Effects of Concurrent Administration of BVDV Modified Live Viral Vaccine and RB51 on Immune Responses in Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Vaccination and Sampling
2.2. BVDV Strains
2.3. Virus Neutralization Titers (VNT) to BVDV
2.4. Evaluation of CMI Responses to BVDV Vaccination via PrimeFlow RNA Assay
2.5. Statistical Analysis
3. Results
3.1. BVDV-Specific Virus Neutralizing Titers (VNT)
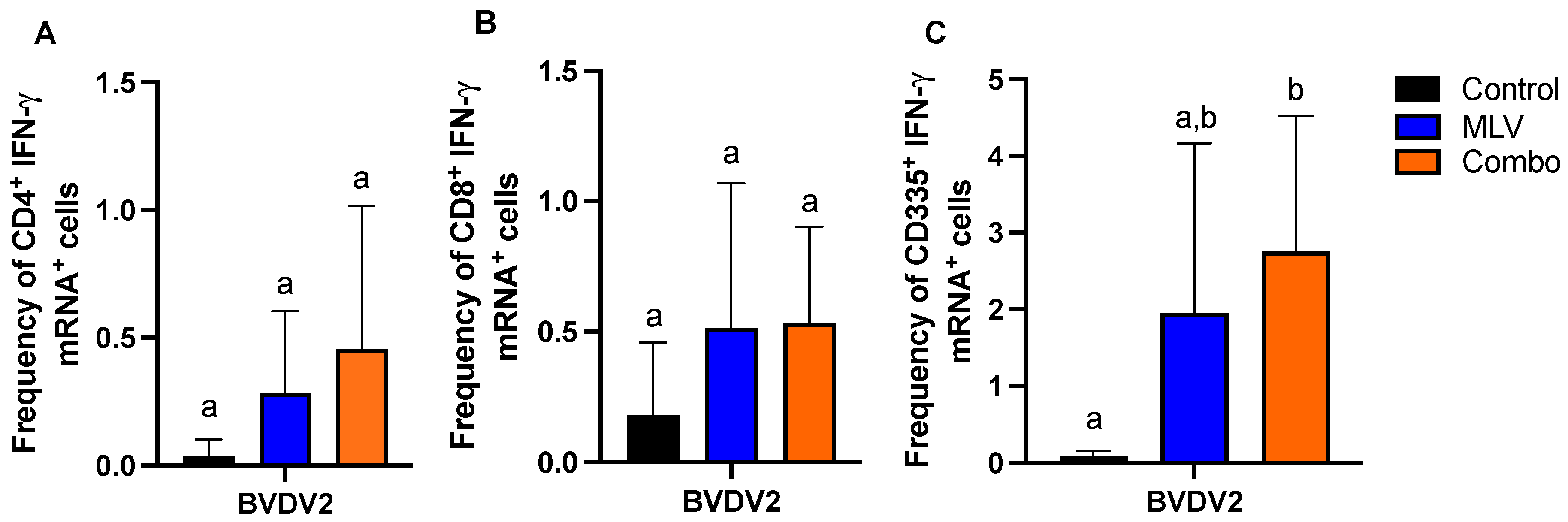
3.2. Evaluation of BVDV-Specific Cell-Mediated Responses
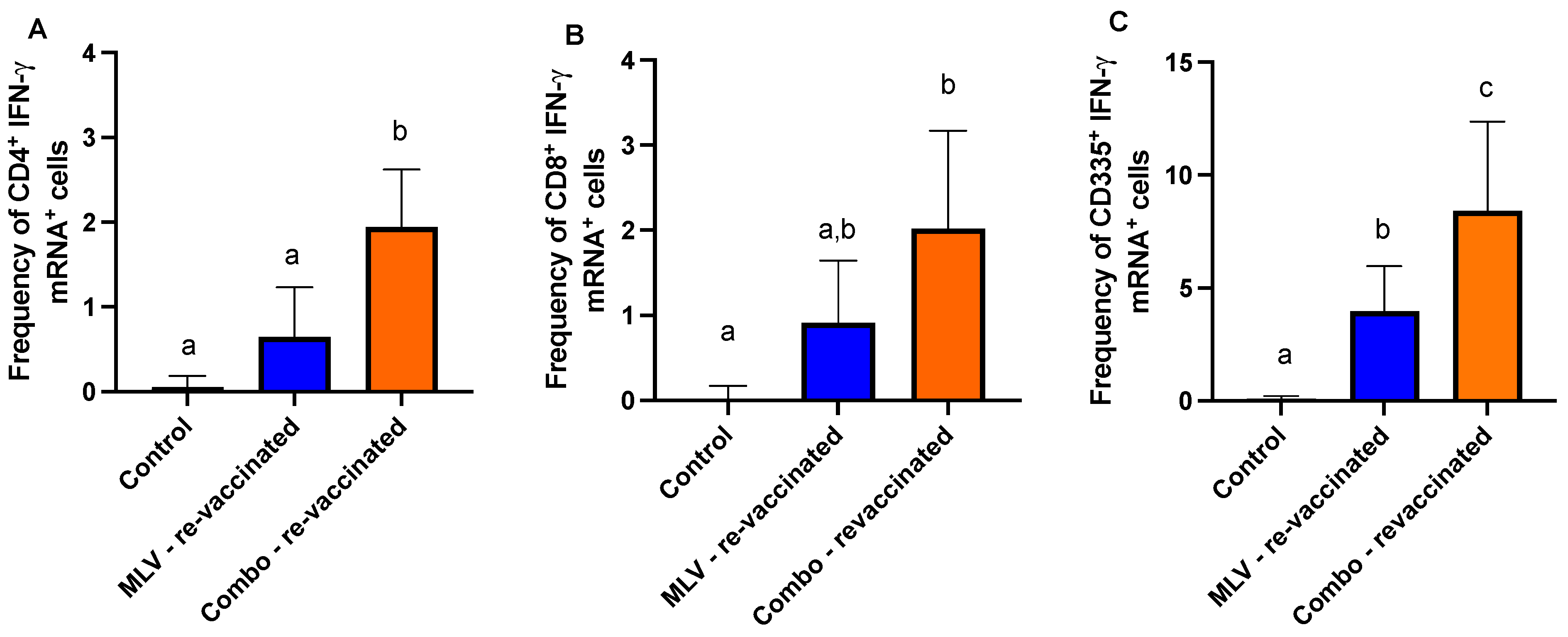
3.3. Evaluation of BVDV-Specific Humoral and Cell-Mediated Responses Following MLV Re-Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BVDV | Bovine viral diarrhea virus |
| RB51 | Brucella abortus strain RB51 |
| PBMC | Peripheral blood mononuclear cells |
| MLV | Modified live vaccine |
| IFN-γ | Interferon gamma |
| TH1 | T helper 1 |
| MDBK | Madin–Darby bovine kidney cells |
| VNT | Virus neutralization titers |
| CPE | Cytopathic effect |
| MOI | Multiplicity of infection |
References
- Al-Kubati, A.A.G.; Hussen, J.; Kandeel, M.; Al-Mubarak, A.I.A.; Hemida, M.G. Recent Advances on the Bovine Viral Diarrhea Virus Molecular Pathogenesis, Immune Response, and Vaccines Development. Front. Vet. Sci. 2021, 8, 665128. [Google Scholar] [CrossRef] [PubMed]
- Harland, R.J.; Potter, A.A.; van Drunen-Littel-van den Hurk, S.; Van Donkersgoed, J.; Parker, M.D.; Zamb, T.J.; Janzen, E.D. The effect of subunit or modified live bovine herpesvirus-1 vaccines on the efficacy of a recombinant Pasteurella haemolytica vaccine for the prevention of respiratory disease in feedlot calves. Can. Vet. J. 1992, 33, 734–741. [Google Scholar]
- Downey-Slinker, E.D.; Ridpath, J.F.; Sawyer, J.E.; Skow, L.C.; Herring, A.D. Antibody titers to vaccination are not predictive of level of protection against a BVDV type 1 b challenge in Bos indicus—Bos taurus steers. Vaccine 2016, 34, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Walz, P.H.; Riddell, K.P.; Newcomer, B.W.; Neill, J.D.; Falkenberg, S.M.; Cortese, V.S.; Scruggs, D.W.; Short, T.H. Comparison of reproductive protection against bovine viral diarrhea virus provided by multivalent viral vaccines containing inactivated fractions of bovine viral diarrhea virus 1 and 2. Vaccine 2018, 36, 3853–3860. [Google Scholar] [CrossRef]
- Falkenberg, S.M.; Dassanayake, R.P.; Neill, J.D.; Walz, P.H.; Casas, E.; Ridpath, J.F.; Roth, J. Measuring CMI responses using the PrimeFlow RNA assay: A new method of evaluating BVDV vaccination response in cattle. Vet. Immunol. Immunopathol. 2020, 221, 110024. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Immunology of BVDV vaccines. Biologicals 2013, 41, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Neill, J.D.; Endsley, J.; Roth, J.A. Effect of passive immunity on the development of a protective immune response against bovine viral diarrhea virus in calves. Am. J. Vet. Res. 2003, 64, 65–69. [Google Scholar] [CrossRef]
- Van Anne, T.R.; Rinehart, C.L.; Buterbaugh, R.E.; Bauer, M.J.; Young, A.J.; Blaha, M.L.; Klein, A.L.; Chase, C.C.L. Cell-mediated and humoral immune responses to bovine herpesvirus type 1 and bovine viral diarrhea virus in calves following administration of a killed-virus vaccine and bovine herpesvirus type 1 challenge. Am. J. Vet. Res. 2018, 79, 1166–1178. [Google Scholar] [CrossRef]
- Crawford, L.; Falkenberg, S.; Putz, E.J.; Olsen, S.; Boggiatto, P.M. Effects of concurrent administration of modified live viral vaccines with RB51 on immune responses to RB51. Front. Vet. Sci. 2023, 10, 1105485. [Google Scholar] [CrossRef]
- Falkenberg, S.M.; Dassanayake, R.P.; Terhaar, B.; Ridpath, J.F.; Neill, J.D.; Roth, J.A. Evaluation of Antigenic Comparisons Among BVDV Isolates as it Relates to Humoral and Cell Mediated Responses. Front. Vet. Sci. 2021, 8, 685114. [Google Scholar] [CrossRef]
- Mosena, A.C.S.; Falkenberg, S.M.; Ma, H.; Casas, E.; Dassanayake, R.P.; Walz, P.H.; Canal, C.W.; Neill, J.D. Multivariate analysis as a method to evaluate antigenic relationships between BVDV vaccine and field strains. Vaccine 2020, 38, 5764–5772. [Google Scholar] [CrossRef] [PubMed]
- Bauermann, F.V.; Flores, E.F.; Falkenberg, S.M.; Weiblen, R.; Ridpath, J.F. Lack of evidence for the presence of emerging HoBi-like viruses in North American fetal bovine serum lots. J. Vet. Diagn. Investig. 2014, 26, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Walz, P.H.; Givens, M.D.; Rodning, S.P.; Riddell, K.P.; Brodersen, B.W.; Scruggs, D.; Short, T.; Grotelueschen, D. Evaluation of reproductive protection against bovine viral diarrhea virus and bovine herpesvirus-1 afforded by annual revaccination with modified-live viral or combination modified-live/killed viral vaccines after primary vaccination with modified-live viral vaccine. Vaccine 2017, 35, 1046–1054. [Google Scholar]
- Falkenberg, S.M.; Dassanayake, R.P.; Palmer, M.V.; Silveira, S.; Roth, J.A.; Gauger, E.; Kaiser, T.J.; Guidarini, C.; Neill, J.D.; Ridpath, J.F. Changes in circulating lymphocytes and lymphoid tissue associated with vaccination of colostrum deprived calves. Vaccine 2020, 38, 7268–7277. [Google Scholar] [CrossRef]
- Cheville, N.F.; Jensen, A.E.; Halling, S.M.; Tatum, F.M.; Morfitt, D.C.; Hennager, S.G.; Frerichs, W.M.; Schurig, G. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am. J. Vet. Res. 1992, 53, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.C.; Bricker, B.; Palmer, M.V.; Jensen, A.E.; Cheville, N.F. Responses of cattle to two dosages of Brucella abortus strain RB51: Serology, clearance and efficacy. Res. Vet. Sci. 1999, 66, 101–105. [Google Scholar] [CrossRef]
- Boysen, P.; Storset, A.K. Bovine natural killer cells. Vet. Immunol. Immunopathol. 2009, 130, 163–177. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Mahan, S.; Entrican, G.; Hope, J.C. Interactions between natural killer cells and dendritic cells favour T helper1-type responses to BCG in calves. Vet. Res. 2016, 47, 2–10. [Google Scholar] [CrossRef]
- Goodier, M.R.; Riley, E.M. Regulation of the human NK cell compartment by pathogens and vaccines. Clin. Transl. Immunol. 2021, 10, e1244. [Google Scholar] [CrossRef]
- Rydyznski, C.E.; Waggoner, S.N. Boosting vaccine efficacy the natural (killer) way. Trends Immunol. 2015, 36, 536–546. [Google Scholar] [CrossRef]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.; Gomes, A.; Reeves, M.; Male, V. NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development. Vaccines 2020, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Cevik, H.; Feldman, H.A.; Canaday, L.M.; Lakes, N.; Waggoner, S.N. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol. Sci. 2021, 42, 789–801. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, L.S.; Falkenberg, S.; Olsen, S.C.; Boggiatto, P.M. Effects of Concurrent Administration of BVDV Modified Live Viral Vaccine and RB51 on Immune Responses in Cattle. Viruses 2025, 17, 553. https://doi.org/10.3390/v17040553
Crawford LS, Falkenberg S, Olsen SC, Boggiatto PM. Effects of Concurrent Administration of BVDV Modified Live Viral Vaccine and RB51 on Immune Responses in Cattle. Viruses. 2025; 17(4):553. https://doi.org/10.3390/v17040553
Chicago/Turabian StyleCrawford, Lauren S., Shollie Falkenberg, Steven C. Olsen, and Paola M. Boggiatto. 2025. "Effects of Concurrent Administration of BVDV Modified Live Viral Vaccine and RB51 on Immune Responses in Cattle" Viruses 17, no. 4: 553. https://doi.org/10.3390/v17040553
APA StyleCrawford, L. S., Falkenberg, S., Olsen, S. C., & Boggiatto, P. M. (2025). Effects of Concurrent Administration of BVDV Modified Live Viral Vaccine and RB51 on Immune Responses in Cattle. Viruses, 17(4), 553. https://doi.org/10.3390/v17040553







