Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics Statement
2.3. SARS-CoV-2 Antiviral Antibody Measurements
2.4. Cell Lines
2.5. Generation of SARS-CoV-2 Isolates
2.6. Serum Neutralization Assays
2.7. Immunofluorescence Staining for SARS-CoV-2 N Protein
2.8. Data Visualization and Statistics
2.8.1. Calculation of Average Slope of Antibody Levels over Time
2.8.2. Calculation of Overall Changes in Circulating Antibody Levels
2.8.3. Calculation of Correlation Between Circulating Anti-SARS-CoV-2 Antibodies and Neutralization Activity
3. Results
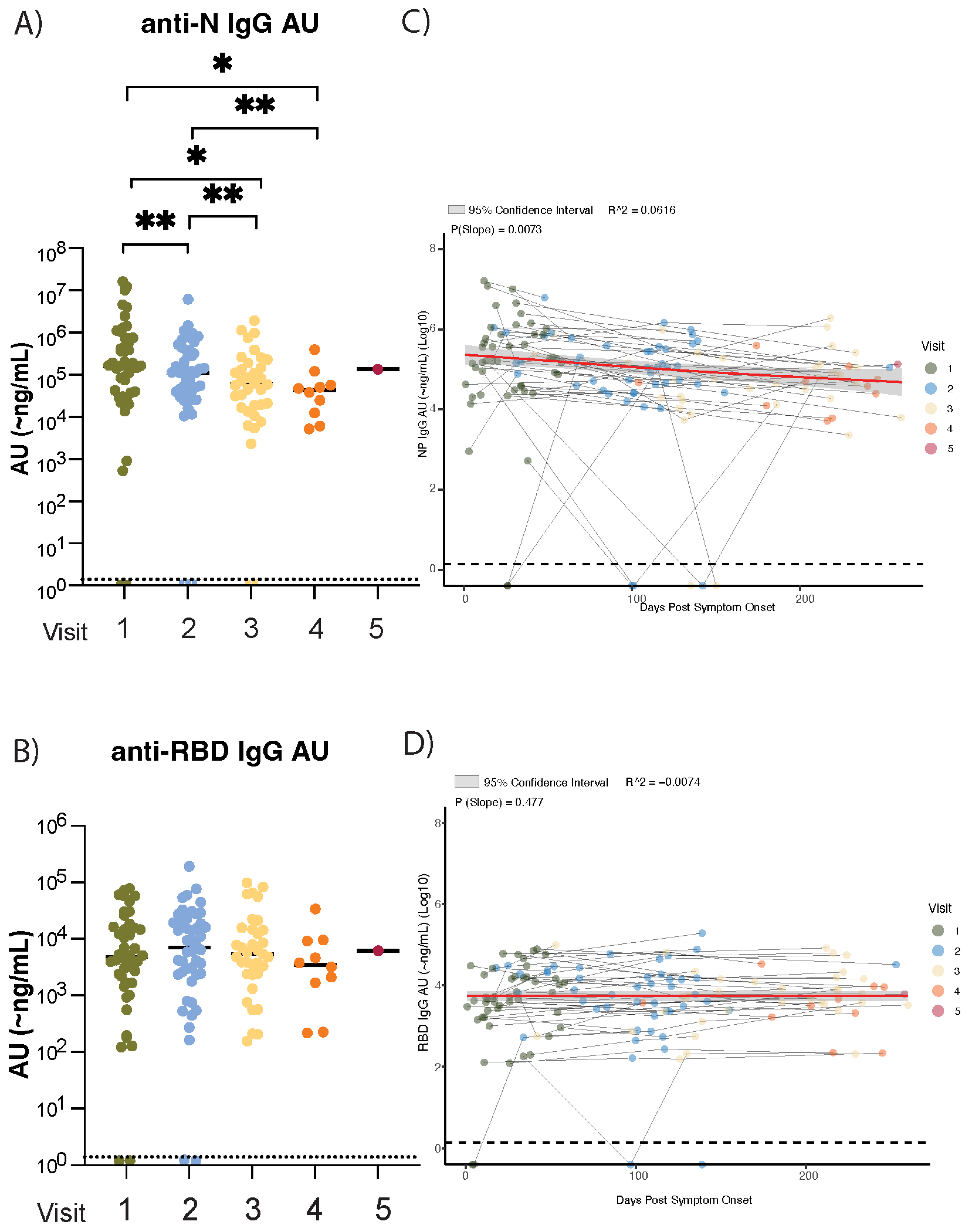
3.1. Waning of Anti-Nucleocapsid (N) but Not Anti-Spike Receptor-Binding Domain (RBD) SARS-CoV-2 IgG Plasma Antibody Levels in the Nine Months After Initial SARS-CoV-2 Infection
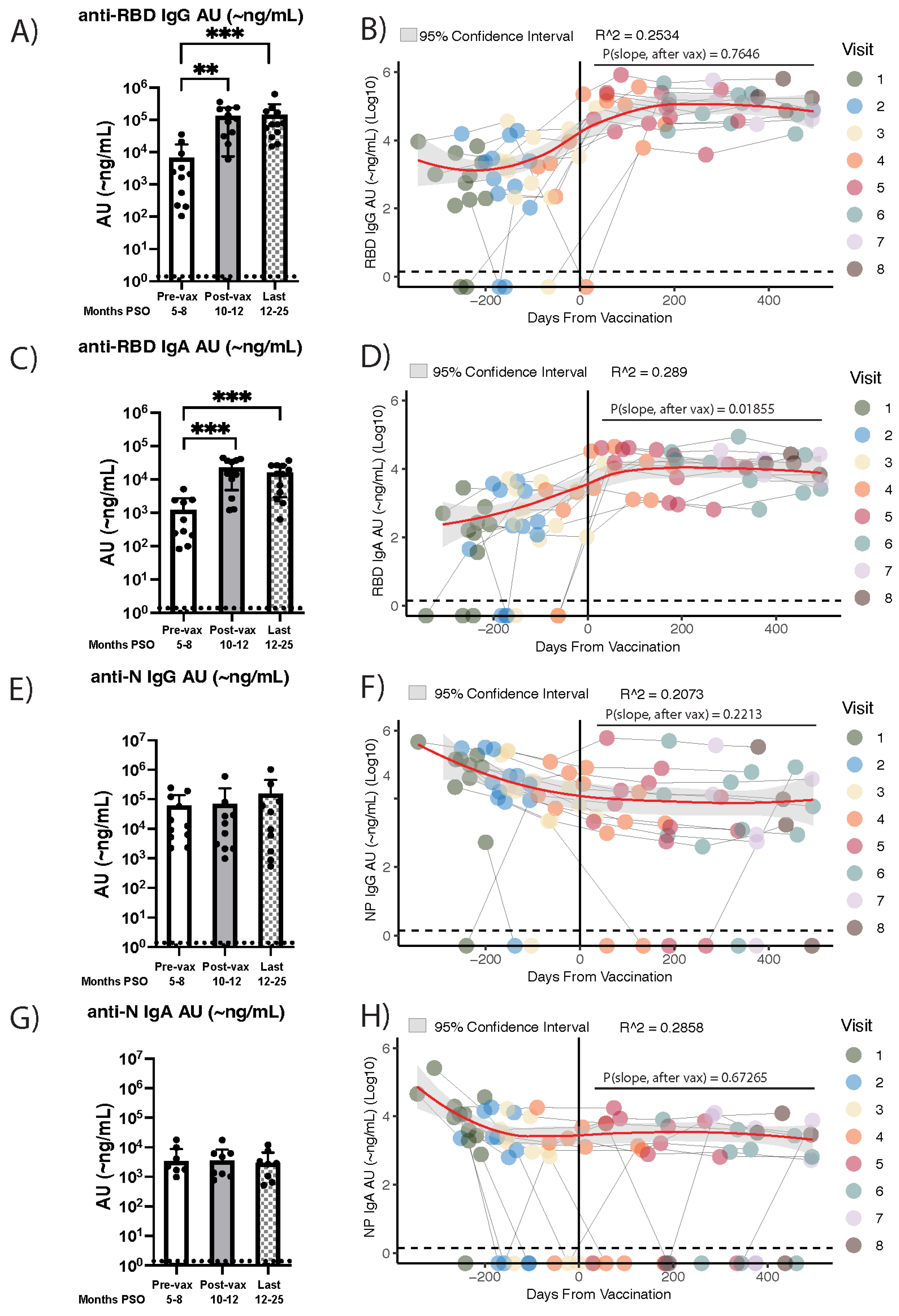
3.2. Vaccine Recipients That Were Infected with SARS-CoV-2 an Average of Eight Months Prior Exhibit Stable Anti-RBD IgG and IgA Antibody Levels for More than a Year Post-Vaccination
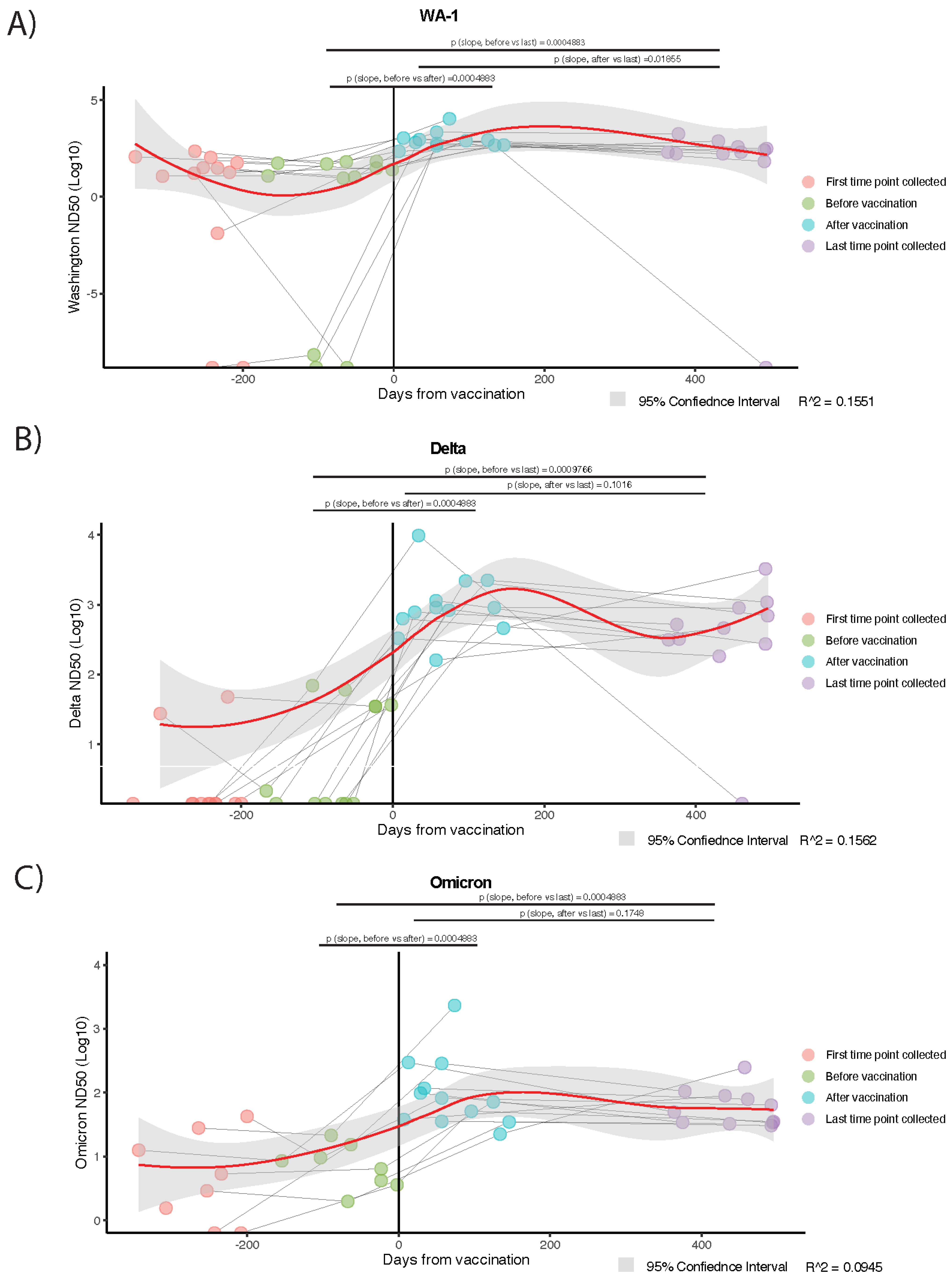
3.3. Temporal Trajectories of Plasma Neutralization Activity Against SARS-CoV-2 Are Analogous, Overall, to Trends Observed in Circulating Anti-RBD IgG and IgA Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| N | Nucleocapsid |
| S | Spike |
| RBD | Receptor-binding domain |
| EBV | Epstein–Barr virus |
| CMV | Cytomegalovirus |
| HA | Hemagglutinin |
| ND50 | 50% Neutralization Dose |
| AU | Arbitrary unit |
References
- Yang, Z.-R.; Jiang, Y.-W.; Li, F.-X.; Liu, D.; Lin, T.-F.; Zhao, Z.-Y.; Wei, C.; Jin, Q.-Y.; Li, X.-M.; Jia, Y.-X.; et al. Efficacy of SARS-CoV-2 Vaccines and the Dose–Response Relationship with Three Major Antibodies: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet Microbe 2023, 4, e236–e246. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Ashmawy, R.; Hamdy, N.A.; Elhadi, Y.A.M.; Reyad, O.A.; Elmalawany, D.; Almaghraby, A.; Shaaban, R.; Taha, S.H.N. Efficacy and Effectiveness of SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Nanduri, S. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1163–1166. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three Exposures to the Spike Protein of SARS-CoV-2 by Either Infection or Vaccination Elicit Superior Neutralizing Immunity to All Variants of Concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.-Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 Infection Leads to Robust Humoral Response and Antibodies That Effectively Neutralize Variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 Reinfections: Overview of Efficacy and Duration of Natural and Hybrid Immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Congrave-Wilson, Z.; Cheng, W.A.; Lee, Y.; Perez, S.; Turner, L.; Marentes Ruiz, C.J.; Mendieta, S.; Skura, A.; Jumarang, J.; Del Valle, J.; et al. Twelve-Month Longitudinal Serology in SARS-CoV-2 Naïve and Experienced Vaccine Recipients and Unvaccinated COVID-19-Infected Individuals. Vaccines 2022, 10, 813. [Google Scholar] [CrossRef]
- Abril, A.G.; Alejandre, J.; Mariscal, A.; Alserawan, L.; Rabella, N.; Roman, E.; Lopez-Contreras, J.; Navarro, F.; Serrano, E.; Nomdedeu, J.F.; et al. Titers of IgG and IgA against SARS-CoV-2 Proteins and Their Association with Symptoms in Mild COVID-19 Infection. Sci. Rep. 2024, 14, 12725. [Google Scholar] [CrossRef]
- Deshpande, G.R.; Kaduskar, O.; Deshpande, K.; Bhatt, V.; Yadav, P.; Gurav, Y.; Potdar, V.; Khutwad, K.; Vidhate, S.; Salunke, A.; et al. Longitudinal Clinico-Serological Analysis of Anti-Nucleocapsid and Anti-Receptor Binding Domain of Spike Protein Antibodies against SARS-CoV-2. Int. J. Infect. Dis. 2021, 112, 103. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Siqueira, L.O.; Sousa, B.G.; Cleto, C.E.; Wermelinger, L.S.; Caetano, B.L.L.; Pacheco, A.R.; Costa, S.M.; Almeida, F.C.L.; Ferreira, G.C.; Salmon, D.; et al. IgA Quantification as a Good Predictor of the Neutralizing Antibodies Levels after Vaccination against SARS-CoV-2. J. Clin. Virol. Plus 2022, 2, 100121. [Google Scholar] [CrossRef]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Fedele, G.; Schiavoni, I.; Trentini, F.; Leone, P.; Olivetta, E.; Fallucca, A.; Fiore, S.; Di Martino, A.; Abrignani, S.; Baldo, V.; et al. A 12-Month Follow-up of the Immune Response to SARS-CoV-2 Primary Vaccination: Evidence from a Real-World Study. Front. Immunol. 2023, 14, 1272119. [Google Scholar] [CrossRef]
- Elslande, J.V.; Oyaert, M.; Lorent, N.; Weygaerde, Y.V.; Pottelbergh, G.V.; Godderis, L.; Ranst, M.V.; André, E.; Padalko, E.; Lagrou, K.; et al. Lower Persistence of Anti-Nucleocapsid Compared to Anti-Spike Antibodies up to One Year after SARS-CoV-2 Infection. Diagn. Microbiol. Infect. Dis. 2022, 103, 115659. [Google Scholar] [CrossRef] [PubMed]
- Dodge, M.C.; Ye, L.; Duffy, E.R.; Cole, M.; Gawel, S.H.; Werler, M.M.; Daghfal, D.; Andry, C.; Kataria, Y. Kinetics of SARS-CoV-2 Serum Antibodies Through the Alpha, Delta, and Omicron Surges Among Vaccinated Health Care Workers at a Boston Hospital. Open Forum Infect. Dis. 2023, 10, ofad266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Slotkin, R.; Sheth, A.H.; Pischel, L.; Kyriakides, T.C.; Emu, B.; McNamara, C.; Shi, Q.; Delgobbo, J.; Xu, J.; et al. Serum Neutralizing Antibody Titers 12 Months After COVID-19 Messenger RNA Vaccination: Correlation to Clinical Variables in an Adult, US Population. Clin. Infect. Dis. 2022, 76, ciac416. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.-Y.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of Immune Responses to mRNA Booster Vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef]
- Hertz, T.; Levy, S.; Ostrovsky, D.; Oppenheimer, H.; Zismanov, S.; Kuzmina, A.; Friedman, L.M.; Trifkovic, S.; Brice, D.; Chun-Yang, L.; et al. Correlates of Protection for Booster Doses of the SARS-CoV-2 Vaccine BNT162b2. Nat. Commun. 2023, 14, 4575. [Google Scholar] [CrossRef]
- Atti, A.; Insalata, F.; Carr, E.J.; Otter, A.D.; Foulkes, S.; Wu, M.Y.; Cole, M.J.; Linley, E.; Semper, A.; Brooks, T.; et al. Antibody Correlates of Protection against Delta Infection after Vaccination: A Nested Case-Control within the UK-Based SIREN Study. J. Infect. 2023, 87, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody Titers and Protection against a SARS-CoV-2 Infection. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An Extended Interval between Vaccination and Infection Enhances Hybrid Immunity against SARS-CoV-2 Variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef] [PubMed]
- Kulemzin, S.V.; Guselnikov, S.V.; Nekrasov, B.G.; Molodykh, S.V.; Kuvshinova, I.N.; Murasheva, S.V.; Belovezhets, T.N.; Gorchakov, A.A.; Chikaev, A.N.; Chikaev, N.A.; et al. Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity Against Omicron Lineage VOCs than Revaccination or Reinfection. Vaccines 2024, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Strnad, P.; Wienand, O.; Krause, U.; Plecko, T.; Effenberger-Klein, A.; Giel, K.E.; Junne, F.; Galante-Gottschalk, A.; Ehehalt, S.; et al. The Humoral Immune Response More than One Year after SARS-CoV-2 Infection: Low Detection Rate of Anti-Nucleocapsid Antibodies via Euroimmun ELISA. Infection 2023, 51, 83–90. [Google Scholar] [CrossRef]
- Jarlhelt, I.; Pérez-Alós, L.; Bayarri-Olmos, R.; Hansen, C.B.; Petersen, M.S.; Weihe, P.; Armenteros, J.J.A.; Madsen, J.R.; Nielsen, J.P.S.; Hilsted, L.M.; et al. Distinguishing SARS-CoV-2 Infection and Vaccine Responses up to 18 Months Post-Infection Using Nucleocapsid Protein and Receptor-Binding Domain Antibodies. Microbiol. Spectr. 2023, 11, e01796-23. [Google Scholar] [CrossRef]
- Stirrup, O.; Tut, G.; Krutikov, M.; Bone, D.; Lancaster, T.; Azmi, B.; Monakhov, I.; Moss, P.; Hayward, A.; Copas, A.; et al. Anti-Nucleocapsid Antibody Levels Following Initial and Repeat SARS-CoV-2 Infections in a Cohort of Long-Term Care Facility Residents in England (VIVALDI). Wellcome Open Res. 2024, 9, 45. [Google Scholar] [CrossRef]
- Yuen, R.R.; Steiner, D.; Pihl, R.M.F.; Chavez, E.; Olson, A.; Smith, E.L.; Baird, L.A.; Korkmaz, F.; Urick, P.; Sagar, M.; et al. Novel ELISA Protocol Links Pre-Existing SARS-CoV-2 Reactive Antibodies with Endemic Coronavirus Immunity and Age and Reveals Improved Serologic Identification of Acute COVID-19 via Multi-Parameter Detection. Front. Immunol. 2021, 12, 614676. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal Analysis of Antibody Dynamics in COVID-19 Convalescents Reveals Neutralizing Responses up to 16 Months after Infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. J. 2020, 26, 1478. [Google Scholar] [CrossRef]
- Bai, Z.; Cao, Y.; Liu, W.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Gaeta, A.; Angeloni, A.; Napoli, A.; Pucci, B.; Cinti, L.; Roberto, P.; Colaiacovo, F.; Berardelli, E.; Farina, A.; Antonelli, G.; et al. Anti-N SARS-CoV-2 Assays for Evaluation of Natural Viral Infection. J. Immunol. Methods 2023, 518, 113486. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, H.R.; Parai, D.; Dash, G.C.; Peter, A.; Sahoo, S.K.; Pattnaik, M.; Rout, U.K.; Nanda, R.R.; Pati, S.; Bhattacharya, D. IgG Antibody Response against Nucleocapsid and Spike Protein Post-SARS-CoV-2 Infection. Infection 2021, 49, 1045–1048. [Google Scholar] [CrossRef]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and Duration of Detectable SARS-CoV-2 Nucleocapsid Antibodies in Staff and Residents of Long-Term Care Facilities over the First Year of the Pandemic (VIVALDI Study): Prospective Cohort Study in England. Lancet Healthy Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef]
- Alfego, D.; Sullivan, A.; Poirier, B.; Williams, J.; Adcock, D.; Letovsky, S. A Population-Based Analysis of the Longevity of SARS-CoV-2 Antibody Seropositivity in the United States. EClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef]
- Loesche, M.; Karlson, E.W.; Talabi, O.; Zhou, G.; Boutin, N.; Atchley, R.; Loevinsohn, G.; Chang, J.B.P.; Hasdianda, M.A.; Okenla, A.; et al. Longitudinal SARS-CoV-2 Nucleocapsid Antibody Kinetics, Seroreversion, and Implications for Seroepidemiologic Studies. Emerg. Infect. Dis. J. 2022, 28, 1859. [Google Scholar] [CrossRef]
- Lea, C.S.; Simeonsson, K.; Kipp, A.M.; McNeill, C.; Wilcox, L.; Irish, W.; Morris, H.; Diaz, O.M.; Fallon, J.T.; Roper, R.L. Waning of SARS-CoV-2 Seropositivity among Healthy Young Adults over Seven Months. Vaccines 2022, 10, 1532. [Google Scholar] [CrossRef]
- Haynes, J.M.; Dodd, R.Y.; Crowder, L.A.; Notari, E.P.; Stramer, S.L. Trajectory and Demographic Correlates of Antibodies to SARS-CoV-2 Nucleocapsid in Recently Infected Blood Donors, United States. Emerg. Infect. Dis. J. 2023, 29, 1323. [Google Scholar] [CrossRef]
- Mvula, M.; Mtonga, F.; Mandolo, J.; Jowati, C.; Kalirani, A.; Chigamba, P.; Lisimba, E.; Mitole, N.; Chibwana, M.G.; Jambo, K.C. Longevity of Hybrid Immunity against SARS-CoV-2 in Adults Vaccinated with an Adenovirus-Based COVID-19 Vaccine. BMC Infect. Dis. 2024, 24, 959. [Google Scholar] [CrossRef] [PubMed]
- Martín Pérez, C.; Aguilar, R.; Jiménez, A.; Salmerón, G.; Canyelles, M.; Rubio, R.; Vidal, M.; Cuamba, I.; Barrios, D.; Díaz, N.; et al. Correlates of Protection and Determinants of SARS-CoV-2 Breakthrough Infections 1 Year after Third Dose Vaccination. BMC Med. 2024, 22, 103. [Google Scholar] [CrossRef]
- Alsoussi, W.B.; Malladi, S.K.; Zhou, J.Q.; Liu, Z.; Ying, B.; Kim, W.; Schmitz, A.J.; Lei, T.; Horvath, S.C.; Sturtz, A.J.; et al. SARS-CoV-2 Omicron Boosting Induces de Novo B Cell Response in Humans. Nature 2023, 617, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 Infection Induces Long-Lived Bone Marrow Plasma Cells in Humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Movsisyan, M.; Chopikyan, A.; Kasparova, I.; Hakobjanyan, G.; Carrat, F.; Sukiasyan, M.; Rushanyan, M.; Chalabyan, M.; Shariff, S.; Kantawala, B.; et al. Kinetics of Anti-Nucleocapsid IgG Response in COVID-19 Immunocompetent Convalescent Patients. Sci. Rep. 2022, 12, 12403. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Panek, A.; Wąsowicz, P.; Anyszek, T.; Martin, E. High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines 2024, 12, 471. [Google Scholar] [CrossRef]
- Harrache, A.; Saker, K.; Mokdad, B.; Generenaz, L.; Saade, C.; Pons, S.; Fassier, J.-B.; Bal, A.; Trabaud, M.-A.; Rabilloud, M.; et al. Anti-RBD IgG Dynamics Following Infection or Vaccination. Vaccine 2024, 42, 126464. [Google Scholar] [CrossRef]
- do Nascimento, T.A.; Nogami, P.Y.; de Oliveira, C.F.; Neto, W.F.F.; da Silva, C.P.; Ribeiro, A.C.S.; de Sousa, A.W.; Freitas, M.N.O.; Chiang, J.O.; Silva, F.A.; et al. Equal Maintenance of Anti-SARS-CoV-2 Antibody Levels Induced by Heterologous and Homologous Regimens of the BNT162b2, ChAdOx1, CoronaVac and Ad26.COV2.S Vaccines: A Longitudinal Study Up to the 4th Dose of Booster. Vaccines 2024, 12, 792. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Hsu, F.-C.; Wu, J.-J.; Lin, Y.-L.; Liu, M.-T.; Yang, C.-H.; Kuo, H.-S.; Chen, Y.-J.; Cheng, C.-Y.; Lin, H.-H.; et al. Longitudinal Neutralizing Antibody Responses after SARS-CoV-2 Infection: A Convalescent Cohort Study in Taiwan. J. Microbiol. Immunol. Infect. 2023, 56, 506–515. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillon, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-Specific Plasma Cells Are Not Durably Established in the Bone Marrow Long-Lived Compartment after mRNA Vaccination. Nat. Med. 2025, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Mao, N.; Yi, C.; Simayi, A.; Feng, J.; Feng, Y.; He, M.; Ding, S.; Wang, Y.; Wang, Y.; et al. Impact of Vaccination on Kinetics of Neutralizing Antibodies against SARS-CoV-2 by Serum Live Neutralization Test Based on a Prospective Cohort. Emerg. Microbes Infect. 2023, 12, 2146535. [Google Scholar] [CrossRef]
- Kuijper, L.H.; Kreher, C.; Elias, G.; Claireaux, M.; Kerster, G.; Bos, A.V.; Duurland, M.C.; Konijn, V.A.L.; Paul, A.G.A.; de Jong, N.; et al. Longevity of Antibody Responses Is Associated with Distinct Antigen-Specific B Cell Subsets Early after Infection. Front. Immunol. 2024, 15, 1505719. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, K.M.; Fatah, M.H.; Tawfeeq, H.M.; Rostam, H.M. SARS-CoV-2 Reinfection in Patients Negative for Immunoglobulin G Following Recovery from COVID-19. New Microbes New Infect. 2021, 43, 100926. [Google Scholar] [CrossRef] [PubMed]
- Rutten, L.; Swart, M.; Koornneef, A.; Bouchier, P.; Blokland, S.; Sadi, A.; Juraszek, J.; Vijayan, A.; Schmit-Tillemans, S.; Verspuij, J.; et al. Impact of SARS-CoV-2 Spike Stability and RBD Exposure on Antigenicity and Immunogenicity. Sci. Rep. 2024, 14, 5735. [Google Scholar] [CrossRef]
- Graham, B.S.; Gilman, M.S.A.; McLellan, J.S. Structure-Based Vaccine Antigen Design. Annu. Rev. Med. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Ravichandran, S.; Lee, Y.; Grubbs, G.; Coyle, E.M.; Klenow, L.; Akasaka, O.; Koga, M.; Adachi, E.; Saito, M.; Nakachi, I.; et al. Longitudinal Antibody Repertoire in “Mild” versus “Severe” COVID-19 Patients Reveals Immune Markers Associated with Disease Severity and Resolution. Sci. Adv. 2021, 7, eabf2467. [Google Scholar] [CrossRef]
- Barin, B.; Kasap, U.; Selçuk, F.; Volkan, E.; Uluçkan, Ö. Comparison of SARS-CoV-2 Anti-Spike Receptor Binding Domain IgG Antibody Responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 Vaccines, and a Single Booster Dose: A Prospective, Longitudinal Population-Based Study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef]
- Sughayer, M.A.; Souan, L.; Abu Alhowr, M.M.; Al Rimawi, D.; Siag, M.; Albadr, S.; Owdeh, M.; Al Atrash, T. Comparison of the Effectiveness and Duration of Anti-RBD SARS-CoV-2 IgG Antibody Response between Different Types of Vaccines: Implications for Vaccine Strategies. Vaccine 2022, 40, 2841–2847. [Google Scholar] [CrossRef]
- Abdel-Qader, D.H.; Abdel-Qader, H.; Silverthorne, J.; Kongkaew, C.; Meslamani, A.Z.A.; Hayajneh, W.; Alwahadneh, A.M.; Hamadi, S.; Abu-Qatouseh, L.; Awad, R.; et al. Real-World Effectiveness of Four Types of COVID-19 Vaccines. Vaccines 2023, 11, 985. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Doherty, M.; Wallace, Z.S.; Sparks, J.A.; Lu, N.; Li, X.; Zeng, C.; Lei, G.; Zhang, Y. Comparative Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 Vaccines against COVID-19. BMC Med. 2023, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal Observation and Decline of Neutralizing Antibody Responses in the Three Months Following SARS-CoV-2 Infection in Humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Lau, E.H.; Hui, D.S.; Tsang, O.T.; Chan, W.-H.; Kwan, M.Y.; Chiu, S.S.; Cheng, S.M.; Ko, R.L.; Li, J.K.; Chaothai, S.; et al. Long-Term Persistence of SARS-CoV-2 Neutralizing Antibody Responses after Infection and Estimates of the Duration of Protection. EClinicalMedicine 2021, 41, 101174. [Google Scholar] [CrossRef] [PubMed]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in Symptomatic COVID-19 Is Persistent and Critical for Survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.; Matassoli, F.; Leggat, D.; Hoover, A.; Srikanth, A.; Wu, W.; Henry, A.R.; Wang, J.; Lin, B.C.; Teng, I.-T.; et al. Adjuvanted SARS-CoV-2 Spike Protein Vaccination Elicits Long-Lived Plasma Cells in Nonhuman Primates. Sci. Transl. Med. 2024, 16, eadd5960. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Maeda, K.; Matsuda, K.; Takamatsu, Y.; Kinoshita, N.; Kutsuna, S.; Hayashida, T.; Gatanaga, H.; Ohmagari, N.; Oka, S.; et al. Neutralization Activity of IgG Antibody in COVID-19-convalescent Plasma against SARS-CoV-2 Variants. Sci. Rep. 2023, 13, 1263. [Google Scholar] [CrossRef]
- Kompaniyets, L. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef]
- Ko, J.Y.; Danielson, M.L.; Town, M.; Derado, G.; Greenlund, K.J.; Kirley, P.D.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; Ryan, P.A.; et al. Risk Factors for Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization: COVID-19–Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin. Infect. Dis. 2021, 72, e695–e703. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
| SARS-CoV-2 Positive Individuals | |
|---|---|
| Total (N)= | 46 |
| Age in years (median, range) | 44 (25–82) |
| Sex (male) | 17 (37%) |
| Race | |
| Black | 2 (15.4%) |
| Asian | 3 (23.1%) |
| White | 37 (80.4%) |
| Other or not available | 5 (10.9%) |
| Ethnicity (Hispanic) | 5 (10.9%) |
| Illness features | |
| Hospitalized | 13 (28.2%) |
| Length of hospitalization | |
| Median (range) | 41 days (5–71) |
| ICU status (YES) | 9 (19.6%) |
| Average follow-up time after symptom onset (days) | |
| Last sample > 30 and ≤90 days | 6 (46.2%) |
| >90 and ≤180 days | 4 (8.7%) |
| >180 and ≤450 days | 19 (41.3%) |
| >550 days | 17 (37%) |
| Medical comorbidities | |
| Lung disease | 2 (6.1%) |
| Autoimmune disease | 6 (46.2%) |
| Immunosuppressed | 10 (21.7%) |
| Hypertension | 2 (6.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mcconney, C.S.; Kenney, D.; Ennis, C.S.; Smith-Mahoney, E.L.; Ayuso, M.J.; Zhong, J.; Douam, F.; Sagar, M.; Snyder-Cappione, J.E. Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year. Viruses 2025, 17, 640. https://doi.org/10.3390/v17050640
Mcconney CS, Kenney D, Ennis CS, Smith-Mahoney EL, Ayuso MJ, Zhong J, Douam F, Sagar M, Snyder-Cappione JE. Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year. Viruses. 2025; 17(5):640. https://doi.org/10.3390/v17050640
Chicago/Turabian StyleMcconney, Christina S., Devin Kenney, Christina S. Ennis, Erika L. Smith-Mahoney, Maria Jose Ayuso, Jiabao Zhong, Florian Douam, Manish Sagar, and Jennifer E. Snyder-Cappione. 2025. "Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year" Viruses 17, no. 5: 640. https://doi.org/10.3390/v17050640
APA StyleMcconney, C. S., Kenney, D., Ennis, C. S., Smith-Mahoney, E. L., Ayuso, M. J., Zhong, J., Douam, F., Sagar, M., & Snyder-Cappione, J. E. (2025). Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year. Viruses, 17(5), 640. https://doi.org/10.3390/v17050640







