Pathogenesis Induced by Influenza Virus Infection: Role of the Early Events of the Infection and the Innate Immune Response
Abstract
1. Introduction
2. Influenza A Virus
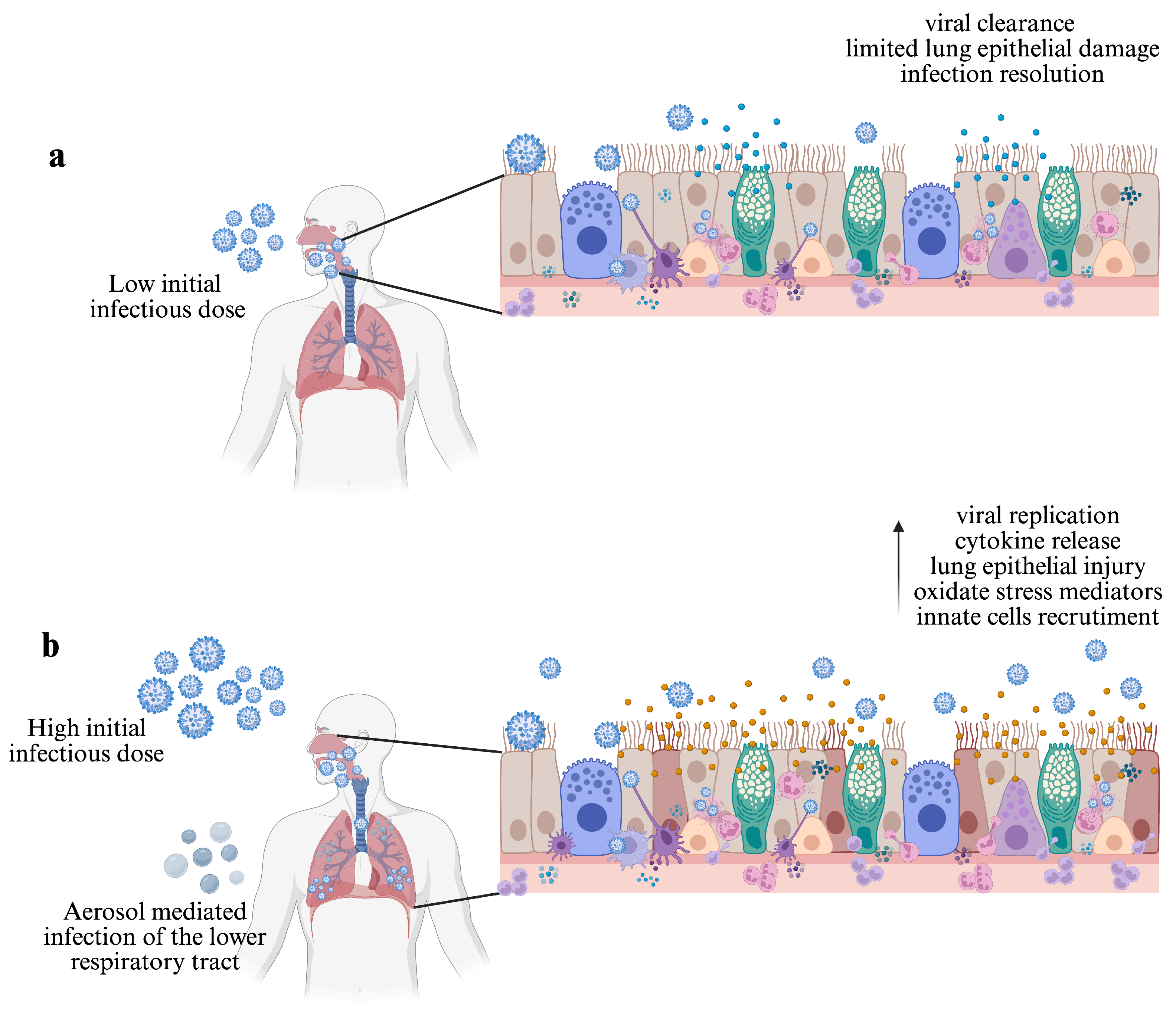
3. Route of Transmission and the Initial Infectious Dose
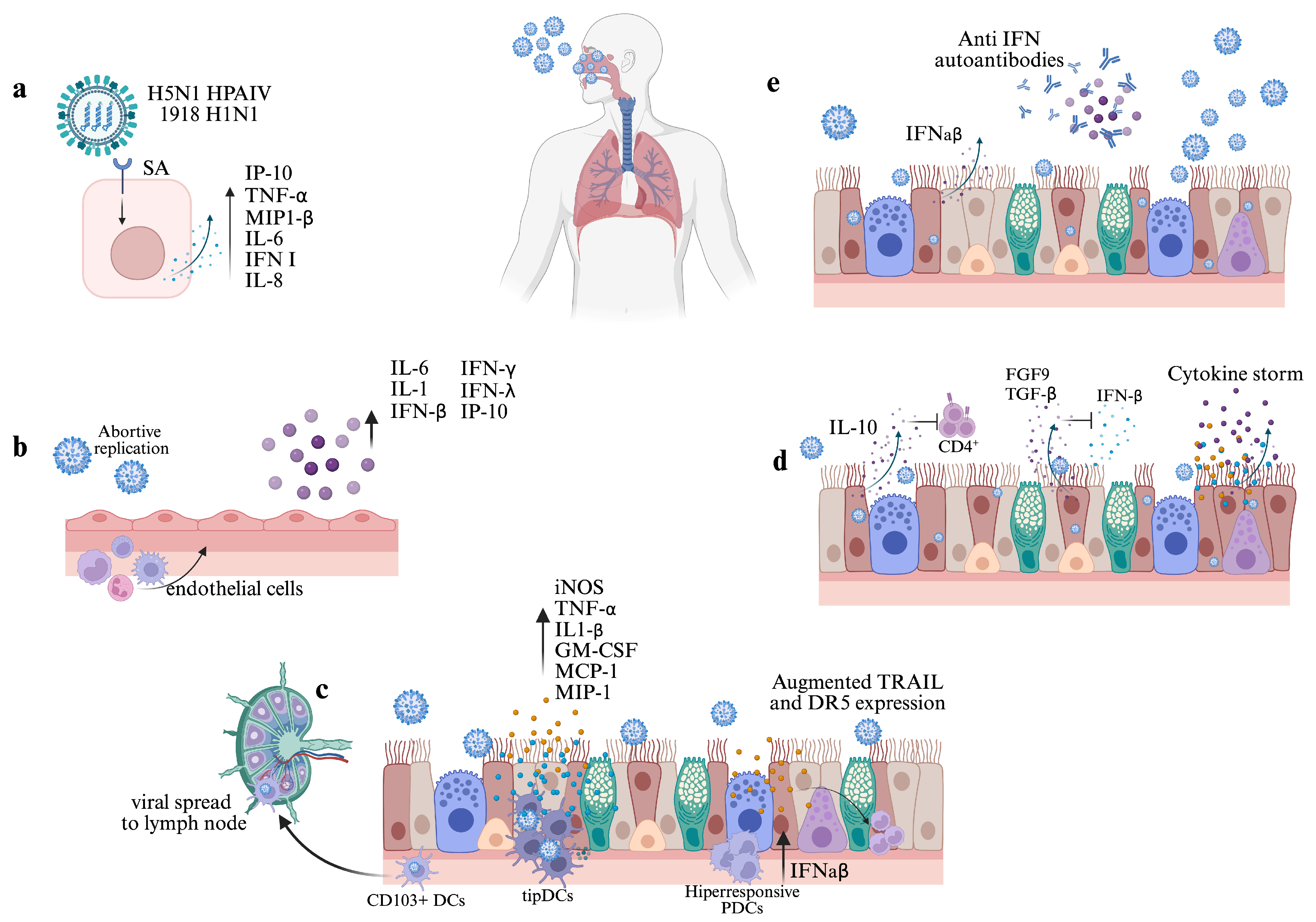
4. Effect of the Viral Infection and Replication
4.1. Infection on Epithelial Cells
4.2. Infection in Other Cells of the Respiratory Tract
5. Early Cytokine Response
6. Type I IFN and Other Cytokine Autoantibodies
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kash, J.C.; Taubenberger, J.K. The Role of Viral, Host, and Secondary Bacterial Factors in Influenza Pathogenesis. Am. J. Pathol. 2015, 185, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.; Vitale, F. Influenza vaccination in high-risk groups: A revision of existing guidelines and rationale for an evidence-based preventive strategy. J. Prev. Med. Hyg. 2016, 57, E13. [Google Scholar] [PubMed]
- Uyeki, T.M. High-risk Groups for Influenza Complications. JAMA 2020, 324, 2334. [Google Scholar] [CrossRef] [PubMed]
- Luliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Adlhoch, C.; Baldinelli, F.; Fusaro, A.; Terregino, C. Avian influenza, a new threat to public health in Europe? Clin. Microbiol. Infect. 2022, 28, 149–151. [Google Scholar] [CrossRef]
- Wille, M.; Barr, I.G. Resurgence of avian influenza virus. Science 2022, 376, 459–460. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, P.; Li, Y.; Zhang, Y.; Zheng, Y.; Yu, M.; Suzuki, Y.; Zhang, H.; Ping, J. Genetic and biological properties of H10N3 avian influenza viruses: A potential pandemic candidate? Transbound. Emerg. Dis. 2022, 69, e3171–e3182. [Google Scholar] [CrossRef]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.R.S. Influenza virus-induced lung injury: Pathogenesis and implications for treatment. Eur. Respir. J. 2015, 45, 1463–1478. [Google Scholar] [CrossRef]
- Boyd, D.F.; Wilson, T.L.; Thomas, P.G. One hundred years of (influenza) immunopathology. Adv. Virus Res. 2020, 107, 247–284. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S. Overview of Influenza Viruses. In Swine Influenza; Springer: Berlin/Heidelberg, Germany, 2013; Volume 370, pp. 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Ramos, I.; Fernandez-Sesma, A. Cell Receptors for Influenza a Viruses and the Innate Immune Response. Front. Microbiol. 2012, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Matsumae, H.; Katoh, M.; Eisfeld, A.J.; Neumann, G.; Hase, T.; Ghosh, S.; Shoemaker, J.E.; Lopes, T.J.; Watanabe, T.; et al. A comprehensive map of the influenza A virus replication cycle. BMC Syst. Biol. 2013, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Denney, L.; Ho, L.-P. The role of respiratory epithelium in host defence against influenza virus infection. Biomed. J. 2018, 41, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, E.J.; Kuba, M.; Barr, I.G. Innate Immune Responses to Influenza Virus Infections in the Upper Respiratory Tract. Viruses 2021, 13, 2090. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef]
- Ward, R.L.; Akin, E.W.; D’Alessio, D.J. Minimum infective dose of animal viruses. Crit. Rev. Environ. Control 1984, 14, 297–310. [Google Scholar] [CrossRef]
- Granados, A.; Peci, A.; McGeer, A.; Gubbay, J.B. Influenza and rhinovirus viral load and disease severity in upper respiratory tract infections. J. Clin. Virol. 2017, 86, 14–19. [Google Scholar] [CrossRef]
- Totura, A.; Livingston, V.; Frick, O.; Dyer, D.; Nichols, D.; Nalca, A. Small Particle Aerosol Exposure of African Green Monkeys to MERS-CoV as a Model for Highly Pathogenic Coronavirus Infection. Emerg. Infect. Dis. 2020, 26, 2835–2843. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Bhopal, R.; Nguyen Tien, H. Review of infective dose, routes of transmission and outcome of COVID-19 caused by the SARS-COV-2: Comparison with other respiratory viruses. Epidemiol. Infect. 2021, 149, e96. [Google Scholar] [CrossRef]
- Doyle, W.J.; Skoner, D.P.; White, M.; Hayden, F.; Kaplan, A.P.; Kaliner, M.A.; Shibayama, Y.; Fireman, P. Pattern of nasal secretions during experimental influenza virus infection. Rhinology 1996, 34, 2–8. [Google Scholar]
- Skoner, D.P.; Doyle, W.J.; Seroky, J.; Fireman, P. Lower airway responses to influenza A virus in healthy allergic and nonallergic subjects. Am. J. Respir. Crit. Care Med. 1996, 154, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Fritz, R.; Lobo, M.C.; Alvord, W.; Strober, W.; Straus, S.E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J. Clin. Investig. 1998, 101, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.W.; Platts-Mills, T.A.E.; Lobo, M.; Hayden, F. Respiratory Nitric Oxide Levels in Experimental Human Influenza. Chest 1998, 114, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.A.; Doyle, W.J.; Fireman, P.; Skoner, D.P. Effect of experimental influenza A infection on systemic immune and inflammatory parameters in allergic and nonallergic adult subjects. Ann. Allergy Asthma Immunol. 2001, 87, 496–500. [Google Scholar] [CrossRef]
- Paulo, A.C.; Correia-Neves, M.; Domingos, T.; Murta, A.G.; Pedrosa, J. Influenza Infectious Dose May Explain the High Mortality of the Second and Third Wave of 1918–1919 Influenza Pandemic. PLoS ONE 2010, 5, e11655. [Google Scholar] [CrossRef][Green Version]
- Yezli, S.; Otter, J.A. Minimum Infective Dose of the Major Human Respiratory and Enteric Viruses Transmitted Through Food and the Environment. Food Environ. Virol. 2011, 3, 1–30. [Google Scholar] [CrossRef]
- Memoli, M.J.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Proudfoot, K.; Fargis, S.; Stein, M.; Dunfee, R.L.; Shaw, P.A.; et al. Validation of the Wild-type Influenza A Human Challenge Model H1N1pdMIST: An A(H1N1)pdm09 Dose-Finding Investigational New Drug Study. Clin. Infect. Dis. 2014, 60, 693–702. [Google Scholar] [CrossRef]
- Marois, I.; Cloutier, A.; Garneau, É.; Richter, M.V. Initial infectious dose dictates the innate, adaptive, and memory responses to influenza in the respiratory tract. J. Leukoc. Biol. 2012, 92, 107–121. [Google Scholar] [CrossRef]
- Moskophidis, D.; Kioussis, D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 1998, 188, 223–232. [Google Scholar] [CrossRef]
- Rashid, F.; Xie, Z.; Li, M.; Xie, Z.; Luo, S.; Xie, L. Roles and functions of IAV proteins in host immune evasion. Front. Immunol. 2023, 14, 1323560. [Google Scholar] [CrossRef]
- Ramos, I.; Smith, G.; Ruf-Zamojski, F.; Martínez-Romero, C.; Fribourg, M.; Carbajal, E.A.; Hartmann, B.M.; Nair, V.D.; Marjanovic, N.; Monteagudo, P.L.; et al. Innate Immune Response to Influenza Virus at Single-Cell Resolution in Human Epithelial Cells Revealed Paracrine Induction of Interferon Lambda 1. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Teunis, P.F.M.; Brienen, N.; Kretzschmar, M.E.E. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics 2010, 2, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.H.; Nagy, T.; Barber, J.; Brooks, P.; Tompkins, S.M.; Tripp, R.A. Aerosol inoculation with a sub-lethal influenza virus leads to exacerbated morbidity and pulmonary disease pathogenesis. Viral Immunol. 2011, 24, 131–142. [Google Scholar] [CrossRef]
- Sanders, C.J.; Doherty, P.C.; Thomas, P.G. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2010, 343, 13–21. [Google Scholar] [CrossRef]
- Klomp, M.; Ghosh, S.; Mohammed, S.; Nadeem Khan, M. From virus to inflammation, how influenza promotes lung damage. J. Leukoc. Biol. 2021, 110, 115–122. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Meischel, T.; Villalon-Letelier, F.; Saunders, P.M.; Reading, P.C.; Londrigan, S.L. Influenza A virus interactions with macrophages: Lessons from epithelial cells. Cell. Microbiol. 2020, 22, e13170. [Google Scholar] [CrossRef]
- Ramos, I.; Bernal-Rubio, D.; Durham, N.; Belicha-Villanueva, A.; Lowen, A.C.; Steel, J.; Fernandez-Sesma, A. Effects of Receptor Binding Specificity of Avian Influenza Virus on the Human Innate Immune Response. J. Virol. 2011, 85, 4421–4431. [Google Scholar] [CrossRef]
- Kobasa, D.; Takada, A.; Shinya, K.; Hatta, M.; Halfmann, P.; Theriault, S.; Suzuki, H.; Nishimura, H.; Mitamura, K.; Sugaya, N.; et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 2004, 431, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Rijsbergen, L.C.; Leijten, L.; Benavides, F.F.; Noack, D.; Lamers, M.M.; Haagmans, B.L.; de Vries, R.D.; de Swart, R.L.; van Riel, D. The pro-inflammatory response to influenza A virus infection is fueled by endothelial cells. Life Sci. Alliance 2023, 6, e202201837. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Walsh, K.B.; Cahalan, S.; Fremgen, D.M.; Roberts, E.; Scott, F.; Martinborough, E.; Peach, R.; Oldstone, M.B.A.; Rosen, H. Endothelial Cells Are Central Orchestrators of Cytokine Amplification during Influenza Virus Infection. Cell 2011, 146, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.Q.; Dutia, B.M. The role of macrophages in influenza A virus infection. Future Virol. 2014, 9, 847–862. [Google Scholar] [CrossRef]
- Lamichhane, P.P.; Samarasinghe, A.E. The Role of Innate Leukocytes during Influenza Virus Infection. J. Immunol. Res. 2019, 2019, 8028725. [Google Scholar] [CrossRef]
- Ruscitti, C.; Radermecker, C.; Marichal, T. Journey of monocytes and macrophages upon influenza A virus infection. Curr. Opin. Virol. 2024, 66, 101409. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Poon, L.L.; Lau, A.S.; Luk, W.; Lau, Y.L.; Shortridge, K.F.; Gordon, S.; Guan, Y.; Peiris, J.S. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: A mechanism for the unusual severity of human disease? Lancet 2002, 360, 1831–1837. [Google Scholar] [CrossRef]
- Zhou, J.; Law, H.K.W.; Cheung, C.Y.; Ng, I.H.Y.; Peiris, J.S.M.; Lau, Y.L. Differential Expression of Chemokines and Their Receptors in Adult and Neonatal Macrophages Infected with Human or Avian Influenza Viruses. J. Infect. Dis. 2006, 194, 61–70. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Goulding, J.; Didierlaurent, A.M.; Lyonga, D.; Vekaria, S.; Edwards, L.; Gwyer, E.; Sedgwick, J.D.; Barclay, A.N.; Hussell, T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008, 9, 1074–1083. [Google Scholar] [CrossRef]
- Ettensohn, D.B.; Frampton, M.W.; Nichols, J.E.; Roberts, N.J. Human Alveolar Macrophages May Not Be Susceptible to Direct Infection by a Human Influenza Virus. J. Infect. Dis. 2016, 214, 1658–1665. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Moki, T.; Takizawa, T.; Shiratsuchi, A.; Nakanishi, Y. Evidence for Phagocytosis of Influenza Virus-Infected, Apoptotic Cells by Neutrophils and Macrophages in Mice. J. Immunol. 2007, 178, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; McCullough, K. Dendritic Cells in Innate and Adaptive Immune Responses against Influenza Virus. Viruses 2009, 1, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The Role of Dendritic Cells During Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Moltedo, B.; Li, W.; Yount, J.S.; Moran, T.M. Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection. PLoS Pathog. 2011, 7, e1002345. [Google Scholar] [CrossRef]
- Aldridge, J.R.; Moseley, C.E.; Boltz, D.A.; Negovetich, N.J.; Reynolds, C.; Franks, J.; Brown, S.A.; Doherty, P.C.; Webster, R.G.; Thomas, P.G. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. USA 2009, 106, 5306–5311. [Google Scholar] [CrossRef]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef]
- Langlois, R.A.; Legge, K.L. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J. Immunol. 2010, 184, 4440–4446. [Google Scholar] [CrossRef]
- Chan, M.C.; Cheung, C.Y.; Chui, W.H.; Tsao, S.W.; Nicholls, J.M.; Chan, Y.O.; Chan, R.W.; Long, H.T.; Poon, L.L.; Guan, Y.; et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005, 6, 135. [Google Scholar] [CrossRef]
- Toapanta, F.R.; Ross, T.M. Impaired immune responses in the lungs of aged mice following influenza infection. Respir. Res. 2009, 10, 112. [Google Scholar] [CrossRef]
- Latino, I.; Gonzalez, S.F. Spatio-temporal profile of innate inflammatory cells and mediators during influenza virus infection. Curr. Opin. Physiol. 2021, 19, 175–186. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Ortiz de Lejarazu, R.; Pumarola, T.; Rello, J.; Almansa, R.; Ramírez, P.; Martin-Loeches, I.; Varillas, D.; Gallegos, M.C.; Serón, C. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care 2009, 13, R201. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Zhao, B.; Wang, J.; Zhu, Z.; Teng, Z.; Shao, J.; Shen, J.; Gao, Y.; Yuan, Z.; et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS ONE 2011, 6, e28680. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Torres, L.; Metzger, D.W. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J. Virol. 2010, 84, 5007–5014. [Google Scholar] [CrossRef]
- McKinstry, K.K.; Strutt, T.M.; Buck, A.; Curtis, J.D.; Dibble, J.P.; Huston, G.; Tighe, M.; Hamada, H.; Sell, S.; Dutton, R.W.; et al. IL-10 Deficiency Unleashes an Influenza-Specific Th17 Response and Enhances Survival against High-Dose Challenge. J. Immunol. 2009, 182, 7353–7363. [Google Scholar] [CrossRef]
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom pathogenesis during acute influenza: Interleukin-6 and Other cytokine responses. J. Med. Virol. 2001, 64, 262–268. [Google Scholar] [CrossRef]
- Denney, L.; Branchett, W.; Gregory, L.G.; Oliver, R.A.; Lloyd, C.M. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal Immunol. 2017, 11, 523–535. [Google Scholar] [CrossRef]
- Hiller, B.E.; Yin, Y.; Perng, Y.C.; de Araujo Castro, Í.; Fox, L.E.; Locke, M.C.; Monte, K.J.; López, C.B.; Ornitz, D.M.; Lenschow, D.J. Fibroblast growth factor-9 expression in airway epithelial cells amplifies the type I interferon response and alters influenza A virus pathogenesis. PLoS Pathog. 2022, 18, e1010228. [Google Scholar] [CrossRef]
- Wan, X.; Li, J.; Wang, Y.; Yu, X.; He, X.; Shi, J.; Deng, G.; Zeng, X.; Tian, G.; Li, Y.; et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl. Sci. Rev. 2021, 9, nwab137. [Google Scholar] [CrossRef]
- Ross, C.; Svenson, M.; Hansen, M.B.; Vejlsgaard, G.L.; Bendtzen, K. High avidity IFN-neutralizing antibodies in pharmaceutically prepared human IgG. J. Clin. Investig. 1995, 95, 1974–1978. [Google Scholar] [CrossRef]
- Svenson, M.; Hansen, M.B.; Ross, C.; Diamant, M.; Rieneck, K.; Nielsen, H.; Bendtzen, K. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood 1998, 91, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.K. Anticytokine autoantibody-associated immunodeficiency. Annu. Rev. Immunol. 2014, 32, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.J.; Vanhoorelbeke, K.; Leypoldt, F.; Kaya, Z.; Bieber, K.; McLachlan, S.M.; Komorowski, L.; Luo, J.; Cabral-Marques, O.; Hammers, C.M.; et al. Mechanisms of Autoantibody-Induced Pathology. Front. Immunol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Janssen, N.A.F.; van de Veerdonk, F.L. Anticytokine Autoantibodies in Infectious Diseases: A Practical Overview. Int. J. Mol. Sci. 2023, 25, 515. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Shi, D.; Chen, J.; Zhao, M.; Tang, Y.; Zhao, C.; Jin, Y.; Tian, D.; Liao, Y.; Wang, X.; Wang, W.; et al. Prevalence of Neutralizing Autoantibodies Against Type I Interferon in a Multicenter Cohort of Severe or Critical COVID-19 Cases in Shanghai. J. Clin. Immunol. 2024, 44, 80. [Google Scholar] [CrossRef]
- Hoshino, A.; Takenaka, H.; Mizukoshi, O.; Imanishi, J.; Kishida, T.; Tovey, M.G. Effect of anti-interferon serum of influenza virus infection in mice. Antivir. Res. 1983, 3, 59–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Pizzorno, A.; Miorin, L.; Bastard, P.; Gervais, A.; Le Voyer, T.; Bizien, L.; Manry, J.; Rosain, J.; Philippot, Q.; et al. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J. Exp. Med. 2022, 219, e20220514. [Google Scholar] [CrossRef]
- Feng, A.; Yang, E.Y.; Moore, A.R.; Dhingra, S.; Chang, S.E.; Yin, X.; Pi, R.; Mack, E.K.; Völkel, S.; Geßner, R.; et al. Autoantibodies are highly prevalent in non-SARS-CoV-2 respiratory infections and critical illness. JCI Insight 2023, 8, e163150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Bandala, A.H.; Gutierrez-Xicotencatl, L.; Esquivel-Guadarrama, F. Pathogenesis Induced by Influenza Virus Infection: Role of the Early Events of the Infection and the Innate Immune Response. Viruses 2025, 17, 694. https://doi.org/10.3390/v17050694
Márquez-Bandala AH, Gutierrez-Xicotencatl L, Esquivel-Guadarrama F. Pathogenesis Induced by Influenza Virus Infection: Role of the Early Events of the Infection and the Innate Immune Response. Viruses. 2025; 17(5):694. https://doi.org/10.3390/v17050694
Chicago/Turabian StyleMárquez-Bandala, Alicia Helena, Lourdes Gutierrez-Xicotencatl, and Fernando Esquivel-Guadarrama. 2025. "Pathogenesis Induced by Influenza Virus Infection: Role of the Early Events of the Infection and the Innate Immune Response" Viruses 17, no. 5: 694. https://doi.org/10.3390/v17050694
APA StyleMárquez-Bandala, A. H., Gutierrez-Xicotencatl, L., & Esquivel-Guadarrama, F. (2025). Pathogenesis Induced by Influenza Virus Infection: Role of the Early Events of the Infection and the Innate Immune Response. Viruses, 17(5), 694. https://doi.org/10.3390/v17050694






