Global Circulation Dynamics and Its Determinants of Dengue Virus: A Network Evolution and Model Study from 1990 to 2019
Abstract
1. Introduction
2. Methods
2.1. Data Collection and Processing
2.1.1. Sequence Data
2.1.2. Case Data
2.1.3. Socioeconomic Data
2.1.4. Population Data
2.1.5. Forest Data
2.1.6. Climate Data
2.1.7. Airline Data
2.2. Mutation Network Construction
2.2.1. Sampling Based on the Dengue Cases
2.2.2. Calculate Pairwise Distance for the Sequences
2.2.3. Mutation Network Construction Procedure
2.3. Measurement of the Circulation Indicators
2.4. Classification of Circulation Roles Based on Circulation Indicators
2.5. Dynamic Change of Three Circulation Roles
2.6. Dynamic Change of Persistence Time
2.7. Correlation Analysis
2.8. Machine Learning Models
3. Results
3.1. Global Distribution for Different Circulation Roles and Persistence
3.2. Spatial and Temporal Dynamics for Different Circulation Roles and Persistence
3.2.1. DENV-1
3.2.2. DENV-2
3.2.3. DENV-3
3.2.4. DENV-4
3.2.5. Persistence Time
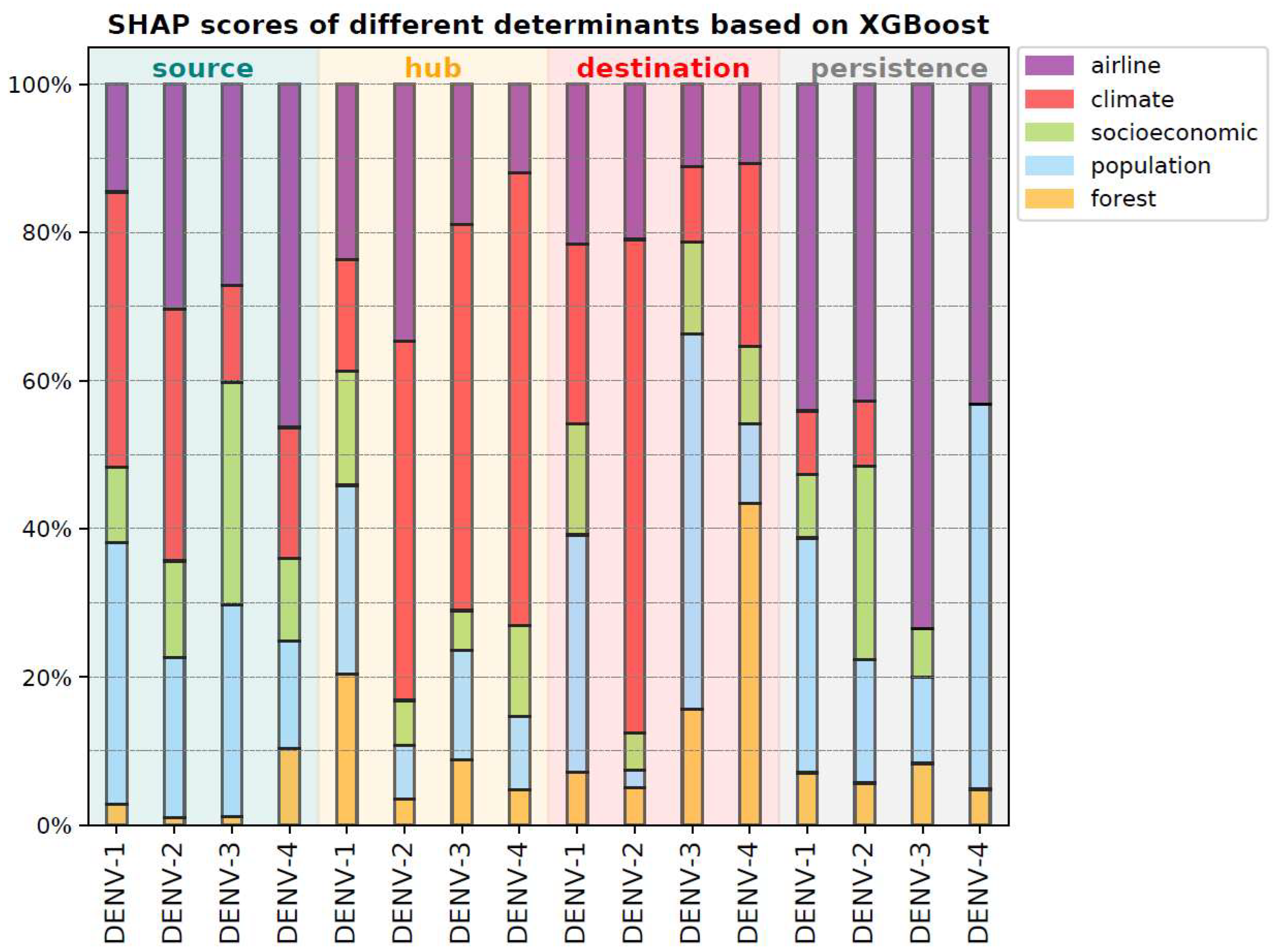
3.3. Determinants for Different Circulation Roles and Persistence
4. Discussions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paz-Bailey, G.; Adams, L.E.; Deen, J.; Anderson, K.B.; Katzelnick, L.C. Dengue. Lancet 2024, 403, 667–682. [Google Scholar] [CrossRef]
- Pollett, S.; Melendrez, M.C.; Berry, I.M.; Duchene, S.; Salje, H.; Cummings, D.A.T.; Jarman, R.G. Understanding dengue virus evolution to support epidemic surveillance and counter-measure development. Infect. Genet. Evol 2018, 62, 279–295. [Google Scholar] [CrossRef]
- Chauhan, N.; Kishan, K.G.; Tejeswara, R.A.; Guchhait, P. Dengue virus: Pathogenesis and potential for small molecule inhibitors. Biosci. Rep. 2024, 44, BSR20240134. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Einav, S. Towards Predicting Progression to Severe Dengue. Trends Microbiol. 2020, 28, 478–486. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Vo, H.T.M.; Duong, V.; Auerswald, H.; Ly, S.; Sakuntabhai, A.; Dussart, P.; Cantaert, T.; Ravetch, J.V. Antibody fucosylation predicts disease severity in secondary dengue infection. Science 2021, 372, 1102–1105. [Google Scholar] [CrossRef]
- Akshatha, H.S.; Pujar, G.V.; Sethu, A.K.; Bhagyalalitha, M.; Singh, M. Dengue structural proteins as antiviral drug targets: Current status in the drug discovery & development. Eur. J. Med. Chem. 2021, 221, 113527. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Wilder-Smith, A. Dengue vaccine development: Challenges and prospects. Curr. Opin. Infect. Dis. 2022, 35, 390–396. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, E.; Lusso, S.B.; Goya, S.; Rojo, G.L.; Natale, M.I.; Viegas, M.; Mistchenko, A.S.; Valinotto, L.E. Dengue Virus 1 Outbreak in Buenos Aires, Argentina, 2016. Emerg. Infect. Dis. 2017, 23, 1684–1685. [Google Scholar] [CrossRef]
- Castonguay-Vanier, J.; Klitting, R.; Sengvilaipaseuth, O.; Piorkowski, G.; Baronti, C.; Sibounheuang, B.; Vongsouvath, M.; Chanthongthip, A.; Thongpaseuth, S.; Mayxay, M.; et al. Molecular epidemiology of dengue viruses in three provinces of Lao PDR, 2006–2010. PLoS Neglected Trop. Dis. 2018, 12, e0006203. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.A.; Alagarasu, K.; Kakade, M.B.; More, A.M.; Gadekar, K.A.; Jadhav, S.M.; Parashar, D.; Shah, P.S. Emergence of dengue virus type 1 and type 3 as dominant serotypes during 2017 in Pune and Nashik regions of Maharashtra, Western India. Infect. Genet. Evol. 2018, 66, 272–283. [Google Scholar] [CrossRef]
- Poltep, K.; Phadungsombat, J.; Nakayama, E.E.; Kosoltanapiwat, N.; Hanboonkunupakarn, B.; Wiriyarat, W.; Shioda, T.; Leaungwutiwong, P. Genetic Diversity of Dengue Virus in Clinical Specimens from Bangkok, Thailand, during 2018–2020: Co-Circulation of All Four Serotypes with Multiple Genotypes and/or Clades. Trop. Med. Infect. Dis. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Allicock, O.M.; Sahadeo, N.; Lemey, P.; Auguste, A.J.; Suchard, M.A.; Rambaut, A.; Carrington, C.V.F. Determinants of dengue virus dispersal in the Americas. Virus Evol. 2020, 6, veaa074. [Google Scholar] [CrossRef]
- Tian, H.; Sun, Z.; Faria, N.R.; Yang, J.; Cazelles, B.; Huang, S.; Xu, B.; Yang, Q.; Pybus, O.G.; Xu, B. Increasing airline travel may facilitate co-circulation of multiple dengue virus serotypes in Asia. PLoS Neglected Trop. Dis. 2017, 11, e0005694. [Google Scholar] [CrossRef]
- Allicock, O.M.; Lemey, P.; Tatem, A.J.; Pybus, O.G.; Bennett, S.N.; Mueller, B.A.; Suchard, M.A.; Foster, J.E.; Rambaut, A.; Carrington, C.V. Phylogeography and population dynamics of dengue viruses in the Americas. Mol. Biol. Evol. 2012, 29, 1533–1543. [Google Scholar] [CrossRef]
- Brito, A.F.; Machado, L.C.; Oidtman, R.J.; Siconelli, M.J.L.; Tran, Q.M.; Fauver, J.R.; Carvalho, R.D.O.; Dezordi, F.Z.; Pereira, M.R.; de Castro-Jorge, L.A.; et al. Lying in wait: The resurgence of dengue virus after the Zika epidemic in Brazil. Nat. Commun. 2021, 12, 2619. [Google Scholar] [CrossRef]
- Galarion, M.J.; Schwem, B.; Pangilinan, C.; Dela Tonga, A.; Petronio-Santos, J.A.; Delos Reyes, E.; Destura, R. Genotypic persistence of dengue virus in the Philippines. Infect. Genet. Evol. 2019, 69, 134–141. [Google Scholar] [CrossRef]
- Teissier, Y.; Paul, R.; Aubry, M.; Rodo, X.; Dommar, C.; Salje, H.; Sakuntabhai, A.; Cazelles, B.; Cao-Lormeau, V.M. Long-term persistence of monotypic dengue transmission in small size isolated populations, French Polynesia, 1978–2014. PLoS Neglected Trop. Dis. 2020, 14, e0008110. [Google Scholar] [CrossRef]
- Cattarino, L.; Rodriguez-Barraquer, I.; Imai, N.; Cummings, D.A.T.; Ferguson, N.M. Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 2020, 12, eaax4144. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Lourenço, J.; Wang, L.; Cazelles, B.; Dong, L.; Li, B.; Liu, Y.; Jit, M.; Bosse, N.I.; et al. Measuring the effects of COVID-19-related disruption on dengue transmission in southeast Asia and Latin America: A statistical modelling study. Lancet Infect. Dis. 2022, 22, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Altassan, K.K.; Morin, C.; Shocket, M.S.; Ebi, K.; Hess, J. Dengue fever in Saudi Arabia: A review of environmental and population factors impacting emergence and spread. Travel Med. Infect. Dis. 2019, 30, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tsheten, T.; Gray, D.J.; Clements, A.C.A.; Wangdi, K. Epidemiology and challenges of dengue surveillance in the WHO South-East Asia Region. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 583–599. [Google Scholar] [CrossRef]
- Grenfell, B.T.; Pybus, O.G.; Gog, J.R.; Wood, J.L.N.; Daly, J.M.; Mumford, J.A.; Holmes, E.C. Unifying the epidemiological and evolutionary dynamics of pathogens. Science 2004, 303, 327–332. [Google Scholar] [CrossRef]
- Bedford, T.; Riley, S.; Barr, I.G.; Broor, S.; Chadha, M.; Cox, N.J.; Daniels, R.S.; Gunasekaran, C.P.; Hurt, A.C.; Kelso, A.; et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 2015, 523, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C. The phylogeography of human viruses. Mol. Ecol. 2004, 13, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Cunha, M.; Diagne, M.M.; Sembene, P.M.; Zanotto, P.M.A.; Faye, O.; Faye, O.; Sall, A.A. Origin and Spread of the Dengue Virus Type 1, Genotype V in Senegal, 2015–2019. Viruses 2021, 13, 57. [Google Scholar] [CrossRef]
- Jombart, T.; Eggo, R.M.; Dodd, P.J.; Balloux, F. Reconstructing disease outbreaks from genetic data: A graph approach. Heredity 2011, 106, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Chen, C.; Long, H.; Bai, J.; Zeng, J.; Cao, Z.; Zhang, B.; Shen, W.; Tang, F.; et al. A Mutation Network Method for Transmission Analysis of Human Influenza H3N2. Viruses 2020, 12, 1125. [Google Scholar] [CrossRef]
- Qiu, Z.; Cao, Z.; Zou, M.; Tang, K.; Zhang, C.; Tang, J.; Zeng, J.; Wang, Y.; Sun, Q.; Wang, D.; et al. The effectiveness of governmental nonpharmaceutical interventions against COVID-19 at controlling seasonal influenza transmission: An ecological study. BMC Infect. Dis. 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Qiu, Z.; Tang, F.; Liang, S.; Wang, Y.; Long, H.; Chen, C.; Zhang, B.; Zhang, C.; Wang, Y.; et al. Drivers and forecasts of multiple waves of the coronavirus disease 2019 pandemic: A systematic analysis based on an interpretable machine learning framework. Transbound. Emerg. Dis. 2022, 69, e1584–e1594. [Google Scholar] [CrossRef]
- Resch, W.; Zaslavsky, L.; Kiryutin, B.; Rozanov, M.; Bao, Y.; Tatusova, T.A. Virus variation resources at the National Center for Biotechnology Information: Dengue virus. BMC Microbiol. 2009, 9, 65. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef]
- Emanuel, K.A. Atmosphere Convection; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Retief, J.D. Phylogenetic Analysis Using PHYLIP; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Long, H.; Zhang, C.; Chen, C.; Tang, J.; Zhang, B.; Wang, Y.; Pang, J.; Su, W.; Li, K.; Di, B.; et al. Assessment of the global circulation and endemicity of dengue. Transbound. Emerg. Dis. 2022, 69, 2148–2155. [Google Scholar] [CrossRef]
- Vazquez, C.; Alcantara, L.C.J.; Fonseca, V.; Lima, M.; Xavier, J.; Adelino, T.; Fritsch, H.; Castro, E.; de Oliveira, C.; Schuab, G.; et al. Retrospective Spatio-Temporal Dynamics of Dengue Virus 1, 2 and 4 in Paraguay. Viruses 2023, 15, 1275. [Google Scholar] [CrossRef]
- Li, L.; Guo, X.; Zhang, X.; Zhao, L.; Li, L.; Wang, Y.; Xie, T.; Yin, Q.; Jing, Q.; Hu, T.; et al. A unified global genotyping framework of dengue virus serotype-1 for a stratified coordinated surveillance strategy of dengue epidemics. Infect. Dis. Poverty 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, X.; Deng, A.; Zhang, H.; Zhang, Y.; Tan, Q.; Peng, Z.; Li, J.; Song, T. Dengue Fever Outbreaks Caused by Varied Serotype Dengue Virus—Guangdong Province, China, 2019. China CDC Wkly 2020, 2, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, Q.; Liu, X.; Zhao, N.; Yin, W. Dengue Fever in Mainland China, 2005–2020: A Descriptive Analysis of Dengue Cases and Aedes Data. Int. J. Environ. Res. Public Health 2022, 19, 3910. [Google Scholar] [CrossRef]
- Li, Y.; Dou, Q.; Lu, Y.; Xiang, H.; Yu, X.; Liu, S. Effects of ambient temperature and precipitation on the risk of dengue fever: A systematic review and updated meta-analysis. Environ. Res. 2020, 191, 110043. [Google Scholar] [CrossRef]
- Gurevitz, J.M.; Antman, J.G.; Laneri, K.; Morales, J.M. Temperature, traveling, slums, and housing drive dengue transmission in a non-endemic metropolis. PLoS Neglected Trop. Dis. 2021, 15, e0009465. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Neglected Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Morin Cory, W.; Comrie Andrew, C.; Ernst, K. Climate and Dengue Transmission: Evidence and Implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Massad, E.; Amaku, M.; Coutinho, F.A.B.; Struchiner, C.J.; Burattini, M.N.; Khan, K.; Liu-Helmersson, J.; Rocklöv, J.; Kraemer, M.U.G.; Wilder-Smith, A. Estimating the probability of dengue virus introduction and secondary autochthonous cases in Europe. Sci. Rep. 2018, 8, 4629. [Google Scholar] [CrossRef]
- Wu, P.-C.; Lay, J.-G.; Guo, H.-R.; Lin, C.-Y.; Lung, S.-C.; Su, H.-J. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009, 407, 2224–2233. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Escoto, A.C.; Huang, A.T.; Garcia-Carreras, B.; Chowdhury, N.; Berry, I.M.; Chavez, C.; Buchy, P.; Duong, V.; Dussart, P.; et al. Antigenic evolution of dengue viruses over 20 years. Science 2021, 374, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Lovera, D.; Martinez-Cuellar, C.; Galeano, F.; Amarilla, S.; Vazquez, C.; Arbo, A. Clinical manifestations of primary and secondary dengue in Paraguay and its relation to virus serotype. J. Infect. Dev. Ctries. 2019, 13, 1127–1134. [Google Scholar] [CrossRef] [PubMed]


| Serotype | Decade | Median (Interquartile Range) | |||
|---|---|---|---|---|---|
| Source | Hub | Destination | Non-Role | ||
| DENV-1 | 2000s | 1 (0, 1.5) | 1 * (0, 1.25) | 0 (0, 1) | 0 (0, 0) |
| 2010s | 1 * (0, 2) | 1 * (0, 2) | 1 * (0, 1.75) | 0 (0, 0) | |
| DENV-2 | 2000s | 0 (0, 1.5) | 0 (0, 0) | 2 * (2, 2.75) | 0 (0, 0) |
| 2010s | 1 * (0, 3) | 1 (0.75, 2.5) | 0 (0, 2) | 0 (0, 0) | |
| DENV-3 | 2000s | 1 * (1, 2) | 0 (0, 0) | 0.5 (0.25, 0.75) | 0 (0, 0) |
| 2010s | 0 (0, 2) | 0 (0, 2) | 1 (0, 1.5) | 0 (0, 0) | |
| DENV-4 | 2000s | 1 (0, 2) | 0 (0, 0) | 5 (5, 5) | 0 (0, 0) |
| 2010s | 3 (2, 3.5) | 0.5 (0, 1.25) | 1 (0, 2.5) | 0 (0, 1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, H.; Zeng, J.; Chen, Y.; Tang, K.; Zhang, C.; Sun, Q.; Gao, L.; Lin, Y.; He, J.; Yang, C.; et al. Global Circulation Dynamics and Its Determinants of Dengue Virus: A Network Evolution and Model Study from 1990 to 2019. Viruses 2025, 17, 1078. https://doi.org/10.3390/v17081078
Long H, Zeng J, Chen Y, Tang K, Zhang C, Sun Q, Gao L, Lin Y, He J, Yang C, et al. Global Circulation Dynamics and Its Determinants of Dengue Virus: A Network Evolution and Model Study from 1990 to 2019. Viruses. 2025; 17(8):1078. https://doi.org/10.3390/v17081078
Chicago/Turabian StyleLong, Haoyu, Jinfeng Zeng, Yilin Chen, Kang Tang, Chi Zhang, Qianru Sun, Lei Gao, Yuhui Lin, Junting He, Chunhui Yang, and et al. 2025. "Global Circulation Dynamics and Its Determinants of Dengue Virus: A Network Evolution and Model Study from 1990 to 2019" Viruses 17, no. 8: 1078. https://doi.org/10.3390/v17081078
APA StyleLong, H., Zeng, J., Chen, Y., Tang, K., Zhang, C., Sun, Q., Gao, L., Lin, Y., He, J., Yang, C., Lin, X., Su, W., Li, K., Di, B., Kang, M., Yang, C., & Du, X. (2025). Global Circulation Dynamics and Its Determinants of Dengue Virus: A Network Evolution and Model Study from 1990 to 2019. Viruses, 17(8), 1078. https://doi.org/10.3390/v17081078






