Predictive Factors of Cytomegalovirus Colonic Reactivation in Patients with Active Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
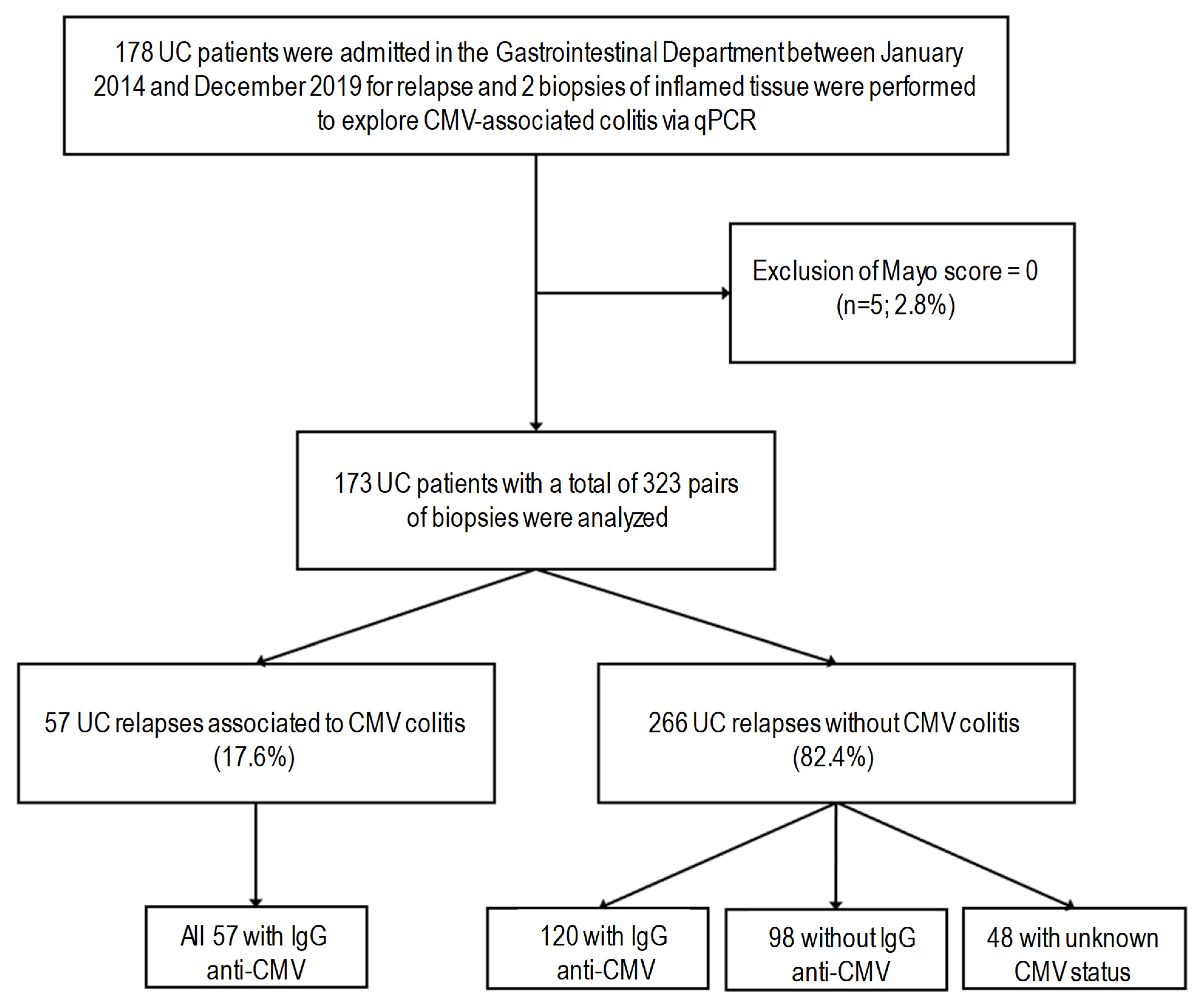
2.1. Study Design and Patients
2.2. CMV qPCR and CMV Serology
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Multivariate Analyses in the CMV Seropositive Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins-McMillen, D.; Buehler, J.; Peppenelli, M.; Goodrum, F. Molecular Determinants and the Regulation of Human Cytomegalovirus Latency and Reactivation. Viruses 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Varani, S.; Landini, M.P. Cytomegalovirus-Induced Immunopathology and Its Clinical Consequences. Herpesviridae 2011, 2, 6. [Google Scholar] [CrossRef]
- Nakase, H.; Honzawa, Y.; Toyonaga, T.; Yamada, S.; Minami, N.; Yoshino, T.; Matsuura, M. Diagnosis and Treatment of Ulcerative Colitis with Cytomegalovirus Infection: Importance of Controlling Mucosal Inflammation to Prevent Cytomegalovirus Reactivation. Intest. Res. 2014, 12, 5–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jentzer, A.; Veyrard, P.; Roblin, X.; Saint-Sardos, P.; Rochereau, N.; Paul, S.; Bourlet, T.; Pozzetto, B.; Pillet, S. Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC). Microorganisms 2020, 8, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hendler, S.A.; Barber, G.E.; Okafor, P.N.; Chang, M.S.; Limsui, D.; Limketkai, B.N. Cytomegalovirus Infection Is Associated with Worse Outcomes in Inflammatory Bowel Disease Hospitalizations Nationwide. Int. J. Colorectal. Dis. 2020, 35, 897–903. [Google Scholar] [CrossRef]
- Roblin, X.; Pillet, S.; Oussalah, A.; Berthelot, P.; Del Tedesco, E.; Phelip, J.-M.; Chambonnière, M.-L.; Garraud, O.; Peyrin-Biroulet, L.; Pozzetto, B. Cytomegalovirus Load in Inflamed Intestinal Tissue Is Predictive of Resistance to Immunosuppressive Therapy in Ulcerative Colitis. Am. J. Gastroenterol. 2011, 106, 2001–2008. [Google Scholar] [CrossRef]
- Pillet, S.; Pozzetto, B.; Jarlot, C.; Paul, S.; Roblin, X. Management of Cytomegalovirus Infection in Inflammatory Bowel Diseases. Dig. Liver Dis. 2012, 44, 541–548. [Google Scholar] [CrossRef]
- Pillet, S. Cytomegalovirus and Ulcerative Colitis: Place of Antiviral Therapy. World J. Gastroenterol. 2016, 22, 2030. [Google Scholar] [CrossRef]
- Nguyen, M.; Bradford, K.; Zhang, X.; Shih, D.Q. Cytomegalovirus Reactivation in Ulcerative Colitis Patients. Ulcers 2011, 2011, 282507. [Google Scholar] [CrossRef]
- Domènech, E.; Vega, R.; Ojanguren, I.; Hernández, A.; Garcia-Planella, E.; Bernal, I.; Rosinach, M.; Boix, J.; Cabré, E.; Gassull, M.A. Cytomegalovirus Infection in Ulcerative Colitis: A Prospective, Comparative Study on Prevalence and Diagnostic Strategy. Inflamm. Bowel. Dis. 2008, 14, 1373–1379. [Google Scholar] [CrossRef]
- Grossberg, L.B.; Ezaz, G.; Grunwald, D.; Cohen, J.; Falchuk, K.R.; Feuerstein, J.D. A National Survey of the Prevalence and Impact of Cytomegalovirus Infection among Hospitalized Patients with Ulcerative Colitis. J. Clin. Gastroenterol. 2018, 52, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, S1–S106. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef] [PubMed]
- Tun, G.S.Z.; Raza, M.; Hale, M.F.; Lobo, A.J. Polymerase Chain Reaction for Detection of Mucosal Cytomegalovirus Infection in Patients with Acute Ulcerative Colitis. Ann. Gastroenterol. 2019, 32, 81–87. [Google Scholar] [CrossRef]
- Hirayama, Y.; Ando, T.; Hirooka, Y.; Watanabe, O.; Miyahara, R.; Nakamura, M.; Yamamura, T.; Goto, H. Characteristic Endoscopic Findings and Risk Factors for Cytomegalovirus-Associated Colitis in Patients with Active Ulcerative Colitis. World J. Gastrointest. Endosc. 2016, 8, 301–309. [Google Scholar] [CrossRef]
- Kishore, J.; Ghoshal, U.; Ghoshal, U.C.; Krishnani, N.; Kumar, S.; Singh, M.; Ayyagari, A. Infection with Cytomegalovirus in Patients with Inflammatory Bowel Disease: Prevalence, Clinical Significance and Outcome. J. Med. Microbiol. 2004, 53, 1155–1160. [Google Scholar] [CrossRef]
- Yang, H.; Wu, K.; Zhang, H.; Owyang, Q.; Miao, Y.; Gu, F.; Hu, N.; Zou, K.; Sheng, J.; Li, J.; et al. IgA, Albumin, and Eosinopenia as Early Indicators of Cytomegalovirus Infection in Patients with Acute Ulcerative Colitis. BMC Gastroenterol. 2020, 20, 294. [Google Scholar] [CrossRef]
- Clos-Parals, A.; Rodríguez-Martínez, P.; Cañete, F.; Mañosa, M.; Ruiz-Cerulla, A.; José Paúles, M.; Llaó, J.; Gordillo, J.; Fumagalli, C.; Garcia-Planella, E.; et al. Prognostic Value of the Burden of Cytomegalovirus Colonic Reactivation Evaluated by Immunohistochemical Staining in Patients with Active Ulcerative Colitis. J. Crohns Colitis 2019, 13, 385–388. [Google Scholar] [CrossRef]
- Lee, H.-S.; Park, S.H.; Kim, S.-H.; Kim, J.; Choi, J.; Lee, H.J.; Kim, W.S.; Lee, J.-M.; Kwak, M.S.; Hwang, S.W.; et al. Risk Factors and Clinical Outcomes Associated with Cytomegalovirus Colitis in Patients with Acute Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 912–918. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Bettenworth, D.; Meister, T.; Heidemann, J.; Lenze, F.; Schmidt, H.H.; Heinzow, H.S. Novel Score Predicts Risk for Cytomegalovirus Infection in Ulcerative Colitis. J. Clin. Virol. 2018, 105, 103–108. [Google Scholar] [CrossRef]
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic Scoring Indices for Evaluation of Disease Activity in Ulcerative Colitis. Cochrane Database Syst. Rev. 2018, 1, CD011450. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, J.D.; Jones, A.; Enders, F.T.; Killian, J.M.; Loftus, E.V.; Smyrk, T.C.; Bruining, D.H. A Model for Identifying Cytomegalovirus in Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Olaisen, M.; Rydning, A.; Martinsen, T.C.; Nordrum, I.S.; Mjønes, P.; Fossmark, R. Cytomegalovirus Infection and Postoperative Complications in Patients with Ulcerative Colitis Undergoing Colectomy. Scand. J. Gastroenterol. 2014, 49, 845–852. [Google Scholar] [CrossRef]
- Maconi, G.; Colombo, E.; Zerbi, P.; Sampietro, G.M.; Fociani, P.; Bosani, M.; Cassinotti, A.; Casini, V.; Russo, A.; Ardizzone, S.; et al. Prevalence, Detection Rate and Outcome of Cytomegalovirus Infection in Ulcerative Colitis Patients Requiring Colonic Resection. Dig. Liver Dis. 2005, 37, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Okamoto, H.; Suda, T.; Ajioka, Y.; Hatakeyama, K. Clinicopathologic Characteristics of Clinically Relevant Cytomegalovirus Infection in Inflammatory Bowel Disease. J. Gastroenterol. 2007, 42, 823–829. [Google Scholar] [CrossRef]
- Kopylov, U.; Papamichael, K.; Katsanos, K.; Waterman, M.; Bar-Gil Shitrit, A.; Boysen, T.; Portela, F.; Peixoto, A.; Szilagyi, A.; Silva, M.; et al. Impact of Infliximab and Cyclosporine on the Risk of Colectomy in Hospitalized Patients with Ulcerative Colitis Complicated by Cytomegalovirus-A Multicenter Retrospective Study. Inflamm. Bowel Dis. 2017, 23, 1605–1613. [Google Scholar] [CrossRef]
- Nakase, H.; Chiba, T. TNF-Alpha Is an Important Pathogenic Factor Contributing to Reactivation of Cytomegalovirus in Inflamed Mucosa of Colon in Patients with Ulcerative Colitis: Lesson from Clinical Experience. Inflamm. Bowel Dis. 2010, 16, 550–551. [Google Scholar] [CrossRef]
- Pillet, S.; Jarlot, C.; Courault, M.; Del Tedesco, E.; Chardon, R.; Saint-Sardos, P.; Presles, E.; Phelip, J.-M.; Berthelot, P.; Pozzetto, B.; et al. Infliximab Does Not Worsen Outcomes During Flare-Ups Associated with Cytomegalovirus Infection in Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2015, 21, 1580–1586. [Google Scholar] [CrossRef]
- Zagórowicz, E.; Bugajski, M.; Wieszczy, P.; Pietrzak, A.; Magdziak, A.; Mróz, A. Cytomegalovirus Infection in Ulcerative Colitis Is Related to Severe Inflammation and a High Count of Cytomegalovirus-Positive Cells in Biopsy Is a Risk Factor for Colectomy. J. Crohns Colitis 2016, 10, 1205–1211. [Google Scholar] [CrossRef]
- Gauss, A.; Rosenstiel, S.; Schnitzler, P.; Hinz, U.; Rehlen, T.; Kadmon, M.; Ehehalt, R.; Stremmel, W.; Zawierucha, A. Intestinal Cytomegalovirus Infection in Patients Hospitalized for Exacerbation of Inflammatory Bowel Disease: A 10-Year Tertiary Referral Center Experience. Eur. J. Gastroenterol. Hepatol. 2015, 27, 712–720. [Google Scholar] [CrossRef]
- Campos, S.T.; Portela, F.A.; Tomé, L. Cytomegalovirus, Inflammatory Bowel Disease, and Anti-TNFα. Int. J. Colorectal. Dis. 2017, 32, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Tosca, J.; Garcia, N.; Pascual, I.; Bosca-Watts, M.M.; Anton, R.; Sanahuja, A.; Mas, P.; Mora, F.; Minguez, M. Clinical Assessment of Risk Factors for Infection in Inflammatory Bowel Disease Patients. Int. J. Colorectal. Dis. 2020, 35, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Pillet, S.; Berthelot, P.; Del Tedesco, E.; Phelip, J.-M.; Chambonnière, M.-L.; Peyrin-Biroulet, L.; Pozzetto, B. Prevalence of Cytomegalovirus Infection in Steroid-Refractory Crohn’s Disease. Inflamm. Bowel Dis. 2012, 18, E1396–E1397. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Williet, N.; Pouvaret, A.; Del Tedesco, E.; Saint-Sardos, P.; Pozzetto, B.; Roblin, X. Distribution of Cytomegalovirus DNA Load in the Inflamed Colon of Ulcerative Colitis Patients. Am. J. Gastroenterol. 2016, 111, 439–441. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Roda, G.; Narula, N.; Pinotti, R.; Skamnelos, A.; Katsanos, K.H.; Ungaro, R.; Burisch, J.; Torres, J.; Colombel, J.-F. Systematic Review with Meta-Analysis: Proximal Disease Extension in Limited Ulcerative Colitis. Aliment. Pharmacol. Ther. 2017, 45, 1481–1492. [Google Scholar] [CrossRef]
- Gugliesi, F.; Coscia, A.; Griffante, G.; Galitska, G.; Pasquero, S.; Albano, C.; Biolatti, M. Where Do We Stand after Decades of Studying Human Cytomegalovirus? Microorganisms 2020, 8, 685. [Google Scholar] [CrossRef]
- Suzuki, H.; Kato, J.; Kuriyama, M.; Hiraoka, S.; Kuwaki, K.; Yamamoto, K. Specific Endoscopic Features of Ulcerative Colitis Complicated by Cytomegalovirus Infection. World J. Gastroenterol. 2010, 16, 1245–1251. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Bettenworth, D.; Ross, M.; Heidemann, J.; Lehmann, P.V.; Lügering, A. Cytomegalovirus (CMV)-Specific Perforin and Granzyme B ELISPOT Assays Detect Reactivation of CMV Infection in Inflammatory Bowel Disease. Cells 2012, 1, 35–50. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Mengoli, C.; Betti, E.; Comolli, G.; Cassaniti, I.; Piralla, A.; Kruzliak, P.; Caprnda, M.; Pozzi, L.; Corazza, G.R.; et al. Human Cytomegalovirus and Epstein-Barr Virus Specific Immunity in Patients with Ulcerative Colitis. Clin. Exp. Med. 2021, 21, 379–388. [Google Scholar] [CrossRef]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Baba, R.; Herbein, G. Immune Landscape of CMV Infection in Cancer Patients: From “Canonical” Diseases Toward Virus-Elicited Oncomodulation. Front. Immunol. 2021, 12, 730765. [Google Scholar] [CrossRef]
| Variable | CMV DNA Load in Biopsy (IU/100,000 Cells) | p-Value | ||
|---|---|---|---|---|
| ≤5 | 6–375 | >375 | ||
| Pair of biopsies: number | 120 | 25 | 32 | - |
| Gender: number of males (%) | 51 (42.5) | 11 (44) | 9 (28.1) | 0.308 |
| Age at biopsy in years: mean (SD) | 45.45 (18.49) | 46.6 (19.35) | 47.75 (15.35) | 0.804 |
| Duration of the disease at the biopsy: mean (SD) | 37.58 (17.37) | 38.8 (18.96) | 41.16 (15.72) | 0.579 |
| Disease description Number of relapses per patient during the study *: number (%) | 0.504 | |||
| 1 | 56 (46.7) | 13 (52) | 16 (50) | |
| 2 | 29 (24.2) | 3 (12) | 11 (34.4) | |
| 3 | 20 (16.7) | 3 (12) | 1 (3.1) | |
| >3 | 14 (12.4) | 6 (24) | 4 (12.5) | |
| Mayo endoscopic score: number (%) | 0.014 | |||
| 1 | 24 (20) | 2 (8) | 2 (6.2) | |
| 2 | 47 (39.2) | 6 (24) | 8 (25) | |
| 3 | 49 (40.8) | 17 (68) | 22 (68.8) | |
| Presence of ulcers: number (%) | 50 (41.7) | 16 (64) | 23 (71.9) | 0.003 |
| Montreal classification: number (%) | 0.453 | |||
| E1 (proctitis) | 19 (15.8) | 4 (16) | 1 (3.1) | |
| E2 (left-sided colitis) | 47 (39.2) | 10 (40) | 14 (43.8) | |
| E3 (pancolitis) | 54 (45) | 11 (44) | 17 (53.1) | |
| Viral load expressed as IU/100,000 cells: mean (SD) | 0.46 (1.18) | 56.36 (68.57) | 14,990.47 (31,785.28) | <0.001 |
| Therapeutic used at the time of flare-up: number (%) | ||||
| Steroid dependence | 45 (37.5) | 9 (36) | 15 (46.9) | 0.594 |
| Steroid refractory | 16 (13.3) | 3 (12) | 5 (15.6) | 0.917 |
| 5-ASA | 21 (17.5) | 7 (28) | 5 (15.6) | 0.419 |
| Purine synthesis inhibitors | 10 (8.3) | 1 (4) | 5 (15.6) | 0.282 |
| Anti-TNFα monoclonal therapy | 54 (45) | 7 (28) | 13 (40.6) | 0.289 |
| Anti-integrin monoclonal therapy | 23 (19.2) | 6 (24) | 2 (6.2) | 0.152 |
| Biological parameters: mean (SD) | ||||
| Hemoglobin (g/dL) | 13.29 (2.08) (n = 87) | 12.54 (1.67) (n = 20) | 12.92 (2.1) (n = 26) | 0.300 |
| White blood cells (109/L) | 8.36 (2.98) (n = 87) | 7.73 (1.87) (n = 20) | 9.59 (2.1) (n = 25) | 0.054 |
| Lymphocyte (109/L) | 2.16 (0.7) (n = 88) | 1.91 (0.62) (n = 20) | 2.49 (0.91) (n = 25) | 0.031 |
| Neutrophils (109/L) | 5.3 (2.79) (n = 87) | 4.86 (1.81) (n = 20) | 3.1 (1.98) (n = 25) | 0.232 |
| Eosinophils (109/L) | 0.19 (0.2) (n = 80) | 0.2 (0.21) (n = 19) | 0.14 (0.22) (n = 24) | 0.467 |
| Platelets (109/L) | 322.88 (122.75) (n = 80) | 343.47 (132.47) (n = 19) | 313.46 (85.58) (n = 24) | 0.700 |
| CRP (mg/L) | 15.11 (30.01) (n = 87) | 17.99 (18.13) (n = 20) | 17.52 (18.17) (n = 24) | 0.889 |
| ASAT (IU/L) | 23.87 (11.9) (n = 86) | 20 (9.98) (n = 20) | 24.25 (10.79) (n = 24) | 0.363 |
| ALAT (IU/L) | 22.26 (17.36) (n = 87) | 22.4 (28.53) (n = 20) | 26.4 (18.74) (n = 25) | 0.643 |
| Alkaline phosphatase (IU/L) | 74.09 (27.62) (n = 86) | 77.95 (28.85) (n = 20) | 75.26 (46.54) (n = 23) | 0.887 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Mayo endoscopic score ≥ 2 | 2.553 | 1.353–4.818 | 0.004 |
| Anti-TNFα monoclonal antibodies | 0.384 | 0.158–0.935 | 0.035 |
| Anti-integrin monoclonal antibodies | 0.359 | 0.111–1.156 | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jentzer, A.; Cantais, A.; Roblin, X.; Barrau, M.; Garcin, A.; Bourlet, T.; Pozzetto, B.; Pillet, S. Predictive Factors of Cytomegalovirus Colonic Reactivation in Patients with Active Ulcerative Colitis. Viruses 2025, 17, 555. https://doi.org/10.3390/v17040555
Jentzer A, Cantais A, Roblin X, Barrau M, Garcin A, Bourlet T, Pozzetto B, Pillet S. Predictive Factors of Cytomegalovirus Colonic Reactivation in Patients with Active Ulcerative Colitis. Viruses. 2025; 17(4):555. https://doi.org/10.3390/v17040555
Chicago/Turabian StyleJentzer, Alexandre, Aymeric Cantais, Xavier Roblin, Mathilde Barrau, Arnauld Garcin, Thomas Bourlet, Bruno Pozzetto, and Sylvie Pillet. 2025. "Predictive Factors of Cytomegalovirus Colonic Reactivation in Patients with Active Ulcerative Colitis" Viruses 17, no. 4: 555. https://doi.org/10.3390/v17040555
APA StyleJentzer, A., Cantais, A., Roblin, X., Barrau, M., Garcin, A., Bourlet, T., Pozzetto, B., & Pillet, S. (2025). Predictive Factors of Cytomegalovirus Colonic Reactivation in Patients with Active Ulcerative Colitis. Viruses, 17(4), 555. https://doi.org/10.3390/v17040555








