Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells and Viruses
2.3. Antiviral Activity
2.4. Cytotoxicity Assay
2.5. Virucidal Effect
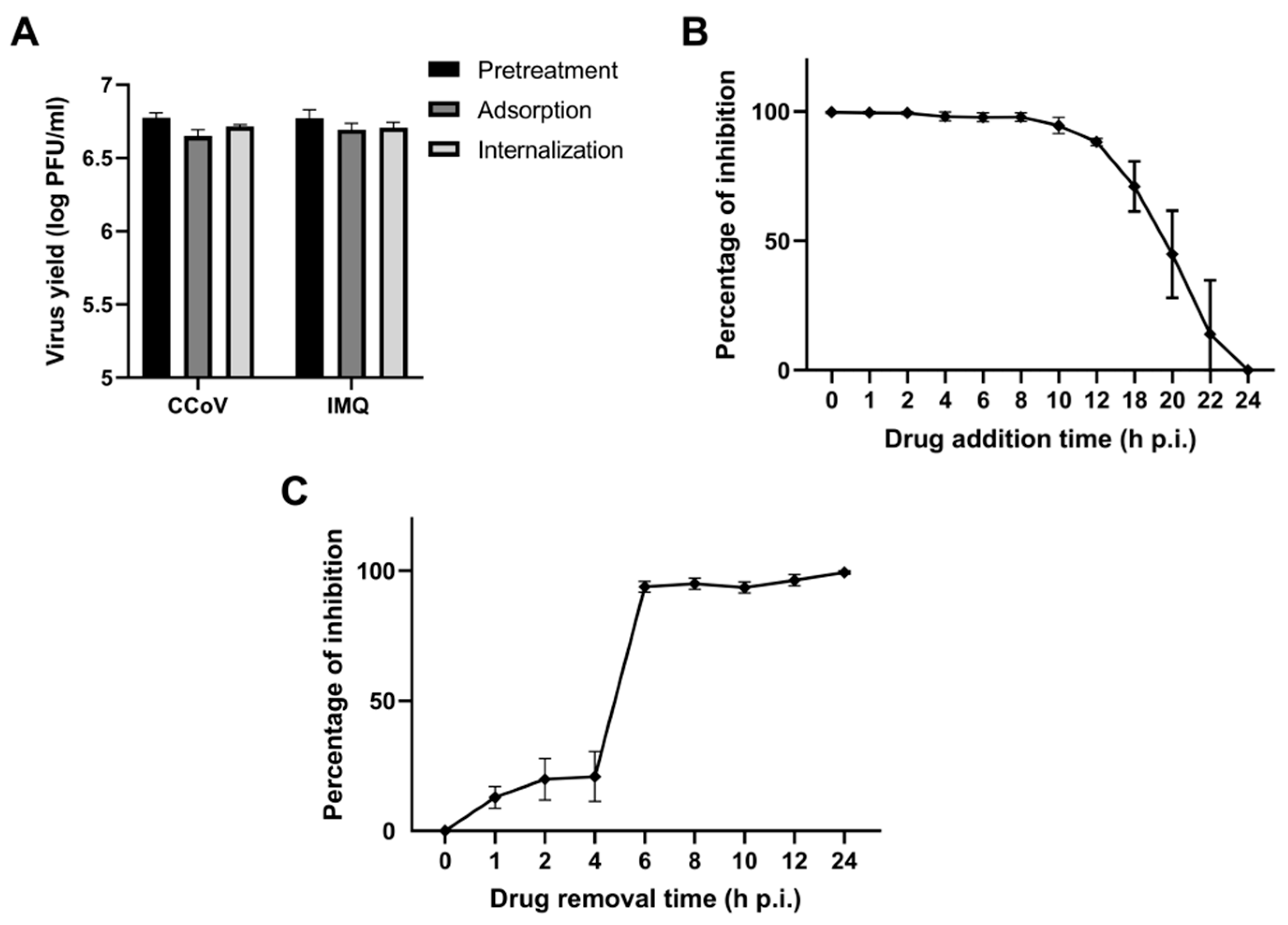
2.6. Pretreatment, Adsorption and Internalization Assays
2.7. Time-of-Addition and Time-of-Removal Assays
2.8. Immunofluorescence Assay (IF)
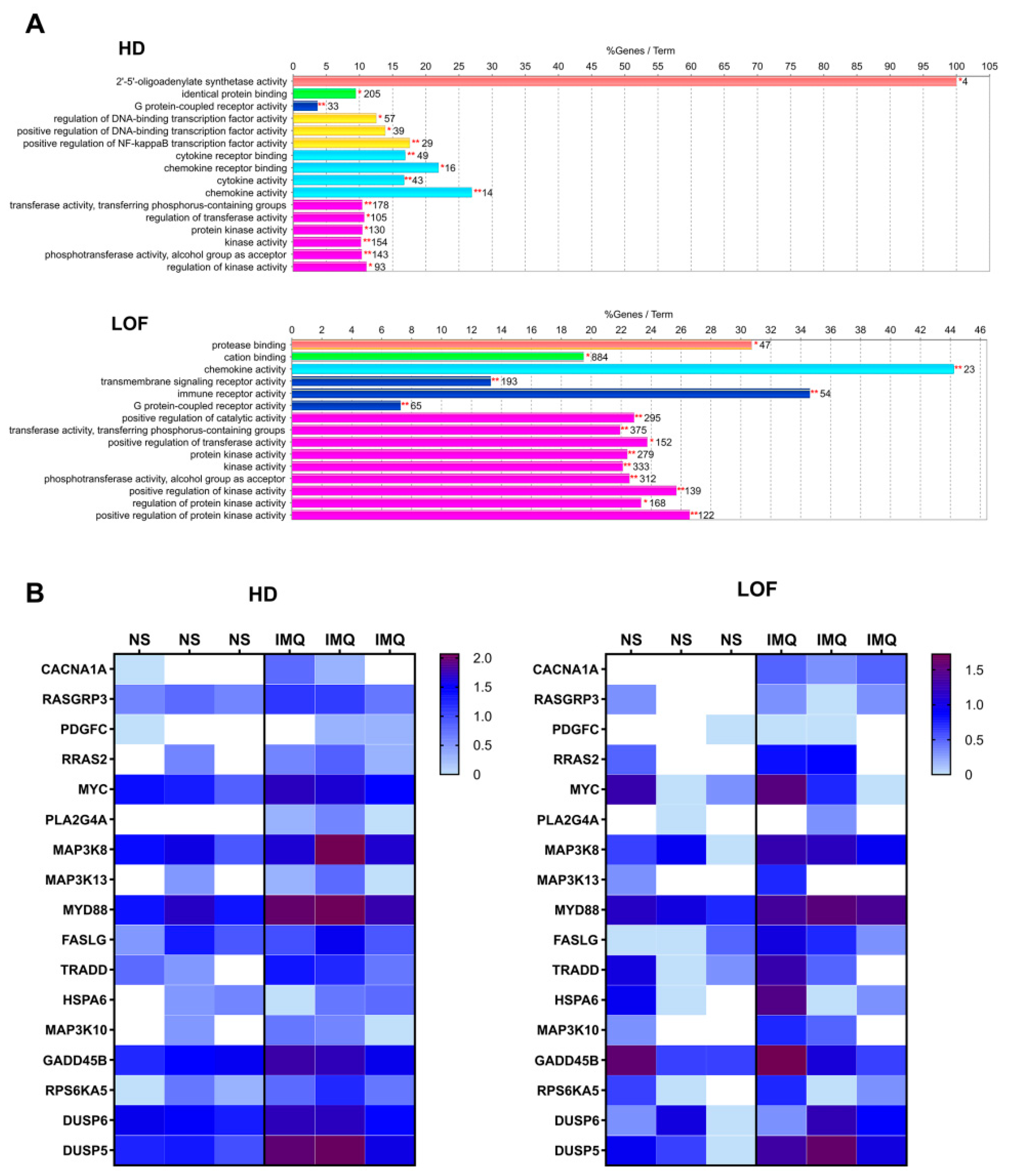
2.9. Raw RNA-Seq Data Analysis
2.10. Western Blot Analysis
2.11. Transfections and Reporter Gene Assays
2.12. Cytokine Determination
2.13. Statistical Analysis
3. Results
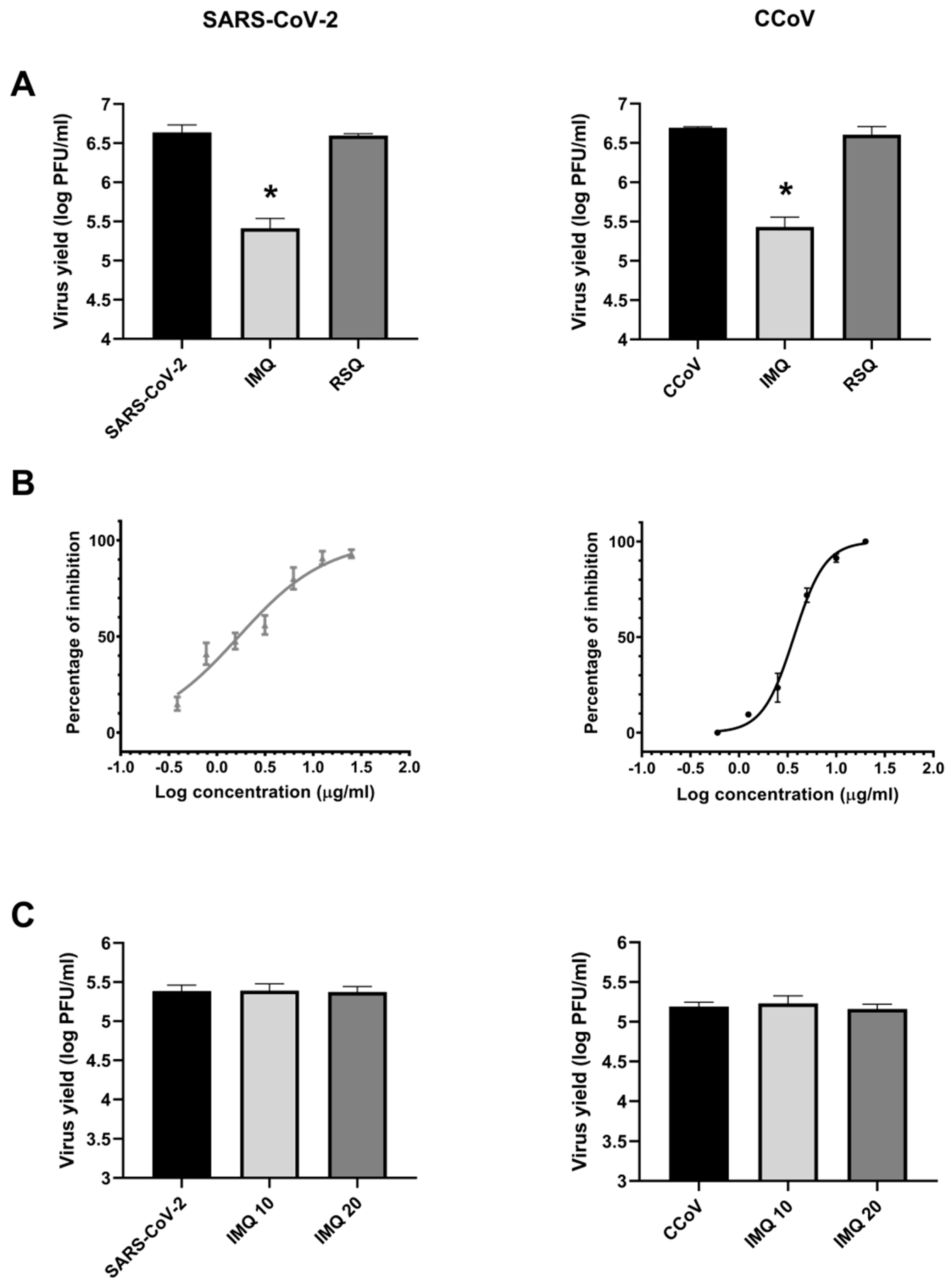
3.1. Antiviral Activity of IMQ Against Coronavirus In Vitro
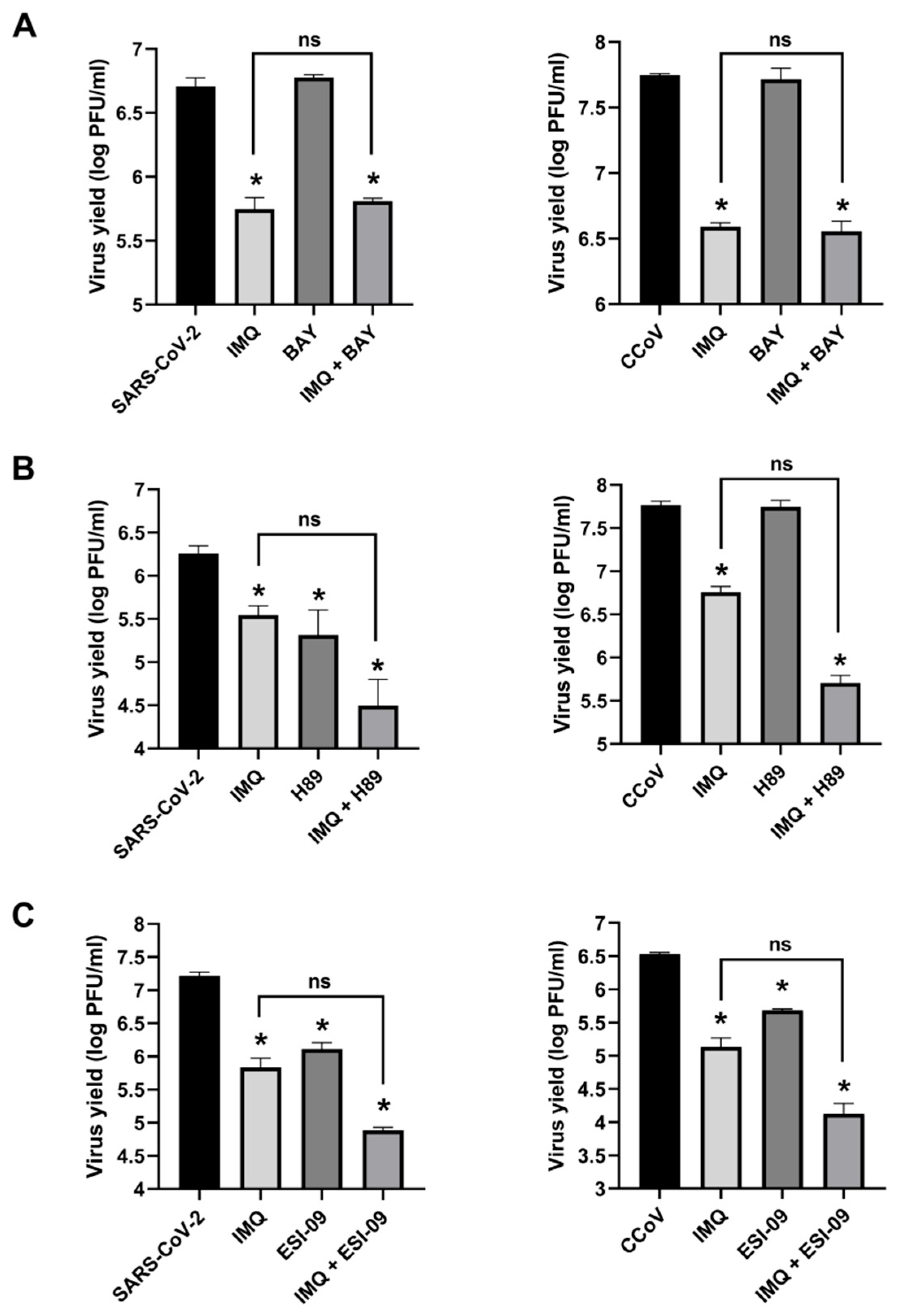
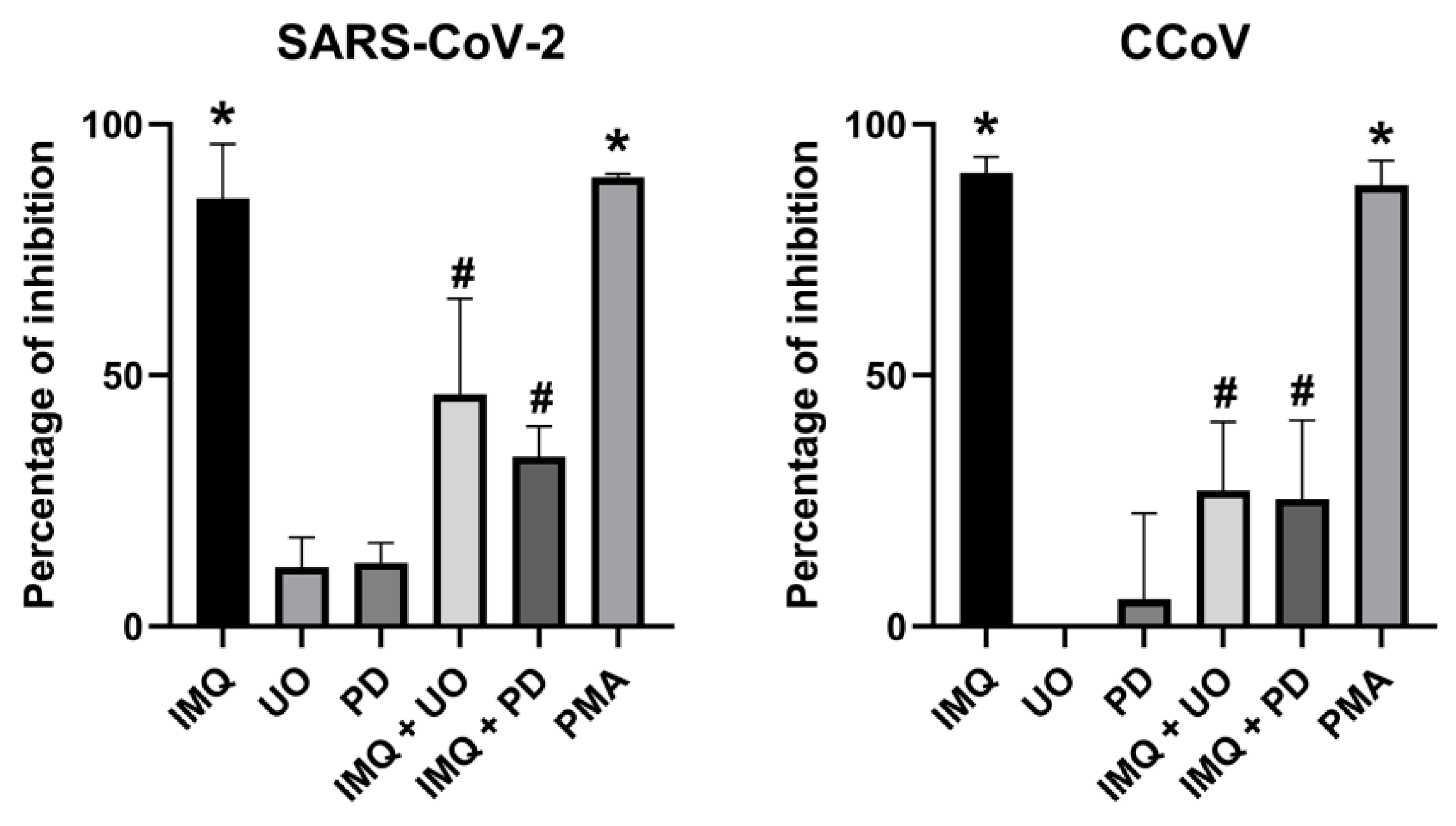
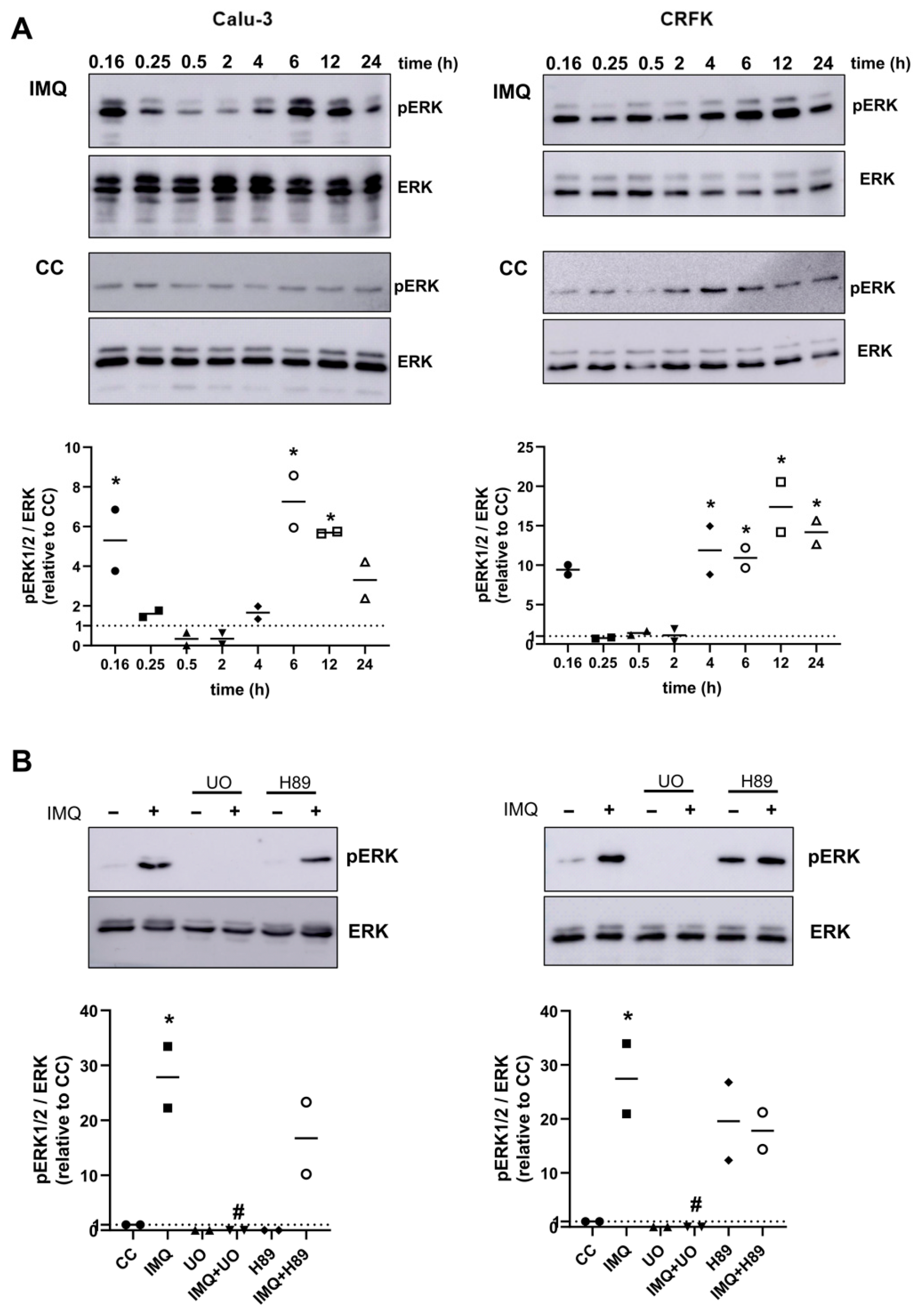
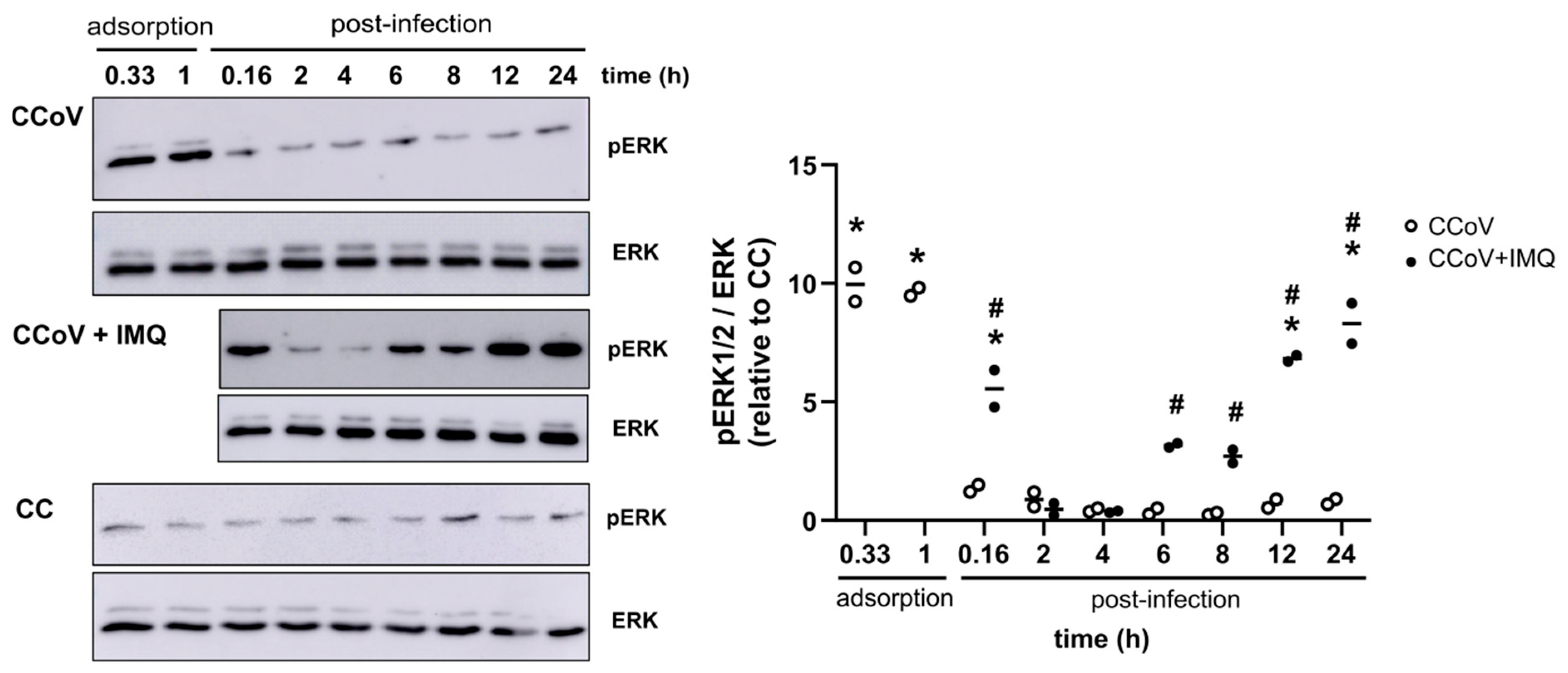
3.2. Anti-Coronavirus Activity of IMQ Was Independent of the NF-κB and PKA/EPAC Pathway and Dependent on the MEK/ERK Pathway
3.3. Role of the MEK/ERK Pathway in the Anti-Coronavirus Activity of IMQ
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Doorn, H.R.; Yu, H. Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 284–288. [Google Scholar] [CrossRef]
- Muthukutty, P.; MacDonald, J.; Yoo, S.Y. Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines 2024, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2019 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 2019, 27, 111–121. [Google Scholar]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.; Li, J.; et al. Mutations in the SARS-CoV-2 RNA-dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.C.; et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 2023, 613, 558–564. [Google Scholar] [CrossRef]
- Igari, H.; Sakao, S.; Ishige, T.; Saito, K.; Murata, S.; Yahaba, M.; Taniguchi, T.; Suganami, A.; Matsushita, K.; Tamura, Y.; et al. Dynamic diversity of SARS-CoV-2 genetic mutations in a lung transplantation patient with persistent COVID-19. Nat. Commun. 2024, 15, 3604. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Shimizu, K.; Usuku, S.; Senda, R.; Okubo, I.; Morita, H.; Nagata, S.; Fujisaki, S.; Miura, H.; Kishida, N.; et al. An outbreak of influenza A(H1N1)pdm09 antigenic variants exhibiting cross-resistance to oseltamivir and peramivir in an elementary school in Japan, September 2024. Euro Surveill. 2024, 29, 2400786. [Google Scholar] [CrossRef]
- Signore, A.V.; Joseph, T.; Ranadheera, C.; Erdelyan, C.N.G.; Alkie, T.N.; Raj, S.; Pama, L.; Ayilara, I.; Hisanaga, T.; Lung, O.; et al. Neuraminidase reassortment and oseltamivir resistance in clade 2.3.4.4b A(H5N1) viruses circulating among Canadian poultry, 2024. Emerg. Microbes Infect. 2025, 14, 2469643. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Salata, C.; Gribaudo, G. Tackling the future pandemics: Broad-spectrum antiviral agents (BSAAs) based on A-type proanthocyanidins. Molecules 2022, 27, 8353. [Google Scholar] [CrossRef]
- Edwards, A.M.; Baric, R.S.; Saphire, E.O.; Ulmer, J.B. Stopping pandemics before they start: Lessons learned from SARS-CoV-2. Science 2022, 375, 1133–1139. [Google Scholar] [CrossRef]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the next viral threat with broad-spectrum antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Maarifi, G.; Martin, M.F.; Zebboudj, A.; Boulay, A.; Nouaux, P.; Fernandez, J.; Lagisquet, J.; Garcin, D.; Gaudin, R.; Arhel, N.J.; et al. Identifying enhancers of innate immune signaling as broad-spectrum antivirals active against emerging viruses. Cell Chem. Biol. 2022, 29, 1113–1125.e6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Latreille, E.; Lee, W.L. Modulation of the host response as a therapeutic strategy in severe lung infections. Viruses 2023, 15, 1462. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cohen, J.I. The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside. Virology 2015, 479–480, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qiu, F.; Gu, R.; Xu, E. Functional Involvement of Signal Transducers and Activators of Transcription in the Pathogenesis of Influenza A Virus. Int. J. Mol. Sci. 2024, 25, 13589. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Z. Repurposing Anticancer Drugs Targeting the MAPK/ERK Signaling Pathway for the Treatment of Respiratory Virus Infections. Int. J. Mol. Sci. 2024, 25, 6946. [Google Scholar] [CrossRef]
- Zhang, Y.; Ba, J.; Luan, J.; Qi, Z.; Liu, B. Orthoflavivirus infection and the mTOR signaling pathway. Front. Microbiol. 2025, 16, 1565350. [Google Scholar] [CrossRef]
- Schön, M.P.; Schön, M. The small-molecule immune response modifier imiquimod—Its mode of action and clinical use in the treatment of skin cancer. Expert Opin. Ther. Targets 2006, 10, 69–76. [Google Scholar] [CrossRef]
- To, E.E.; Erlich, J.; Liong, F.; Luong, R.; Liong, S.; Bozinovski, S.; Seow, H.J.; O’Leary, J.J.; Brooks, D.A.; Vlahos, R.; et al. Intranasal and epicutaneous administration of Toll-like receptor 7 (TLR7) agonists provides protection against influenza A virus-induced morbidity in mice. Sci. Rep. 2019, 9, 2366. [Google Scholar] [CrossRef]
- Salinas, F.M.; Nebreda, A.D.; Vázquez, L.; Gentilini, M.V.; Marini, V.; Benedetti, M.; Jodar, M.S.N.; Viegas, M.; Shayo, C.; Bueno, C.A. Imiquimod suppresses respiratory syncytial virus (RSV) replication via PKA pathway and reduces RSV inducedinflammation and viral load in mice lungs. Antivir. Res. 2020, 179, 104817. [Google Scholar] [CrossRef]
- Kan, Y.; Okabayashi, T.; Yokota, S.; Yamamoto, S.; Fujii, N.; Yamashita, T. Imiquimod Suppresses Propagation of Herpes Simplex Virus 1 by Upregulation of Cystatin A via the Adenosine Receptor A1 Pathway. J. Virol. 2012, 86, 10338–10346. [Google Scholar] [CrossRef]
- Vicente, J.; Benedetti, M.; Martelliti, P.; Vázquez, L.; Gentilini, M.V.; Figueredo, F.A.P.; Jodar, M.S.N.; Viegas, M.; Barquero, A.A.; Bueno, C.A. The Flavonoid Cyanidin Shows Immunomodulatory and Broad-Spectrum Antiviral Properties, Including SARS-CoV-2. Viruses 2023, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Michelini, F.M.; Alché, L.E.; Bueno, C.A. Virucidal, antiviral and immunomodulatory activities of β-escin and Aesculus hippocastanum extract. J. Pharm. Pharmacol. 2018, 70, 1561–1571. [Google Scholar] [CrossRef]
- Figueredo, F.A.P.; Vicente, J.; Barquero, A.A.; Bueno, C.A. Aesculus hippocastanum extract and the main bioactive constituent β-escin as antivirals agents against coronaviruses, including SARS-CoV-2. Sci. Rep. 2024, 14, 6418. [Google Scholar] [CrossRef]
- Salinas, F.M.; Vázquez, L.; Gentilini, M.V.; O’Donohoe, A.; Regueira, E.; Jodar, M.S.N.; Viegas, M.; Michelini, F.M.; Hermida, G.; Alché, L.E.; et al. Aesculus hippocastanum L. seed extract shows virucidal and antiviral activities against respiratory syncytial virus (RSV) and reduces lung inflammation in vivo. Antivir. Res. 2019, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, S.; Daga, S.; Fallerini, C.; Baldassarri, M.; Benetti, E.; Picchiotti, N.; Fava, F.; Gallì, A.; Zibellini, S.; Bruttini, M.; et al. Rare variants in Toll-like receptor 7 results in functional impairment and downregulation of cytokine-mediated signaling in COVID-19 patients. Genes Immun. 2021, 23, 51–56. [Google Scholar] [CrossRef]
- Boulton, S.; Crupi, M.J.F.; Singh, S.; Carter-Timofte, M.E.; Azad, T.; Organ, B.C.; He, X.; Gill, R.; Neault, S.; Jamieson, T.; et al. Inhibition of exchange proteins directly activated by cAMP as a strategy for broad-spectrum antiviral development. J. Biol. Chem. 2023, 299, 104749. [Google Scholar] [CrossRef]
- Tao, X.; Mei, F.; Agrawal, A.; Peters, C.J.; Ksiazek, T.G.; Cheng, X.; Tseng, C.T. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J. Virol. 2014, 88, 3902–3910. [Google Scholar] [CrossRef]
- Bharatha, M.; Nandana, M.B.; Praveen, R.; Nayaka, S.; Velmurugan, D.; Vishwanath, B.S.; Rajaiah, R. Unconjugated bilirubin and its derivative ameliorate IMQ-induced psoriasis-like skin inflammation in mice by inhibiting MMP9 and MAPK pathway. Int. Immunopharmacol. 2024, 130, 111679. [Google Scholar] [CrossRef]
- Brunetti, J.E.; Foscaldi, S.; Quintana, V.M.; Scolaro, L.A.; López, N.; Castilla, V. Role of the ERK1/2 Signaling Pathway in the Replication of Junín and Tacaribe Viruses. Viruses 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, S.; Barati, M.T.; Rane, S.; Lin, Q.; Tan, Y.; Cai, L.; Rane, M.J. ERK and p38 MAPK inhibition controls NF-E2 degradation and profibrotic signaling in renal proximal tubule cells. Life Sci. 2021, 287, 120092. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Viemann, D.; Schöning, J.; Schloer, S.; Zambrano, A.M.; Brunotte, L.; Faist, A.; Schöfbänker, M.; Hrincius, E.; Hoffmann, H.; et al. The MEK1/2-inhibitor ATR-002 efficiently blocks SARS-CoV-2 propagation and alleviates pro-inflammatory cytokine/chemokine responses. Cell. Mol. Life Sci. 2022, 79, 65. [Google Scholar] [CrossRef]
- Cohen, M.S. Early treatment to prevent progression of SARS-CoV-2 infection. Lancet Respir. Med. 2022, 10, 930–931. [Google Scholar] [CrossRef]
- D’Abramo, A.; Vita, S.; Nicastri, E. The unmet need for COVID-19 treatment in immunocompromised patients. BMC Infect. Dis. 2022, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- WHO. Therapeutics and COVID-19: Living Guideline (v13.1). Available online: https://app.magicapp.org/#/guideline/7789 (accessed on 15 April 2025).
- Nieto-Fontarigo, J.J.; Tillgren, S.; Cerps, S.; Sverrild, A.; Hvidtfeldt, M.; Ramu, S.; Menzel, M.; Sander, A.F.; Porsbjerg, C.; Uller, L. Imiquimod Boosts Interferon Response, and Decreases ACE2 and Pro-Inflammatory Response of Human Bronchial Epithelium in Asthma. Front. Immunol. 2021, 12, 743890. [Google Scholar] [CrossRef]
- Szeto, M.D.; Maghfour, J.; Sivesind, T.E.; Anderson, J.; Olayinka, J.T.; Mamo, A.; Runion, T.M.; Dellavalle, R.P. Interferon and Toll-Like Receptor 7 Response in COVID-19: Implications of Topical Imiquimod for Prophylaxis and Treatment. Dermatology 2021, 237, 847–856. [Google Scholar] [CrossRef]
- Papatheodorou, I.; Fonseca, N.A.; Keays, M.; Tang, Y.A.; Barrera, E.; Bazant, W.; Burke, M.; Füllgrabe, A.; Fuentes, A.M.; George, N.; et al. Expression Atlas: Gene and protein expression across multiple studies and organisms. Nucleic Acids Res. 2018, 46, D246–D251. [Google Scholar] [CrossRef] [PubMed]
- Khair, M.H.M.M.; Selvarajah, G.T.; Omar, A.R.; Mustaffa-Kamal, F. Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes. J. Vet. Sci. 2022, 23, e27. [Google Scholar] [CrossRef]
- Schön, M.P.; Schön, M.; Klotz, K.N. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J. Investig. Dermatol. 2006, 126, 1338–1347. [Google Scholar] [CrossRef]
- Eishingdrelo, H.; Kongsamut, S. Minireview: Targeting GPCR Activated ERK Pathways for Drug Discovery. Curr. Chem. Genom. Transl. Med. 2013, 7, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; Uniyal, A.; Gadepalli, A.; Tiwari, V.; Belinskaia, D.A.; Shestakova, N.N.; Venugopala, K.N.; Deb, P.K.; Tiwari, V. Adenosine receptor signalling: Probing the potential pathways for the ministration of neuropathic pain. Eur. J. Pharmacol. 2020, 889, 173619. [Google Scholar] [CrossRef] [PubMed]
- Eblen, S.T. Extracellular-Regulated Kinases: Signaling from Ras to ERK Substrates to Control Biological Outcomes. Adv. Cancer Res. 2018, 138, 99–142. [Google Scholar] [CrossRef] [PubMed]

| SARS-CoV-2 | CCoV | |
|---|---|---|
| Cell Line | Calu-3 | CRFK |
| EC50 (μg/mL) | 1.69 ± 1.11 | 3.66 ± 1.03 |
| CC50 (μg/mL) | 372.6 ± 1.06 | 95.97 ± 1.10 |
| SI (CC50/CE50) | 219.43 | 26.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, J.; Peñaranda Figueredo, F.A.; Mantovani, S.; Papademetrio, D.L.; Nemirovsky, S.I.; Barquero, A.A.; Shayo, C.; Bueno, C.A. Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway. Viruses 2025, 17, 801. https://doi.org/10.3390/v17060801
Vicente J, Peñaranda Figueredo FA, Mantovani S, Papademetrio DL, Nemirovsky SI, Barquero AA, Shayo C, Bueno CA. Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway. Viruses. 2025; 17(6):801. https://doi.org/10.3390/v17060801
Chicago/Turabian StyleVicente, Josefina, Freddy Armando Peñaranda Figueredo, Stefania Mantovani, Daniela Laura Papademetrio, Sergio Ivan Nemirovsky, Andrea Alejandra Barquero, Carina Shayo, and Carlos Alberto Bueno. 2025. "Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway" Viruses 17, no. 6: 801. https://doi.org/10.3390/v17060801
APA StyleVicente, J., Peñaranda Figueredo, F. A., Mantovani, S., Papademetrio, D. L., Nemirovsky, S. I., Barquero, A. A., Shayo, C., & Bueno, C. A. (2025). Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway. Viruses, 17(6), 801. https://doi.org/10.3390/v17060801






