Non-Compromised Efficacy of the First Commercial Ready-to-Use Genotype 2d Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Parameters (Only in Study A)
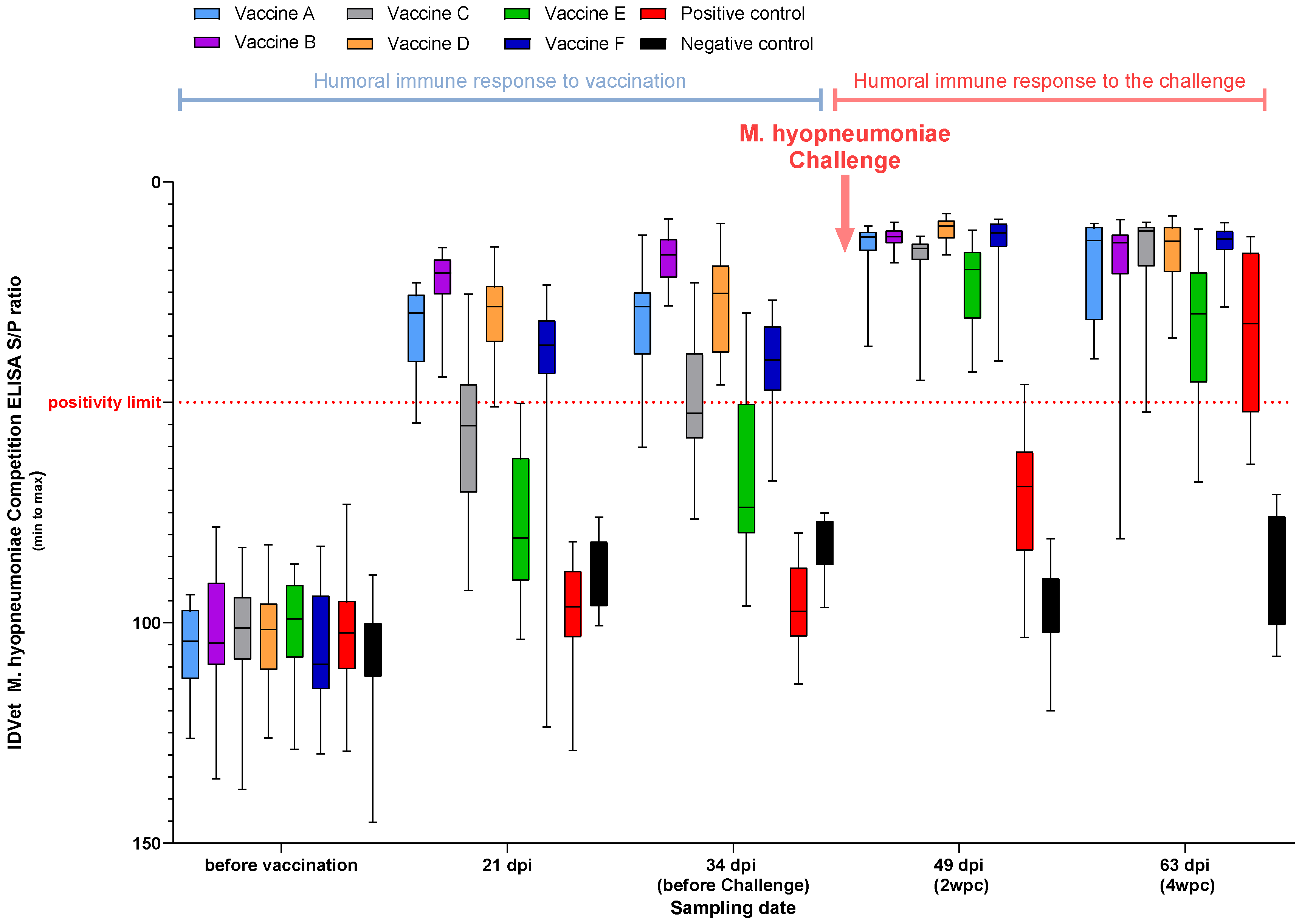
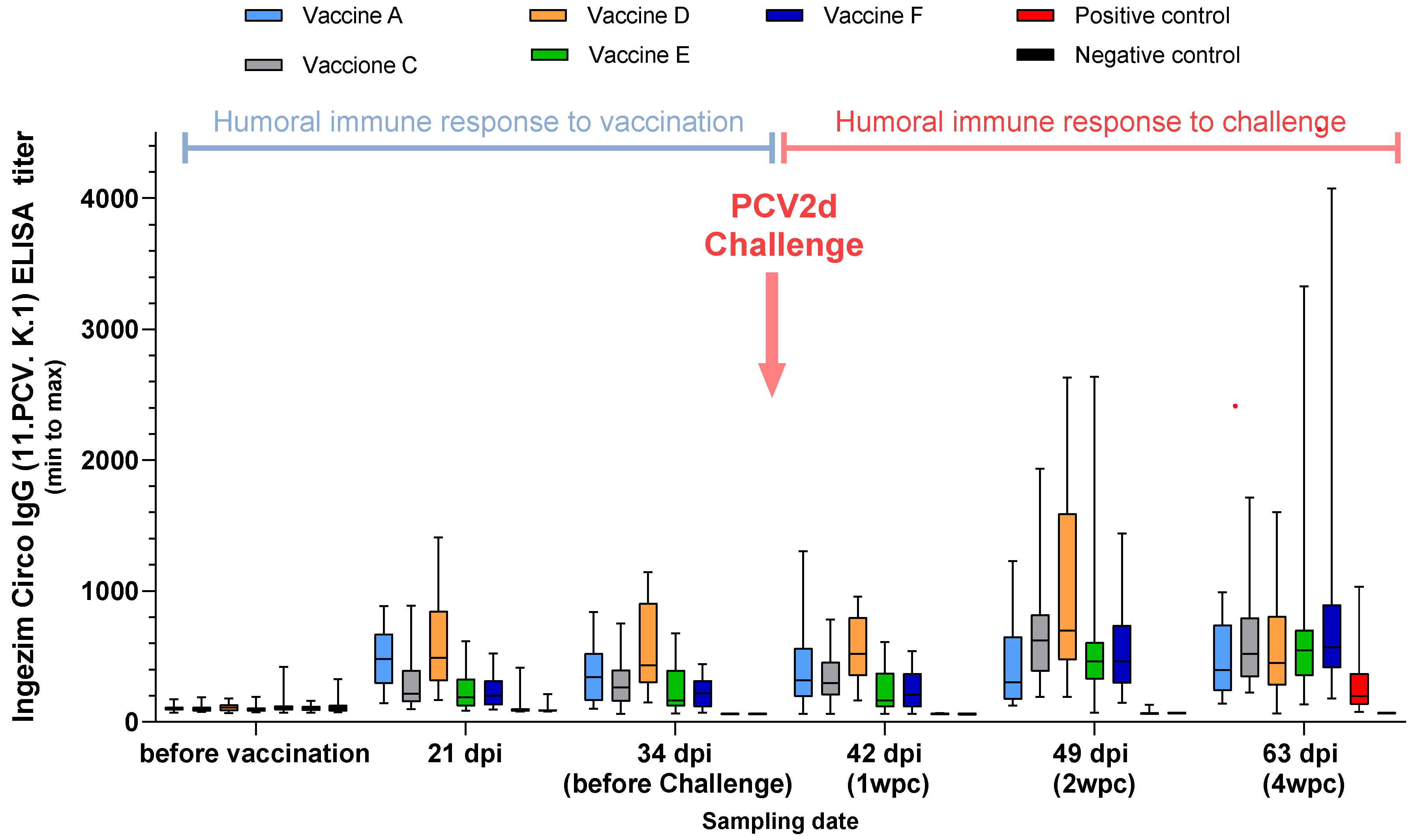
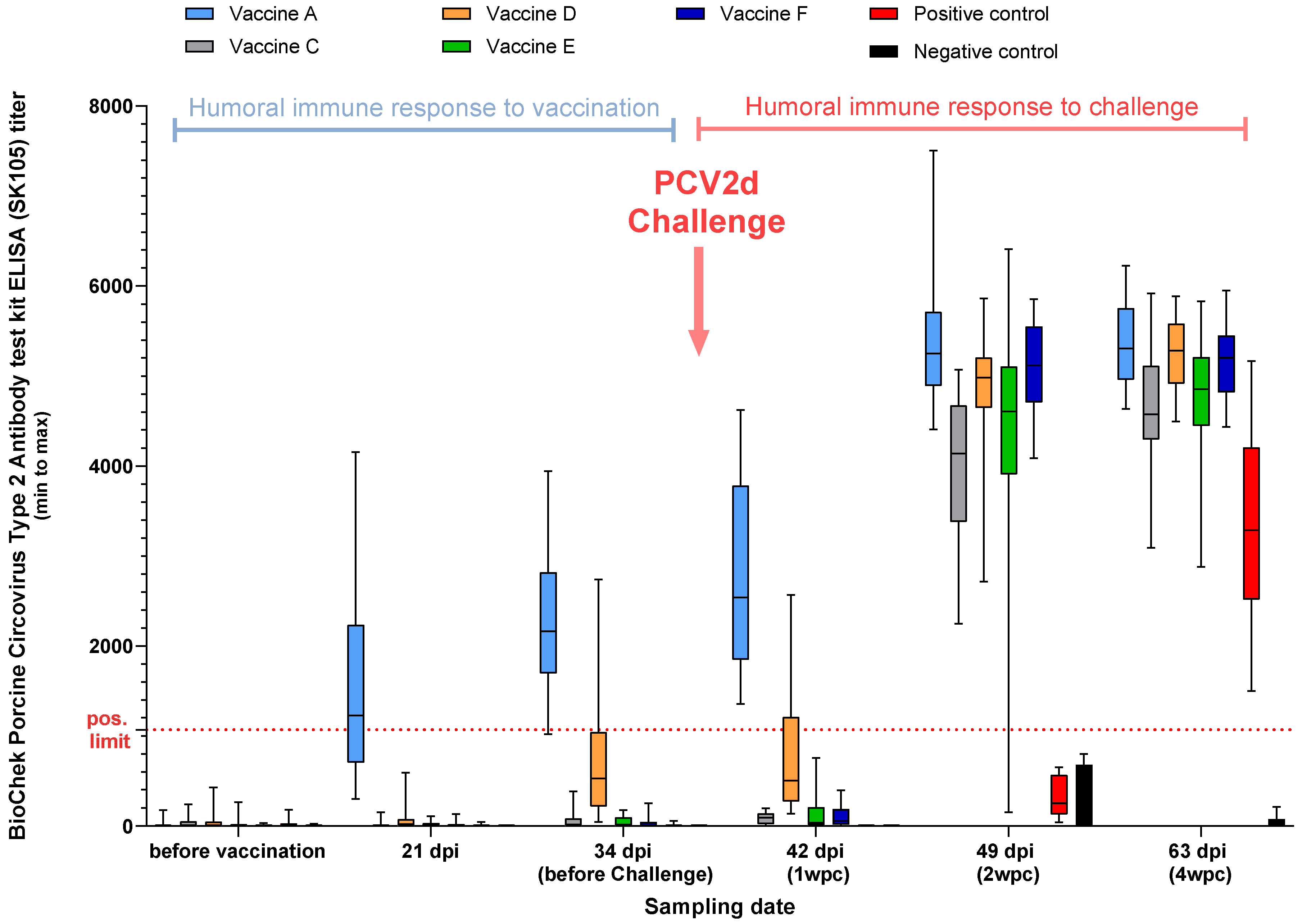
2.2. Serology
2.3. qPCR (Only in Study B)
2.4. Challenge
2.5. Mhyo
2.6. Statistics
3. Results
3.1. Clinical Observations
3.2. Serology
3.2.1. M. Hyopneumoniae ELISA (Study A and Study B)
3.2.2. PCV2 ELISA, Study B
3.2.3. IgG/IgM Ratio, Study B
3.3. Mhyo Related Lung Lesions (Study A)
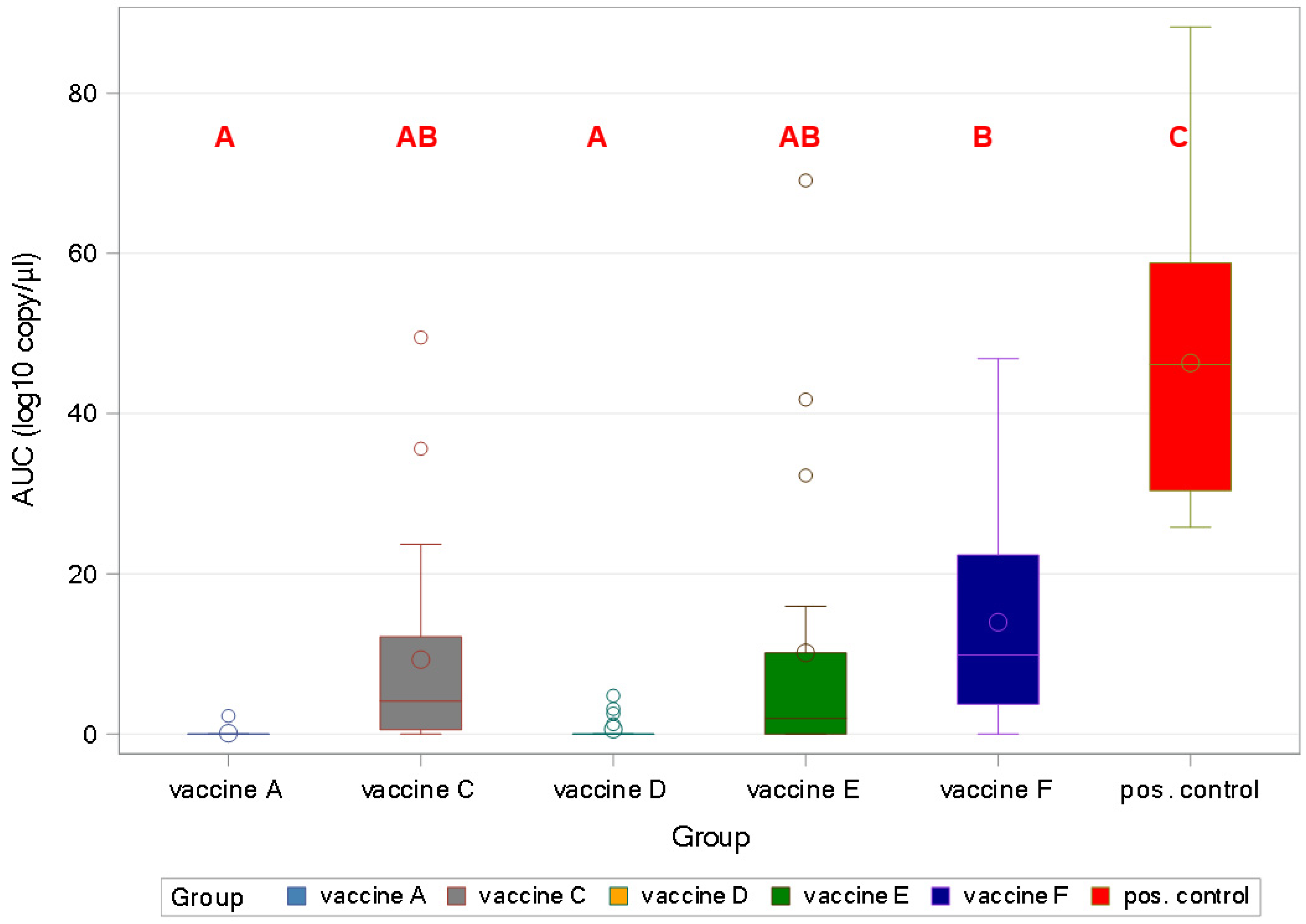
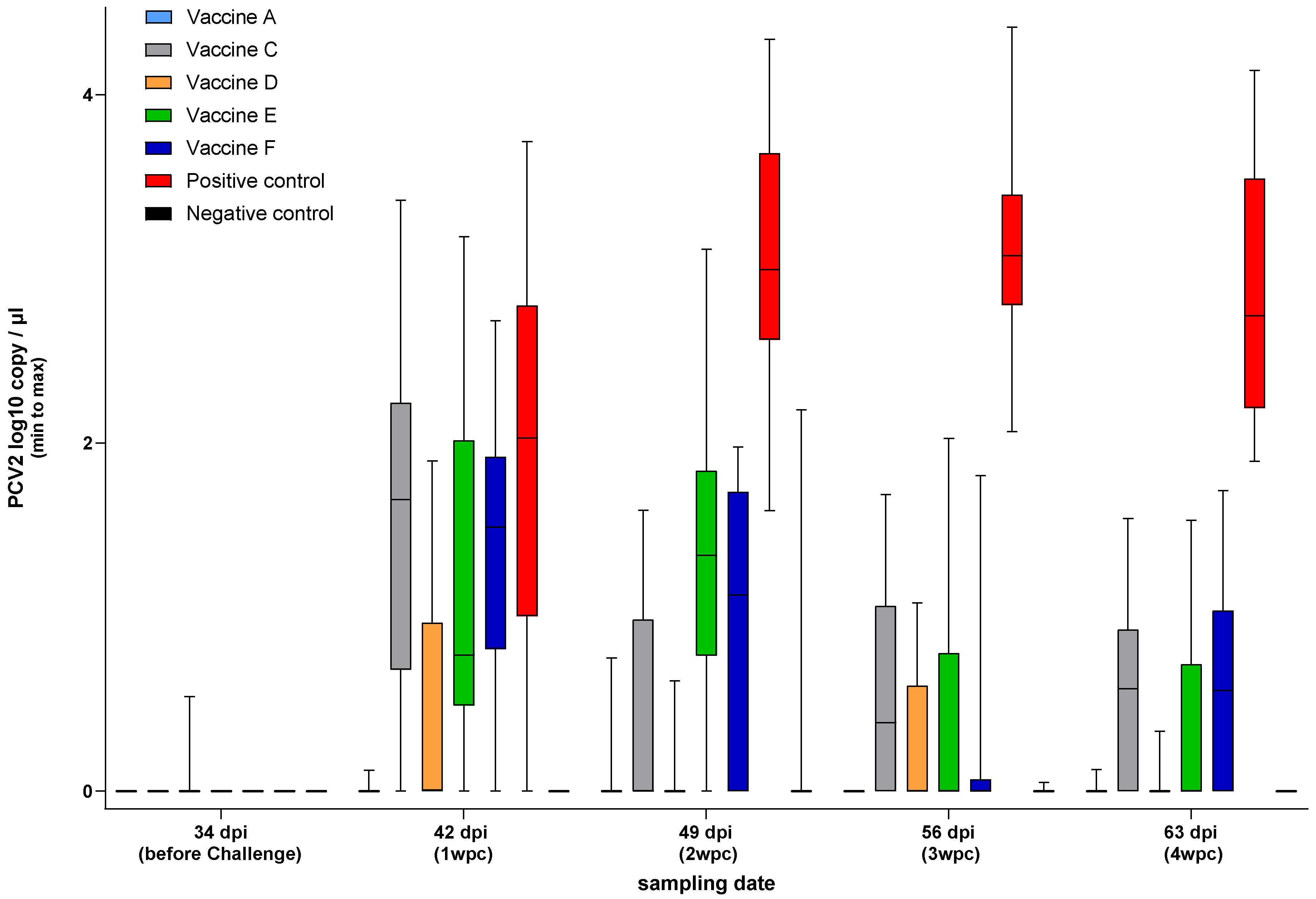
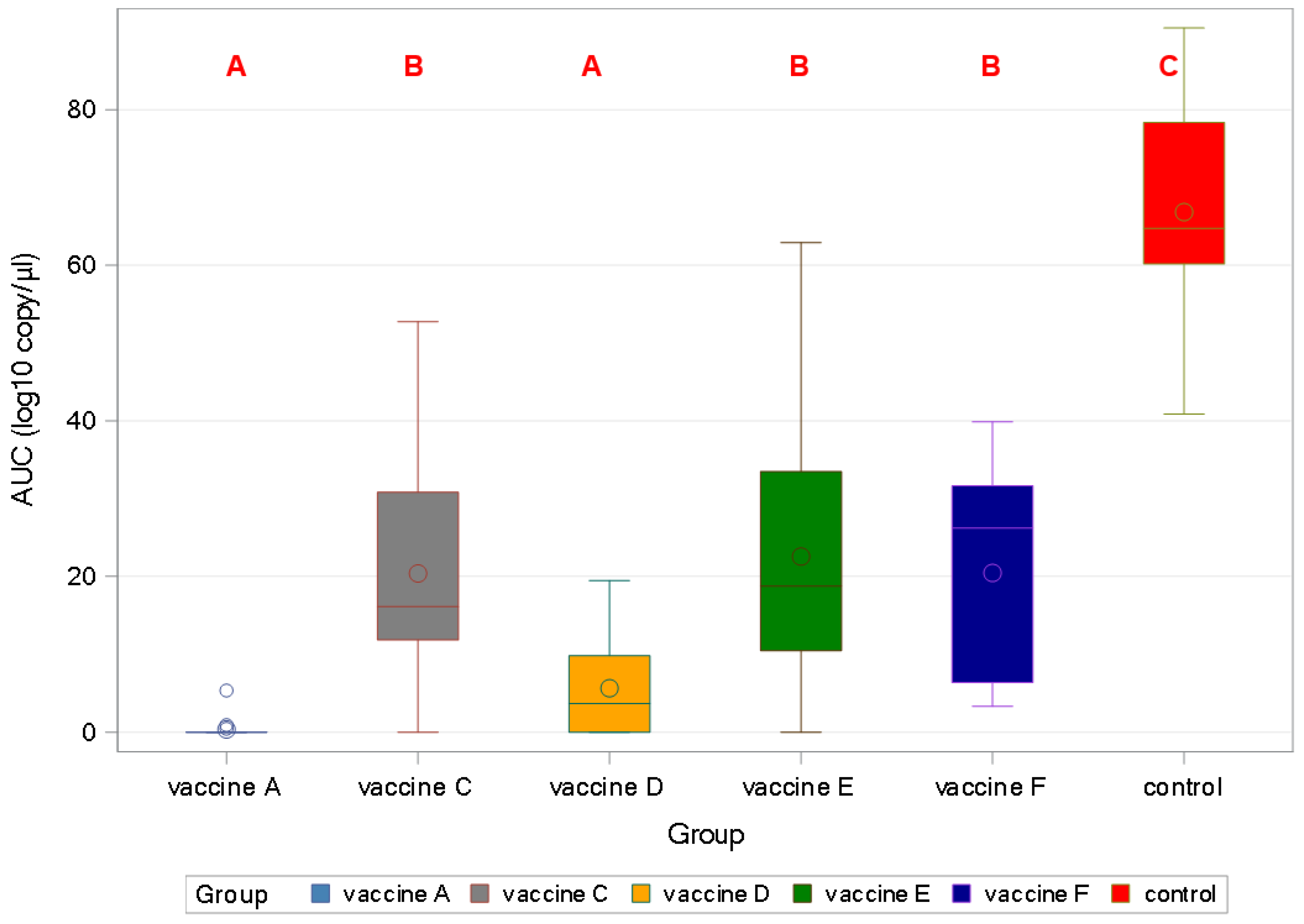
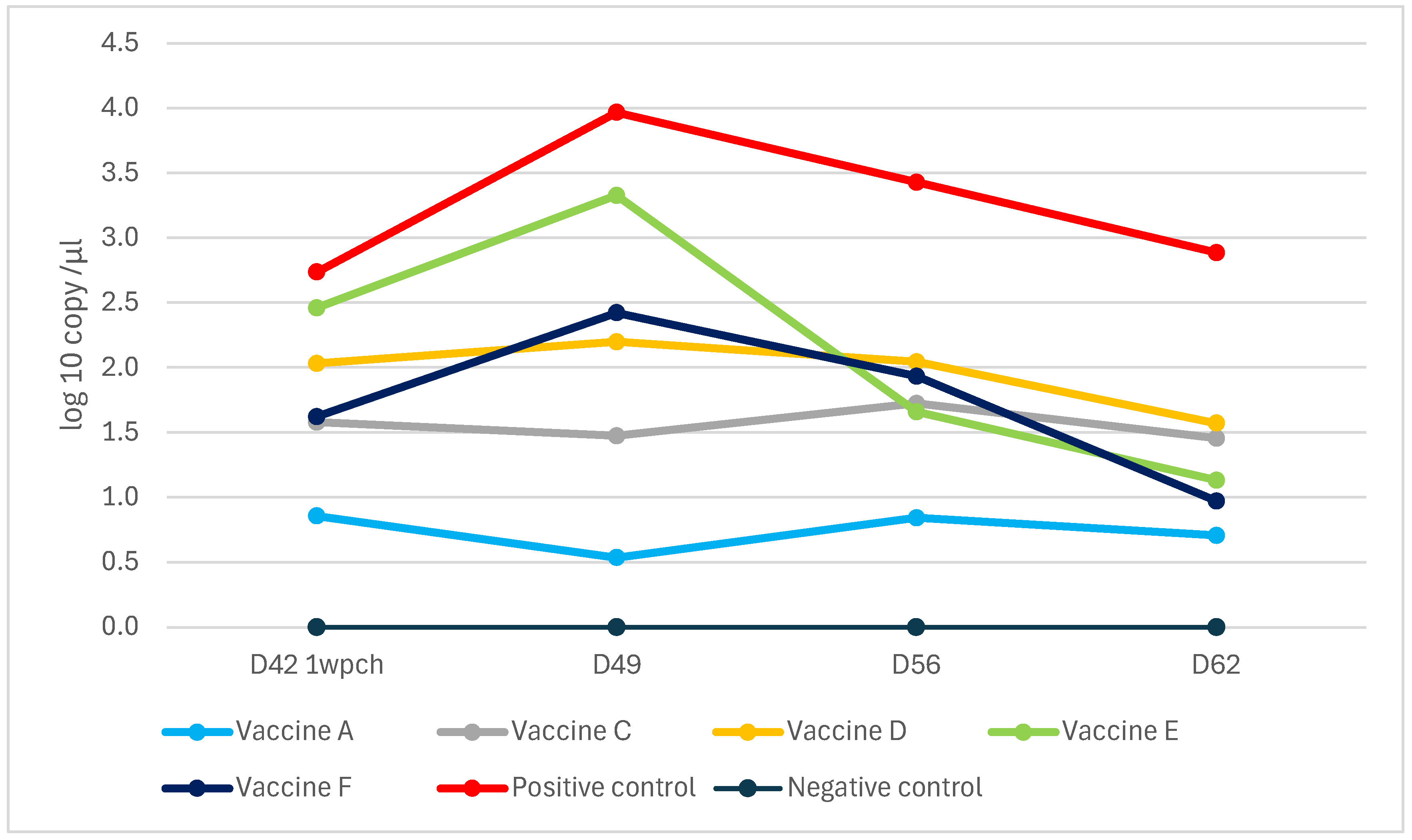
3.4. PCV2 qPCR—Study B
3.4.1. PCV2 Viraemia
3.4.2. PCV2 Shedding
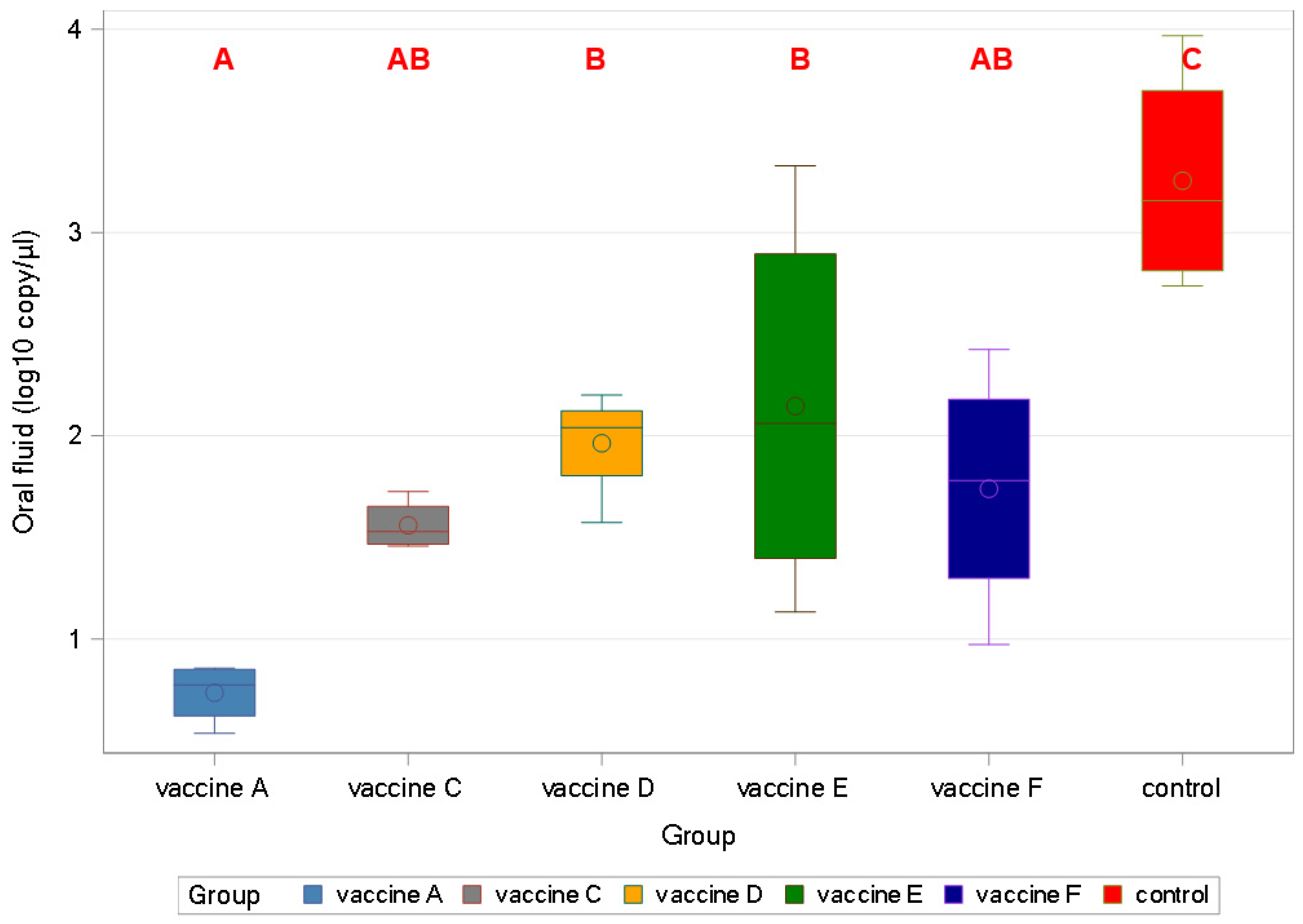
3.4.3. Oral Fluid qPCR Results
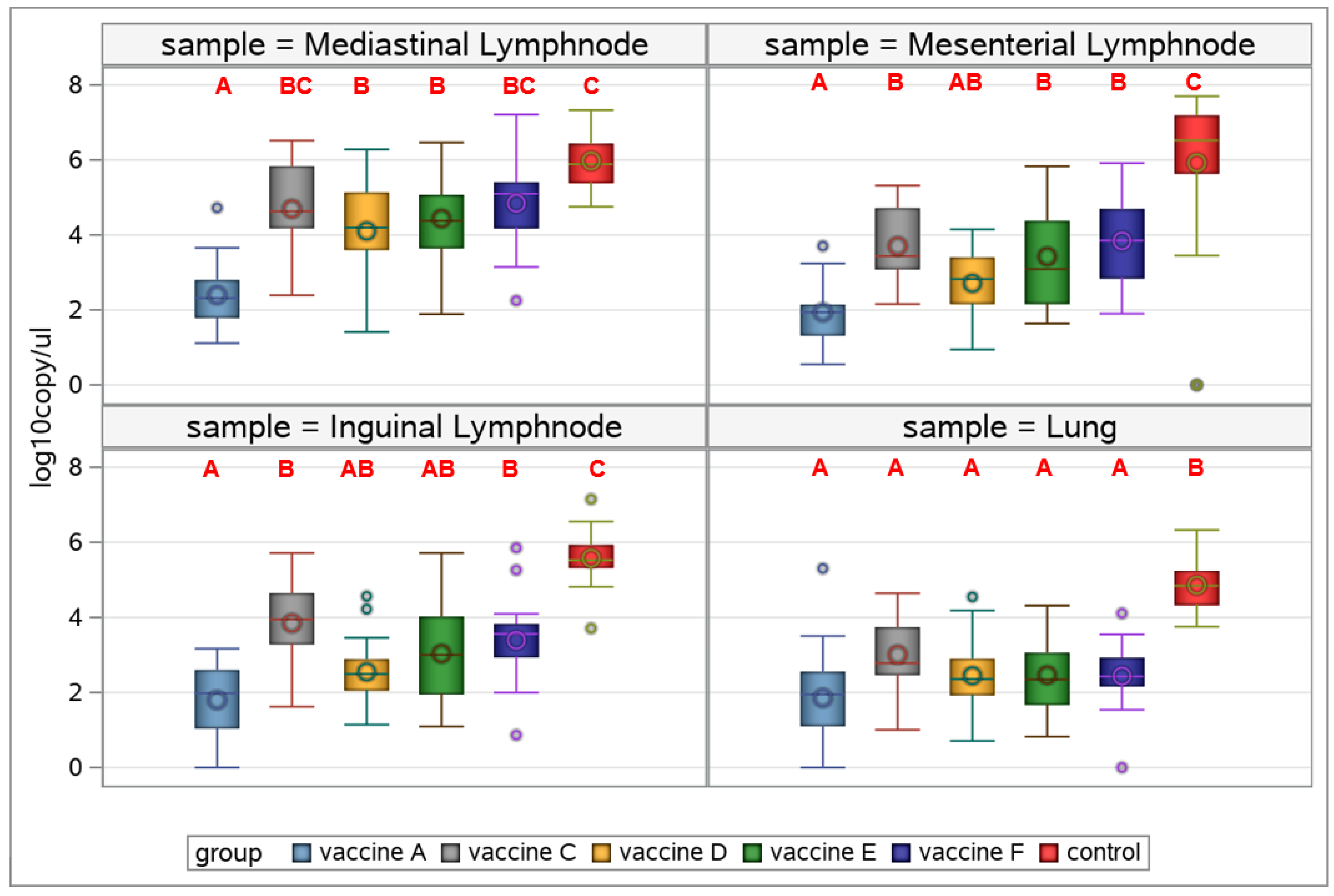
3.4.4. Viral Load in Organ Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EP | Enzootic pneumonia |
| PRDC | Porcine respiratory disease complex |
| PCV2 | Porcine circovirus type 2 |
| PCVAD | Porcine circovirus-associated diseases |
References
- Pallares, F.J.; Anon, J.A.; Rodriguez-Gomez, I.M.; Gomez-Laguna, J.; Fabre, R.; Sanchez-Carvajal, J.M.; Ruedas-Torres, I.; Carrasco, L. Prevalence of mycoplasma-like lung lesions in pigs from commercial farms from Spain and Portugal. Porc. Health Manag. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Assavacheep, P.; Thanawongnuwech, R. Porcine respiratory disease complex: Dynamics of polymicrobial infections and management strategies after the introduction of the African swine fever. Front. Vet. Sci. 2022, 9, 1048861. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Zhang, L.; Zheng, J.; Yang, L.; Niu, G.; Zhang, H.; Ren, Y.; Qian, J.; Sun, C.; et al. Phylogenetic and Structural Analysis of Porcine Circovirus Type 2 from 2016 to 2021 in Jilin Province, China. Microorganisms 2023, 11, 983. [Google Scholar] [CrossRef]
- Pleguezuelos, P.; Sibila, M.; Ramirez, C.; Lopez-Jimenez, R.; Perez, D.; Huerta, E.; Llorens, A.M.; Perez, M.; Correa-Fiz, F.; Mancera Gracia, J.C.; et al. Efficacy Studies against PCV-2 of a New Trivalent Vaccine including PCV-2a and PCV-2b Genotypes and Mycoplasma hyopneumoniae When Administered at 3 Weeks of Age. Vaccines 2022, 10, 2108. [Google Scholar] [CrossRef]
- Poulsen Nautrup, B.; Van Vlaenderen, I.; Mellencamp, M.A. A Chimeric Vaccine against Porcine Circovirus Type 2: Meta-Analysis of Comparative Clinical Trials. Vaccines 2023, 11, 584. [Google Scholar] [CrossRef]
- Puig, A.; Bernal, I.; Sabate, D.; Ballara, I.; Montane, J.; Nodar, L.; Angelats, D.; Jorda, R. Comparison of effects of a single dose of MHYOSPHERE(R) PCV ID with three commercial porcine vaccine associations against Mycoplasma hyopneumoniae (Mhyo) and porcine circovirus type 2 (PCV2) on piglet growth during the nursery period under field conditions. Vet. Res. Commun. 2022, 46, 1167–1173. [Google Scholar] [CrossRef]
- Firth, C.; Charleston, M.A.; Duffy, S.; Shapiro, B.; Holmes, E.C. Insights into the evolutionary history of an emerging livestock pathogen: Porcine circovirus 2. J. Virol. 2009, 83, 12813–12821. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Legnardi, M.; Drigo, M.; Segales, J. An updated phylogeography and population dynamics of porcine circovirus 2 genotypes: Are they reaching an equilibrium? Front. Microbiol. 2024, 15, 1500498. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Qi, J.; Hao, F.; Xu, L.; Guo, K. Genetic Diversity and Prevalence of Porcine Circovirus Type 2 in China During 2000–2019. Front. Vet. Sci. 2021, 8, 788172. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Y.; Sun, W.; Chen, H.; Zhu, D.; Lu, C.; Yin, Y.; Rai, K.R.; Chen, J.L.; Chen, Y. Epidemiology and Genetic Diversity of PCV2 Reveals That PCV2e Is an Emerging Genotype in Southern China: A Preliminary Study. Viruses 2022, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Bandrick, M.; Balasch, M.; Heinz, A.; Taylor, L.; King, V.; Toepfer, J.; Foss, D. A bivalent porcine circovirus type 2 (PCV2), PCV2a-PCV2b, vaccine offers biologically superior protection compared to monovalent PCV2 vaccines. Vet. Res. 2022, 53, 12. [Google Scholar] [CrossRef] [PubMed]
- Brunborg, I.M.; Moldal, T.; Jonassen, C.M. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods 2004, 122, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Szigeti, K.; Homonnay, Z.G.; Tamas, V.; Smits, H.; Krejci, R. Maternally Derived Antibody Levels Influence on Vaccine Protection against PCV2d Challenge. Animals 2021, 11, 2231. [Google Scholar] [CrossRef]
- Palya, V.; Homonnay, Z.G.; Mato, T.; Kiss, I. Characterization of a PCV2d-2 isolate by experimental infection of pigs. Virol. J. 2018, 15, 185. [Google Scholar] [CrossRef]
- Hannan, P.C.; Bhogal, B.S.; Fish, J.P. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonic pig lung homogenate containing mycoplasmas, bacteria and viruses. Res. Vet. Sci. 1982, 33, 76–88. [Google Scholar] [CrossRef]
- Šidák, Z. Rectangular Confidence Regions for the Means of Multivariate Normal Distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
- Cho, H.; Oh, T.; Suh, J.; Chae, C. A Comparative Field Evaluation of the Effect of Growth Performance Between Porcine Circovirus Type 2a (PCV2a)- and PCV2b-Based Bivalent Vaccines Containing PCV2 and Mycoplasma hyopneumoniae. Front. Vet. Sci. 2022, 9, 859344. [Google Scholar] [CrossRef]
- Sibila, M.; Guevara, G.; Cuadrado, R.; Pleguezuelos, P.; Perez, D.; Perez de Rozas, A.; Huerta, E.; Llorens, A.; Valero, O.; Perez, M.; et al. Comparison of Mycoplasma hyopneumoniae and porcine circovirus 2 commercial vaccines efficacy when applied separate or combined under experimental conditions. Porc. Health Manag. 2020, 6, 11. [Google Scholar] [CrossRef]
- Michiels, A.; Arsenakis, I.; Boyen, F.; Krejci, R.; Haesebrouck, F.; Maes, D. Efficacy of one dose vaccination against experimental infection with two Mycoplasma hyopneumoniae strains. BMC Vet. Res. 2017, 13, 274. [Google Scholar] [CrossRef]
- Cvjetkovic, V.; Sipos, S.; Szabo, I.; Sipos, W. Clinical efficacy of two vaccination strategies against Mycoplasma hyopneumoniae in a pig herd suffering from respiratory disease. Porc. Health Manag. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Krejci, R.; Kalina, J.; Kuncova, M. A Meta-Analysis of the Relationship Between Lung Lesion Scores in Slaughter Pigs and their Daily Weight Gain. Int. Anim. Health J. 2022, 9, 24–28. [Google Scholar]
- Fort, M.; Fernandes, L.T.; Nofrarias, M.; Diaz, I.; Sibila, M.; Pujols, J.; Mateu, E.; Segales, J. Development of cell-mediated immunity to porcine circovirus type 2 (PCV2) in caesarean-derived, colostrum-deprived piglets. Vet. Immunol. Immunopathol. 2009, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Xiao, C.T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naive pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Olvera, A.; Sibila, M.; Calsamiglia, M.; Segales, J.; Domingo, M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods 2004, 117, 75–80. [Google Scholar] [CrossRef]
- Grau-Roma, L.; Hjulsager, C.K.; Sibila, M.; Kristensen, C.S.; Lopez-Soria, S.; Enoe, C.; Casal, J.; Botner, A.; Nofrarias, M.; Bille-Hansen, V.; et al. Infection, excretion and seroconversion dynamics of porcine circovirus type 2 (PCV2) in pigs from post-weaning multisystemic wasting syndrome (PMWS) affected farms in Spain and Denmark. Vet. Microbiol. 2009, 135, 272–282. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, K.S.; Hong, K.C.; Kim, J.M. Genetic association of polymorphisms in porcine RGS16 with porcine circovirus viral load in naturally infected Yorkshire pigs. J. Anim. Sci. Technol. 2021, 63, 1223–1231. [Google Scholar] [CrossRef]
- Sousa, K.R.S.; de Melo Ferreira Dantas, W.; de Oliveira, L.L.; Cardoso, S.A.; Dos Santos Araujo, R.; Guimaraes, S.E.F. Effect of vaccination against Mycoplasma hyopneumoniae on divergent pig genetic groups. Res. Vet. Sci. 2024, 180, 105417. [Google Scholar] [CrossRef]



| Group | G1 | G2 (Only in Study A) | G3 | G4 | G5 | G6 | G7 Challenged Control | G8 Negative Unchallenged Control * |
|---|---|---|---|---|---|---|---|---|
| Product name | Cirbloc M Hyo | Hyogen | FLEXcombo | Porcilis PCV M Hyo | CircoMax Myco | Mhyosphere PCV ID | ||
| Vaccine A | Vaccine B | Vaccine C | Vaccine D | Vaccine E | Vaccine F | |||
| Composition | PCV2d capsid protein + inactivated M. hyo strain 2940 | Inactivated M. hyo strain 2940 | PCV-2 recombinant ORF2 protein + Inactivated M. hyo J Strain Isolate B-3745 | PCV2 ORF2 subunit antigen + Inactivated M. hyo J Strain | Inactivated recombinant chimeric PCV1 containing PCV2a and 2b ORF2 subunit antigens, plus inactivated M. hyo strain P-5722-3 | Inactivated recombinant M. hyocpPCV2 strain Nexhyon | PBS | PBS |
| Dose | 2 mL | 2 mL | 2 mL | 2 mL | 2 mL | 0.2 mL | 2 mL | 2 mL |
| Administration route | i.m. | i.m. | i.m. | i.m. | i.m. | i.d. | i.m. | i.m. |
| Animals | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 10 |
| 42 dpi | 49 dpi | 56 dpi | 63 dpi | |
|---|---|---|---|---|
| Vaccine A | 0% | 5% | 15% | 0% |
| Vaccine B | 0% | 4.8% | 9.5% | 0% |
| Vaccine C | 0% | 14.3% | 19% | 0% |
| Vaccine D | 0% | 10% | 15% | 0% |
| Vaccine E | 0% | 14.3% | 14.3% | 0% |
| Vaccine F | 0% | 10% | 15% | 0% |
| Positive control | 0% | 26.3% | 89.5% | 0% |
| Negative control | 0% | 0% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pálmai, N.; Széplaki, N.-Á.; Molnár, B.; Smits, H.; Krejci, R.; Kiss, I. Non-Compromised Efficacy of the First Commercial Ready-to-Use Genotype 2d Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine. Viruses 2025, 17, 554. https://doi.org/10.3390/v17040554
Pálmai N, Széplaki N-Á, Molnár B, Smits H, Krejci R, Kiss I. Non-Compromised Efficacy of the First Commercial Ready-to-Use Genotype 2d Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine. Viruses. 2025; 17(4):554. https://doi.org/10.3390/v17040554
Chicago/Turabian StylePálmai, Nimród, Nikoletta-Ágnes Széplaki, Bálint Molnár, Han Smits, Roman Krejci, and István Kiss. 2025. "Non-Compromised Efficacy of the First Commercial Ready-to-Use Genotype 2d Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine" Viruses 17, no. 4: 554. https://doi.org/10.3390/v17040554
APA StylePálmai, N., Széplaki, N.-Á., Molnár, B., Smits, H., Krejci, R., & Kiss, I. (2025). Non-Compromised Efficacy of the First Commercial Ready-to-Use Genotype 2d Porcine Circovirus Type 2 and Mycoplasma hyopneumoniae Vaccine. Viruses, 17(4), 554. https://doi.org/10.3390/v17040554







