizvi, I.; Ingole, R.; Jain, A.; Malhotra, H.S.; Kumar, N.; Batra, D. Cortical Laminar Necrosis in Dengue Encephalitis—A Case Report. BMC Neurol. 2017, 17, 79. [Google Scholar] [CrossRef]
- Sahu, R.; Verma, R.; Jain, A.; Garg, R.K.; Singh, M.K.; Malhotra, H.S.; Sharma, P.K.; Parihar, A. Neurologic Complications in Dengue Virus Infection. Neurology 2014, 83, 1601–1609. [Google Scholar] [CrossRef]
- Kulkarni, R.; Pujari, S.; Gupta, D. Neurological Manifestations of Dengue Fever. Ann. Indian Acad. Neurol. 2021, 24, 693–702. [Google Scholar] [CrossRef]
- Sanjay, S.; Wagle, A.M.; Au Eong, K.G. Optic Neuropathy Associated with Dengue Fever. Eye 2008, 22, 722–724. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Lam, S.K. Dengue Virus Infection--the Malaysian Experience. Ann. Acad. Med. Singap. 1997, 26, 815–819. [Google Scholar]
- Murthy, J.M.K. Neurological Complications of Dengue Infection. Neurol. India 2010, 58, 581. [Google Scholar] [CrossRef]
- Pancharoen, C.; Thisyakorn, U. Neurological Manifestations in Dengue Patients. Southeast Asian J. Trop. Med. Public Health 2001, 32, 341–345. [Google Scholar] [PubMed]
- Liou, L.-M.; Lan, S.-H.; Lai, C.-L. Dengue Fever with Ischemic Stroke. Neurologist 2008, 14, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Pandian, J.D. Stroke in Patients with Dengue. J. Stroke Cerebrovasc. Dis. 2010, 19, 253–256. [Google Scholar] [CrossRef]
- Munoz, X.; Muñoz, X.; Obach, V.; Hurtado, B.; de Frutos, P.G.; Chamorro, A.; Sala, N. Association of Specific Haplotypes of GAS6 Gene with Stroke. Thromb. Haemost. 2007, 98, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Sahu, R.; Singh, A.; Atam, V. Dengue Infection Presenting as Ischemic Stroke: An Uncommon Neurological Manifestation. Neurol. India 2013, 61, 317. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, M.K.; Stoll, G.; Bieber, M.; Vögtle, T.; Hofmann, S.; Klaus, V.; Kraft, P.; Seyhan, M.; Kollikowski, A.M.; Papp, L.; et al. CD84 Links T Cell and Platelet Activity in Cerebral Thrombo-Inflammation in Acute Stroke. Circ. Res. 2020, 127, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Azmin, S.; Sahathevan, R.; Suehazlyn, Z.; Law, Z.K.; Rabani, R.; Nafisah, W.Y.; Tan, H.J.; Norlinah, M.I. Post-Dengue Parkinsonism. BMC Infect. Dis. 2013, 13, 179. [Google Scholar] [CrossRef]
- Hopkins, H.K.; Traverse, E.M.; Barr, K.L. Viral Parkinsonism: An Underdiagnosed Neurological Complication of Dengue Virus Infection. PLoS Negl. Trop. Dis. 2022, 16, e0010118. [Google Scholar] [CrossRef] [PubMed]
- Apoorva; Kumar, A.; Singh, S.K. Dengue Virus NS1 Hits Hard at the Barrier Integrity of Human Cerebral Microvascular Endothelial Cells via Cellular MicroRNA Dysregulations. Tissue Barriers 2024, 2424628. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Pandey, N.; Makani, J.; Hussain, I.; Dhiman, N.R.; Kumar, A.; Joshi, D. A Comprehensive and Critical Narrative Review on Movement Disorders Linked through Dengue Virus Based Infection. Neurol. Sci. 2025, 46, 4193–4207. [Google Scholar] [CrossRef]
- Chow, J.H.; Khanna, A.K.; Kethireddy, S.; Yamane, D.; Levine, A.; Jackson, A.M.; McCurdy, M.T.; Tabatabai, A.; Kumar, G.; Park, P.; et al. Aspirin Use Is Associated with Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients with Coronavirus Disease 2019. Anesth. Analg. 2021, 132, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Mazloomzadeh, S.; Khaleghparast, S.; Ghadrdoost, B.; Mousavizadeh, M.; Baay, M.R.; Noohi, F.; Sharifnia, H.; Ahmadi, A.; Tavan, S.; Malekpour Alamdari, N.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients with COVID-19 Admitted to the Intensive Care Unit. JAMA 2021, 325, 1620. [Google Scholar] [CrossRef]
- Sivaloganathan, H.; Ladikou, E.E.; Chevassut, T. COVID-19 Mortality in Patients on Anticoagulants and Antiplatelet Agents. Br. J. Haematol. 2020, 190, e192. [Google Scholar] [CrossRef] [PubMed]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators; Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Nie, N.; Jiang, M.; Yang, X.; Wang, Q.; Deng, J.; Kang, J.; Li, X.; Zhang, L.; Zhang, Y.; et al. Efficacy and Safety of Combined Nebulization of Unfractionated Heparin, Acetylcysteine, Budesonide and Ipratropium Bromide in Hospitalised Patients with COVID-19 Pneumonia: A Randomized Controlled Clinical Trial. BMC Pulm. Med. 2025, 25, 346. [Google Scholar] [CrossRef]
- Camprubí-Rimblas, M.; Tantinyà, N.; Bringué, J.; Guillamat-Prats, R.; Artigas, A. Anticoagulant Therapy in Acute Respiratory Distress Syndrome. Ann. Transl. Med. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Targeting Factor Xa and Thrombin: Impact on Coagulation and Beyond. Thromb. Haemost. 2014, 111, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lother, S.A.; Tennenhouse, L.; Rabbani, R.; Abou-Setta, A.M.; Askin, N.; Turgeon, A.F.; Murthy, S.; Houston, B.L.; Houston, D.S.; Mendelson, A.A.; et al. The Association of Antiplatelet Agents with Mortality among Patients with Non–COVID-19 Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis. Res. Pract. Thromb. Haemost. 2024, 8, 102526. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet Gene Expression and Function in Patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Chow, J.H.; Rahnavard, A.; Gomberg-Maitland, M.; Chatterjee, R.; Patodi, P.; Yamane, D.P.; Levine, A.R.; Davison, D.; Hawkins, K.; Jackson, A.M.; et al. Association of Early Aspirin Use with In-Hospital Mortality in Patients with Moderate COVID-19. JAMA Netw. Open 2022, 5, e223890. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Aspirin in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Siepmann, T.; Sedghi, A.; Simon, E.; Winzer, S.; Barlinn, J.; de With, K.; Mirow, L.; Wolz, M.; Gruenewald, T.; Schroettner, P.; et al. Increased Risk of Acute Stroke among Patients with Severe COVID-19: A Multicenter Study and Meta-analysis. Eur. J. Neurol. 2021, 28, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Sawajan, N.; Rungrotmongkol, T.; Sanachai, K.; Ershadian, M.; Sukasem, C. Pharmacogenetics and Precision Medicine Approaches for the Improvement of COVID-19 Therapies. Front. Pharmacol. 2022, 13, 835136. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Htun, H.L.; Leo, Y.S.; Lye, D.C. Safety of Temporary Interruption of Antiplatelet Therapy in Dengue Fever with Thrombocytopenia. J. Infect. 2021, 82, 270–275. [Google Scholar] [CrossRef]
- Roy, S. Thrombopoietin Receptor Agonists: Can These Be the Future Answer to the Deadly Thrombocytopenia in Dengue Fever? Cureus 2019, 11, e4361. [Google Scholar] [CrossRef]
- Aboulafia, D.; Vishnu, P. Long-Term Safety and Efficacy of Romiplostim for Treatment of Immune Thrombocytopenia. J. Blood Med. 2016, 7, 99–106. [Google Scholar] [CrossRef]
- Mitchell, W.B.; Pinheiro, M.P.; Boulad, N.; Kaplan, D.; Edison, M.N.; Psaila, B.; Karpoff, M.; White, M.J.; Josefsson, E.C.; Kile, B.T.; et al. Effect of Thrombopoietin Receptor Agonists on the Apoptotic Profile of Platelets in Patients with Chronic Immune Thrombocytopenia. Am. J. Hematol. 2014, 89, E228–E234. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, S.-S.; Jeong, S.-H.; Ahn, J.-S.; Yang, D.-H.; Lee, J.-J.; Kim, H.-J. Efficacy and Safety of Eltrombopag in Adult Refractory Immune Thrombocytopenia. Blood Res. 2015, 50, 19. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Cheng, G.; Saleh, M.N.; Psaila, B.; Kovaleva, L.; Meddeb, B.; Kloczko, J.; Hassani, H.; Mayer, B.; Stone, N.L.; et al. Eltrombopag for the Treatment of Chronic Idiopathic Thrombocytopenic Purpura. N. Engl. J. Med. 2007, 357, 2237–2247. [Google Scholar] [CrossRef]
- Ayaz, S.I.; Sharkey, C.M.; Kwiatkowski, G.M.; Wilson, S.S.; John, R.S.; Tolomello, R.; Mahajan, A.; Millis, S.; Levy, P.D. Intravenous Enalaprilat for Treatment of Acute Hypertensive Heart Failure in the Emergency Department. Int. J. Emerg. Med. 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Goa, K.L. Enalapril. Drugs 1992, 43, 346–381. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fonseca, J.P.; Durán, A.; Valero, N.; Mosquera, J. Losartan and Enalapril Decrease Viral Absorption and Interleukin 1 Beta Production by Macrophages in an Experimental Dengue Virus Infection. Arch. Virol. 2015, 160, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Pulavendran, S.; Rudd, J.M.; Maram, P.; Thomas, P.G.; Akhilesh, R.; Malayer, J.R.; Chow, V.T.K.; Teluguakula, N. Combination Therapy Targeting Platelet Activation and Virus Replication Protects Mice against Lethal Influenza Pneumonia. Am. J. Respir. Cell Mol. Biol. 2019, 61, 689–701. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, V.L.; Martins, J.R.; Queiroz-Junior, C.M.; Hottz, E.D.; Teixeira, M.M.; Costa, V.V. Mechanisms of Thromboinflammation in Viral Infections—A Narrative Review. Viruses 2025, 17, 1207. https://doi.org/10.3390/v17091207
Batista VL, Martins JR, Queiroz-Junior CM, Hottz ED, Teixeira MM, Costa VV. Mechanisms of Thromboinflammation in Viral Infections—A Narrative Review. Viruses. 2025; 17(9):1207. https://doi.org/10.3390/v17091207
Chicago/Turabian StyleBatista, Viviane Lima, Jenniffer Ramos Martins, Celso Martins Queiroz-Junior, Eugenio Damaceno Hottz, Mauro Martins Teixeira, and Vivian Vasconcelos Costa. 2025. "Mechanisms of Thromboinflammation in Viral Infections—A Narrative Review" Viruses 17, no. 9: 1207. https://doi.org/10.3390/v17091207
APA StyleBatista, V. L., Martins, J. R., Queiroz-Junior, C. M., Hottz, E. D., Teixeira, M. M., & Costa, V. V. (2025). Mechanisms of Thromboinflammation in Viral Infections—A Narrative Review. Viruses, 17(9), 1207. https://doi.org/10.3390/v17091207






