PKM2 Facilitates Classical Swine Fever Virus Replication by Enhancing NS5B Polymerase Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus and Antibodies
2.2. Plasmid Construction
2.3. siRNA Transfection
2.4. Cell Viability Assay
2.5. Transient Transfection of Plasmid
2.6. Virus Titration by Indirect Immunofluorescence Assay (IFA)
2.7. Western Blot
2.8. Confocal Microscopy
2.9. Infection of Animals
2.10. Immunohistochemistry (IHC)
2.11. Co-Immunoprecipitation (Co-IP) Assays
2.12. GST Pull-Down
2.13. Luciferase Assay
2.14. Statistical Analysis
3. Results
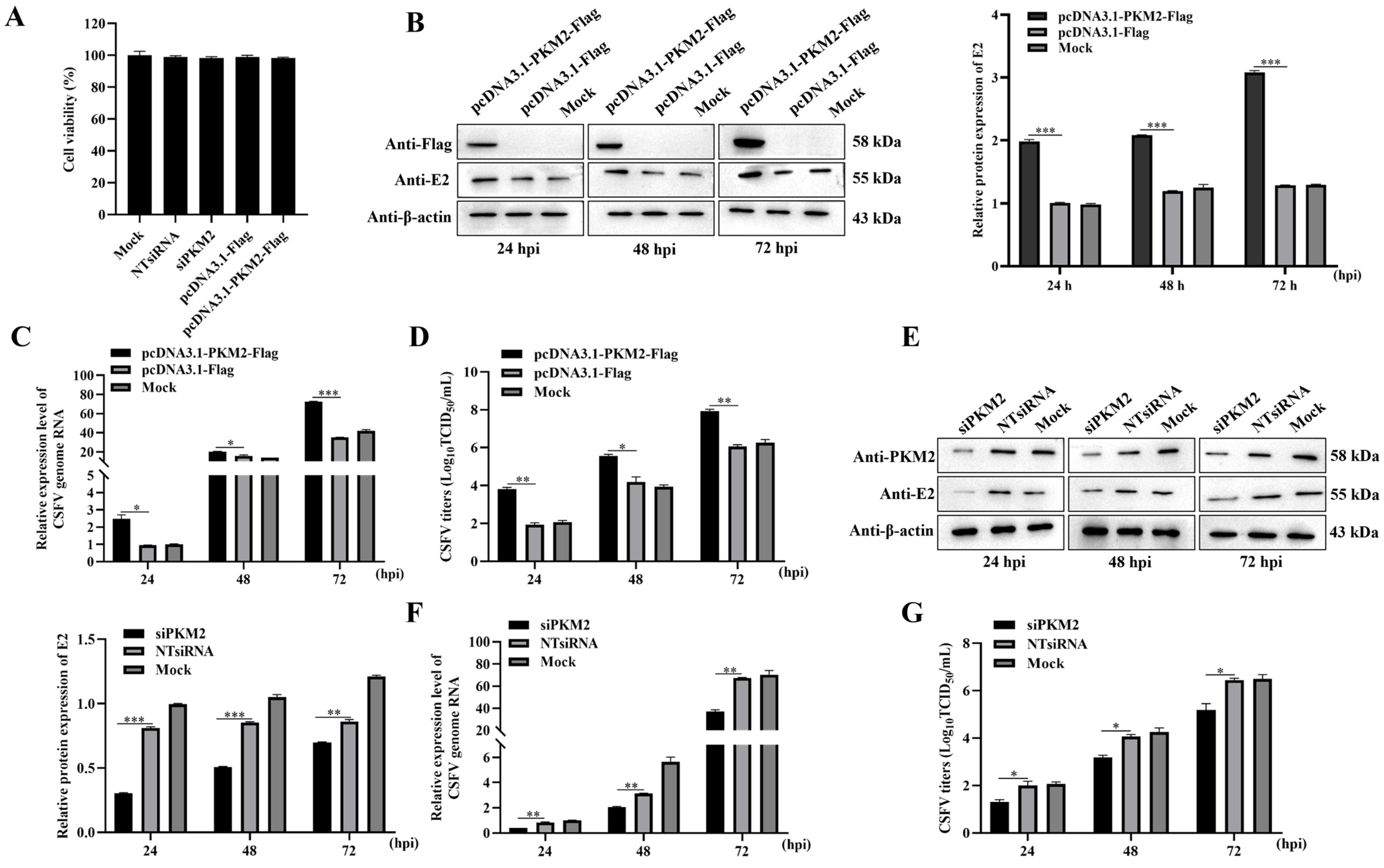
3.1. PKM2 Level Influences the Production of CSFV
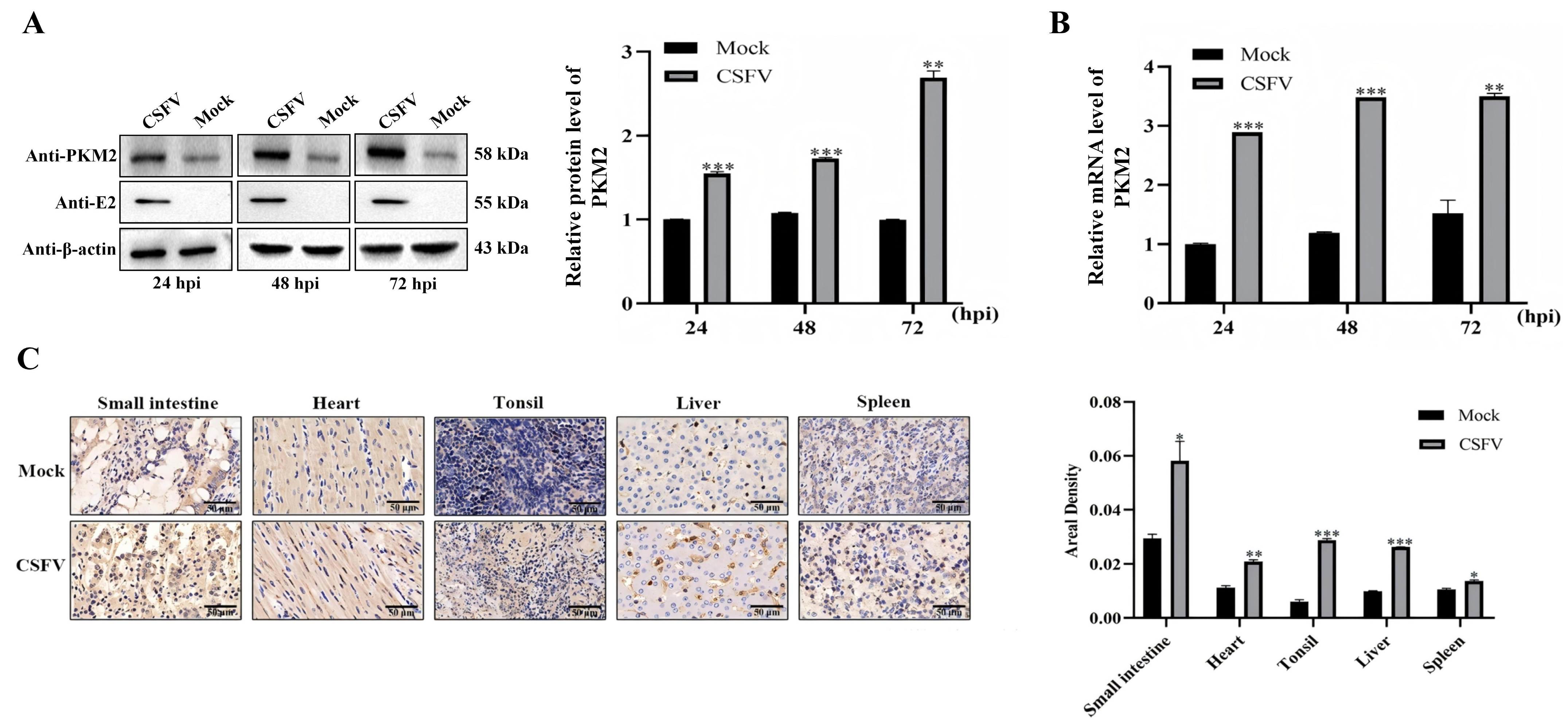
3.2. CSFV Infection Upregulates the Expression of PKM2
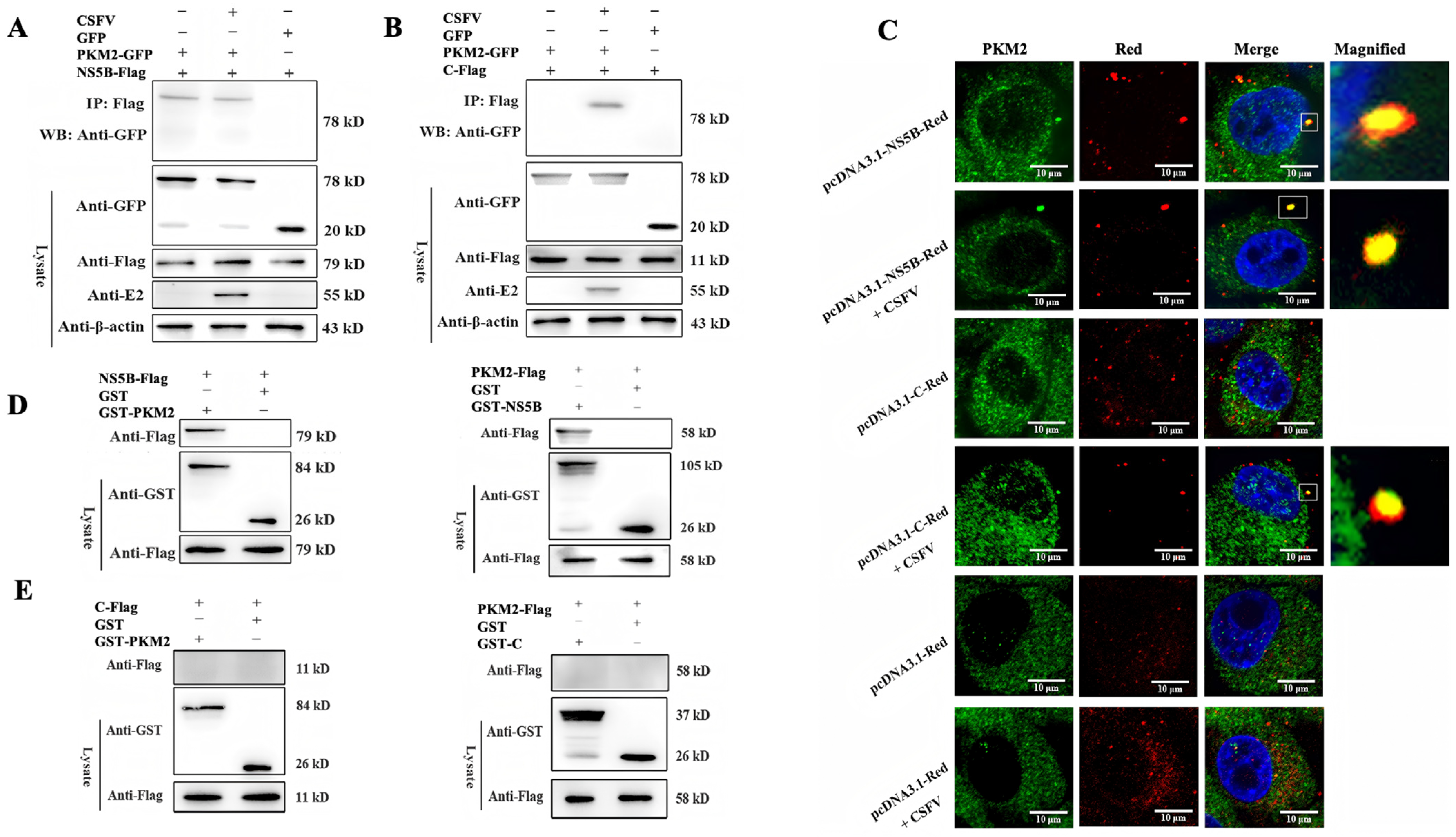
3.3. PKM2 Binds to CSFV NS5B Independent of Viral C Protein
3.4. PKM2 Increases the Replication of the CSFV Genome
3.5. Evidence for a PKM2 Effect on NS5B Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSFV | classical swine fever virus |
| PKM2 | pyruvate kinase M2 |
| NS5B | non-structural protein 5B |
| RdRp | RNA-dependent RNA polymerase |
References
- ICTV. Available online: https://ictv.global/taxonomy (accessed on 28 November 2024).
- Ji, W.; Guo, Z.; Ding, N.Z.; He, C.Q. Studying classical swine fever virus: Making the best of a bad virus. Virus Res. 2015, 197, 35–47. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Yang, Q.; Naveed, A.M.; Yu, S.; Qiu, H.J. Complex Virus-Host Interactions Involved in the Regulation of Classical Swine Fever Virus Replication: A Minireview. Viruses 2017, 9, 171. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, J.; Wang, W.; Wang, Y.; Chen, J.; Chen, J.; Li, B. Specific interaction between the classical swine fever virus NS5B protein and the viral genome. Eur. J. Biochem. 2004, 271, 3888–3896. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Kaiser, A.; Thiel, H.J. NS3 serine protease of bovine viral diarrhea virus: Characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology 2000, 273, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, Y.; Chen, J.; Li, B. Characterization of RNA-dependent RNA polymerase activity of CSFV NS5B proteins expressed in Escherichia coli. Virus Genes 2003, 27, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander, H.M. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Zhang, K.; Liu, Q.; Guo, D. Isoform-specific interaction of pyruvate kinase with hepatitis C virus NS5B. FEBS Lett. 2008, 582, 2155–2160. [Google Scholar] [CrossRef]
- Miyake, Y.; Ishii, K.; Honda, A. Influenza Virus Infection Induces Host Pyruvate Kinase M Which Interacts with Viral RNA-Dependent RNA Polymerase. Front. Microbiol. 2017, 8, 162. [Google Scholar] [CrossRef]
- Chuang, C.; Prasanth, K.R.; Nagy, P.D. The Glycolytic Pyruvate Kinase Is Recruited Directly into the Viral Replicase Complex to Generate ATP for RNA Synthesis. Cell Host Microbe 2017, 22, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Zhao, M.; Yuan, J.; Xu, H.; Ding, H.; Chen, J. Metabolic Profiles in Cell Lines Infected with Classical Swine Fever Virus. Front. Microbiol. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Q.; Liu, X.; Wei, W.; Zou, L.; Zhao, F.; Zeng, S.; Yi, L.; Ding, H.; Zhao, M.; et al. PKM2 induces mitophagy through the AMPK-mTOR pathway promoting CSFV proliferation. J. Virol. 2024, 98, e01751-23. [Google Scholar] [CrossRef]
- Liu, S. Effects of Host PKM Protein on CSFVProliferation and Interaction with NS5B. Master’ Thesis, Northwest A&F University, Yangling, China, 2021. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; You, X.; Hu, A.; Liu, M.; Yao, H.; He, J.; Zhang, X.; Ning, P. AIF1 was identified as an up-regulated gene contributing to CSFV Shimen infection in porcine alveolar macrophage 3D4/21 cells. PeerJ 2020, 8, e8543. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.S.; Jeon, J.H.; Choi, Y.K.; Jang, S.Y.; Park, S.Y.; Kim, S.W.; Byun, J.K.; Kim, M.K.; Lee, S.; Shin, E.C.; et al. Pyruvate dehydrogenase kinase regulates hepatitis C virus replication. Sci. Rep. 2016, 6, 30846. [Google Scholar] [CrossRef]
- Mouree, K.R.; Kishimoto, N.; Iga, N.; Kirihara, C.; Yamamoto, K.; Takamune, N.; Misumi, S. Virion-Packaged Pyruvate Kinase Muscle Type 2 Affects Reverse Transcription Efficiency of Human Immunodeficiency Virus Type 1 by Blocking Virion Recruitment of tRNA(Lys3). Biol. Pharm. Bull. 2018, 41, 612–618. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Schindler, M.; Bailer, N.; Renkema, G.H.; Saksela, K.; Knoop, V.; Müller-Trutwin, M.C.; Santiago, M.L.; Bibollet-Ruche, F.; Dittmar, M.T.; et al. Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors. J. Virol. 2004, 78, 6864–6874. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, J.; Song, Y. Safety of RNA-Dependent RNA Polymerase Inhibitors, Molnupiravir and VV116, for Oral Treatment of COVID-19: A Meta-Analysis. Iran. J. Med. Sci. 2024, 49, 275–285. [Google Scholar]
- Li, W.; Zhang, Y.; Kao, C.C. The classic swine fever virus (CSFV) core protein can enhance de novo-initiated RNA synthesis by the CSFV polymerase NS5B. Virus Genes 2014, 49, 106–115. [Google Scholar] [CrossRef]
- She, Y.; Liao, Q.; Chen, X.; Ye, L.; Wu, Z. Hepatitis C virus (HCV) NS2 protein up-regulates HCV IRES-dependent translation and down-regulates NS5B RdRp activity. Arch. Virol. 2008, 153, 1991–1997. [Google Scholar] [CrossRef]
- She, Y.; Han, T.; Ye, L.; Wu, Z. Hepatitis C virus NS2/3 protease regulates HCV IRES-dependent translation and NS5B RdRp activity. Arch. Virol. 2009, 154, 1465–1473. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Qi, W.; Sun, Z.; Xie, Z.; Jia, W.; Ning, Z. Senecavirus A-induced glycolysis facilitates virus replication by promoting lactate production that attenuates the interaction between MAVS and RIG-I. PLoS Pathog. 2023, 19, e1011371. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.M. Pyruvate Kinase, Inflammation and Period.d.dontal Disease. Pathogens 2021, 10, 784. [Google Scholar] [CrossRef]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liang, Z.; Lan, H.; Teng, X.; Wang, C. The role of PKM2 in cancer progression and its structural and biological basis. J. Physiol. Biochem. 2024, 80, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Min, J.K.; Kim, J.G.; Cap, K.C.; Islam, R.; Hossain, A.J.; Dogsom, O.; Hamza, A.; Mahmud, S.; Choi, D.R.; et al. Multiple functions of pyruvate kinase M2 in various cell types. J. Cell Physiol. 2022, 237, 128–148. [Google Scholar] [CrossRef]
- Ren, X.; Song, H.; Wang, Y.; Wang, Y.; Zhang, Q.; Yue, X.; Wu, Z.; Li, C.; Gao, L.; Ma, C.; et al. TIPE1 limits virus replication by disrupting PKM2/ HIF-1α/ glycolysis feedback loop. Free Radic. Biol. Med. 2024, 221, 52–63. [Google Scholar] [CrossRef]
- Lo, A.K.; Dawson, C.W.; Young, L.S.; Ko, C.W.; Hau, P.K.; Lo, K.W. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J. Pathol. 2015, 237, 238–248. [Google Scholar] [CrossRef]
- Lin, C.; Wu, J.W.; Hsiao, K.; Su, M.S. The hepatitis C virus NS4A protein: Interactions with the NS4B and NS5A proteins. J. Virol. 1997, 71, 6465–6471. [Google Scholar] [CrossRef]
- Wongtrakul, J.; Thongtan, T.; Pannengpetch, S.; Wikan, N.; Kantamala, D.; Kumrapich, B.; Suwan, W.; Smith, D.R. Phosphoproteomic analysis of dengue virus infected U937 cells and identification of pyruvate kinase M2 as a differentially phosphorylated phosphoprotein. Sci. Rep. 2020, 10, 14493. [Google Scholar] [CrossRef]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, W.; Zhang, J.; Zhang, J.; Zhang, H.; Zhu, Y.; Meng, X.; Yi, Z.; Wang, R. Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication. Virol. Sin. 2021, 36, 1532–1542. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Liu, S.; Luo, Y.; Ji, T.; Zhang, Y.; Deng, W. PKM2 Facilitates Classical Swine Fever Virus Replication by Enhancing NS5B Polymerase Function. Viruses 2025, 17, 648. https://doi.org/10.3390/v17050648
Song M, Liu S, Luo Y, Ji T, Zhang Y, Deng W. PKM2 Facilitates Classical Swine Fever Virus Replication by Enhancing NS5B Polymerase Function. Viruses. 2025; 17(5):648. https://doi.org/10.3390/v17050648
Chicago/Turabian StyleSong, Mengzhao, Shanchuan Liu, Yan Luo, Tiantian Ji, Yanming Zhang, and Wen Deng. 2025. "PKM2 Facilitates Classical Swine Fever Virus Replication by Enhancing NS5B Polymerase Function" Viruses 17, no. 5: 648. https://doi.org/10.3390/v17050648
APA StyleSong, M., Liu, S., Luo, Y., Ji, T., Zhang, Y., & Deng, W. (2025). PKM2 Facilitates Classical Swine Fever Virus Replication by Enhancing NS5B Polymerase Function. Viruses, 17(5), 648. https://doi.org/10.3390/v17050648





