The Seasonality and Spatial Landscape of the Historical Climate-Based Suitability of Aedes-Borne Viruses in Four Atlantic Archipelagos
Abstract
1. Introduction
2. Materials and Methods
2.1. Aedes-Borne Viral Outbreak Data
2.2. Transmission Suitability, Altitude, and Climate Data
2.3. Transmission Suitability Long-Term Trends
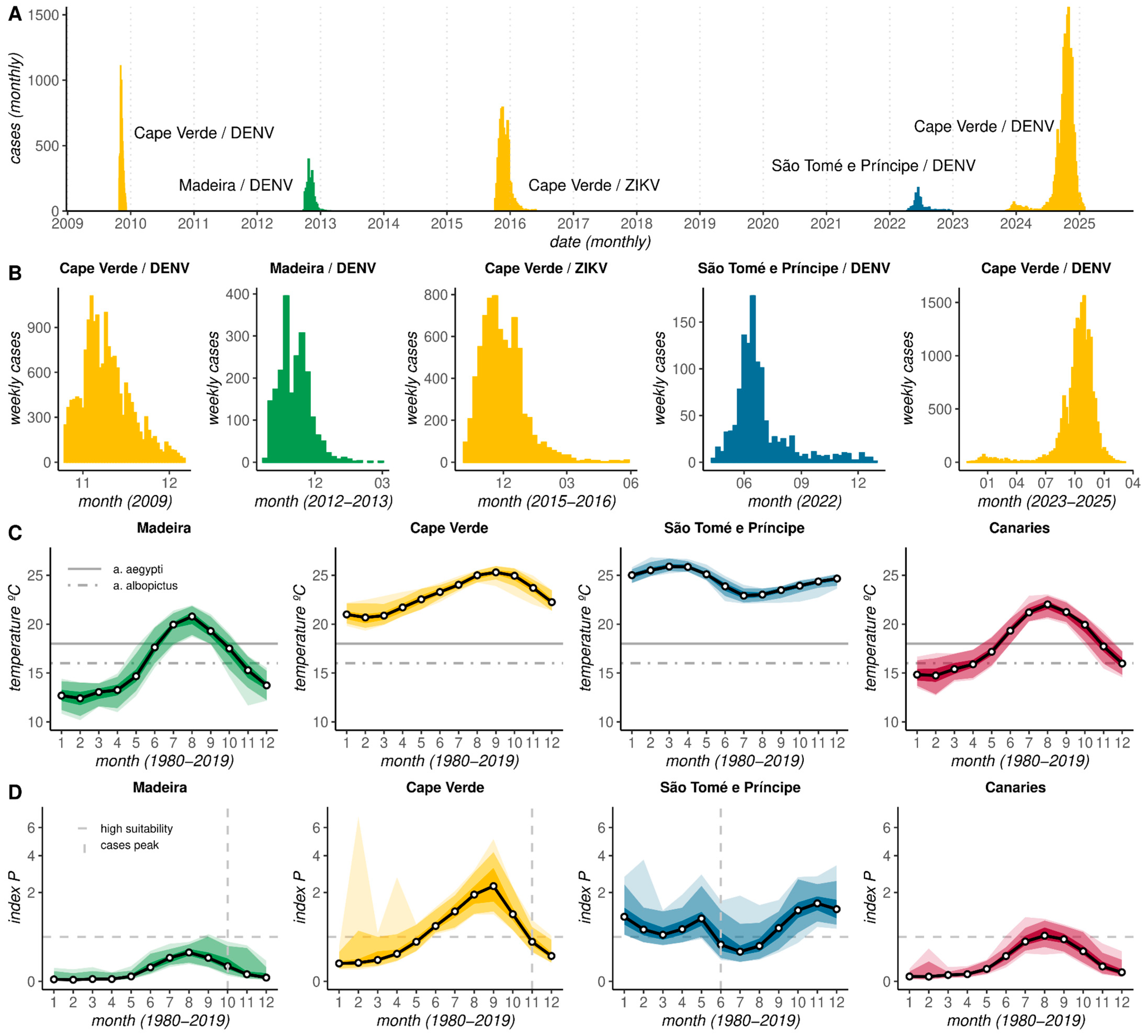
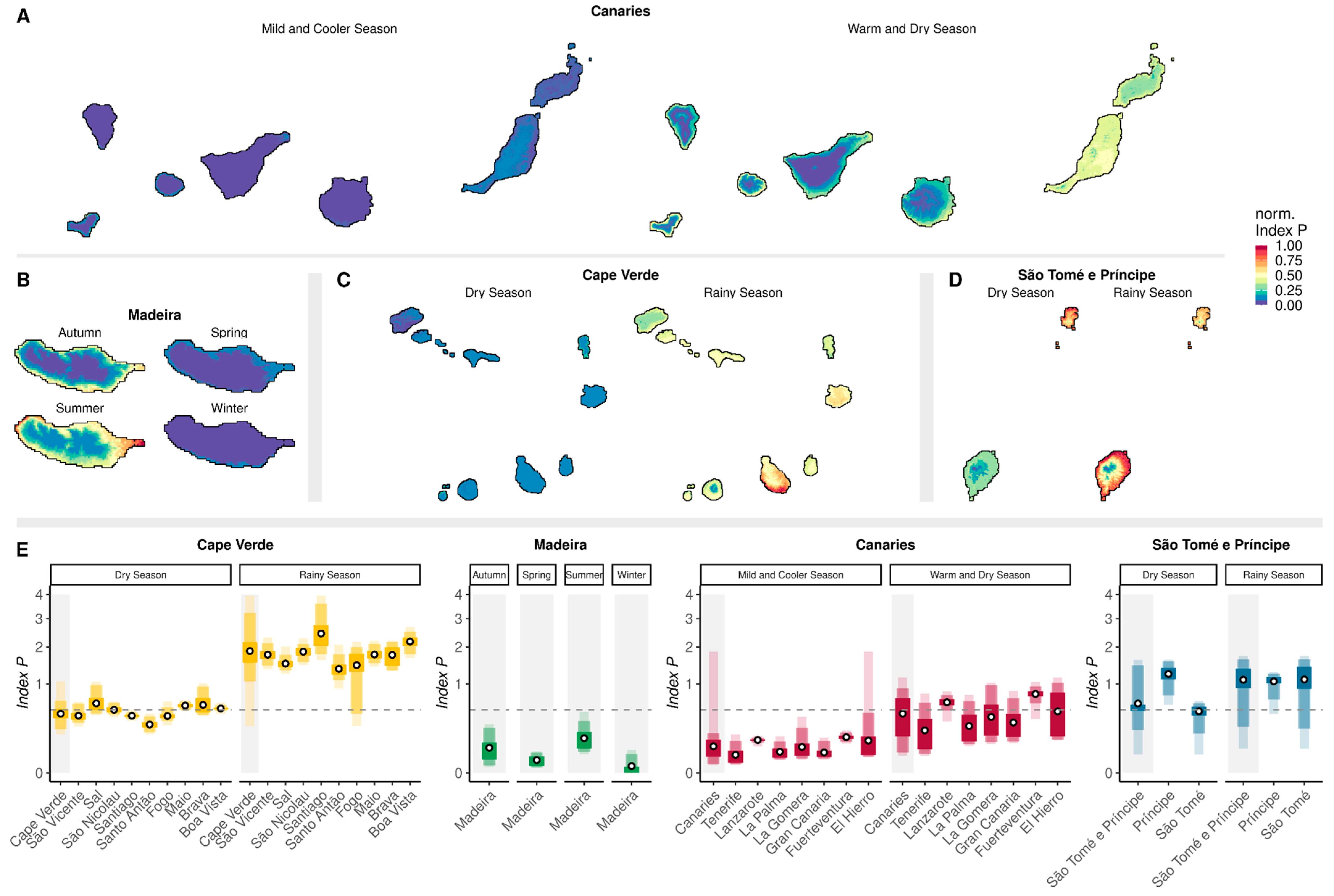
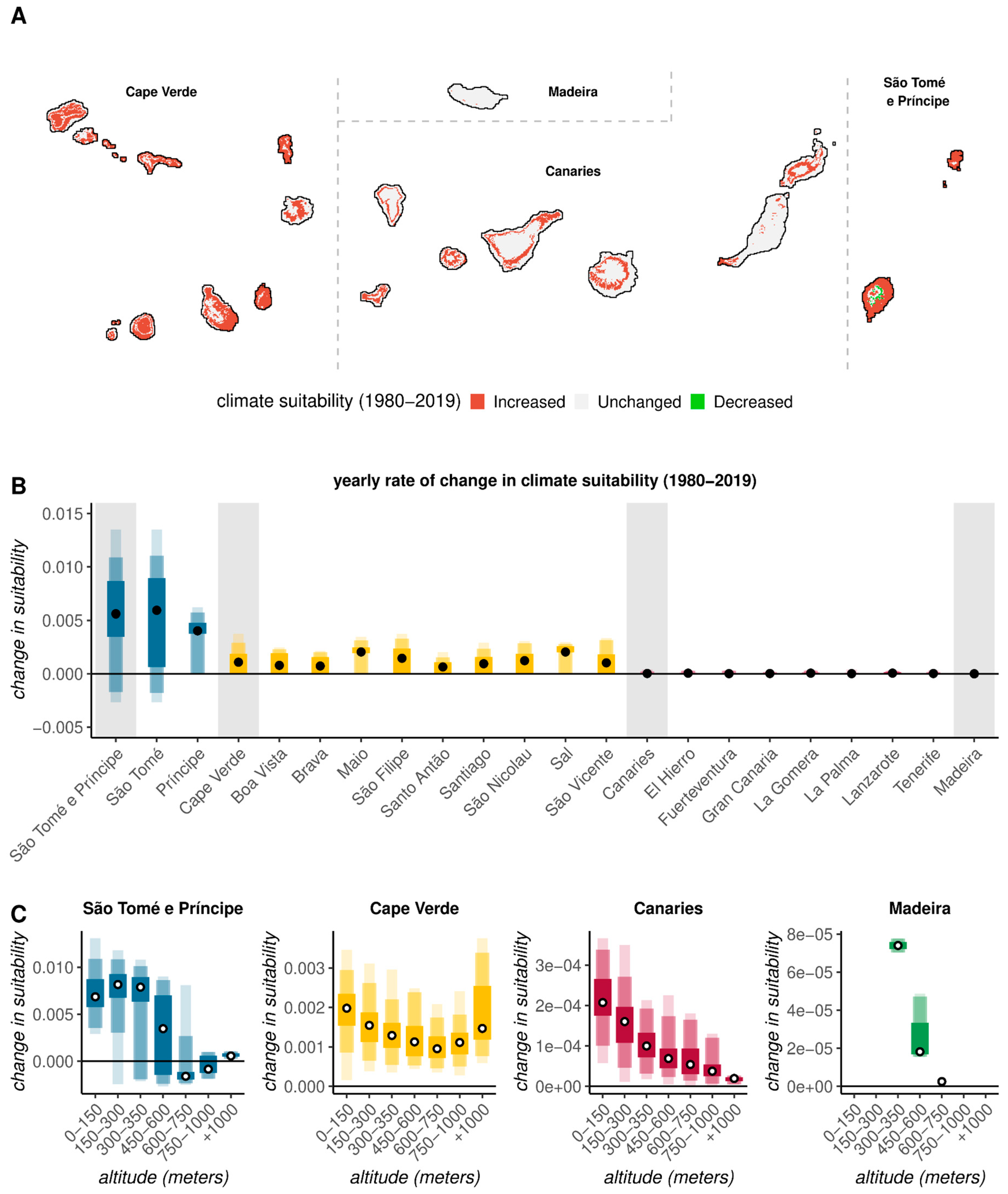
3. Results
3.1. Aedes-Borne Outbreak Dynamics and Climate-Based Suitability Across the Archipelagos
3.2. Geographic Variation in Climate-Based Suitability Across the Archipelagos
3.3. Long-Term Trends in Climate-Based Suitability Across the Archipelagos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| CHIKV | Chikungunya virus |
| ZIKV | Zika virus |
| YFV | Yellow fever virus |
References
- Alfsnes, K.; Eldholm, V.; Gaunt, M.W.; de Lamballerie, X.; Gould, E.A.; Pettersson, J.H.-O. Tracing and tracking the emergence, epidemiology and dispersal of dengue virus to Africa during the 20th century. One Health 2021, 13, 100337. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Li, Y. Global evolutionary history and spatio-temporal dynamics of dengue virus type 2. Sci. Rep. 2017, 7, 45505. [Google Scholar] [CrossRef] [PubMed]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 17 October 2024).
- Nakase, T.; Giovanetti, M.; Obolski, U.; Lourenço, J. Population at risk of dengue virus transmission has increased due to coupled climate factors and population growth. Commun. Earth Environ. 2024, 5, 475. [Google Scholar] [CrossRef]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Quigley, J.E.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Shi, W.-F.; Qin, C.-F. The evolution of Zika virus from Asia to the Americas. Nat. Rev. Microbiol. 2019, 17, 131–139. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017, 58, 69–76. [Google Scholar] [CrossRef]
- Lumsden, W.H. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–1953. II. General description and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 33–57. [Google Scholar] [CrossRef]
- Bettis, A.A.; Jackson, M.L.; Yoon, I.-K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Neglected Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-borne arboviruses of African origin: Review of key viruses and vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.G.; Hayden, M.H.; Dadzie, S.K.; Paull, S.H.; Carlton, E.J. Aedes-borne disease outbreaks in West Africa: A call for enhanced surveillance. Acta Trop. 2020, 209, 105468. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, J.; Walekhwa, A.W.; Mwingira, V.; Kijanga, O.; Mramba, F.; Lord, J.S. Aedes albopictus invasion across Africa: The time is now for cross-country collaboration and control. Lancet Glob. Health 2023, 11, e623–e628. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Meltzer, E.; Mendelson, M.; Tooke, A.; Steiner, F.; Gautret, P.; Friedrich-Jaenicke, B.; Libman, M.; Bin, H.; Wilder-Smith, A.; et al. Detection on four continents of dengue fever cases related to an ongoing outbreak in Luanda, Angola, March to May 2013. Eurosurveillance 2013, 18, 20488. [Google Scholar] [CrossRef]
- Tarnagda, Z.; Cissé, A.; Bicaba, B.W.; Diagbouga, S.; Sagna, T.; Ilboudo, A.K.; Tialla, D.; Lingani, M.; Sondo, K.A.; Yougbaré, I.; et al. Dengue Fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018, 24, 170–172. [Google Scholar] [CrossRef]
- Simo Tchetgna, H.; Sado Yousseu, F.; Kamgang, B.; Tedjou, A.; McCall, P.J.; Wondji, C.S. Concurrent circulation of dengue serotype 1, 2 and 3 among acute febrile patients in Cameroon. PLoS Neglected Trop. Dis. 2021, 15, e0009860. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Africa. In Climate Change 2014—Impacts, Adaptation and Vulnerability: Part B: Regional Aspects: Working Group II Contribution to the IPCC Fifth Assessment Report; Cambridge University Press: Cambridge, UK, 2014; pp. 1199–1266. [Google Scholar]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Campos, T.D.L.; Durães-Carvalho, R.; Rezende, A.M.; de Carvalho, O.V.; Kohl, A.; Wallau, G.L.; Pena, L.J. Revisiting Key Entry Routes of Human Epidemic Arboviruses into the Mainland Americas through Large-Scale Phylogenomics. Int. J. Genom. 2018, 2018, 6941735. [Google Scholar] [CrossRef]
- Almeida, A.P.G.; Gonçalves, Y.M.; Novo, M.T.; Sousa, C.A.; Melim, M.; Gracio, A.J.S. Vector monitoring of Aedes aegypti in the Autonomous Region of Madeira, Portugal. Wkly. Releases (1997–2007) 2007, 12, 3311. [Google Scholar] [CrossRef]
- Lourenço, J.; Recker, M. The 2012 Madeira Dengue Outbreak: Epidemiological Determinants and Future Epidemic Potential. PLoS Neglected Trop. Dis. 2014, 8, e3083. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Quam, M.; Sessions, O.; Rocklov, J.; Liu-Helmersson, J.; Franco, L.; Khan, K. The 2012 dengue outbreak in Madeira: Exploring the origins. Eurosurveillance 2014, 19, 20718. [Google Scholar] [CrossRef] [PubMed]
- Aedes Albopictus—Current Known Distribution: May 2024. European Centre for Disease Prevention and Control. 11 June 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-albopictus-current-known-distribution-may-2024 (accessed on 10 December 2024).
- Chou, S.C.; de Arruda Lyra, A.; Gomes, J.L.; Rodriguez, D.A.; Alves Martins, M.; Costa Resende, N.; Tavares, P.d.S.; Dereczynski, C.P.; Pilotto, I.L.; Martins, A.M.; et al. Downscaling projections of climate change in Sao Tome and Principe Islands, Africa. Clim. Dyn. 2020, 54, 4021–4042. [Google Scholar] [CrossRef]
- Yen, T.Y.; dos Santos, M.D.J.T.; Tseng, L.F.; Chang, S.F.; Cheng, C.F.; de Assunção Carvalho, A.V.; Shu, P.Y.; Lien, J.C.; Tsai, K.H. Seroprevalence of antibodies against dengue virus among pregnant women in the Democratic Republic of Sao Tome and Principe. Acta Trop. 2016, 155, 58–62. [Google Scholar] [CrossRef]
- Lázaro, L.; Winter, D.; Toancha, K.; Borges, A.; Gonçalves, A.; Santos, A.; Nascimento, M.D.; Teixeira, N.; Sequeira, Y.S.; Lima, A.K.; et al. Phylogenomics of Dengue Virus Isolates Causing Dengue Outbreak, São Tomé and Príncipe, 2022. Emerg. Infect. Dis. 2024, 30, 384. [Google Scholar] [CrossRef]
- Kamgang, B.; Acântara, J.; Tedjou, A.; Keumeni, C.; Yougang, A.; Ancia, A.; Bigirimana, F.; Clarke, S.E.; Gil, V.S.; Wondji, C. Entomological surveys and insecticide susceptibility profile of Aedes aegypti during the dengue outbreak in Sao Tome and Principe in 2022. PLoS Neglected Trop. Dis. 2024, 18, e0011903. [Google Scholar] [CrossRef]
- Rader, J.A.; Serrato-Capuchina, A.; Anspach, T.; Matute, D.R. The spread of Aedes albopictus (Diptera: Culicidae) in the islands of São Tomé and Príncipe. Acta Trop. 2024, 251, 107106. [Google Scholar] [CrossRef] [PubMed]
- Identificación del Mosquito Aedes Aegypti en Fuerteventura (Centro de Coordinación de Alertas y Emergencias Sanitarias). Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20171226_Aedes-aegypti_en_Fuerteventura_ERR.pdf (accessed on 10 December 2024).
- Aedes Aegypti—Current Known Distribution: May 2024. European Centre for Disease Prevention and Control. 11 June 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-aegypti-current-known-distribution-may-2024 (accessed on 10 December 2024).
- Schaffner, F.; Mathis, A. Dengue and dengue vectors in the WHO European region: Past, present, and scenarios for the future. Lancet Infect. Dis. 2014, 14, 1271–1280. [Google Scholar] [CrossRef]
- Franco, L.; Di Caro, A.; Carletti, F.; Vapalahti, O.; Renaudat, C.; Zeller, H.; Tenorio, A. Recent expansion of dengue virus serotype 3 in West Africa. Eurosurveillance 2010, 15, 19490. [Google Scholar] [CrossRef]
- Campos, M.; Ward, D.; Morales, R.F.; Gomes, A.R.; Silva, K.; Sepúlveda, N.; Gomez, L.F.; Clark, T.G.; Campino, S. Surveillance of Aedes aegypti populations in the city of Praia, Cape Verde: Zika virus infection, insecticide resistance and genetic diversity. Parasites Vectors 2020, 13, 481. [Google Scholar] [CrossRef]
- Lourenço, J.; de Lourdes Monteiro, M.; Valdez, T.; Rodrigues, J.M.; Pybus, O.; Faria, N.R. Epidemiology of the Zika Virus Outbreak in the Cabo Verde Islands, West Africa. PLoS Curr. 2018, 10, ecurrents.outbreaks. [Google Scholar] [CrossRef]
- Fernandes da Moura, A.J.; Varjal de Melo Santos, M.A.; Fontes Oliveira, C.M.; Duarte Guedes, D.R.; de Carvalho-Leandro, D.; da Cruz Brito, M.L.; Ribeiro Rocha, H.D.; Gomez, L.F.; Junqueira Ayres, C.F. Vector competence of the Aedes aegypti population from Santiago Island, Cape Verde, to different serotypes of dengue virus. Parasit. Vectors 2015, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.R.D.; Gomes, E.T.B.; Paiva, M.H.S.; de Melo-Santos, M.A.V.; Alves, J.; Gómez, L.F.; Ayres, C.F.J. Circulation of DENV2 and DENV4 in Aedes aegypti (Diptera: Culicidae) mosquitoes from Praia, Santiago Island, Cabo Verde. J. Insect Sci. 2017, 17, 86. [Google Scholar] [CrossRef]
- Santos, M.D.; Dieng, I.; Fernandes Varela, I.B.; Rosa Carvalho, K.S.D.; Texeira, D.D.; Furtado, U.; Hungria, L.; Souza, L.; Tavares, L.; Barry, M.A.; et al. Re-emergence of dengue virus 3 genotype III in Cabo Verde, 2023. medRxiv 2024. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Cohen, J.M.; Evans, M.V.; Gudapati, P.; Johnson, L.R.; Lippi, C.A.; Miazgowicz, K.; Murdock, C.C.; Rohr, J.R.; Ryan, S.J.; et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglected Trop. Dis. 2017, 11, e0005568. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017, 15, e2003489. [Google Scholar] [CrossRef]
- Thu, H.M.; Aye, K.M.; Thein, S. The effect of temperature and humidity on dengue virus propagation in Aedes aegypti mosquitos. Southeast Asian J. Trop. Med. Public Health 1998, 29, 280–284. [Google Scholar]
- Lega, J.; Brown, H.E.; Barrera, R. Aedes aegypti (Diptera: Culicidae) Abundance Model Improved With Relative Humidity and Precipitation-Driven Egg Hatching. J. Med. Entomol. 2017, 54, 1375–1384. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors 2014, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Zardini, A.; Menegale, F.; Gobbi, A.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Trentini, F.; Montarsi, F.; Caputo, B.; et al. Estimating the potential risk of transmission of arboviruses in the Americas and Europe: A modelling study. Lancet Planet. Health 2024, 8, e30–e40. [Google Scholar] [CrossRef]
- Obolski, U.; Perez, P.N.; Villabona-Arenas, C.J.; Thézé, J.; Faria, N.R.; Lourenço, J. MVSE: An R-package that estimates a climate-driven mosquito-borne viral suitability index. Methods Ecol. Evol. 2019, 10, 1357–1370. [Google Scholar] [CrossRef]
- Nakase, T.; Giovanetti, M.; Obolski, U.; Lourenço, J. Global transmission suitability maps for dengue virus transmitted by Aedes aegypti from 1981 to 2019. Sci. Data 2023, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Petrone, M.E.; Earnest, R.; Lourenço, J.; Kraemer, M.U.G.; Paulino-Ramirez, R.; Grubaugh, N.D.; Tapia, L. Asynchronicity of endemic and emerging mosquito-borne disease outbreaks in the Dominican Republic. Nat. Commun. 2021, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Perez-Guzman, P.N.; Carlos Junior Alcantara, L.; Obolski, U.; de Lima, M.M.; Ashley, E.A.; Smithuis, F.; Horby, P.; Maude, R.J.; Lin, Z.; Kyaw, A.M.M.; et al. Measuring Mosquito-borne Viral Suitability in Myanmar and Implications for Local Zika Virus Transmission. PLoS Curr. 2018, 10, ecurrents-outbreaks. [Google Scholar] [CrossRef]
- Pereira, G.M.Z.; Mota, P.F.; do Carmo Said, R.F.; Fonseca, V.; Gräf, T.; de Bruycker Nogueira, F.; Nardy, V.B.; Xavier, J.; Maia, M.L.; Abreu, A.L.; et al. Return of the founder Chikungunya virus to its place of introduction into Brazil is revealed by genomic characterization of exanthematic disease cases. Emerg. Microbes Infect. 2019, 9, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Africa Weekly Emergency Situation Update Vol. 2, No. 45 (ReliefWeb) Website. Available online: https://reliefweb.int/report/cape-verde/africa-weekly-emergency-situation-update-vol-2-no-45 (accessed on 18 January 2025).
- Boletim Nacional—Ministério da Saúde. Available online: https://minsaude.st/boletim-nacional/ (accessed on 21 April 2025).
- Boletim da Dengue em Cabo Verde (Instituto Nacional de Saúde Pública). Website. Available online: https://insp.gov.cv/boletim-da-dengue/ (accessed on 18 January 2025).
- Lourenço, J.; Thompson, R.N.; Thézé, J.; Obolski, U. Characterising West Nile virus epidemiology in Israel using a transmission suitability index. Eurosurveillance 2020, 25, 1900629. [Google Scholar] [CrossRef]
- Lourenço, J.; Barros, S.C.; Zé-Zé, L.; Damineli, D.S.C.; Giovanetti, M.; Osório, H.C.; Amaro, F.; Henriques, A.M.; Ramos, F.; Luís, T.; et al. West Nile virus transmission potential in Portugal. Commun. Biol. 2022, 5, 6. [Google Scholar] [CrossRef]
- Costa, É.A.; Giovanetti, M.; Silva Catenacci, L.; Fonseca, V.; Aburjaile, F.F.; Chalhoub, F.L.L.; Xavier, J.; Iani, F.C.d.M.; Vieira, M.A.d.C.e.S.; Henriques, D.F.; et al. West Nile Virus in Brazil. Pathogens 2021, 10, 896. [Google Scholar] [CrossRef]
- Lourenço, J.; Pinotti, F.; Nakase, T.; Giovanetti, M.; Obolski, U. Letter to the editor: Atypical weather is associated with the 2022 early start of West Nile virus transmission in Italy. Eurosurveillance 2022, 27, 2200662. [Google Scholar] [CrossRef]
- Geraldes, M.A.; Cunha, M.V.; Godinho, C.; Faustino de Lima, R.; Giovanetti, M.; Lourenço, J. The historical ecological background of West Nile virus in Portugal indicates One Health opportunities. Sci. Total Environ. 2024, 944, 173875. [Google Scholar] [CrossRef]
- Carreto, C.; Gutiérrez-Romero, R.; Rodríguez, T. Climate-driven mosquito-borne viral suitability index: Measuring risk transmission of dengue, chikungunya and Zika in Mexico. Int. J. Health Geogr. 2022, 21, 15. [Google Scholar] [CrossRef]
- Adelino, T.É.R.; Giovanetti, M.; Fonseca, V.; Xavier, J.; de Abreu, Á.S.; do Nascimento, V.A.; Demarchi, L.H.F.; Oliveira, M.A.A.; da Silva, V.L.; de Mello, A.L.E.S.; et al. Field and classroom initiatives for portable sequence-based monitoring of dengue virus in Brazil. Nat. Commun. 2021, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Yue, S.; Pilon, P.; Phinney, B.; Cavadias, G. The influence of autocorrelation on the ability to detect trend in hydrological series. Hydrol. Process. 2002, 16, 1807–1829. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Ward, D.; Gomes, A.R.; Tetteh, K.K.A.; Sepúlveda, N.; Gomez, L.F.; Campino, S.; Clark, T.G. Sero-epidemiological study of arbovirus infection following the 2015–2016 Zika virus outbreak in Cabo Verde. Sci. Rep. 2022, 12, 11719. [Google Scholar] [CrossRef] [PubMed]
- DePina, A. Clinical and Epidemiological Characterization of Dengue Outbreak in Cabo Verde, 2009–2010. J. Trop. Dis. Public Health 2019, 7, 296. Available online: https://www.academia.edu/109095890/Clinical_and_Epidemiological_Characterization_of_Dengue_Outbreak_in_Cabo_Verde_2009_2010 (accessed on 20 December 2024).
- Santos, J.M.; Capinha, C.; Rocha, J.; Sousa, C.A. The current and future distribution of the yellow fever mosquito (Aedes aegypti) on Madeira Island. PLoS Neglected Trop. Dis. 2022, 16, e0010715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraldes, M.A.; Giovanetti, M.; Cunha, M.V.; Lourenço, J. The Seasonality and Spatial Landscape of the Historical Climate-Based Suitability of Aedes-Borne Viruses in Four Atlantic Archipelagos. Viruses 2025, 17, 799. https://doi.org/10.3390/v17060799
Geraldes MA, Giovanetti M, Cunha MV, Lourenço J. The Seasonality and Spatial Landscape of the Historical Climate-Based Suitability of Aedes-Borne Viruses in Four Atlantic Archipelagos. Viruses. 2025; 17(6):799. https://doi.org/10.3390/v17060799
Chicago/Turabian StyleGeraldes, Martim A., Marta Giovanetti, Mónica V. Cunha, and José Lourenço. 2025. "The Seasonality and Spatial Landscape of the Historical Climate-Based Suitability of Aedes-Borne Viruses in Four Atlantic Archipelagos" Viruses 17, no. 6: 799. https://doi.org/10.3390/v17060799
APA StyleGeraldes, M. A., Giovanetti, M., Cunha, M. V., & Lourenço, J. (2025). The Seasonality and Spatial Landscape of the Historical Climate-Based Suitability of Aedes-Borne Viruses in Four Atlantic Archipelagos. Viruses, 17(6), 799. https://doi.org/10.3390/v17060799






