Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild–Domestic Canid Interface in Aburrá Valley, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. RNA Extraction and cDNA Synthesis
2.3. PCR and Sequencing
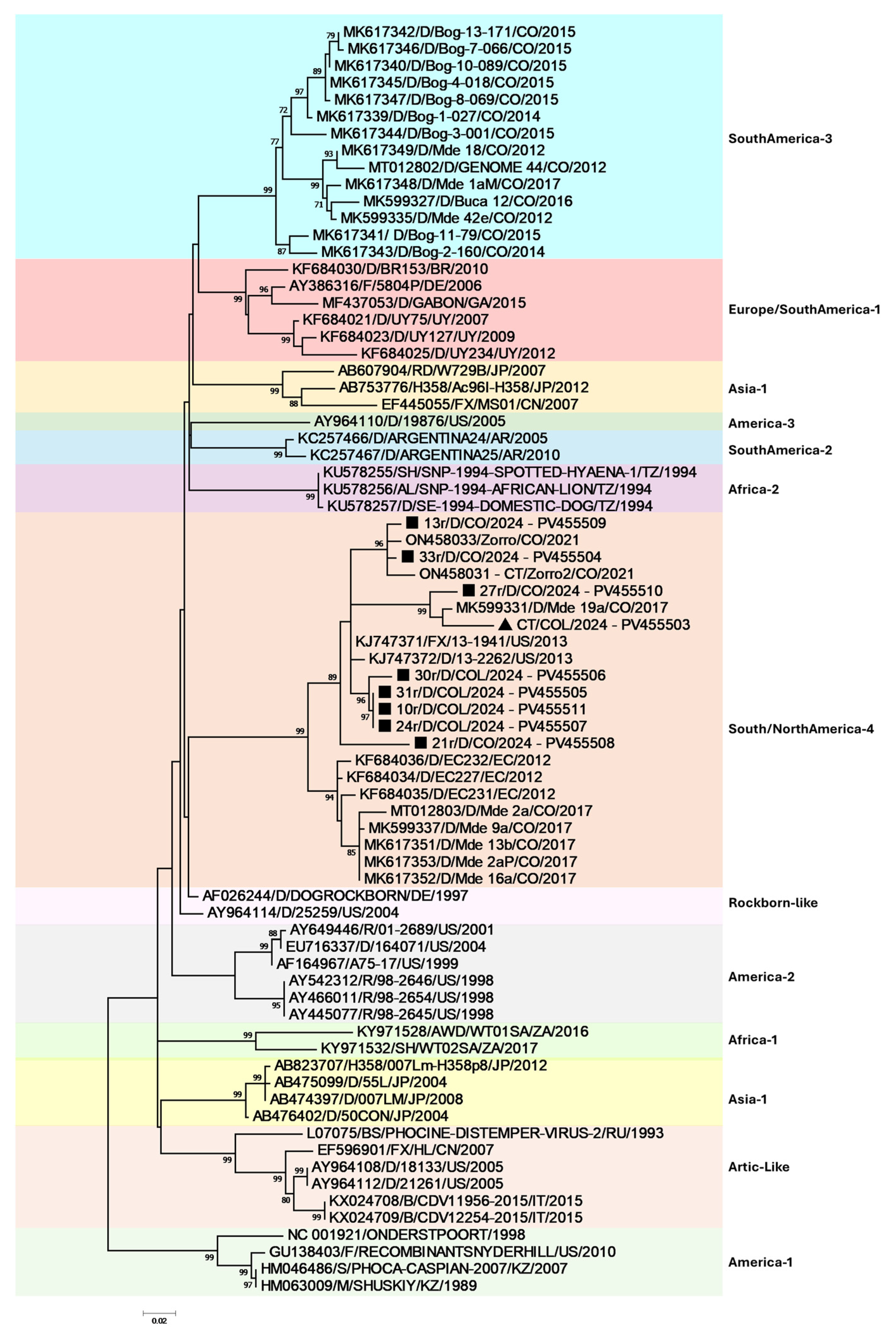
2.4. Phylogenetic Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamb, R.A.; Parks, G.D. Paramyxoviridae. In Fields Virology, 6th ed.; Wolters Kluwer Health Adis (ESP): Alphen aan den Rijn, The Netherlands, 2013. [Google Scholar]
- MacLachlan, N.; Dubovi, E.; Fenner, F. Paramyxoviridae. In Fenner’s Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 299–325. [Google Scholar]
- de Vries, R.D.; Duprex, W.P.; de Swart, R.L. Morbillivirus infections: An introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Pfeffermann, K.; Dorr, M.; Zirkel, F.; von Messling, V. Morbillivirus Pathogenesis and Virus-Host Interactions. Adv. Virus Res. 2018, 100, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Wipf, A.; Perez-Cutillas, P.; Ortega, N.; Huertas-López, A.; Martínez-Carrasco, C.; Candela, M.G. Geographical Distribution of Carnivore Hosts and Genotypes of Canine Distemper Virus (CDV) Worldwide: A Scoping Review and Spatial Meta-Analysis. Transbound. Emerg. Dis. 2025, 2025, 6632068. [Google Scholar] [CrossRef]
- Anis, E.; Newell, T.K.; Dyer, N.; Wilkes, R.P. Phylogenetic analysis of the wild-type strains of canine distemper virus circulating in the United States. Virol. J. 2018, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Rajak, K.; Chakravarti, S.; Yadav, A.; Kumar, A.; Gupta, V.; Chander, V.; Mathesh, K.; Chandramohan, S.; Sharma, A. Phylogenetic analysis of haemagglutinin gene deciphering a new genetically distinct lineage of canine distemper virus circulating among domestic dogs in India. Transbound. Emerg. Dis. 2019, 66, 1252–1267. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Diaz, F.J.; Ruiz-Saenz, J. Phylogenomic Analysis of Two Co-Circulating Canine Distemper Virus Lineages in Colombia. Pathogens 2019, 9, 26. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Forero-Munoz, N.R.; Diaz, F.J.; Martins, E.; Barato, P.; Ruiz-Saenz, J. Phylogenetic evidence of the intercontinental circulation of a Canine distemper virus lineage in the Americas. Sci. Rep. 2019, 9, 15747. [Google Scholar] [CrossRef]
- Espinal, M.A.; Diaz, F.J.; Ruiz-Saenz, J. Phylogenetic evidence of a new canine distemper virus lineage among domestic dogs in Colombia, South America. Vet. Microbiol. 2014, 172, 168–176. [Google Scholar] [CrossRef]
- Giacinti, J.A.; Pearl, D.L.; Ojkic, D.; Campbell, G.D.; Jardine, C.M. Genetic characterization of canine distemper virus from wild and domestic animal submissions to diagnostic facilities in Canada. Prev. Vet. Med. 2022, 198, 105535. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Zhai, J.; Zhang, P.; Irwin, D.M.; Shen, X.; Chen, W.; Shen, Y. A new canine distemper virus lineage identified from red pandas in China. Transbound. Emerg. Dis. 2022, 69, e944–e952. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Marin, S.; Martinez-Gutierrez, M.; Suarez, J.A.; Ruiz-Saenz, J. Canine Distemper Virus (CDV) Transit Through the Americas: Need to Assess the Impact of CDV Infection on Species Conservation. Front. Microbiol. 2020, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.P. Canine Distemper Virus in Endangered Species: Species Jump, Clinical Variations, and Vaccination. Pathogens 2023, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Saenz, J.; Barragan, V.; Grijalva-Rosero, C.J.; Diaz, E.A.; Páez-Rosas, D. Seroconversion in Galapagos Sea Lions (Zalophus wollebaeki) Confirms the Presence of Canine Distemper Virus in Rookeries of San Cristóbal Island. Animals 2023, 13, 3657. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruiz-Saenz, J. Evolution and Interspecies Transmission of Canine Distemper Virus-An Outlook of the Diverse Evolutionary Landscapes of a Multi-Host Virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Cleaveland, S.; Matthiopoulos, J.; Halliday, J.; Packer, C.; Craft, M.E.; Hampson, K.; Czupryna, A.; Dobson, A.P.; Dubovi, E.J. Dynamics of a morbillivirus at the domestic–wildlife interface: Canine distemper virus in domestic dogs and lions. Proc. Natl. Acad. Sci. USA 2015, 112, 1464–1469. [Google Scholar] [CrossRef]
- Fuques, E.; Tomás, G.; Grecco, S.; Condon, E.; Techera, C.; Marandino, A.; Sarute, N.; Aldaz, J.; Enciso, J.; Benech, A.; et al. Origin and spreading of canine morbillivirus in South America. Virus Res. 2022, 319, 198858. [Google Scholar] [CrossRef]
- Echeverry-Bonilla, D.F.; Buriticá-Gaviria, E.F.; Orjuela-Acosta, D.; Chinchilla-Cardenas, D.J.; Ruiz-Saenz, J. The First Report and Phylogenetic Analysis of Canine Distemper Virus in Cerdocyon thous from Colombia. Viruses 2022, 14, 1947. [Google Scholar] [CrossRef]
- Barrett, T.; Visser, I.; Mamaev, L.; Goatley, L.; Van Bressem, M.-F.; Osterhaus, A. Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 1993, 193, 1010–1012. [Google Scholar] [CrossRef]
- Riley, M.C.; Wilkes, R.P. Sequencing of emerging canine distemper virus strain reveals new distinct genetic lineage in the United States associated with disease in wildlife and domestic canine populations. Virol. J. 2015, 12, 219. [Google Scholar] [CrossRef]
- Kapil, S.; Yeary, T.J. Canine Distemper Spillover in Domestic Dogs from Urban Wildlife. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Loots, A.K.; Mitchell, E.; Dalton, D.L.; Kotze, A.; Venter, E.H. Advances in canine distemper virus pathogenesis research: A wildlife perspective. J. Gen. Virol. 2017, 98, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Maganga, G.D.; Labouba, I.; Ngoubangoye, B.; Nkili-Meyong, A.A.; Obame Ondo, D.; Leroy, E.M.; Berthet, N. Molecular characterization of complete genome of a canine distemper virus associated with fatal infection in dogs in Gabon, Central Africa. Virus Res. 2018, 247, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kodi, H.; Putty, K.; Ganji, V.K.; Bhagyalakshmi, B.; Reddy, Y.N.; Satish, K.; Prakash, M.G. H gene-based molecular characterization of field isolates of canine distemper virus from cases of canine gastroenteritis. Indian J. Anim. Res. 2021, 55, 561–567. [Google Scholar]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Odigie, A.E.; Madubuike, K.G.; Lucente, M.S.; Ezeifeka, C.A.; Patruno, G.; Lorusso, E.; Elia, G.; et al. Detection of Selected Canine Viruses in Nigerian Free-Ranging Dogs Traded for Meat Consumption. Animals 2023, 13, 1119. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; de Villiers, L.; Coetzee, L.M.; de Villiers, M.; Nyathi, F.N.; Garbade, M.; Hansen, C.; Berjaoui, S.; Ripà, P.; Lorusso, A.; et al. Unveiling the molecular epidemiology of canine distemper virus in Namibia: An expected pathogen showing an unexpected origin. Heliyon 2024, 10, e34805. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Marinho, P.H.; Venticinque, E.M.; Fonseca, C.R. Spatial and temporal ecology of Cerdocyon thous: A mesopredator canid coping with habitat loss, fragmentation, and chronic anthropogenic disturbances. Landsc. Ecol. 2024, 39, 157. [Google Scholar] [CrossRef]
- Fiorello, C.V.; Noss, A.J.; Deem, S.L.; Maffei, L.; Dubovi, E.J. Serosurvey of small carnivores in the Bolivian Chaco. J. Wildl. Dis. 2007, 43, 551–557. [Google Scholar] [CrossRef]
- Wilson, J.; Rubio, S.; Salvador, L.C.M.; Nemeth, N.M.; Fishburn, J.D.; Gottdenker, N.L. Canine distemper virus phylogenetic structure and ecological correlates of infection in mesocarnivores across anthropogenic land use gradients. Microbiol. Spectr. 2025, 13, e0122524. [Google Scholar] [CrossRef]
- Páez, A.; Saad, C.; Núñez, C.; Boshell, J. Molecular epidemiology of rabies in northern Colombia 1994–2003. Evidence for human and fox rabies associated with dogs. Epidemiol. Infect. 2005, 133, 529–536. [Google Scholar] [CrossRef]
- Cediel-Becerra, N.; Collins, R.; Restrepo-Botero, D.; Pardo, M.C.; Polo, L.; Villamil, L. Lessons learned from the history of rabies vaccination in Colombia using the one health approach. One Health Implement. Res. 2023, 3, 42–54. [Google Scholar] [CrossRef]
- Gilbert, M.; Miquelle, D.G.; Goodrich, J.M.; Reeve, R.; Cleaveland, S.; Matthews, L.; Joly, D.O. Estimating the Potential Impact of Canine Distemper Virus on the Amur Tiger Population (Panthera tigris altaica) in Russia. PLoS ONE 2014, 9, e110811. [Google Scholar] [CrossRef] [PubMed]
- Anis, E.; Holford, A.L.; Galyon, G.D.; Wilkes, R.P. Antigenic analysis of genetic variants of Canine distemper virus. Vet. Microbiol. 2018, 219, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.E.; Gogal, R.M.; Meindl, A.G.; Boyer, N.; Nelson, S.; Everett, S.E.; Vetter, C.A.; Gonzalez, J.M. Influence of age and vaccination interval on canine parvovirus, distemper virus, and adenovirus serum antibody titers. Vet. Immunol. Immunopathol. 2023, 262, 110630. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Marin, S.; Higuita-Gutiérrez, L.F.; Ruiz-Saenz, J. Safety and Immunogenicity of Morbillivirus canis Vaccines for Domestic and Wild Animals: A Scoping Review. Viruses 2024, 16, 1078. [Google Scholar] [CrossRef] [PubMed]
- Rendon-Marin, S.; Ruíz-Saenz, J. Universal peptide-based potential vaccine design against canine distemper virus (CDV) using a vaccinomic approach. Sci. Rep. 2024, 14, 16605. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Rincón-Tabares, D.-S.; Tabares-Guevara, J.H.; Arbeláez, N.; Forero-Duarte, J.E.; Díaz, F.J.; Robledo, S.M.; Hernandez, J.C.; Ruiz-Saenz, J. Evaluation of the Safety and Immunogenicity of a Multiple Epitope Polypeptide from Canine Distemper Virus (CDV) in Mice. Vaccines 2024, 12, 1140. [Google Scholar] [CrossRef]
- Guerrero, Y.M.; Cadena, A. Caracterización, evaluación y uso de hábitats del zorro perruno (Cerdocyon thous) en los llanos orientales de Colombia. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2000, 24, 383–392. [Google Scholar] [CrossRef]
- Muñoz Mazo, S.S. Agentes Infecciosos en el Zorro Cangrejero (Cerdocyon Thous) en las Áreas Protegidas Urbanas del Valle de Aburrá, Colombia; Universidad Nacional de Costa Rica: Heredia, Costa Rica, 2021. [Google Scholar]
- Sillero-Zubiri, C.; Hoffmann, M.; Macdonald, D.W. Canids: Foxes, Wolves, Jackals, and Dogs: Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 2004; Volume 95. [Google Scholar]
- Gómez Barragán, J. Práctica Social, Empresarial y Solidaria: Apoyo en la Vigilancia de Casos de Distemper Canino en la Especie Zorro Cangrejero (Cerdocyon thous) del CAV-AMVA Barbosa, Antioquia. Ph.D. Thesis, Universidad Cooperativa de Colombia, Santander, Colombia, 2022. [Google Scholar]
- Martins, N.B.; de Almeida, J.C.N.; Gonçalves, M.S.S.; Gila, L.I.; Yogui, D.R.; Alves, M.H.; Desbiez, A.L.J.; Brandão, P.E.; da Hora, A.S. Occurrence of Typical Domestic Animal Viruses in Wild Carnivorans: An Emerging Threat to the Conservation of Endangered Species. Transbound. Emerg. Dis. 2024, 2024, 3931047. [Google Scholar] [CrossRef]
- Angwenyi, S.K.S.; Rooney, N.J.; Eisler, M.C. Are Domestic Dogs (Canis familiaris) the Family Scapegoats? A Systematic Review of Canine Distemper Virus in African Wildlife, 1978–2021. J. Wildl. Dis. 2025, 61, 1–16. [Google Scholar] [CrossRef]
- Medina, M.; Beltran, D.; Castellanos, A. Record of canine distemper virus in an Andean Bear, Colombia. Boletín Técnico Ser. Zoológica 2023, 18, 5–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios-Usuga, C.; Ortiz-Pineda, M.C.; Aguirre-Catolico, S.D.; Quiroz, V.H.; Ruiz-Saenz, J. Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild–Domestic Canid Interface in Aburrá Valley, Colombia. Viruses 2025, 17, 649. https://doi.org/10.3390/v17050649
Rios-Usuga C, Ortiz-Pineda MC, Aguirre-Catolico SD, Quiroz VH, Ruiz-Saenz J. Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild–Domestic Canid Interface in Aburrá Valley, Colombia. Viruses. 2025; 17(5):649. https://doi.org/10.3390/v17050649
Chicago/Turabian StyleRios-Usuga, Carolina, Melissa C. Ortiz-Pineda, Sergio Daniel Aguirre-Catolico, Víctor H. Quiroz, and Julian Ruiz-Saenz. 2025. "Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild–Domestic Canid Interface in Aburrá Valley, Colombia" Viruses 17, no. 5: 649. https://doi.org/10.3390/v17050649
APA StyleRios-Usuga, C., Ortiz-Pineda, M. C., Aguirre-Catolico, S. D., Quiroz, V. H., & Ruiz-Saenz, J. (2025). Concurrent Circulation of Canine Distemper Virus (South America-4 Lineage) at the Wild–Domestic Canid Interface in Aburrá Valley, Colombia. Viruses, 17(5), 649. https://doi.org/10.3390/v17050649









