A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens
Abstract
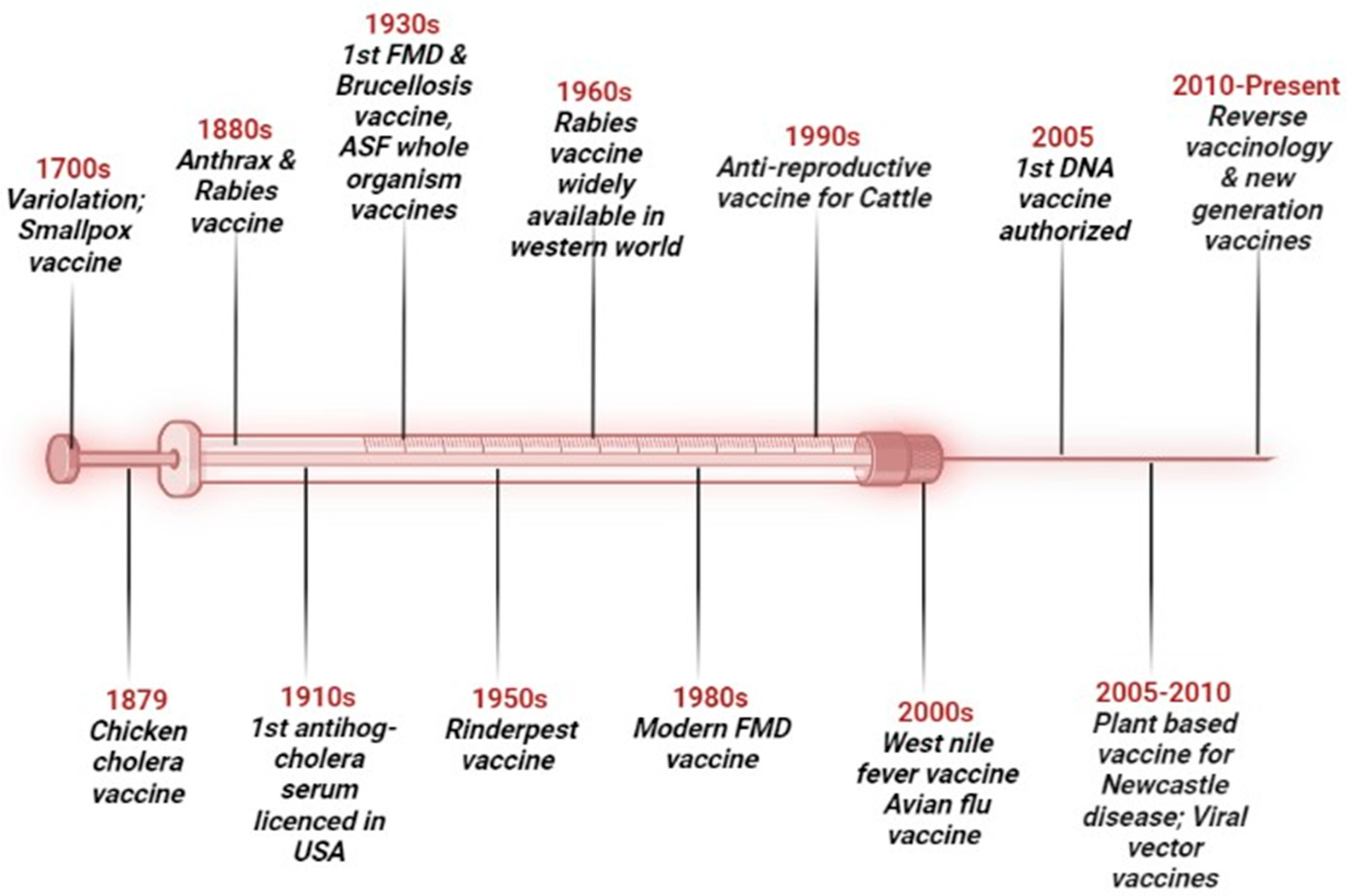
1. Introduction
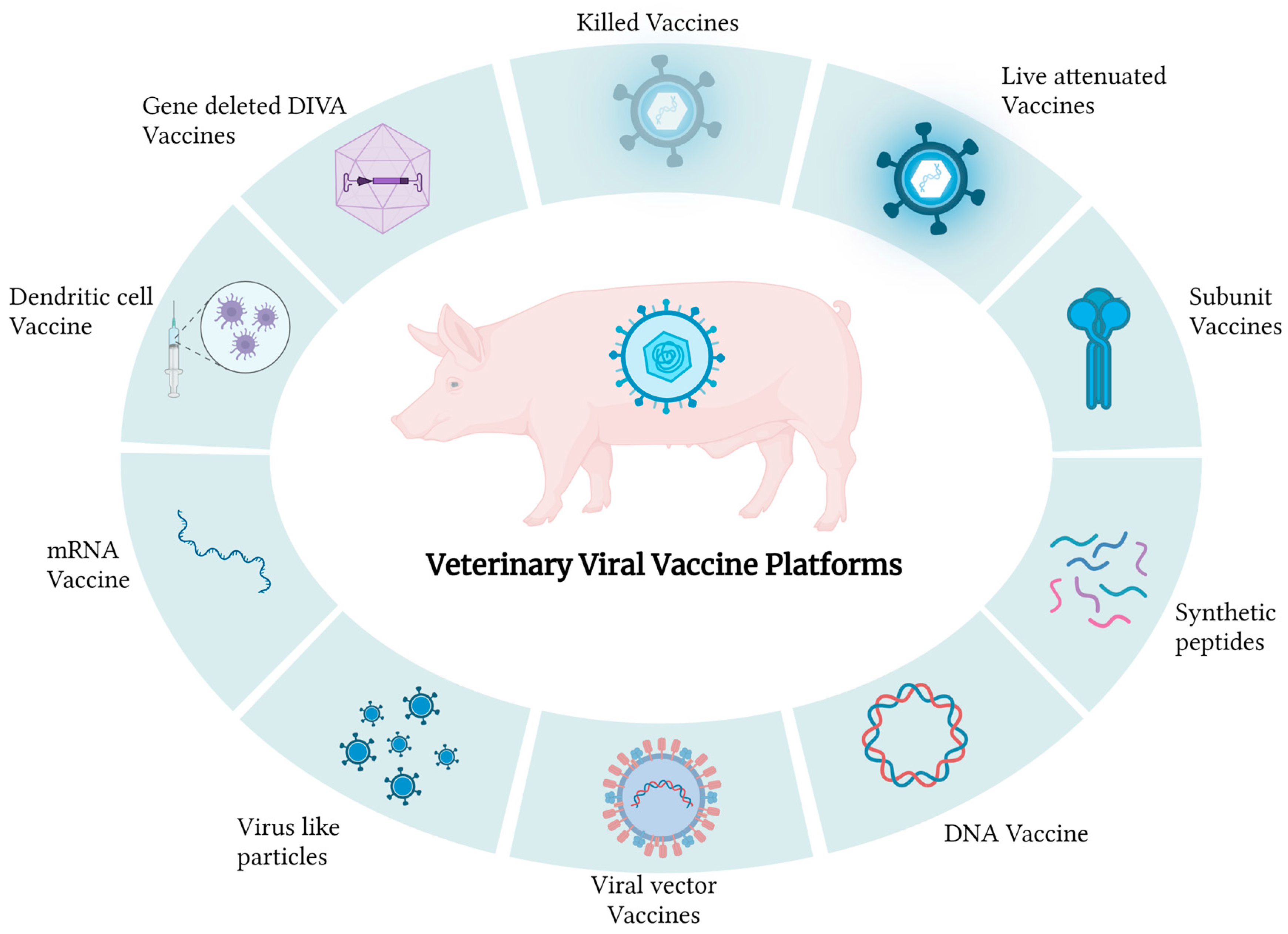
2. Types of Vaccines
3. Whole-Organism Vaccines
3.1. Conventional Killed/Inactivated Vaccines
3.2. Live/Attenuated Vaccines
3.3. Live Attenuated DIVA Vaccines
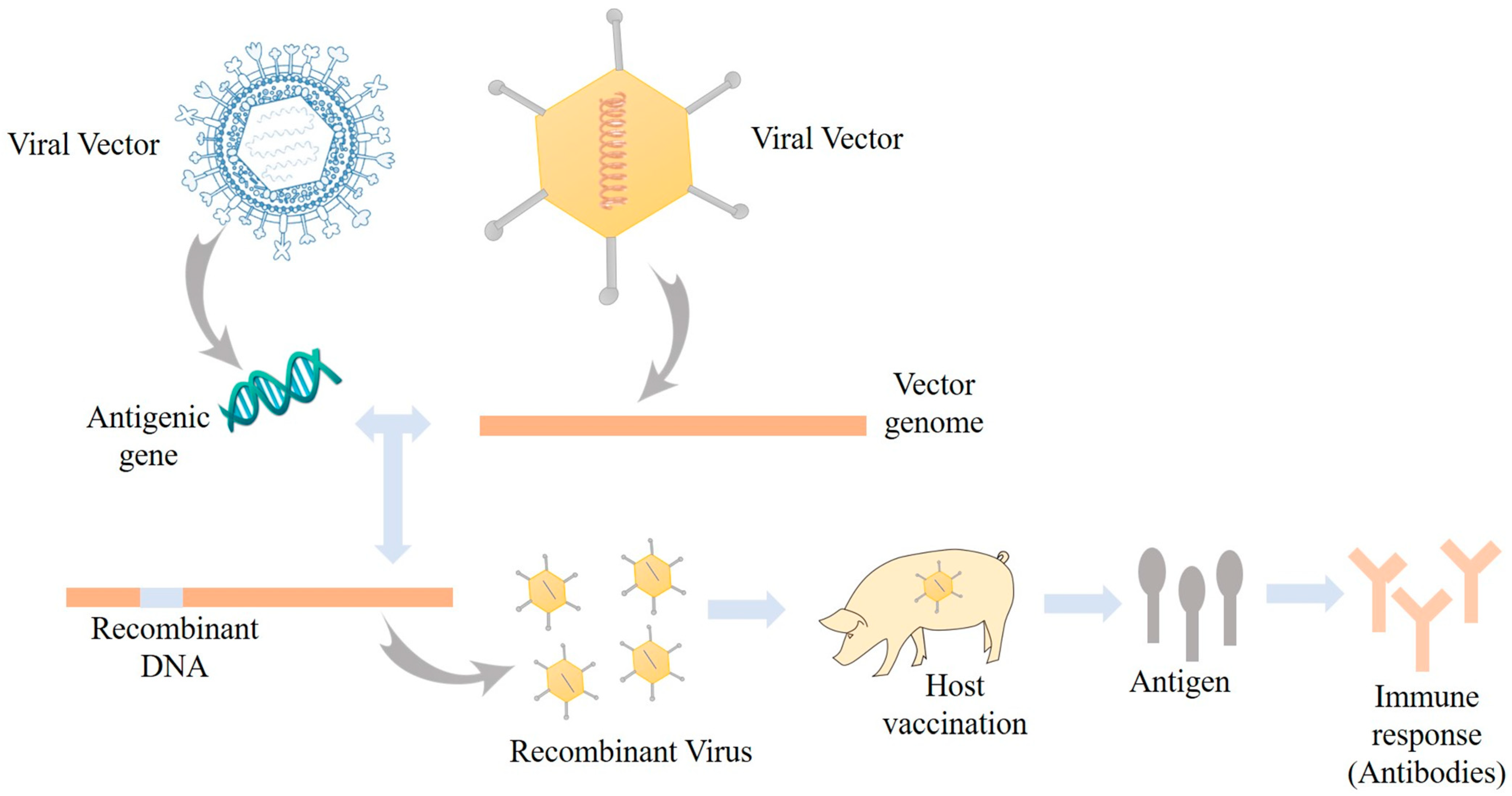
3.4. Viral Vectored Vaccine
4. Subunit Vaccines
4.1. Purified Antigens
4.2. Recombinant Proteins
4.3. Synthetic Peptides
5. Nucleic-Acid-Based Vaccines
5.1. DNA Vaccines
5.2. mRNA Vaccines
6. Emerging New-Generation Vaccine Technologies
6.1. Virosomes and Virus-like Particles (VLPs)
6.2. Mucosal Vaccines
6.3. Dendritic Cell Vaccine
6.4. Multivalent and Polyvalent Vaccines
7. Reverse Genetics and Personalized Vaccines
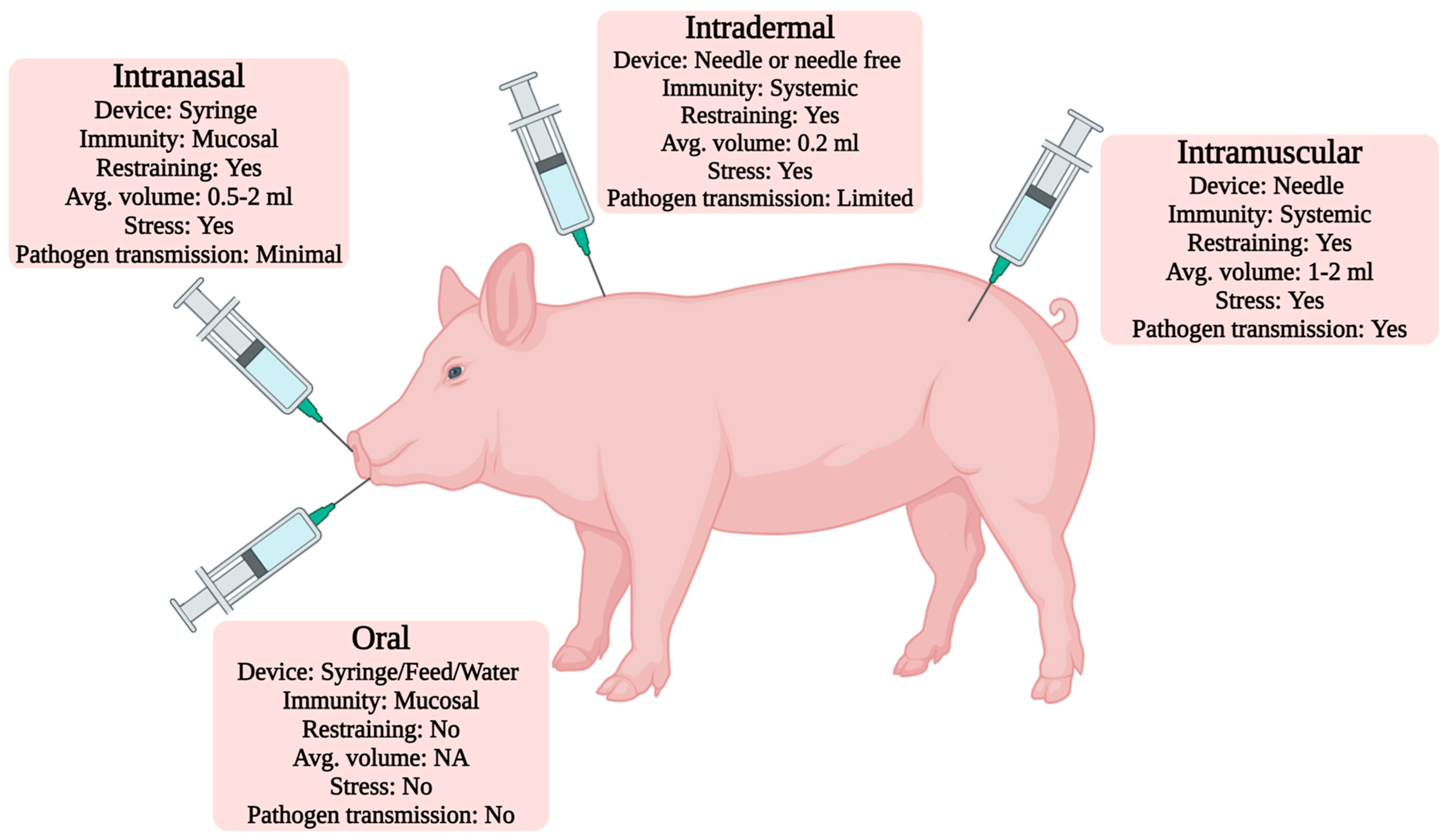
8. Vaccine Administration Routes
9. Adjuvant Systems
10. Nanotechnology Interventions in Swine Vaccinology
11. Herd Immunity
12. Economy, Limitations, and Future Prospective of the Vaccine Industry
13. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN Department of Economic and Social Affairs. World Population to Reach 8 Billion on 15 November 2022. Available online: https://www.un.org/en/desa/world-population-reach-8-billion-15-november-2022#:~:text=The%20latest%20projections%20by%20the,at%20that%20level%20until%202100 (accessed on 1 March 2024).
- Statista Statista Inc. Available online: https://www.statista.com/statistics/263963/number-of-pigs-worldwide-since-1990/ (accessed on 28 February 2023).
- Woonwong, Y.; Tien, D.; Thanawongnuwech, R. The future of the pig industry after the introduction of African swine fever into Asia. Anim. Front. 2020, 10, 30–37. [Google Scholar] [CrossRef]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef]
- Lombard, M.; Pastoret, P.; Moulin, A. A brief history of vaccines and vaccination. Rev. Sci. Tech. 2007, 26, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel vaccine technologies in veterinary medicine: A herald to human medicine vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef]
- Meeusen, E.; Walker, J.; Peters, A.; Pastoret, P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.; Leite, L. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Small, J.; Ertl, H. Viruses—From pathogens to vaccine carriers. Curr. Opin. Virol. 2011, 1, 241–245. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Haigwood, N. DNA vaccine strategies: Candidates for immune modulation and immunization regimens. Methods 2003, 31, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging concepts and technologies in vaccine development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J. Recent advances in vaccine delivery. Curr. Opin. Immunol. 2016, 5, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.; Plotkin, S. The development of vaccines: How the past led to the future. Nat. Rev. Microbiol. 2011, 9, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Black, S.; Lambert, P. Vaccine discovery and translation of new vaccine technology. Lancet 2011, 378, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: Where are we headed? Porc. Health Manag. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Dellagostin, O. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Elveborg, S.; Monteil, V.; Mirazimi, A. Methods of inactivation of highly pathogenic viruses for molecular, serology or vaccine development purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated viral vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–80. [Google Scholar] [CrossRef]
- Brockwell-Staats, C.; Webster, R.G.; Webby, R. Diversity of influenza viruses in swine and the emergence of a novel human pandemic influenza A (H1N1). Influenza Other Respir. Viruses 2009, 3, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Loving, C.; Lager, K.; Vincent, A.; Brockmeier, S.; Gauger, P.; Anderson, T.; Kitikoon, P.; Perez, D.; Kehrli, M., Jr. Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011–2012 H3N2v. J. Virol. 2013, 87, 9895–9903. [Google Scholar] [CrossRef] [PubMed]
- Ma, W. Swine influenza virus: Current status and challenge. Virus Res. 2020, 288, 198118. [Google Scholar] [CrossRef]
- Munoz, F.M.; Cramer, J.P.; Dekker, C.L.; Dudley, M.Z.; Graham, B.S.; Gurwith, M.; Law, B.; Perlman, S.; Polack, F.P.; Spergel, J.M.; et al. Vaccine-associated enhanced disease: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3053–3066. [Google Scholar] [CrossRef]
- Tizard, I.R. Porcine vaccines. Vaccines Vet. 2021, 225–242.e1. [Google Scholar] [CrossRef]
- Lauring, A.; Jones, J.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhao, Z.; Opriessnig, T.; Subramaniam, S.; Zhou, L.; Cao, D.; Cao, Q.; Yang, H.; Meng, X. Computer-aided codon-pairs deoptimization of the major envelope GP5 gene attenuates porcine reproductive and respiratory syndrome virus. Virology 2014, 450, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Gourapura, R.; Meng, X.; Calvert, J.; Roof, M.; Lager, K. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar]
- Opriessnig, T.; Halbur, P.; Yoon, K.; Pogranichniy, R.; Harmon, K.; Evans, R.; Key, K.; Pallares, F.; Thomas, P.; Meng, X. Comparison of molecular and biological characteristics of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (ingelvac PRRS MLV), the parent strain of the vaccine (ATCC VR2332), ATCC VR2385, and two recent field isolates of PRRSV. J. Virol. 2002, 76, 11837–11844. [Google Scholar] [PubMed]
- Nielsen, H.; Oleksiewicz, M.; Forsberg, R.; Stadejek, T.; Bøtner, A.; Storgaard, T. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J. Gen. Virol. 2001, 82, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Kvisgaard, L.; Kristensen, C.; Ryt-Hansen, P.; Pedersen, K.; Stadejek, T.; Trebbien, R.; Andresen, L.; Larsen, L. A recombination between two Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-1) vaccine strains has caused severe outbreaks in Danish pigs. Transbound. Emerg. Dis. 2020, 67, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS modified-live virus vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.; Smiley, R.; Fergen, B.; Eichmeyer, M.; Genzow, M. Influenza A virus shedding reduction observed at 12 weeks post-vaccination when newborn pigs are administered live-attenuated influenza virus vaccine. Influenza Other Respir. Viruses 2019, 13, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, S.; Zhang, H.; Qin, Z.; Shan, H.; Cai, X. Vaccines for African swine fever: An update. Front. Microbiol. 2023, 14, 1139494. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, P.; Forth, J.-H.; Sehl-Ewert, J.; Carrau, T.; Viaplana, E.; Mancera, J.C.; Urniza, A.; Beer, M.; Blome, S. Assessment of African swine fever vaccine candidate ASFV-G-∆MGF in a reversion to virulence study. npj Vaccines 2023, 8, 78. [Google Scholar] [CrossRef]
- Sánchez, E.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A. Approaches and perspectives for development of African swine fever virus vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Revilla, Y.; Pérez-Núñez, D.; Richt, J. African swine fever virus biology and vaccine approaches. Adv. Virus Res. 2018, 100, 41–74. [Google Scholar] [PubMed]
- Rock, D. Challenges for African swine fever vaccine development—“… perhaps the end of the beginning.”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef] [PubMed]
- National Hog Farmer. Vietnam First to Commercially Produce African Swine Fever Vaccine. 2022. Available online: https://www.nationalhogfarmer.com/news/vietnam-first-commercially-produce-african-swine-fever-vaccine (accessed on 2 June 2022).
- Gallardo, C.; Sánchez, E.; Pérez-Núñez, D.; Nogal, M.; de León, P.; Carrascosa, Á.; Nieto, R.; Soler, A.; Arias, M.; Revilla, Y. African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses. Vaccine 2018, 36, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Chapman, D.; Argilaguet, J.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.; Netherton, C.; Moffat, K. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine 2011, 29, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Risatti, G.; Holinka, L.; Krug, P.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.; Gladue, D.; Borca, M. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Goatley, L.; Jabbar, T.; Sanchez-Cordon, P.; Netherton, C.; Chapman, D.; Dixon, L. Deletion of the African swine fever virus gene DP148R does not reduce virus replication in culture but reduces virus virulence in pigs and induces high levels of protection against challenge. J. Virol. 2017, 91, e01428-17. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.; Vidal, E. BA71ΔCD2: A new recombinant live attenuated African swine fever virus with cross-protective capabilities. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Graham, S.; Everett, H.; Haines, F.; Johns, H.; Sosan, O.; Salguero, F.; Clifford, D.; Steinbach, F.; Drew, T.; Crooke, H. Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS ONE 2012, 7, e29310. [Google Scholar] [CrossRef]
- Kamboj, A.; Satia, S.K.; Khulbe, M.; Dumka, S. Recent updates on classical swine fever and its status in India. Pharma Innov. 2022, 11, 1165–1170. [Google Scholar]
- Greiser-Wilke, I.; Moennig, V. Vaccination against classical swine fever virus: Limitations and new strategies. Anim. Health Res. Rev. 2004, 5, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Chen, Y. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 2007, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Coronado, L.; Rios, L.; Frías, M.T.; Amarán, L.; Naranjo, P.; Percedo, M.I.; Perera, C.L.; Prieto, F.; Fonseca-Rodriguez, O.; Perez, L.J. Positive selection pressure on E2 protein of classical swine fever virus drives variations in virulence, pathogenesis and antigenicity: Implication for epidemiological surveillance in endemic areas. Transbound. Emerg. Dis. 2019, 66, 2362–2382. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Niu, D.; Si, H.; Ding, N.; He, C. Vaccination influences the evolution of classical swine fever virus. Infect. Genet. Evol. 2014, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pei, J.; Bai, J.; Zhao, M.; Ju, C.; Yi, L.; Kang, Y.; Zhang, X.; Chen, L.; Li, Y. Genetic diversity and positive selection analysis of classical swine fever virus isolates in south China. Virus Genes 2011, 43, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Staubach, C.; Blome, S.; Guberti, V.; Thulke, H.; Vos, A.; Koenen, F.; Le Potier, M. Controlling of CSFV in European wild boar using oral vaccination: A review. Front. Microbiol. 2015, 6, 1141. [Google Scholar] [CrossRef] [PubMed]
- Milicevic, V.; Dietze, K.; Plavsic, B.; Tikvicki, M.; Pinto, J.; Depner, K. Oral vaccination of backyard pigs against classical swine fever. Vet. Microbiol. 2013, 163, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kunu, W.; Jiwakanon, J.; Porntrakulpipat, S. A bread-based lyophilized C-strain CSF virus vaccine as an oral vaccine in pigs. Transbound. Emerg. Dis. 2019, 66, 1597–1601. [Google Scholar] [CrossRef]
- Bazarragchaa, E.; Isoda, N.; Kim, T.; Tetsuo, M.; Ito, S.; Matsuno, K.; Sakoda, Y. Efficacy of oral vaccine against classical swine fever in wild boar and estimation of the disease dynamics in the quantitative approach. Viruses 2021, 13, 319. [Google Scholar] [CrossRef]
- Henderson, L.M. Overview of marker vaccine and differential diagnostic test technology. Biologicals 2005, 33, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pasick, J. Application of DIVA vaccines and their companion diagnostic tests to foreign animal disease eradication. Anim. Health Res. Rev. 2004, 5, 257–262. [Google Scholar] [CrossRef]
- Kamboj, A.; Saini, M.; Rajan, L.; Patel, C.; Chaturvedi, V.; Gupta, P. Construction of infectious cDNA clone derived from a classical swine fever virus field isolate in BAC vector using in vitro overlap extension PCR and recombination. J. Virol. Methods 2015, 226, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, B.; Niu, X.; Chen, W.; Li, Y.; Wu, K.; Li, X.; Ding, H.; Zhao, M.; Chen, J. The development of classical swine fever marker vaccines in recent years. Vaccines 2022, 10, 603. [Google Scholar] [CrossRef]
- Coronado, L.; Perera, C.L.; Rios, L.; Frías, M.T.; Pérez, L.J. A critical review about different vaccines against classical swine fever virus and their repercussions in endemic regions. Vaccines 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Wernike, K.; Reimann, I.; König, P.; Moß, C.; Beer, M. A decade of research into classical swine fever marker vaccine CP7_E2alf (Suvaxyn® CSF Marker): A review of vaccine properties. Vet. Res. 2017, 48, 51. [Google Scholar] [CrossRef]
- Rasmussen, T.; Uttenthal, Å.; Reimann, I.; Nielsen, J.; Depner, K.; Beer, M. Virulence, immunogenicity and vaccine properties of a novel chimeric pestivirus. J. Gen. Virol. 2007, 88, 481–486. [Google Scholar] [CrossRef] [PubMed]
- von Rosen, T.; Rangelova, D.; Nielsen, J.; Rasmussen, T.; Uttenthal, Å. DIVA vaccine properties of the live chimeric pestivirus strain CP7_E2gif. Vet. Microbiol. 2014, 170, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.L.; Yin, D.; Xing, G.; Huang, Y.M.; Fan, C.M.; Fan, C.F.; Qiu, X.H.; Dong, W.R.; Yan, Y.; Gu, J.Y.; et al. The Inactivated gE/TK Gene-Deleted Vaccine against Pseudorabies Virus Type II Confers Effective Protection in Mice and Pigs. Front. Microbiol. 2022, 13, 943707. [Google Scholar] [CrossRef]
- Dudek, T.; Knipe, D. Replication-defective viruses as vaccines and vaccine vectors. Virology 2006, 344, 230–239. [Google Scholar] [CrossRef]
- Draper, S.; Heeney, J. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.; Blasi, M. The use of viral vectors in vaccine development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Jackson, D.; Symons, R.; Berg, P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Morla, S.; Kumar, S. Reverse Genetics and Its Usage in the Development of Vaccine Against Poultry Diseases. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Thomas, S., Ed.; Springer: New York, NY, USA, 2022; pp. 77–92. [Google Scholar]
- Kumar, R.; Kumar, V.; Kumar, S.J.P.B. Production of recombinant Erns protein of classical swine fever virus and assessment of its enzymatic activity: A recombinant Newcastle disease virus-based approach. Process Biochem. 2018, 66, 113–119. [Google Scholar] [CrossRef]
- Baron, M.D.; Iqbal, M.; Nair, V. Recent advances in viral vectors in veterinary vaccinology. Curr. Opin. Virol. 2018, 29, 1–7. [Google Scholar] [CrossRef]
- Fougeroux, C.; Holst, P. Future prospects for the development of cost-effective adenovirus vaccines. Int. J. Mol. Sci. 2017, 18, 686. [Google Scholar] [CrossRef]
- Ertl, H. Viral vectors as vaccine carriers. Curr. Opin. Virol. 2016, 21, 1–8. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef]
- Tian, D.; Sooryanarain, H.; Matzinger, S.; Gauger, P.; Karuppannan, A.; Elankumaran, S.; Opriessnig, T.; Meng, X. Protective efficacy of a virus-vectored multi-component vaccine against porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and swine influenza virus. J. Gen. Virol. 2017, 98, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, V.; Kekungu, P.; Barman, N.; Kumar, S. Evaluation of surface glycoproteins of classical swine fever virus as immunogens and reagents for serological diagnosis of infections in pigs: A recombinant Newcastle disease virus approach. Arch. Virol. 2019, 164, 3007–3017. [Google Scholar] [CrossRef]
- Ganges, L.; Crooke, H.; Bohórquez, J.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhang, Y.; Yang, Y.; Ren, J.; Zhang, X.; Du, E. Surface displaying of swine IgG1 Fc enhances baculovirus-vectored vaccine efficacy by facilitating viral complement escape and mammalian cell transduction. Vet. Res. 2017, 48, 29. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ma, Z.; Chen, L.; Fan, H. Recombinant swinepox virus expressing glycoprotein E2 of classical swine fever virus confers complete protection in pigs upon viral challenge. Front. Vet. Sci. 2017, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef]
- Pollard, A.; Bijker, E. Publisher Correction: A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Van Oirschot, J. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef]
- Brown, F. Synthetic peptides and purified antigens as vaccines. Int. J. Technol. Assess. Health Care 1994, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Jeon, C. High-throughput recombinant protein expression in Escherichia coli: Current status and future perspectives. Open Biol. 2016, 6, 160196. [Google Scholar] [CrossRef] [PubMed]
- Westers, L.; Westers, H.; Quax, W. Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochim. Biophys. Acta 2004, 1694, 299–310. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, L.; Peng, B.; Wang, Y.; Yang, Z.; Yao, X.; Hu, L.; Lin, X. Prokaryotic expression, purification and antigenicity analysis of African swine fever virus pK205R protein. Pol. J. Vet. Sci. 2016, 19, 41–48. [Google Scholar] [CrossRef]
- Marcekova, Z.; Psikal, I.; Kosinova, E.; Benada, O.; Sebo, P.; Bumba, L. Heterologous expression of full-length capsid protein of porcine circovirus 2 in Escherichia coli and its potential use for detection of antibodies. J. Virol. Methods 2009, 162, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Quiroga, D.; Zapata-Cuellar, L.; Uribe-Flores, J.; Gaona-Bernal, J.; Camacho-Villegas, T.; Manuel-Cabrera, C.; Trujillo-Ortega, M.; Ramírez-Hernández, G.; Herradora-Lozano, M.; Mercado-Garcia, M. An Escherichia coli-expressed porcine reproductive and respiratory syndrome virus chimeric protein induces a specific immunoglobulin G response in immunized piglets. Viral Immunol. 2019, 32, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Xu, H.; Xiang, B.; Dang, R.; Yang, Z. Orally administrated whole yeast vaccine against porcine epidemic diarrhea virus induced high levels of IgA response in mice and piglets. Viral Immunol. 2016, 29, 526–531. [Google Scholar] [CrossRef]
- Legastelois, I.; Buffin, S.; Peubez, I.; Mignon, C.; Sodoyer, R.; Werle, B. Non-conventional expression systems for the production of vaccine proteins and immunotherapeutic molecules. Hum. Vaccin. Immunother. 2017, 13, 947–961. [Google Scholar] [CrossRef]
- Makadiya, N.; Brownlie, R.; van den Hurk, J.; Berube, N.; Allan, B.; Gerdts, V.; Zakhartchouk, A. S1 domain of the porcine epidemic diarrhea virus spike protein as a vaccine antigen. Virol. J. 2016, 13, 57. [Google Scholar] [CrossRef]
- Lorenzo, E.; Méndez, L.; Rodríguez, E.; Gonzalez, N.; Cabrera, G.; Pérez, C.; Pimentel, R.; Sordo, Y.; Molto, M.; Sardina, T. Plasticity of the HEK-293 cells, related to the culture media, as platform to produce a subunit vaccine against classical swine fever virus. AMB Express 2019, 9, 139. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ma, H.; Ren, X.; Hao, G.; Zhang, H.; Zhao, Z.; Fang, K.; Li, X.; Rong, Z. Efficient mucosal vaccination of a novel classical swine fever virus E2-Fc fusion protein mediated by neonatal Fc receptor. Vaccine 2020, 38, 4574–4583. [Google Scholar] [CrossRef]
- Kost, T.; Condreay, J.; Jarvis, D. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cid, R.; Bolívar, J. Platforms for production of protein-based vaccines: From classical to next-generation strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Chen, Y.; Wang, A.; Feng, H.; Wei, Q.; Zhou, E.; Zhang, G. A single dose glycoprotein D-based subunit vaccine against pseudorabies virus infection. Vaccine 2020, 38, 6153–6161. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Z.; Hu, Y.; Yuan, P.; Yang, Y.; Xie, L.; Huang, S.; Liu, J.; Ran, L.; Song, Z. Construction of a recombinant baculovirus expressing swine hepatitis E Virus ORF2 and preliminary research on its immune effect. Pol. J. Vet. Sci. 2018, 21, 47–54. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Peng, G.; Tang, C.; Zhu, S.; Qian, S.; Xu, J.; Qian, P. Glycoprotein E2 of classical swine fever virus expressed by baculovirus induces the protective immune responses in rabbits. Vaccine 2014, 32, 6607–6613. [Google Scholar] [CrossRef]
- Cafferkey, R.; Bowdish, K.J.N. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar]
- Mason, H.S.; Lam, D.M.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 15, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Vermij, P. South African GM label confusion. Nat. Biotechnol. 2006, 24, 233. [Google Scholar]
- Liew, P.; Hair-Bejo, M. Farming of plant-based veterinary vaccines and their applications for disease prevention in animals. Adv. Virol. 2015, 2015, 936940. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Lee, Y.; Park, N.; Park, M.; Kim, N.; Park, S.; Min, K.; Gu, S.; Park, Y.; Song, J. Development of plant-produced E2 protein for use as a green vaccine against classical swine fever virus. J. Plant Biol. 2018, 61, 241–252. [Google Scholar] [CrossRef]
- Park, Y.; An, D.; Choe, S.; Lee, Y.; Park, M.; Park, S.; Gu, S.; Min, K.; Kim, N.; Lee, S. Development of recombinant protein-based vaccine against classical swine fever virus in pigs using transgenic Nicotiana benthamiana. Front. Plant Sci. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, R.; Madera, R.; Peres, Y.; Berquist, B.; Wang, L.; Buist, S.; Burakova, Y.; Palle, S.; Chung, C.; Rasmussen, M. Plant-made E2 glycoprotein single-dose vaccine protects pigs against classical swine fever. Plant Biotechnol. J. 2019, 17, 410–420. [Google Scholar] [CrossRef]
- Kolotilin, I.; Kaldis, A.; Devriendt, B.; Joensuu, J.; Cox, E.; Menassa, R. Production of a subunit vaccine candidate against porcine post-weaning diarrhea in high-biomass transplastomic tobacco. PLoS ONE 2012, 7, e42405. [Google Scholar] [CrossRef]
- Chan, H.T.; Chia, M.; Pang, V.F.; Jeng, C.R.; Do, Y.Y.; Huang, P.L. Oral immunogenicity of porcine reproductive and respiratory syndrome virus antigen expressed in transgenic banana. Plant Biotechnol. J. 2013, 11, 315–324. [Google Scholar] [CrossRef]
- Chia, M.Y.; Hsiao, S.H.; Chan, H.T.; Do, Y.Y.; Huang, P.L.; Chang, H.W.; Tsai, Y.C.; Lin, C.M.; Pang, V.F.; Jeng, C.R. Evaluation of the immunogenicity of a transgenic tobacco plant expressing the recombinant fusion protein of GP5 of porcine reproductive and respiratory syndrome virus and B subunit of Escherichia coli heat-labile enterotoxin in pigs. Vet. Immunol. Immunopathol. 2011, 140, 215–225. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J. Generation and immunogenicity of transgenic potato expressing the GP5 protein of porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2011, 173, 153–158. [Google Scholar] [CrossRef]
- Dyck, M.; Lacroix, D.; Pothier, F.; Sirard, M. Making recombinant proteins in animals–different systems, different applications. Trends Biotechnol. 2003, 21, 394–399. [Google Scholar] [CrossRef]
- Toledo, J.; Sánchez, O.; Montesino, R.; Farnos, O.; Rodríguez, M.; Alfonso, P.; Oramas, N.; Rodríguez, E.; Santana, E.; Vega, E. Highly protective E2–CSFV vaccine candidate produced in the mammary gland of adenoviral transduced goats. J. Biotechnol. 2008, 133, 370–376. [Google Scholar] [CrossRef]
- Barrera, M.; Sánchez, O.; Farnós, O.; Rodríguez, M.P.; Domínguez, P.; Tait, H.; Frías, M.; Ávila, M.; Vega, E.; Toledo, J.R. Early onset and long lasting protection in pigs provided by a classical swine fever E2-vaccine candidate produced in the milk of goats. Vet. Immunol. Immunopathol. 2010, 133, 25–32. [Google Scholar] [CrossRef]
- Bijker, M.S.; Melief, C.J.M.; Offringa, R.; Van Der Burg, S.H. Design and development of synthetic peptide vaccines: Past, present and future. Expert. Rev. Vaccines 2007, 6, 591–603. [Google Scholar] [CrossRef]
- Forner, M.; Cañas-Arranz, R.; Defaus, S.; De León, P.; Rodríguez-Pulido, M.; Ganges, L.; Blanco, E.; Sobrino, F.; Andreu, D. Peptide-based vaccines: Foot-and-mouth disease virus, a paradigm in animal health. Vaccines 2021, 9, 477. [Google Scholar] [CrossRef]
- Cubillos, C.; de la Torre, B.G.; Jakab, A.; Clementi, G.; Borrás, E.; Bárcena, J.; Andreu, D.; Sobrino, F.; Blanco, E. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 2008, 82, 7223–7230. [Google Scholar] [CrossRef]
- Monsó, M.; Tarradas, J.; de la Torre, B.; Sobrino, F.; Ganges, L.; Andreu, D. Peptide vaccine candidates against classical swine fever virus: T cell and neutralizing antibody responses of dendrimers displaying E2 and NS2–3 epitopes. J. Pept. Sci. 2011, 17, 24–31. [Google Scholar] [CrossRef]
- Kutzler, M.; Weiner, D. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Donnelly, J.; Liu, M.; Ulmer, J. Antigen presentation and DNA vaccines. Am. J. Respir. Crit. Care Med. 2000, 162 (Suppl. S3), S190–S193. [Google Scholar] [CrossRef]
- Hewitt, J.; Karuppannan, A.; Tan, S.; Gauger, P.; Halbur, P.; Gerber, P.; De Groot, A.; Moise, L.; Opriessnig, T. A prime-boost concept using a T-cell epitope-driven DNA vaccine followed by a whole virus vaccine effectively protected pigs in the pandemic H1N1 pig challenge model. Vaccine 2019, 37, 4302–4309. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef]
- Ganges, L.; Barrera, M.; Núñez, J.; Blanco, I.; Frias, M.; Rodríguez, F.; Sobrino, F. A DNA vaccine expressing the E2 protein of classical swine fever virus elicits T cell responses that can prime for rapid antibody production and confer total protection upon viral challenge. Vaccine 2005, 23, 3741–3752. [Google Scholar] [CrossRef]
- Du, F.; Cao, Z.; Ye, Z.; He, J.; Zhang, W.; Zhang, K.; Ning, P. Production and immunogenicity of a deoxyribonucleic acid Alphavirus vaccine expressing classical swine fever virus E2-Erns protein and porcine Circovirus Cap-Rep protein. Front. Microbiol. 2022, 13, 1065532. [Google Scholar] [CrossRef]
- Cui, J.; O’Connell, C.M.; Costa, A.; Pan, Y.; Smyth, J.A.; Verardi, P.H.; Burgess, D.J.; Van Kruiningen, H.J.; Garmendia, A.E. A PRRSV GP5-Mosaic vaccine: Protection of pigs from challenge and ex vivo detection of IFNγ responses against several genotype 2 strains. PLoS ONE 2019, 14, e0208801. [Google Scholar]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N.J.M.T. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef]
- Tombácz, I.; Weissman, D.; Pardi, N. Vaccination with messenger RNA: A promising alternative to DNA vaccination. In DNA Vaccines: Methods and Protocols; Humana: New York, NY, USA, 2021; pp. 13–31. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.; Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef]
- Zhou, L.; Wubshet, A.K.; Zhang, J.; Hou, S.; Yao, K.; Zhao, Q.; Dai, J.; Liu, Y.; Ding, Y.; Zhang, J.; et al. The mRNA Vaccine Expressing Single and Fused Structural Proteins of Porcine Reproductive and Respiratory Syndrome Induces Strong Cellular and Humoral Immune Responses in BalB/C Mice. Viruses 2024, 16, 544. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Xu, M.; Wang, H.; Zhang, X.; Liu, W.; Chen, C. Preparation and immunogenicity evaluation of mRNA vaccine against porcine epidemic diarrhea. Sheng wu gong cheng xue bao = Chin. J. Biotechnol. 2023, 39, 2624–2633. [Google Scholar]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e02958-23. [Google Scholar] [CrossRef]
- Pedrera, M.; McLean, R.K.; Medfai, L.; Thakur, N.; Todd, S.; Marsh, G.; Bailey, D.; Donofrio, G.; Muramatsu, H.; Pardi, N.; et al. Evaluation of the immunogenicity of an mRNA vectored Nipah virus vaccine candidate in pigs. Front. Immunol. 2024, 15, 1384417. [Google Scholar] [CrossRef]
- Xia, T.; Yang, H.; Guo, Y.; Guo, T.; Xin, L.; Jiang, Y.; Cui, W.; Zhou, H.; Qiao, X.; Wang, X. Human dendritic cell targeting peptide can be targeted to porcine dendritic cells to improve antigen capture efficiency to stimulate stronger immune response. Front. Immunol. 2022, 13, 950597. [Google Scholar] [CrossRef]
- Renukaradhya GJ, D.V. Manickam C, Binjawadagi B, Benfield D, Mucosal vaccines to prevent porcine reproductive and respiratory syndrome: A new perspective. Anim. Health Res. Rev. 2012, 13, 21–37. [Google Scholar] [CrossRef]
- Daemen, T.; de Mare, A.; Bungener, L.; de Jonge, J.; Huckriede, A.; Wilschut, J. Virosomes for antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 451–463. [Google Scholar] [CrossRef]
- Asadi, K.; Gholami, A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. Int. J. Biol. Macromol. 2021, 182, 648–658. [Google Scholar] [CrossRef]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef]
- Baudoux, P.; Carrat, C.; Besnardeau, L.; Charley, B.; Laude, H. Coronavirus pseudoparticles formed with recombinant M and E proteins induce alpha interferon synthesis by leukocytes. J. Virol. 1998, 72, 8636–8643. [Google Scholar] [CrossRef]
- Zheng, C.; LÜ, F. The Virosome as a Novel Concept for High Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (HP-PRRSV) Vaccines. J. Integr. Agric. 2013, 12, 1215–1224. [Google Scholar]
- Kim, J.; Yoon, J.; Park, J. Construction of porcine epidemic diarrhea virus-like particles and its immunogenicity in mice. Vaccines 2021, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Cheng, J.; Zhou, X.; Lu, H.; Zhang, X.; Xia, X.; Sun, H. Generation and immunogenicity assessment of ELPylated virus-like particles of porcine circovirus type 2. Virol. J. 2020, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, L.; Xu, L.; Li, F.; Deng, H.; Huang, Y.; Gu, S.; Sun, X.; Zhou, Y.; Xu, Z. The construction and immunogenicity analyses of recombinant pseudorabies virus with NADC30-like porcine reproductive and respiratory syndrome virus-like particles co-expression. Front. Microbiol. 2022, 13, 846079. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Lu, Y.; Fang, W.; He, F. A modular and self-adjuvanted multivalent vaccine platform based on porcine circovirus virus-like nanoparticles. J. Nanobiotechnol. 2022, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Diaz-San Segundo, F.; Medina, G.; Stenfeldt, C.; Arzt, J.; de Los Santos, T. Foot-and-mouth disease vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.; Qiu, H. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Do, V.; Jang, J.; Park, J.; Dao, H.; Kim, K.; Hahn, T. Recombinant adenovirus carrying a core neutralizing epitope of porcine epidemic diarrhea virus and heat-labile enterotoxin B of Escherichia coli as a mucosal vaccine. Arch. Virol. 2020, 165, 609–618. [Google Scholar] [CrossRef]
- Alejo, D.M.; Moraes, M.P.; Liao, X.; Dias, C.C.; Tulman, E.R.; Diaz-San Segundo, F.; Rood, D.; Grubman, M.J.; Silbart, L.K. An adenovirus vectored mucosal adjuvant augments protection of mice immunized intranasally with an adenovirus-vectored foot-and-mouth disease virus subunit vaccine. Vaccine 2013, 31, 2302–2309. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Dai, X.; Liu, M.; Khalique, A.; Wang, Z.; Zeng, Y.; Zhang, D.; Ni, X.; Zeng, D. Surface Display of porcine circovirus type 2 antigen protein cap on the spores of bacillus subtilis 168: An effective mucosal vaccine candidate. Front. Immunol. 2022, 13, 1007202. [Google Scholar] [CrossRef]
- Saif, L. Coronavirus immunogens. Vet. Microbiol. 1993, 37, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wang, N.; Tang, Y.; Gao, Y.; Gao, C.; Hao, J.; Jiang, Y.; Wang, X.; Shan, Z.; Li, J. Delivery of antigen to porcine dendritic cells by fusing antigen with porcine dendritic cells targeting peptide. Front. Immunol. 2022, 13, 926279. [Google Scholar] [CrossRef]
- Hernandez-Franco, J.F.; Xie, S.; Thimmapuram, J.; Ragland, D.; HogenEsch, H. Mechanism of activation of porcine dendritic cells by an α-D-glucan nanoparticle adjuvant and a nanoparticle/poly (I:C) combination adjuvant. Front. Immunol. 2022, 13, 990900. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, S.; Qin, T.; Yin, Y.; Yang, Q. Effects of porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Vet. Microbiol. 2015, 179, 131–141. [Google Scholar] [CrossRef]
- Subramaniam, S.; Yugo, D.; Heffron, C.; Rogers, A.; Sooryanarain, H.; LeRoith, T.; Overend, C.; Cao, D.; Meng, X. Vaccination of sows with a dendritic cell-targeted porcine epidemic diarrhea virus S1 protein-based candidate vaccine reduced viral shedding but exacerbated gross pathological lesions in suckling neonatal piglets. J. Gen. Virol. 2018, 99, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, S.; Qin, T.; Yin, Y.; Yu, Q.; Yang, Q. Effects of inactivated porcine epidemic diarrhea virus on porcine monocyte-derived dendritic cells and intestinal dendritic cells. Res. Vet. Sci. 2016, 106, 149–158. [Google Scholar] [CrossRef]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wang, J.; Man, K.; Yang, Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine 2017, 35, 7033–7041. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Franzoni, G.; Netherton, C.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive cellular immunity against African swine fever virus infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef]
- Flores-Mendoza, L.; Silva-Campa, E.; Reséndiz, M.; Osorio, F.; Hernández, J. Porcine reproductive and respiratory syndrome virus infects mature porcine dendritic cells and up-regulates interleukin-10 production. Clin. Vaccine Immunol. 2008, 15, 720–725. [Google Scholar] [CrossRef]
- Subramaniam, S.; Piñeyro, P.; Tian, D.; Overend, C.; Yugo, D.; Matzinger, S.; Rogers, A.; Haac, M.; Cao, Q.; Heffron, C. In vivo targeting of porcine reproductive and respiratory syndrome virus antigen through porcine DC-SIGN to dendritic cells elicits antigen-specific CD4T cell immunity in pigs. Vaccine 2014, 32, 6768–6775. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jin, M.; Yoon, I.; Yoo, H. Efficacy of bivalent vaccines of porcine circovirus type 2 and Mycoplasma hyopneumoniae in specific pathogen-free pigs challenged with porcine circovirus type 2d. J. Vet. Sci. 2022, 23, e49. [Google Scholar] [CrossRef] [PubMed]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Using a concurrent challenge with porcine circovirus 2 and porcine reproductive and respiratory syndrome virus to compare swine vaccination programs. Sci. Rep. 2022, 12, 15524. [Google Scholar] [CrossRef] [PubMed]
- Niederwerder, M.; Bawa, B.; Serão, N.; Trible, B.; Kerrigan, M.; Lunney, J.; Dekkers, J.; Rowland, R. Vaccination with a porcine reproductive and respiratory syndrome (PRRS) modified live virus vaccine followed by challenge with PRRS virus and porcine circovirus type 2 (PCV2) protects against PRRS but enhances PCV2 replication and pathogenesis compared to results for nonvaccinated cochallenged controls. Clin. Vaccine Immunol. 2015, 22, 1244–1254. [Google Scholar] [PubMed]
- Oh, T.; Suh, J.; Cho, H.; Min, K.; Choi, B.; Chae, C. Efficacy test of a plant-based porcine circovirus type 2 (PCV2) virus-like particle vaccine against four PCV2 genotypes (2a, 2b, 2d, and 2e) in pigs. Vet. Microbiol. 2022, 272, 109512. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qiao, X.; Chang, C.; Hua, T.; Wang, J.; Tang, B.; Zhang, D. Reduction of postweaning multisystemic wasting syndrome-associated clinical symptoms by virus-like particle vaccine against porcine parvovirus and porcine circovirus type 2. Viral Immunol. 2020, 33, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.W.; Anderson, T.K.; Kitikoon, P.; Kimble, J.B.; Otis, N.; Gauger, P.C.; Souza, C.K.; Kaplan, B.; Mogler, M.; Strait, E. Bivalent hemagglutinin and neuraminidase influenza replicon particle vaccines protect pigs against influenza a virus without causing vaccine associated enhanced respiratory disease. Vaccine 2022, 40, 5569–5578. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lei, M.; Li, Z.; Li, H.; Liu, Z.; He, Q.; Luo, R. Development of a Genetically Engineered Bivalent Vaccine against Porcine Epidemic Diarrhea Virus and Porcine Rotavirus. Viruses 2022, 14, 1746. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, Z.; Li, D.; Fan, P.; Sun, P.; Bao, H.; Fu, Y.; Li, P.; Bai, X.; Chen, Y. Evaluation of cross-protection against three topotypes of serotype O foot-and-mouth disease virus in pigs vaccinated with multi-epitope protein vaccine incorporated with poly (I: C). Vet. Microbiol. 2014, 168, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Moxon, R.; Reche, P.; Rappuoli, R. Reverse vaccinology. Front. Immunol. 2019, 10, 2776. [Google Scholar] [CrossRef]
- Bruno, L.; Cortese, M.; Rappuoli, R.; Merola, M. Lessons from Reverse Vaccinology for viral vaccine design. Curr. Opin. Virol. 2015, 11, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, A.; Qiang, J.; Chang, C.; Kwang, J. A novel baculovirus vector shows efficient gene delivery of modified porcine reproductive and respiratory syndrome virus antigens and elicits specific immune response. Vaccine 2013, 31, 5471–5478. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Ke, H.; Du, Y.; Zhang, Q.; Yoo, D. Reverse genetics for porcine reproductive and respiratory syndrome virus. In Reverse Genetics of RNA Viruses: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 29–46. [Google Scholar] [CrossRef]
- Seibert, B.; Cardenas-Garcia, S.; Rajao, D.; Perez, D. Reverse Genetics for Influenza Influenza A and B Viruses Driven by Swine Polymerase I Promoter. In Vaccine Technologies for Veterinary Viral Diseases: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 257–281. [Google Scholar]
- Jang, G.; Lee, D.; Lee, C.V. Development of a next-generation vaccine platform for porcine epidemic diarrhea virus using a reverse genetics system. Viruses 2022, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, X.; Li, H.; Yu, X.; Zhao, B.; Liu, B.; Ning, Z. Development of the reverse genetics system for emerging atypical porcine pestivirus using in vitro and intracellular transcription systems. Virus Res. 2020, 283, 197975. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.; Ovsyannikova, I.; Kennedy, R. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, W. Towards a sane and rational approach to management of Influenza H1N1 2009. Virol. J. 2009, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Manual, M.M.V. Administration of Vaccines in Animals. Available online: https://www.msdvetmanual.com/pharmacology/vaccines-and-immunotherapy/adjuvants-in-animals (accessed on 1 October 2023).
- Dalmau, A.; Sánchez-Matamoros, A.; Molina, J.M.; Xercavins, A.; Varvaró-Porter, A.; Muñoz, I.; Moles, X.; Baulida, B.; Fàbrega, E.; Velarde, A. Intramuscular vs. intradermic needle-free vaccination in piglets: Relevance for animal welfare based on an aversion learning test and vocalizations. Front. Vet. Sci. 2021, 8, 715260. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.A.; Có Rives, I.; Moore, A. Skin-Based Vaccination: A Systematic Mapping Review of the Types of Vaccines and Methods Used and Immunity and Protection Elicited in Pigs. Vaccines 2023, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Schat, K. Vaccines and vaccination practices: Key to sustainable animal production. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–332. [Google Scholar]
- Sánchez-Cordón, P.; Chapman, D.; Jabbar, T.; Reis, A.; Goatley, L.; Netherton, C.; Taylor, G.; Montoya, M.; Dixon, L. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; T O’Hagan, D. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol. 2003, 33, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Charerntantanakul, W. Adjuvants for swine vaccines: Mechanisms of actions and adjuvant effects. Vaccine 2020, 38, 6659–6681. [Google Scholar] [CrossRef] [PubMed]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol. 2018, 31, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Renukaradhya, G.J. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet. Res. 2019, 50, 90. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, Y.; Ferdows, B.; Patel, D.; Ouyang, J.; Tang, Z.; Kong, N.; Chen, E.; Tao, W. Emerging vaccine nanotechnology: From defense against infection to sniping cancer. Acta Pharm. Sin. B 2022, 12, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell Infect. Microbiol. 2013, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Khairy, G.; Hesham, A.; Rabaan, A.; El-Shamy, A.; Nagy, A. Nanoparticles as a novel and promising antiviral platform in veterinary medicine. Arch. Virol. 2021, 166, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Gill, P. Nanocarriers, nanovaccines, and nanobacteria as nanobiotechnological concerns in modern vaccines. Sci. Iran. 2013, 20, 1003–1013. [Google Scholar]
- Kirtane, A.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Renu, S.; Feliciano-Ruiz, N.; Ghimire, S.; Han, Y.; Schrock, J.; Dhakal, S.; Patil, V.; Krakowka, S.; Renukaradhya, G. Poly (I: C) augments inactivated influenza virus-chitosan nanovaccine induced cell mediated immune response in pigs vaccinated intranasally. Vet. Microbiol. 2020, 242, 108611. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. Herd immunity. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Rebuli, N.; Bean, N.; Ross, J. Estimating the basic reproductive number during the early stages of an emerging epidemic. Theor. Popul. Biol. 2018, 119, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ridenhour, B.; Kowalik, J.; Shay, D. El número reproductivo básico (R0): Consideraciones para su aplicación en la salud pública. Rev. Panam. Salud Publica 2015, 38, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Santoni, A.; Mantovani, A. Vaccines: An achievement of civilization, a human right, our health insurance for the future. J. Exp. Med. 2019, 216, 7–9. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Rushton, J. Economics of animal vaccination. Rev. Sci. Tech. 2007, 26, 313–326. [Google Scholar] [CrossRef]
- Ulmer, J.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377–1383. [Google Scholar] [CrossRef]
| Vaccine Name | Manufacturer | Type | Administration Method |
|---|---|---|---|
| Ingelvac® PRRS MLV | Boehringer Ingelheim | Live attenuated | Injectable |
| Ingelvac® PRRS ATP | Boehringer Ingelheim | Live attenuated | Injectable |
| ReprosCyc® PRRS EU | Boehringer Ingelheim | Live attenuated | Injectable |
| Porcilis® PRRS | MSD | Live attenuated | Injectable |
| Fostera® PRRS | Zoetis | Live attenuated | Injectable |
| Suvaxyn® PRRS MLV | Zoetis | Live attenuated | Injectable |
| Prime Pac® PRRS RR | Merck | Live attenuated | Injectable |
| Prevacent® PRRS | Elanco | Live attenuated | Injectable |
| UNISTRAIN® PRRS | HIPRA | Live attenuated | Injectable |
| AMERVAC® PRRS | HIPRA | Live attenuated | Injectable |
| Ingelvac Provenza™ | Boehringer Ingelheim | Live attenuated (H1N1, H3N2) | Intranasal (piglets at one day old) |
| Virus | Subunit Vaccine Candidate Protein |
|---|---|
| African swine fever virus | p30, p54, p72, pp62, CD2v |
| Classical swine fever virus | E2 |
| Porcine circovirus (PCV 2) | ORF-2 |
| Foot and mouth disease | VP1, VP2, VP3 |
| Porcine endemic diarrhea | Spike protein (S) |
| Swine influenza | HA |
| Porcine reproductive and respiratory syndrome | GP5 and M proteins |
| Virus | Baculovirus-Expression-System-Based Commercial Subunit Vaccines |
|---|---|
| PCV2 | Ingelvac CircoFLEX® (BIVI) |
| Porcilis® PCV (MSD) | |
| Circumvent® PCV (Merck) | |
| PPV | Reprocyc® ParvoFLEX (BIVI) |
| CSFV | Porcilis Pesti® (MSD Animal Health) |
| BayoVac® (BAYER AG) | |
| Tian Wen Jing (TWJ-E2®) (TECON, Shenzhen, China) |
| NP Candidate | Porcine Vaccine Targets |
|---|---|
| VLPs | PRRSV |
| IAV | |
| FMDV | |
| Encephalomyocarditis virus (EMCV) | |
| Japanese encephalitis virus (JEV) | |
| PCV2 | |
| PPV | |
| Poly lactic-co-glycolic acid (PLGA) | PRRSV |
| IAV | |
| PEDV | |
| IAV | |
| Chitosan | IAV |
| Nano-11 | IAV |
| PEDV | |
| Polyanhydride | IAV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamboj, A.; Dumka, S.; Saxena, M.K.; Singh, Y.; Kaur, B.P.; da Silva, S.J.R.; Kumar, S. A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens. Viruses 2024, 16, 833. https://doi.org/10.3390/v16060833
Kamboj A, Dumka S, Saxena MK, Singh Y, Kaur BP, da Silva SJR, Kumar S. A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens. Viruses. 2024; 16(6):833. https://doi.org/10.3390/v16060833
Chicago/Turabian StyleKamboj, Aman, Shaurya Dumka, Mumtesh Kumar Saxena, Yashpal Singh, Bani Preet Kaur, Severino Jefferson Ribeiro da Silva, and Sachin Kumar. 2024. "A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens" Viruses 16, no. 6: 833. https://doi.org/10.3390/v16060833
APA StyleKamboj, A., Dumka, S., Saxena, M. K., Singh, Y., Kaur, B. P., da Silva, S. J. R., & Kumar, S. (2024). A Comprehensive Review of Our Understanding and Challenges of Viral Vaccines against Swine Pathogens. Viruses, 16(6), 833. https://doi.org/10.3390/v16060833







