Baculovirus-Based Biocontrol: Synergistic and Antagonistic Interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in Integrative Pest Management
Abstract
1. Introduction
2. Information Retrieval Strategy
2.1. Selection and Collection of Studies
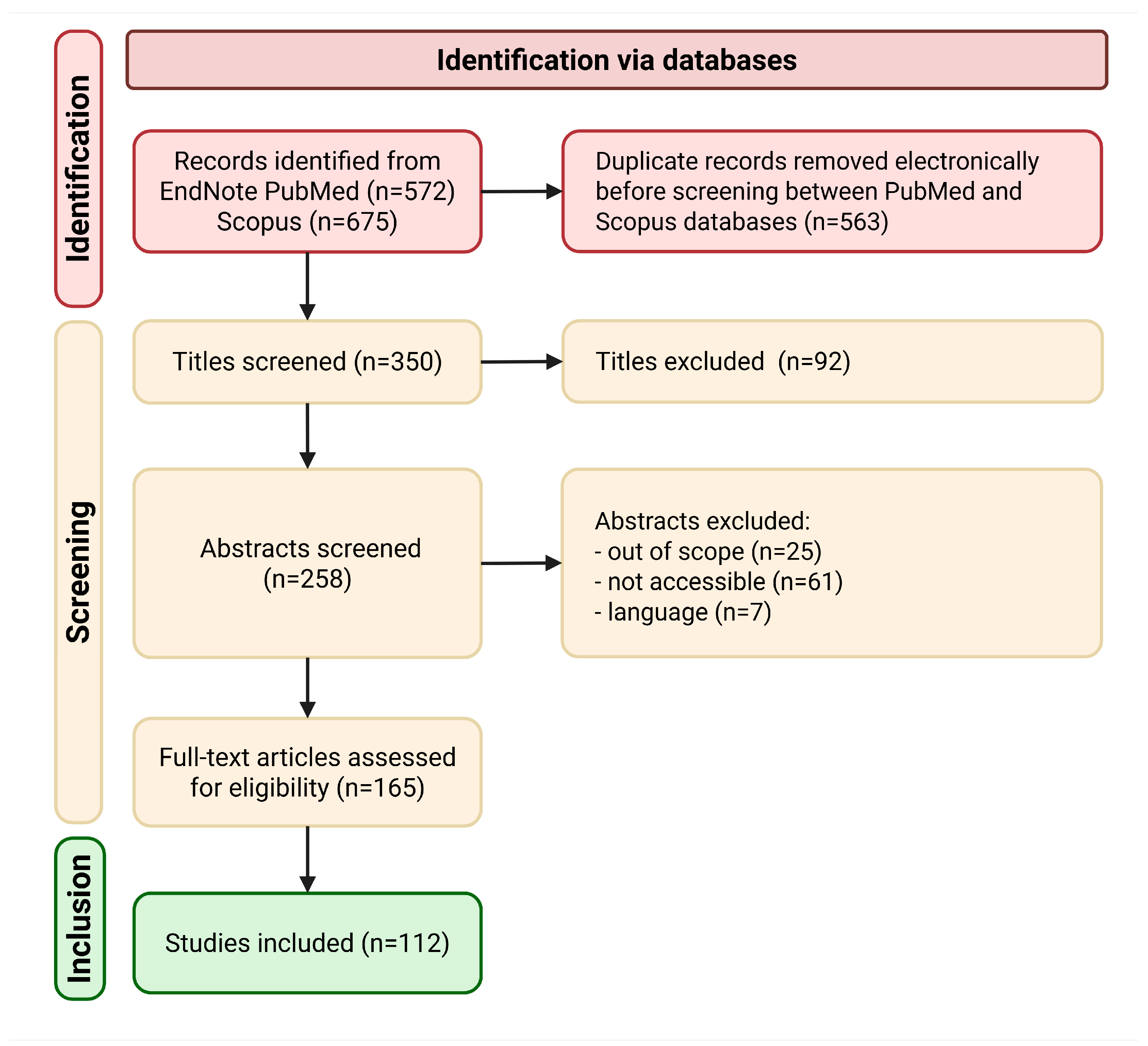
2.1.1. Identification
2.1.2. Screening
2.1.3. Eligibility
2.1.4. Data Analysis
3. Results
3.1. Economic Impact
3.2. Impact and Management of Infestations in Crops
3.3. Mechanisms of Resistance: Metabolic Adaptations and Target-Site Mutations
3.4. Evaluating Baculoviruses as a Sustainable Strategy for Integrated Pest Management (IPM)
3.4.1. Overview of the Baculoviruses
3.4.2. General Factors of Molecular Biology of Baculoviruses
3.4.3. Specific Findings on Baculoviruses in Plutella xylostella
3.4.4. Specific Findings on Spodoptera exigua nucleopolyhedrovirus
3.4.5. Specific Findings of Spodoptera frugiperda nucleopolyhedrovirus SfMNPV for Mitigating Spodoptera frugiperda in Maize
3.5. Interactions Between Baculoviruses and Insecticides
3.5.1. Synergistic Interactions
3.5.2. Antagonism Interactions
3.6. Examples of Baculovirus Resistance Emergence in Target Populations
3.7. Limitations and Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PxNPV | Plutella xylostella nucleopolyhedrovirus |
| PxGV | Plutella xylostella granulovirus |
| SeMNPV | Spodoptera exigua multiple nucleopolyhedrovirus |
| SfMNPV | Spodoptera frugiperda nucleopolyhedrovirus |
| NPV | Nucleopolyhedrovirus |
| GV | Granulovirus |
| PICO | Problem, Intervention, Comparison, Outcome |
| IPM | Integrated Pest Management |
| ODV | Occlusion-Derived Viruses |
| PIF | Per os Infectivity Factor |
| GP64 | Glycoprotein 64 |
| kDa | Kilo daltons |
| ORF | Open Reading Frames |
| bp | Base pairs |
| SINEs | Short Interspersed Nuclear Elements |
| tRNA | Transfer RNA |
| GDP | Gross Domestic Product |
| PTP2 | Protein Tyrosine Phosphatase-2 |
| GABA | Gamma-Aminobutyric Acid |
| ATP | Adenosine Triphosphate |
| DNA | Deoxynucleotide Triphosphate |
References
- Taha, H.A.; Salim, N.S.; Sedeeq, A.M.; Ibraheem, F.F.; ALkuaz, A.A. Impact of some agricultural treatments on the growth characteristics and yield of broccoli. Int. J. Environ. Sci. 2025, 11, 710–719. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Liu, S.; Tian, Y.; Li, W.; Wang, B.; Hu, X.; Sun, D.; Wang, T.; Wu, S. When Tomatoes Hit the Winter: A Counterattack to Overwinter Production in Soft-Shell Solar Greenhouses in North China. Horticulturae 2025, 11, 436. [Google Scholar] [CrossRef]
- Liu, H.-J.; Liu, J.; Zhai, Z.; Dai, M.; Tian, F.; Wu, Y.; Tang, J.; Lu, Y.; Wang, H.; Jackson, D. Maize2035: A decadal vision for intelligent maize breeding. Mol. Plant 2025, 18, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Khare, T.; Zhang, X.; Rahman, M.M.; Hussain, M.; Gill, S.S.; Chen, Z.H.; Zhou, M.; Hu, Z.; Varshney, R.K. Novel strategies for designing climate-smart crops to ensure sustainable agriculture and future food security. J. Sustain. Agric. Environ. 2025, 4, e70048. [Google Scholar] [CrossRef]
- Akhter, W.; Shah, F.M.; Yang, M.; Freed, S.; Razaq, M.; Mkindi, A.G.; Akram, H.; Ali, A.; Mahmood, K.; Hanif, M. Botanical biopesticides have an influence on tomato quality through pest control and are cost-effective for farmers in developing countries. PLoS ONE 2023, 18, e0294775. [Google Scholar] [CrossRef]
- Askri, S.M.H.; Fu, W.; Abd El-Rady, W.A.; Adil, M.F.; Sehar, S.; Ali, A.; Ullah, N.; Munawar, A.; Zhou, W.; Jiang, L. Comparative metabolomics elucidates the early defense response mechanisms to Plutella xylostella infestation in Brassica napus. Plant Physiol. Biochem. 2025, 221, 109678. [Google Scholar] [CrossRef]
- Ullah, F.; Murtaza, G.; Li, X.; Gul, H.; Wang, Y.; Zhao, S.; Abbas, A.; Zhang, Z.; Huang, J.; Desneux, N. Selection-Induced Spinosad Resistance and Associated Fitness Costs in Tuta absoluta: A Key Invasive Tomato Pest. Agronomy 2025, 15, 358. [Google Scholar] [CrossRef]
- Hafeez, A.; Maalik, S.; Mushtaq, S.; Batool, M.; Bano, N.; Ehsan, N. Laboratory Rearing of Herbivore Larva of Spodoptera Frugiperda (Order: Lepidoptera) to Assess Yield Loss of Economically Important Crops. J. Zool. Syst. 2025, 3, 36–46. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Machekano, H.; Chidawanyika, F.; Mutamiswa, R.; Ma, G.; Ma, C.-S. Geographic dispersion of invasive crop pests: The role of basal, plastic climate stress tolerance and other complementary traits in the tropics. Curr. Opin. Insect Sci. 2022, 50, 100878. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Ullah, F.; Zhang, Z.; Zhang, J.; Huang, J.; Chen, L.; Siddiqui, J.A.; Ren, X.; Zhou, S. Characterization of indoxacarb resistance in the fall armyworm: Selection, inheritance, cross-resistance, possible biochemical mechanisms, and fitness costs. Biology 2022, 11, 1718. [Google Scholar] [CrossRef]
- Banazeer, A.; Afzal, M.B.S.; Hassan, S.; Ijaz, M.; Shad, S.A.; Serrão, J.E. Status of insecticide resistance in Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) from 1997 to 2019: Cross-resistance, genetics, biological costs, underlying mechanisms, and implications for management. Phytoparasitica 2022, 50, 465–485. [Google Scholar] [CrossRef]
- Mawcha, K.T.; Malinga, L.; Muir, D.; Ge, J.; Ndolo, D. Recent advances in biopesticide research and development with a focus on microbials. F1000Research 2025, 13, 1071. [Google Scholar] [CrossRef]
- Civolani, S.; Bariselli, M.; Osti, R.; Bernacchia, G. Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges. Insects 2025, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N. Pest Management in Agriculture: Integrated Approaches for Sustainable Control. Front. Agric. 2024, 1, 416–443. [Google Scholar]
- Gelaye, Y.; Negash, B. The role of baculoviruses in controlling insect pests: A review. Cogent Food Agric. 2023, 9, 2254139. [Google Scholar] [CrossRef]
- Simmonds, P.; Adriaenssens, E.M.; Lefkowitz, E.J.; Oksanen, H.M.; Siddell, S.G.; Zerbini, F.M.; Alfenas-Zerbini, P.; Aylward, F.O.; Dempsey, D.M.; Dutilh, B.E. Changes to virus taxonomy and the ICTV Statutes ratified by the International Committee on Taxonomy of Viruses (2024). Arch. Virol. 2024, 169, 236. [Google Scholar] [CrossRef]
- López-Ferber, M.; Caballero, P.; Williams, T. Baculovirus Genetic Diversity and Population Structure. Viruses 2025, 17, 142. [Google Scholar] [CrossRef]
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef]
- Hutchinson, M. Popular methods of Biological control and the future of baculoviruses. Undergrad. Rev. 2021, 16, 100–108. [Google Scholar]
- Smith, G.E.; Summers, M.D. Analysis of baculovirus genomes with restriction endonucleases. Virology 1978, 89, 517–527. [Google Scholar] [CrossRef]
- Miller, L.K. The Baculoviruses; Springer: New York, NY, USA, 1997. [Google Scholar] [CrossRef]
- Payne, C. Insect viruses as control agents. Parasitology 1982, 84, 35–77. [Google Scholar] [CrossRef]
- Ikeda, M.; Hamajima, R.; Kobayashi, M. Baculoviruses: Diversity, evolution and manipulation of insects. Entomol. Sci. 2015, 18, 1–20. [Google Scholar] [CrossRef]
- Méndez, W.A.; Valle, J.; Ibarra, J.E.; Cisneros, J.; Penagos, D.I.; Williams, T. Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Biol. Control 2002, 25, 195–206. [Google Scholar] [CrossRef]
- Gómez, J.; Guevara, J.; Cuartas, P.; Espinel, C.; Villamizar, L. Microencapsulated Spodoptera frugiperda nucleopolyhedrovirus: Insecticidal activity and effect on arthropod populations in maize. Biocontrol Sci. Technol. 2013, 23, 829–846. [Google Scholar] [CrossRef]
- Pineda, S.; Pérez-Robledo, C.; Hernández, R.; Figueroa De La Rosa, J.; Chavarrieta, J.; Martínez, A. Combined and individual effects of a nucleopolyhedrovirus and azadirachtin on the mortality and maize-leaf consumption of Spodoptera frugiperda. Phytoparasitica 2014, 42, 571–578. [Google Scholar] [CrossRef]
- Lasa, R.; Pagola, I.; Ibanez, I.; Belda, J.E.; Williams, T.; Caballero, P. Efficacy of Spodoptera exigua multiple nucleopolyhedrovirus as a biological insecticide for beet armyworm control in greenhouses of southern Spain. Biocontrol Sci. Technol. 2007, 17, 221–232. [Google Scholar] [CrossRef]
- Zamora-Avilés, N.; Murillo, R.; Lasa, R.; Pineda, S.; Figueroa, J.; Bravo-Patiño, A.; Díaz, O.; Corrales, J.; Martínez, A. Genetic and biological characterization of four nucleopolyhedrovirus isolates collected in Mexico for the control of Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, J.; Lin, Y.; Li, X.; Liu, M.; Hafeez, M.; Huang, J.; Zhang, Z.; Chen, L.; Ren, X. Spodoptera exigua multiple nucleopolyhedrovirus increases the susceptibility to insecticides: A promising efficient way for pest resistance management. Biology 2023, 12, 260. [Google Scholar] [CrossRef]
- Carrasco-Baeza, V.M.; Tamayo-Mejía, F.; Ibarra, J.E.; Del Rincón-Castro, M.C. Antagonism between a Baculovirus and Bacillus thuringiensis against Plutella xyllostella 1 Larvae at Laboratory and Field Conditions. Southwest. Entomol. 2023, 48, 879–894. [Google Scholar] [CrossRef]
- Nishikawa-Pacher, A. Research Questions with PICO: A Universal Mnemonic. Publications 2022, 10, 21. [Google Scholar] [CrossRef]
- Gumbeze, W.V.; Irakarama, G.M.; Mukankurunziza, J.; Sindarihora, P.; Hatungimana, C.J.; Mandingaisa, M.; Katsaruware-Chapoto, R.D.; Mngadze, K.C. Agronomic and economic evaluations of nutrient source, defoliation intensity and defoliation frequency on the Broccoli head size under rainfed conditions in Rwanda. Afr. J. Agric. Res. 2024, 20, 952–962. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Martínez-Ortiz, M.A.; Salinas-Moreno, Y.; Ramírez-Díaz, J.L.; Ledesma-Miramontes, A.; Alemán de la Torre, I. Challenges and Opportunities in the Specialization of Maize Cultivation. Agro Prod. 2024, 17, 81–92. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, H.; Zhang, M.; Wu, B.; Qin, X. Global de-trending significantly improves the accuracy of XGBoost-based county-level maize and soybean yield prediction in the Midwestern United States. GISci. Remote Sens. 2024, 61, 2349341. [Google Scholar] [CrossRef]
- Tepato-Barba, J.; González-Hernández, H.; Lomeli-Flores, J.R.; Tamayo-Mejia, F.; Soto-Rojas, L. Effectiveness of Commercial Plant Extracts in Management of Plutella xylostella (Lepidoptera: Plutellidae) on Broccoli in Mexico1. J. Entomol. Sci. 2024, 60, 334–346. [Google Scholar] [CrossRef]
- Mutyambai, D.M.; Niassy, S.; Calatayud, P.-A.; Subramanian, S. Agronomic factors influencing fall armyworm (Spodoptera frugiperda) infestation and damage and its co-occurrence with stemborers in maize cropping systems in Kenya. Insects 2022, 13, 266. [Google Scholar] [CrossRef]
- Sarfraz, M.; Keddie, A.B.; Dosdall, L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Technol. 2005, 15, 763–789. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Prasanna, B.; Kalleshwaraswamy, C.; Jaba, J.; Choudhary, B. Fall armyworm (Spodoptera frugiperda). In Polyphag Pests Crops; Springer: Singapore, 2021; pp. 349–372. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Li, Y.; Zhong, G. Prospects of botanical compounds and pesticides as sustainable management strategies against Spodoptera frugiperda. J. Econ. Entomol. 2022, 115, 1834–1845. [Google Scholar] [CrossRef]
- Usman, M.; Adamu, R.; Onu, I.; Kogi, E.; Musa, N.; Ahmed, I. Economic injury levels of fall armyworm (Spodoptera frugiperda Smith) at early-whorl vegetative growth stage of quality protein maize variety. J. Agric. Environ. 2024, 20, 253–264. [Google Scholar] [CrossRef]
- Soujanya, P.L.; VaniSree, K.; Giri, G.S.; Mahadik, S.; Jat, S.; Sekhar, J.; Jat, H. Intercropping in maize reduces fall armyworm Spodoptera frugiperda (JE Smith) infestation, supports natural enemies, and enhances yield. Agr. Ecosyst. Environ. 2024, 373, 109130. [Google Scholar] [CrossRef]
- Van den Berg, J.; du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef]
- Shelton, A.; Nault, B. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, P.; Ferré, J.; Escriche, B. Susceptibility of Spodoptera exigua to 9 toxins from Bacillus thuringiensis. J. Invertebr. Pathol. 2008, 97, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Frattini, A.; González-Martínez, R.M.; García, J.M.; Minchev, Z.; Pozo, M.J.; Flors, V.; Crava, C.M.; Herrero, S. Constitutive and inducible tomato defenses contribute to Bacillus thuringiensis lethality against Spodoptera exigua. Biol. Control 2024, 198, 105624. [Google Scholar] [CrossRef]
- Anilkumar, G.; LakshmiSoujanya, P.; Kumar, D.S.R.; Kumar, V.M.; Yathish, K.; Sekhar, J.; Jat, H. Integrated approaches for the management of invasive fall armyworm, Spodoptera frugiperda (JE Smith) in maize. J. Plant Dis. Prot. 2024, 131, 793–803. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Nazarian, M.; Darroudi, M.; Marouzi, S.; Harifi-Mood, M.S.; Samarghandian, S.; Farkhondeh, T. Carbamate compounds induced toxic effects by affecting Nrf2 signaling pathways. Toxicol. Rep. 2024, 12, 148–157. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, C.-B.; Jin, B.; Ali, A.S.; Han, X.; Zhang, H.; Zhang, M.-Z.; Zhang, W.-H.; Gu, Y.-C. Recent advances in the natural products-based lead discovery for new agrochemicals. Adv. Agrochem. 2023, 2, 324–339. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical evaluation of carbamate and organophosphate pesticides in human and environmental matrices: A review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Sun, M.; Xu, W.; Zhang, W.; Guang, C.; Mu, W. Microbial elimination of carbamate pesticides: Specific strains and promising enzymes. Appl. Microbiol. Biotechnol. 2022, 106, 5973–5986. [Google Scholar] [CrossRef]
- Gupta, R.C. Carbofuran toxicity. J. Toxicol. Environ. Health Part A Curr. Issues 1994, 43, 383–418. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Gupta, S.; Murthy, R. Comparative toxicity of carbaryl, carbofuran, cypermethrin and fenvalerate in Metaphire posthuma and Eisenia fetida—A possible mechanism. Ecotoxicol. Environ. Saf. 2014, 100, 218–225. [Google Scholar] [CrossRef]
- Umar, A.M.; Aisami, A. Acetylcholinesterase enzyme (AChE) as a biosensor and biomarker for pesticides: A mini review. Bull. Environ. Sci. Sustain. Manag. 2020, 4, 7–12. [Google Scholar] [CrossRef]
- Baharudin, N.S.; Ahmad, H.; Hossain, M.S. Understanding the Degradation of Carbofuran in Agricultural Area: A Review of Fate, Metabolites, and Toxicity. Pertanika J. Sci. Technol. 2024, 32, 285–322. [Google Scholar] [CrossRef]
- Aziz, A.; Martono, E.; Indarti, S.; Trisyono, Y.A. Resistance of Spodoptera exigua Population from Nganjuk against Methomyl, Chlorfenapyr, and Emamectin Benzoate. J. Perlindungan Tanam. Indones. 2023, 27, 76–85. [Google Scholar] [CrossRef]
- Okwute, S.K.; Egharevba, H.O. Insecticidal Agents in Pest Control: Sources, Challenges, and Advantages; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Khan, H.A.A. Resistance risk assessment, cross-resistance potential and realized heritability of resistance to methomyl in Musca domestica Linnaeus. Ecotoxicology 2024, 33, 226–234. [Google Scholar] [CrossRef]
- Sadek, H.E.; Elbehery, H.H.; Mohamed, S.A.-H.; El-wahab, T.A. Genetic expressions and evaluation of insecticidal activity of some essential oil and methomyl lannate 90% against Spodoptera frugiperda. Bull. Natl. Res. Cent. 2024, 48, 15. [Google Scholar] [CrossRef]
- Haikal, A.; Kamal, M.; Hosni, E.M.; Amen, Y. Evaluation of hesperidin as a potential larvicide against Culex pipiens with computational prediction of its mode of action via molecular docking. Sci. Rep. 2025, 15, 2677. [Google Scholar] [CrossRef]
- Erdogan, C.; Toprak, U.; Gurkan, M.O. Biochemical and molecular analyses of insecticide resistance in greenhouse populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in Türkiye. Phytoparasitica 2024, 52, 41. [Google Scholar] [CrossRef]
- Sunanda, M.; Rao, J.C.S.; Neelima, P.; Simhachalam, G. Toxicity and effects of chlorpyrifos in a non-target organism (Fish)-A review. J. At. Mol. 2016, 6, 966–976. [Google Scholar]
- Lam, D.V.; Toan, V.D.; Phuong, T.M. Residual and ecological risk assessment of Chlorpyrifos in coffee growing soil areas: A case study in Lam Ha district, Lam Dong province. J. Hydro-Meteorol. 2023, 3, 100–108. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Degradation of acephate and its intermediate methamidophos: Mechanisms and biochemical pathways. Front. Microbiol. 2020, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Oyugi, A.M.a.; Kibet, J.K.; Adongo, J.O. A review on the biodegradation of acephate insecticides widely used in khat farming and horticultural crops. Int. J. Environ. Anal. Chem. 2024, 1–19. [Google Scholar] [CrossRef]
- Vijverberg, H.P.; vanden Bercken, J. Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit. Rev. Toxicol. 1990, 21, 105–126. [Google Scholar] [CrossRef]
- Shimada, K.; Natsuhara, K.; Oomori, Y.; Miyata, T. Permethrin resistance mechanisms in the beet armyworm (Spodoptera exigua (Hübner)). J. Pestic. Sci. 2005, 30, 214–219. [Google Scholar] [CrossRef][Green Version]
- Anderson, R.L. Toxicity of fenvalerate and permethrin to several nontarget aquatic invertebrates. Environ. Entomol. 1982, 11, 1251–1257. [Google Scholar] [CrossRef]
- Zhorov, B.S.; Dong, K. Pyrethroids in an AlphaFold2 model of the insect sodium channel. Insects 2022, 13, 745. [Google Scholar] [CrossRef]
- Raisch, T.; Raunser, S. The modes of action of ion-channel-targeting neurotoxic insecticides: Lessons from structural biology. Nat. Struct. Mol. Biol. 2023, 30, 1411–1427. [Google Scholar] [CrossRef]
- Zavala-García, M.; Bujanos-Muñiz, R.; Núñez-Colín, C.A.; Raya-Pérez, J.C.; Aguirre-Mancilla, C.L.; Covarrubias-Prieto, J. Efectividad de insecticidas para el control de Plutella xylostella (L.) (Lepidoptera: Plutellidae) y mortalidad del parasitoide Diadegma insulare (Cresson) (Hymenoptera: Ichneumonidae) en plantas brasicáceas. Rev. Colomb. Entomol. 2024, 50, e13202. [Google Scholar] [CrossRef]
- Ruberti, M. One Hundred Years of Pyrethroid Chemistry: A Still-Open Research Effort to Combine Efficacy, Cost-Effectiveness and Environmental Sustainability. Sustainability 2024, 16, 8322. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, L.; Yang, Y.; Zhang, Y.; Lu, M.; Tao, L.; Xu, W. Bifenthrin induces DNA damage and autophagy in Spodoptera frugiperda (Sf9) insect cells. Vitr. Cell Dev. Biol-Anim. 2021, 57, 264–271. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Santos, I.B.; Paula-Moraes, S.V. Spodoptera exigua (Hubner)(Lepidoptera: Noctuidae) fitness and resistance stability to diamide and pyrethroid insecticides in the United States. Insects 2022, 13, 365. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Atere, A.D.; Chimwuba, P.; Joseph, U.G. Implication of Pyrethroid Neurotoxicity for Human Health: A Lesson from Animal Models. Neurotox. Res. 2025, 43, 1. [Google Scholar] [CrossRef]
- Lilin, Z.; Kaifan, X.; Pan, W.; Fan, Y.; Yong, W.; Xiaoping, W.; Shengyun, S. Screening of optimal formulations of insecticide mixtures and validation of the field efficacy against Spodoptera exigua. Chin. J. Pestic. Sci. 2023, 25, 668–677. [Google Scholar] [CrossRef]
- Shargi, H.; Eivazian Kary, N.; Mohammadi, D. Compatibility of Steinernema carpocapsae and Steinernema feltiae with cypermethrin against the beet armyworm Spodoptera exigua. J. App Res. Plant Prot. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.; Dhama, K.; Abdel-Latif, H.M.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Updated risk assessment concerning the risk to wild mammals for the active substance gamma-cyhalothrin. EFSA J. 2021, 19, e06489. [Google Scholar] [CrossRef]
- EFSA; Bellisai, G.; Bernasconi, G.; Carrasco Cabrera, L.; Castellan, I.; del Aguila, M.; Ferreira, L.; Greco, L.; Jarrah, S.; Leuschner, R. Review of the existing maximum residue levels for gamma-cyhalothrin according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2024, 22, e8758. [Google Scholar] [CrossRef]
- Maqsood, S.; Sabri, M.A.; Ali, A.; Abbas, M.; Aziz, A. Comparative toxicity of some insecticides against armyworm, Spodoptera litura L. (Lepidoptera: Noctuidae) under laboratory conditions. J. Entomol. Zool. Stud. 2016, 5, 770–773. [Google Scholar]
- Taillebois, E.; Thany, S.H. The use of insecticide mixtures containing neonicotinoids as a strategy to limit insect pests: Efficiency and mode of action. Pestic. Biochem. Physiol. 2022, 184, 105126. [Google Scholar] [CrossRef]
- Krishnan, N.; Jurenka, R.A.; Bradbury, S.P. Neonicotinoids can cause arrested pupal ecdysis in Lepidoptera. Sci. Rep. 2021, 11, 15787. [Google Scholar] [CrossRef]
- Thany, S.H. Molecular mechanism of action of neonicotinoid insecticides. Int. J. Mol. Sci. 2023, 24, 5484. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Wang, Y.; Li, X.; Lv, J.; Luo, J.; Yang, M. Insight of neonicotinoid insecticides: Exploring Exposure, Mechanisms in Non-Target Organisms, and Removal Technologies. Pharmacol. Res. 2024, 209, 107415. [Google Scholar] [CrossRef]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Ghazali, R.; Batterham, P.; Perry, T. Inhibiting the proteasome reduces molecular and biological impacts of the natural product insecticide, spinosad. Pest. Manag. Sci. 2021, 77, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Tang, P.; Wang, X.; Yang, Y.; Wu, Y. CRISPR-mediated knockout of nicotinic acetylcholine receptor (nAChR) α6 subunit confers high levels of resistance to spinosyns in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2022, 187, 105191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yu, Z.; He, Y.; Wang, F.; Gu, Y.; Davies, T.E.; Fan, Z.; Wang, X.; Wu, Y. Key role of the ryanodine receptor I4790K mutation in mediating diamide resistance in Plutella xylostella. Insect Biochem. Mol. Biol. 2024, 168, 104107. [Google Scholar] [CrossRef]
- Ramírez-Cerón, D.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Soto-Rojas, L.; Ramírez-Alarcón, S.; Segura-Miranda, A. Toxicity and Residual Activity of Insecticides against Diadegma insulare, a Parasitoid of the Diamondback Moth. Insects 2022, 13, 514. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; Chiusolo, A.; Court Marques, D. Peer review of the pesticide risk assessment of the active substance spinosad. EFSA J. 2018, 16, e05252. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Pereira, B.B. Properties, toxicity and current applications of the biolarvicide spinosad. J. Toxicol. Environ. Health Part. B 2020, 23, 13–26. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Li, X.-L.; Tan, X.-F.; Yang, M.-F.; Idrees, A.; Liu, J.-F.; Song, S.-J.; Shen, J. Sublethal effects of emamectin benzoate on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Agriculture 2022, 12, 959. [Google Scholar] [CrossRef]
- Muraro, D.S.; Salmeron, E.; Cruz, J.V.; Amaral, F.S.; Guidolin, A.S.; Nascimento, A.R.; Malaquias, J.B.; Bernardi, O.; Omoto, C. Evidence of field-evolved resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) to emamectin benzoate in Brazil. Crop Prot. 2022, 162, 106071. [Google Scholar] [CrossRef]
- Prasad, S.R.; Krishnayya, P.; Vijayalakshmi, K. Insecticidal efficacy of cryolite against Spodoptera litura Fabricius. Ann. Plant Prot. Sci. 1999, 7, 150–153. [Google Scholar]
- Dudha, N.; Tomar, L.K.; Tyagi, C.; Reddy, Y.P.; Sharma, V.K. Fluoride excess and neuroinflammation. In Vitamins and Minerals in Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2023; pp. 475–493. [Google Scholar] [CrossRef]
- Kanno, R.H.; Bolzan, A.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Guidolin, A.S.; Nascimento, A.R.; Omoto, C. Low risk of resistance evolution of Spodoptera frugiperda to chlorfenapyr in Brazil. J. Pest. Sci. 2020, 93, 365–378. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Teng, M.; Zhang, J.; Qian, L.; Duan, M.; Zhao, F.; Zhao, W.; Wang, Z.; Wang, C. Bioaccumulation, metabolism and the toxic effects of chlorfenapyr in zebrafish (Danio rerio). J. Agric. Food Chem. 2021, 69, 8110–8119. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yan, X.; Yu, B.; He, X.; Lu, L.; Ren, Y. A comprehensive review of the current knowledge of chlorfenapyr: Synthesis, mode of action, resistance, and environmental toxicology. Molecules 2023, 28, 7673. [Google Scholar] [CrossRef]
- Pathan, A.R.K.; Jakhar, B.L.; Dhaka, S.R.; Nitharwal, M.; Jatav, H.S.; Dudwal, R.G.; Yadav, A.K.; Choudhary, S.K.; Gauttam, V.; Rajput, V.D. Persistence and dissipation kinetics of novaluron 9.45% + lambda-cyhalothrin 1.9% ZC insecticides in tomato crop under semi-arid region. Environ. Geochem. Health 2023, 45, 9293–9302. [Google Scholar] [CrossRef]
- Aly, M.F.K.; Ali, A. Toxicity and biochemical effects of some insecticides on the cotton leafworm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae) under laboratory. Egypt. J. Crop Prot. 2024, 19, 27–46. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhao, R.; Gao, J.; Zhang, L.; Zhang, S.; Liang, P.; Gao, X.-W.; Gu, S.-H. Genetic architecture and insecticide resistance in Chinese populations of Spodoptera frugiperda. J. Pest. Sci. 2023, 96, 1595–1610. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, N.; Wang, Z.; Liang, Y.; Liu, X.; Wang, P.; Liu, D. The stereoselective bioactivity and mechanism of indoxacarb against Spodoptera frugiperda. Pest. Manag. Sci. 2025, 81, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, H.; Wu, J.; Zheng, F.; Xu, K.; Lin, Y.; Zhang, Z.; Xu, H. Drip application of chlorantraniliprole effectively controls invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) and its distribution in maize in China. Crop Prot. 2021, 143, 105474. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, Z.; Li, Y.; Hao, R.; Chen, Y.; Chen, B.; Qin, X.; Tao, X.; Gui, F. Effects of elevated CO2 concentration on host adaptability and chlorantraniliprole susceptibility in Spodoptera frugiperda. Insects 2022, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-M.; Zhao, Y.-X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.-F.; Wu, S.-F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef]
- Landwehr, A. Benefits of baculovirus use in IPM strategies for open field and protected vegetables. Front. Sustain. Food Syst. 2021, 4, 593796. [Google Scholar] [CrossRef]
- Raj, M.N.; Samal, I.; Paschapur, A.; Subbanna, A. Entomopathogenic viruses and their potential role in sustainable pest management. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–72. [Google Scholar] [CrossRef]
- Anggraini, E.; Hong, L.W.; Vadamalai, G.; Kong, L.L.; Mat, M. The effect and possible mitigation of UV radiation on baculoviruses as bioinsecticides. Biodivers. J. Biol. Divers. 2022, 23, 3721–3735. [Google Scholar] [CrossRef]
- Schmutterer, H. Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu. Rev. Entomol. 1990, 35, 271–297. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Brzozowski, L.; Mazourek, M. A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 2018, 10, 2023. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, H.; Hu, M.; Wang, X.; Zhang, L. Discovery of novel potential insecticide-resistance mutations in Spodoptera frugiperda. Insects 2024, 15, 186. [Google Scholar] [CrossRef]
- Hu, B.; Deng, Y.; Lu, T.; Ren, M.; Liu, K.; Rao, C.; Guo, H.; Su, J. Inhibition of transcriptional regulation of detoxification genes contributes to insecticide resistance management in Spodoptera exigua. Commun. Biol. 2025, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Ullah, Z.; Gul, H.; Li, X.; Pan, Y.; Zhang, H.; Zhang, Z.; Huang, J.; Emmanouil, R.; Guedes, R.N.C. Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole. Int. J. Mol. Sci. 2025, 26, 5180. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, M.; Liu, X.; Shi, W.; Qi, J.; Zhou, S.; Wang, G. The Spodoptera frugiperda L-aminoacylase degrades fatty acid-amino acid conjugates and promotes larvae growth on Zea mays. Commun. Biol. 2025, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-B.; Cui, L.-L.; Zhang, B.-Z.; Jiang, Y.-T.; Lv, Y.-P.; Zhang, P.; Peng, Y.-Y.; Xiong, Y.-S.; Ji, X.; Liu, R.-Q. miR-10-5p mediated susceptibility to chlorantraniliprole by targeting for SfGSTe1 in Spodoptera frugiperda (Smith). Sci. Rep. 2025, 15, 19833. [Google Scholar] [CrossRef]
- Madesh, K.; Komala, G.; Chandralekha, R.; Tripathi, P. Mode of action of novel insecticides. In Advanced Trends in Plant Protection; PK Publishers & Distributors: Delhi, India, 2024; pp. 255–291. [Google Scholar]
- Martínez-Balerdi, M.; Caballero, J.; Aguirre, E.; Caballero, P.; Beperet, I. Baculoviruses as Microbial Pesticides: Potential, Challenges, and Market Overview. Viruses 2025, 17, 917. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Harish, S.; Murugan, M.; Kannan, M.; Parthasarathy, S.; Prabhukarthikeyan, S.; Elango, K. Entomopathogenic viruses. In Microbial Approaches for Insect Pest Management; Springer: Singapore, 2021; pp. 1–57. [Google Scholar] [CrossRef]
- Williams, T.; Bergoin, M.; van Oers, M.M. Diversity of large DNA viruses of invertebrates. J. Invertebr. Pathol. 2017, 147, 4–22. [Google Scholar] [CrossRef]
- Kuzio, J.; Jaques, R.t.; Faulkner, P. Identification of p74, a gene essential for virulence of baculovirus occlusion bodies. Virology 1989, 173, 759–763. [Google Scholar] [CrossRef]
- Nugnes, M.V.; Targovnik, A.M.; Mengual-Martí, A.; Miranda, M.V.; Cerrudo, C.S.; Herrero, S.; Belaich, M.N. The membrane-anchoring region of the acmnpv p74 protein is expendable or interchangeable with homologs from other species. Viruses 2021, 13, 2416. [Google Scholar] [CrossRef]
- Rohrmann, G.F. Baculovirus Molecular Biology; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019. Available online: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=bacvir (accessed on 3 February 2025).
- Cerrudo, C.S.; Motta, L.F.; Cuccovia Warlet, F.U.; Lassalle, F.M.; Simonin, J.A.; Belaich, M.N. Protein-gene orthology in baculoviridae: An exhaustive analysis to redefine the ancestrally common coding sequences. Viruses 2023, 15, 1091. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Liu, C.; Qu, L.; Wang, D. Genome analysis of an alphabaculovirus isolated from the larch looper, Erannis ankeraria. Viruses 2021, 14, 34. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Ohkawa, T.; Washburn, J.O.; Volkman, L.E. Effects of Ac150 on virulence and pathogenesis of Autographa californica multiple nucleopolyhedrovirus in noctuid hosts. J. Gen. Virol. 2005, 86, 1619–1627. [Google Scholar] [CrossRef]
- Han, G.; Zhang, N.; Jiang, H.; Meng, X.; Qian, K.; Zheng, Y.; Xu, J.; Wang, J. Diversity of short interspersed nuclear elements (SINEs) in Lepidopteran insects and evidence of horizontal SINE transfer between baculovirus and Lepidopteran hosts. BMC Genom. 2021, 22, 226. [Google Scholar] [CrossRef]
- Jiménez-Hernández, S.Y.; Rangel-Núñez, J.C.; Ibarra, J.E.; Del Rincón-Castro, M.C. Biological, morphological, and molecular characterization of the baculovirus PlxyMNPV_LBIV-11, and its virulence towards Plutella xylostella, Trichoplusia ni, and Spodoptera frugiperda larvae. Arch. Microbiol. 2022, 204, 598. [Google Scholar] [CrossRef] [PubMed]
- Gasque, S.N.; Han, Y.; van der Ham, I.; van Leeuwen, D.; van Oers, M.M.; Haverkamp, A.; Ros, V.I. Baculovirus entry into the central nervous system of Spodoptera exigua caterpillars is independent of the viral protein tyrosine phosphatase. Open Biol. 2024, 14, 230278. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, G.B.; Karimi, J. Whole genome analysis of a novel Spodoptera exigua nucleopolyhedrovirus isolate (SeMNPV-IR) to Iran. Biologia 2023, 78, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Kamita, S.G.; Nagasaka, K.; Chua, J.W.; Shimada, T.; Mita, K.; Kobayashi, M.; Maeda, S.; Hammock, B.D. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a Lepidopteran host. Proc. Natl. Acad. Sci. USA 2005, 102, 2584–2589. [Google Scholar] [CrossRef]
- Han, Y.; Van Houte, S.; Van Oers, M.M.; Ros, V.I. Baculovirus PTP2 functions as a pro-apoptotic protein. Viruses 2018, 10, 181. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Mikhailov, V.S.; Rohrmann, G.F. Baculovirus DNA replication and processing. Curr. Drug Targets 2007, 8, 1096–1102. [Google Scholar] [CrossRef]
- Rangel-Núñez, J.C.; Ibarra, J.E.; Del Rincon-Castro, M.C. Transcriptomics and interactomics during the primary infection of an SfMNPV baculovirus on Spodoptera frugiperda larvae. Front. Cell Infect. Microbiol. 2023, 13, 1291433. [Google Scholar] [CrossRef]
- Pavan, J.; Patel, N.B.; Rajarushi, C.; Gouda, M.; Raghunandan, B. Exploring the influence of weather parameters on natural nucleopolyhedrovirus (NPV) infection in fall armyworm, Spodoptera frugiperda (JE Smith) larvae in maize ecosystems. J. Entomol. Res. 2024, 48, 310–316. [Google Scholar] [CrossRef]
- Ferrelli, M.L.; Salvador, R. Effects of mixed baculovirus infections in biological control: A comprehensive historical and technical analysis. Viruses 2023, 15, 1838. [Google Scholar] [CrossRef]
- Malik, M.A.; Ahmad, S.J.N.; Arif, M.J.; Ahmad, J.N. Management of diamond back moth (Plutella xylostella) using indigenous isolated Granulovirus and Azadirachta indica. Pak. J. Zool. 2020, 52, 573–583. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Chaudhary, M.; Kumar, P. Myco-Jaal: A novel formulation of Beauveria bassiana for managing diamondback moth (Plutella xylostella) in subtropical and tropical crucifer production systems. In Proceedings of the Sixth International Workshop on Management of the Diamondback Moth and Other Crucifer Insect Pests, Nakhon Pathom, Thailand, 21–25 March 2011; Srinivasan, R., Shelton, A.M., Collins, H.L., Eds.; AVRDC-World Vegetable Center: Bangkok, Thailand, 2011; Volume 11, pp. 153–158. [Google Scholar]
- McCutchen, W.F.; Flexner, L. Joint actions of baculoviruses and other control agents. In Biopesticides: Use and Delivery; Humana Press: Totowa, NJ, USA, 1999; pp. 341–355. [Google Scholar] [CrossRef]
- Presa-Parra, E.; Navarro-De-La-Fuente, L.; Williams, T.; Lasa, R. Can low concentration flufenoxuron treatment increase the pathogenicity or production of nucleopolyhedrovirus occlusion bodies in Spodoptera exigua (Hübner) or Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae)? Acta Zoológica Mex. 2023, 39, 1–15. [Google Scholar] [CrossRef]
- Geervliet, J.; Vlak, J.; Smits, P. Effects of Spodoptera exigua nuclear polyhedrosis virus and Bacillus thuringiensis subsp. aizawai mixtures on mortality of beet armyworm, Spodoptera exigua. Meded. Van De Fac. Landbouwwet. Rijksuniv. Gent 1991, 56, 305–311. [Google Scholar]
- Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of Lepidopteran nucleopolyhedroviruses AcMNPV and SpliNPV with insecticides. Insects 2020, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, J.; Zhou, X.; Pu, X. Study on physical properties of four pH responsive Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) microcapsules as controlled release carriers. Sci. Rep. 2022, 12, 21873. [Google Scholar] [CrossRef]
- Gelernter, W.; Toscano, N.; Kido, K.; Federici, B. Comparison of a nuclear polyhedrosis virus and chemical insecticides for control of the beet armyworm (Lepidoptera: Noctuidae) on head lettuce. J. Econ. Entomol. 1986, 79, 714–717. [Google Scholar] [CrossRef]
- Pavan, J.; Patel, N.; Raghunandan, B.; Baldaniya, A. Efficacy of nucleopolyhedrovirus (SfMNPV) and insecticides alone and in combination against fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) infesting maize. Biol. Int. J. 2022, 14, 1120–1125. [Google Scholar] [CrossRef]
- Pavan, J.; Patel, N.B.; Raghunandan, B.; Baldaniya, A.; Bhatt, N. Comparative efficacy of nucleopolyhedrovirus (NPV) alone and in conjunction with chemical insecticides against fall armyworm, Spodoptera frugiperda (JE Smith) (Noctuidae: Lepidoptera) under laboratory conditions. Int. J. Trop. Insect Sci. 2024, 44, 1475–1486. [Google Scholar] [CrossRef]
- Abd El-Samei, E.; Hamama, H.; El-Enien, M.; Awad, H. Interaction of spinosad and Bacillus thuringiensis on certain toxicological, biochemical and molecular aspects in the Egyptian cotton leaf worm, Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Afr. Entomol. 2019, 27, 508–522. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, H.; Sufyan, M.; Gogi, M.D.; Arif, M.J.; Ranjha, M.H.; Arshid, M.; Waseem, M.; Mustafa, T.; Qasim, M. Comparative bio-efficacy of nuclear polyhedrosis virus (NPV) and Spinosad against American bollwormm, Helicoverpa armigera (Hubner). Rev. Bras. Entomol. 2019, 63, 277–282. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Nasir, F.; Qayyum, M.A.; Tahir, M. Insecticidal efficacy of Azadirachta indica, nucleopolyhedrovirus and chlorantraniliprole singly or combined against field populations of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2012, 72, 53–61. [Google Scholar] [CrossRef][Green Version]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. BioControl 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Trang, T.; Chaudhari, S. Bioassay of nuclear polyhedrosis virus (NPV) and in combination with insecticide on Spodoptera litura (Fab). Omonrice 2002, 10, 45–53. [Google Scholar]
- Abd El-Wahab, A.; El-Bendary, H. Nano silica as a promising nano pesticide to control three different aphid species under semi-field conditions in Egypt. Egypt. Acad. J. Biol. Sci. F Toxicol. Pest. Control 2016, 8, 35–49. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.-M.; Liu, H.-Y.; Xin, Z.; Xue, M. Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 1825–1831. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Zarmakoupi, C.; Kitsiou, F.; Eliopoulos, P.A.; Patakioutas, G. Evaluation of Commercial Virus Biopesticides for the Control of Moth Pests in Laboratory Conditions: The Cases of Thaumetopoea pityocampa and Helicoverpa armigera. Appl. Sci. 2024, 14, 506. [Google Scholar] [CrossRef]
- Kolodny-Hirsch, D.; Sitchawat, T.; Jansiri, T.; Chenrchaivachirakul, A.; Ketunuti, U. Field evaluation of a commercial formulation of the Spodoptera exigua (Lepidoptera: Noctuidae) nuclear polyhedrosis virus for control of beet armyworm on vegetable crops in Thailand. Biocontrol Sci. Technol. 1997, 7, 475–488. [Google Scholar] [CrossRef]
- Maciel, R.M.; Luski, P.G.; Sutil, W.P.; Gonçalves, J.; Hayashida, R.; de Queiroz, A.P.; Neves, P.M.; Bueno, A.d.F. The use of baculovirus Spodoptera SfMNPV alone and combined with herbicides and adjuvant to control Spodoptera frugiperda (Smith, 1797)(Lepidoptera: Noctuidae). BioControl 2024, 188, 105408. [Google Scholar] [CrossRef]
- Santos, A.M.; Uribe, L.A.; Ruiz, J.C.; Tabima, L.; Gómez, J.A.; Villamizar, L.F. Spodoptera frugiperda Nucleopolyhedrovirus Sf NPV003: Compatibility with agrochemicals and storage stability. Cienc. Tecnol. Agropecu. 2014, 15, 219–228. [Google Scholar] [CrossRef]
- Han, G.; Li, C.; Liu, Q.; Xu, J. Synergistic Effect of Combining Plutella xylostella Granulovirus and Bacillus thuringiensis at Sublethal Dosages on Controlling of Diamondback Moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015, 108, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Valderrama, J.; Cuartas-Otálora, P.; Espinel-Correal, C.; Barrera-Cubillos, G.; Villamizar-Rivero, L. Fungal and viral entomopathogens as a combined strategy for the biological control of fall armyworm larvae in maize. CABI Agric. Biosci. 2022, 3, 24. [Google Scholar] [CrossRef]
- Marhaen, L.S.; Aprianto, F.; Hasyim, A.; Lukman, L. Potensi campuran Spodoptera exigua Nucleopolyhedrovirus (SeNPV) dengan insektisida botani untuk meningkatkan mortalitas ulat bawang Spodoptera exigua (Hubner)(Lepidoptera: Noctuidae) di laboratorium. J. Hortik. 2016, 26, 103–112. [Google Scholar] [CrossRef]
- Mehrvar, A.; Ghanbari, S.; Söylemezoğlu, G.; Toprak, U. A novel tank-mix formulation increases the efficacy of alphabaculoviruses on different phylloplanes. J. Econ. Entomol. 2025, 118, 83–92. [Google Scholar] [CrossRef]
- Hayakawa, T.; Shimojo, E.-i.; Mori, M.; Kaido, M.; Furusawa, I.; Miyata, S.; Sano, Y.; Matsumoto, T.; Hashimoto, Y.; Granados, R.R. Enhancement of baculovirus infection in Spodoptera exigua (Lepidoptera: Noctuidae) larvae with Autographa californica nucleopolyhedrovirus or Nicotiana tabacum engineered with a granulovirus enhancin gene. Appl. Entomol. Zool. 2000, 35, 163–170. [Google Scholar] [CrossRef]
- Shapiro, M.; Shepard, B.M. The gypsy moth (Lepidoptera: Lymantriidae) nucleopolyhedrovirus as a synergist for baculoviruses against beet armyworm, fall armyworm and corn earworm (Lepidoptera: Noctuidae). J. Agric. Urban. Entomol. 2006, 23, 243–251. [Google Scholar]
- Lasa, R.; Caballero, P.; Williams, T. Juvenile hormone analogs greatly increase the production of a nucleopolyhedrovirus. BioControl 2007, 41, 389–396. [Google Scholar] [CrossRef]
- Lasa, R.; Ruiz-Portero, C.; Alcázar, M.D.; Belda, J.E.; Caballero, P.; Williams, T. Efficacy of optical brightener formulations of Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) as a biological insecticide in greenhouses in southern Spain. BioControl 2007, 40, 89–96. [Google Scholar] [CrossRef]
- Caballero, P.; Murillo, R.; Muñoz, D.; Williams, T. El nucleopoliedrovirus de Spodoptera exigua (Lepidoptera: Noctuidae) como bioplaguicida: Análisis de avances recientes en España. Rev. Colomb. Entomol. 2009, 35, 105–115. [Google Scholar] [CrossRef]
- Abot, A.; Moscardi, F.; Fuxa, J.; Sosa-Gomez, D.; Richter, A. Development of resistance by Anticarsia gemmatalisfrom Brazil and the United States to a nuclear polyhedrosis virus under laboratory selection pressure. BioControl 1996, 7, 126–130. [Google Scholar] [CrossRef]
- Briese, D.; Mende, H. Differences in susceptibility to a granulosis virus between field populations of the potato moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). Bull. Entomol. Res. 1981, 71, 11–18. [Google Scholar] [CrossRef]
- Zichová, T.; Stará, J.; Kundu, J.K.; Eberle, K.E.; Jehle, J.A. Resistance to Cydia pomonella granulovirus follows a geographically widely distributed inheritance type within Europe. BioControl 2013, 58, 525–534. [Google Scholar] [CrossRef]
- Smits, P.; Vlak, J. Biological activity of Spodoptera exigua nuclear polyhedrosis virus against S. exigua larvae. J. Inverteb. Pathol. 1988, 51, 107–114. [Google Scholar] [CrossRef]
- Fang, Z.; Shao, J.; Weng, Q. De novo transcriptome analysis of Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) genes in latently infected Se301 cells. Virol. Sin. 2016, 31, 425–436. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; Lynn, D.E.; Herrero, S.; Vlak, J.M.; van Oers, M.M. Host-range expansion of Spodoptera exigua multiple nucleopolyhedrovirus to Agrotis segetum larvae when the midgut is bypassed. J. Gen. Virol. 2010, 91, 898–906. [Google Scholar] [CrossRef]
- Qiao, F.-J.; Xing, L.-S.; Li, C.-Y.; Zheng, G.-L.; Zhang, B. Sensitivity of Spodoptera exigua larvae to nucleopolyhedrovirus and their feeding preference under different nutritional conditions. J. Environ. Entomol. 2021, 43, 1122–1128. [Google Scholar] [CrossRef]
- Li, J.; Jing, Z.; Yu, Q.; Zheng, G.; Zhang, B.; Xing, L.; Zhang, H.; Wan, F.; Li, C. Antiviral function of peptidoglycan recognition protein in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2023, 30, 1092–1104. [Google Scholar] [CrossRef]
- Bentivenha, J.P.; Rodrigues, J.G.; Lima, M.F.; Marçon, P.; Popham, H.J.; Omoto, C. Baseline susceptibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) to SfMNPV and evaluation of cross-resistance to major insecticides and Bt proteins. J. Econ. Entomol. 2019, 112, 91–98. [Google Scholar] [CrossRef]

| Phases | Description |
|---|---|
| 1 | Definition of the study theme and guiding question (PICO) |
| 2 | Establishment of selection criteria (inclusion and exclusion of articles) |
| 3 | Selection of databases and descriptors for literature access |
| 4 | Data collection |
| 5 | Analysis of results |
| Chemical Family * | Molecular Target | Active Compound (Treatment) | Crop | Effectiveness Plague | Effective Physiology in Plagues | Effectiveness (%) | Resistance | Non-target Insect Toxicity | Human Toxicity | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1A Carbamates | 1 Acetylcholinesterase (AChE) inhibitors | Carbosulfan | Broccoli | P. xylostella, | Nerve and Muscle | 70–80 | Yes | High | High | [50,51,52,53] |
| Carbofuran | Tomato | S. exigua | Nerve and Muscle | 80–90 | Yes | High | High | [54,55,56,57] | ||
| Methomyl | Tomato, Maize | S. exigua,
S. frugiperda | Nerve and Muscle | 80–90 | Yes | High | High | [58,59,60,61] | ||
| 1B Organophosphates | Chlorpyrifosmethyl | Tomato | S. exigua | Nerve and Muscle | Up to 90 | Yes | High | High | [62,63,64,65] | |
| Methamidophos | Tomato | S. exigua | Nerve and Muscle | 85–90 | Yes | High | High | [66] | ||
| Acephate | Tomato | S. exigua | Nerve and Muscle | 85–90 | Yes | High | High | [67] | ||
| 3A Pyrethroids | 3 Sodium channel modulators | Permethrin | Broccoli, Tomato | P. xylostella,
S. exigua | Nerve and Muscle | 70–85 | Yes | High | High | [11,68,69,70,71,72,73,74] |
| Bifenthrin | Tomato | S. exigua | Nerve and Muscle | 85–95 | Yes | High | High | [75,76] | ||
| Cypermethrin | Tomato | S. exigua | Nerve and Muscle | 85–95 | Yes | High | High | [77,78,79,80] | ||
| Gamma Cyhalothrin | Tomato | S. exigua | Nerve and Muscle | 85–95 | Yes | High | High | [81,82,83] | ||
| 4A Neonicotinoids | 4 Nicotinic acetylcholine receptor (nAChR) competitive modulators | Neonicotinoids | Broccoli | P. xylostella | Nerve and Muscle | 75–85 | Yes | Low | Low | [84,85,86,87,88] |
| 5 Spinosyns | 5 Nicotinic acetylcholine receptor (nAChR) allosteric modulators site I | Spinosad | Broccoli | P. xylostella | Nerve and Muscle | 80–90 | Yes | Low | Low | [89,90,91,92,93,94] |
| 6 Avermectins and Milbemycins | 6 Glutamate-gated chloride channel (GluCl) allosteric modulators | Emamectin benzoate | Maize Tomato | S. frugiperda S. exigua | Nerve and Muscle | 90 | Yes | High | High | [95,96] |
| 8C Fluorides | 8 Miscellaneous non-specific (multisite) inhibitors | Cryolite | Tomato | S. exigua | Unknown or Non-specific | 70–80 | Yes | Low | Low | [97,98] |
| 11A Bacillus thuringiensis | 11 Microbial disruptors of insect midgut membranes | Bacillus thuringiensis | Tomato | S. exigua | Midgut | 70–90 | Yes | High | Low | [47,48] |
| 13 Pyrroles, Dinitrophenols, Sulfluramid | 13 Uncouplers of oxidative phosphorylation via disruption of proton gradient | Chlorfenapyr | Maize Tomato | S. frugiperda, S. exigua | Respiration | 85.18 | Yes | High | High | [99,100,101] |
| 15 Benzoylureas | 15 Inhibitors of chitin biosynthesis affecting CHS1 | Novaluron | Tomato | S. exigua | Growth and Development | 70–85 | Yes | High | Low | [102,103] |
| 22 Oxadiazines | 22 Voltage-dependent sodium channel blockers | Indoxacarb | Maize | S. frugiperda | Nerve and Muscle | 94.23 | Yes | High | High | [10,104,105] |
| 28 Diamides | 28 Ryanodine receptor modulators | Chlorantraniliprole | Maize | S. frugiperda | Nerve and Muscle | 90 | Yes | High | Moderate | [106,107,108] |
| 31 Granuloviruses and Nucleopolyhedroiruses | 31 Baculoviruses | Baculovirus | Broccoli | P. xylostella | Midgut | 60–75 | No | None | None | [30,109,110,111] |
| Unclassified | Unknown or uncertain mode of action | Azadirachtin | Tomato | S. exigua | Unknown or Non-specific | 70–85 | Yes | Low | Low | [5,112,113] |
| Active Compound | Plutella xylostella nucleopolyhedrovirus (PxGV) * (PxNPV) | Spodoptera exigua nucleopolyhedrovirus (SeMNPV) | Spodoptera frugiperda nucleopolyhedrovirus (SfMNPV) | References | |||
|---|---|---|---|---|---|---|---|
| Synergy | Antagonism | Synergy | Antagonism | Synergy | Antagonism | ||
| Methomyl | - | - | Yes | - | - | - | [143] |
| Chlorpyrifos methyl | - | - | - | - | Yes | - | [161] |
| Deltamethrin | - | - | - | Yes | - | - | [159] |
| Lambda Cyhalothrin | - | - | - | - | Yes | - | [161] |
| Spinosad | Yes | Yes | Yes | Yes | Yes | Yes | [24,29] |
| Spinetoram | - | - | Yes | Yes | - | [29,149] | |
| Emamectin benzoate | - | - | Yes | Yes | - | [29,150] | |
| Bacillus thuringiensis | Yes * | Yes | Yes | - | - | - | [30,145,162] |
| Beauveria bassiana | Yes * | - | - | - | - | - | [142] |
| Metarhizium rileyi | - | - | - | - | Yes | - | [163] |
| extract of Vitex trifolia | - | - | Yes | - | - | - | [164] |
| Chlorfenapyr | - | - | Yes | - | - | - | [29] |
| Flufenoxuron | - | - | - | Yes | - | Yes | [144] |
| Novaluron | Yes | - | - | - | Yes | - | [161] |
| Tebufenozide | Yes | - | Yes | - | - | - | [159] |
| Methoxyfenozide | - | - | - | Yes | - | - | [29] |
| Indoxacarb | Yes * | - | Yes | - | - | - | [29,142] |
| Chlorantraniliprole | Yes | - | Yes | - | Yes | - | [29,150] |
| SpliNPV, AcMNPV, or LdMNPV | Yes | - | Yes | - | Yes | - | [140,165,166,167] |
| Azadirachtin | Yes * | - | Yes | - | Yes | - | [141,146] |
| Fipronil | Yes * | - | - | - | - | - | [142] |
| Methoprene | - | - | Yes | - | - | - | [168] |
| Stilbene derivatives optical brighteners | - | - | Yes | - | - | - | [169,170] |
| Microencapsulation Formulations | - | - | Yes | - | Yes | - | [147,161] |
| Aggressive surfactants or solvents | - | - | - | - | - | Yes | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Munguía, A.M.; García-Munguía, C.A.; Guerra-Ávila, P.L.; Sánchez-Mendoza, E.A.; Rubalcava-Castillo, F.A.; García-Munguía, A.; Robles-López, M.R.; Cisneros-Guzmán, L.F.; Martínez-Alba, M.G.; Olvera-Gonzalez, E.; et al. Baculovirus-Based Biocontrol: Synergistic and Antagonistic Interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in Integrative Pest Management. Viruses 2025, 17, 1077. https://doi.org/10.3390/v17081077
García-Munguía AM, García-Munguía CA, Guerra-Ávila PL, Sánchez-Mendoza EA, Rubalcava-Castillo FA, García-Munguía A, Robles-López MR, Cisneros-Guzmán LF, Martínez-Alba MG, Olvera-Gonzalez E, et al. Baculovirus-Based Biocontrol: Synergistic and Antagonistic Interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in Integrative Pest Management. Viruses. 2025; 17(8):1077. https://doi.org/10.3390/v17081077
Chicago/Turabian StyleGarcía-Munguía, Alberto Margarito, Carlos Alberto García-Munguía, Paloma Lucía Guerra-Ávila, Estefany Alejandra Sánchez-Mendoza, Fabián Alejandro Rubalcava-Castillo, Argelia García-Munguía, María Reyna Robles-López, Luis Fernando Cisneros-Guzmán, María Guadalupe Martínez-Alba, Ernesto Olvera-Gonzalez, and et al. 2025. "Baculovirus-Based Biocontrol: Synergistic and Antagonistic Interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in Integrative Pest Management" Viruses 17, no. 8: 1077. https://doi.org/10.3390/v17081077
APA StyleGarcía-Munguía, A. M., García-Munguía, C. A., Guerra-Ávila, P. L., Sánchez-Mendoza, E. A., Rubalcava-Castillo, F. A., García-Munguía, A., Robles-López, M. R., Cisneros-Guzmán, L. F., Martínez-Alba, M. G., Olvera-Gonzalez, E., Torre, R. R. R.-d. l., & García-Munguía, O. (2025). Baculovirus-Based Biocontrol: Synergistic and Antagonistic Interactions of PxGV, PxNPV, SeMNPV, and SfMNPV in Integrative Pest Management. Viruses, 17(8), 1077. https://doi.org/10.3390/v17081077






