Abstract
The spread of Lassa virus in West Africa is reliant on the abundance and distribution of its rodent host reservoirs. While the impact of environmental change on viral spread has been studied for many zoonotic viruses, there is still a limited understanding of how seasonal impacts, land-use conversion, and biodiversity loss influence the expansion of Lassa virus among reservoirs. This systematic review synthesizes existing research on the association between environmental variables and Lassa virus circulation in West Africa to inform future research, public health interventions, and One Health policy. We searched international and African scientific databases using a set of pre-defined search terms to obtain publications reporting on Lassa virus in West Africa between 1969 and 2023. A total of 9465 articles were retrieved from this search and 70 studies met inclusion criteria for this review. Through systematic data extraction, we identified seasonal precipitation, land-use change, and host expansion as key environmental drivers of Lassa virus in reservoir hosts; however, we also highlight notable gaps in knowledge that limit our current understanding of these complex relationships. This review underscores the need for interdisciplinary research and strategies to mitigate the impacts of environmental change on Lassa virus transmission and protect vulnerable populations in West Africa.
Keywords:
Lassa fever; Lassa virus; biodiversity; conservation; land use; climate; ecology; transmission dynamics 1. Introduction
Lassa fever (LF), a viral hemorrhagic disease endemic to West Africa, is intricately linked to ecological, environmental, and climatic factors, underscoring the complex relationships between the disease, its reservoir hosts, and the environment. The etiologic agent for the disease, Lassa virus (LASV), belongs to the Arenaviridae family and is predominately transmitted to humans by the multimammate rat, Mastomys natalensis, through contact with urine, feces, saliva, and blood [1,2,3]. LASV infections in humans are largely dependent on the spatial distribution of M. natalensis, a known agricultural pest and commensal rodent species predominately found in human settlements, with a preference for scavenging in indoor environments and in surrounding agricultural cultivations due to the availability of food sources [4]. Understanding how environmental factors influence LASV host dynamics is critical for evaluating the disease’s epidemiology and developing effective public health interventions.
LASV was first discovered in the Borno State of Nigeria in 1969 and is now endemic to multiple West African countries, with notable past and current outbreaks occurring in Nigeria, Guinea, Liberia, and Sierra Leone [5]. Infected rodents and sporadic human cases have more recently been reported in Mali, Togo, and Benin, suggesting the endemic range may be expanding [6,7]. Critical disease underreporting has resulted in a distinct underestimation of infections and case fatality rates [8]. As a result, disease estimates rely on mathematical modeling studies, which have estimated that approximately 37.7 million individuals in 14 West African countries reside in areas with suitable conditions for zoonotic transmission of LASV, with a projected annual incidence of 897,700 human infections, half of which are predicted to occur in Nigeria [2,9]. Based on these projections, it is estimated that 18,000 people die each year from LF; however, global climate change, mounting evidence of new rodent hosts, urbanization and expansion of agricultural practices in West Africa threaten to expand the ecological niche for LASV and influence disease spread into previously non-endemic areas [2,10,11,12,13,14]. Lassa virus is categorized as a Category A bioterrorism agent by the US Center for Disease Control, and Lassa fever was listed as a World Health Organization priority disease for research in 2016 due to its status as a hemorrhagic virus capable of widespread death and disease [15,16]. LF was further determined to be a priority zoonotic disease for West Africa by the Economic Community of West African States (ECOWAS) [16]. Despite these critical calls for action, LF remains a crucially understudied hemorrhagic disease largely due to a lack of available diagnostic assays and limited clinical research infrastructure [6,17].
LF epidemics have historically been seasonally dependent, with human cases typically peaking in January–February during the dry season in West Africa, and secondary peaks occurring in March [11,18]. The seasonal epidemiology of LF is largely attributed to the population dynamics of the primary rodent reservoir, M. natalensis, with differing evidence on whether this is due to seasonal fluctuations of virus prevalence in the host species, increased rodent abundance indoors during the dry season, host breeding cycles, or a balance of all three factors [4,18].
Further, the transmission of LASV is influenced by land-use patterns, with M. natalensis populations predominating in rural human homes and surrounding agricultural fields and having a lesser abundance in forested or urban areas [8]. The distribution and LASV seropositivity of M. natalensis are strongly positively correlated with increased habitat fragmentation, agricultural expansion, and savanna conversion, indicating a high dependency on human settlements and vulnerability to habitat expansion due to increased human development [11,19,20]. Projected climatic and land-use changes in West Africa are expected to create more favorable habitats for M. natalensis, driving species growth and potentially increasing the risk of LASV spillovers to humans [19].
The mechanisms by which seasonal precipitation, land-use conversion, and host dynamics drive LF outbreaks in West Africa are not fully understood, highlighting a need for further research to explore these complex relationships. This study aimed to systematically synthesize and critically evaluate the environmental drivers of LASV in West Africa and identify knowledge gaps in current research to target future efforts. A thorough understanding of how ecological and environmental variables relate to seasonal outbreaks of LASV is essential for guiding future public health efforts in the face of climate change, landscape alteration, and human settlement expansion.
2. Materials and Methods
2.1. Literature Search and Eligibility Criteria
A systematic literature review of LASV in West Africa was performed by two independent reviewers in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Guidelines (PRISMA 2020 Checklist available in Supplementary Files). Between January and February 2024, six databases were systematically searched: Google Scholar, PubMed, Web of Science, BIOSIS, Embase, and African Journals Online. The primary keywords “Lassa”, “virus” and “fever” were utilized in all search strings and matched with all combinations of secondary and tertiary keywords (Table S1). Secondary search terms were chosen to extract relevant articles on previously identified environmental drivers of LASV emergence and included the following: “Environment”, “Biodiversity”, “Biodiversity loss”, “Land use”, “Land use change”, “Climate”, “Climate change”, “Mastomys natalensis”, “Hylomyscus pamfi”, “Mastomys erythroleucus”, “Ecology”, and “Host”. Tertiary search terms included known geographical occurrences of Lassa fever and West African countries, and included the following: “West Africa”, “Sierra Leone”, “Guinea”, “Liberia”, “Nigeria”, “Côte D’Ivoire”, “Central African Republic”, “Mali”, “Congo”, and “Senegal”. Given the vast number of search results generated by Google Scholar, only the first 400 search results were extracted for each search string. By extracting only the first 400 results, we were able to perform a robust but feasible literature search and ensure the most pertinent studies were included for review. This amount was chosen to ensure an equal sample was taken across all search strings, including larger searches that returned greater than 500 results, as well as smaller searches that yielded less than 500 returns. Article publication years were limited to 1969–2023 as LASV was not discovered until 1969 [5]. For quality control, a secondary PubMed search was performed by an independent researcher to ensure all articles meeting inclusion criteria were screened.
Grey literature, including non-peer reviewed articles, case reports, letters to the editor, editorials, and reviews that did not include secondary data analysis were excluded. Studies published outside of the included date range, studies reporting on imported cases of Lassa fever, and studies not pertaining to LASV in West Africa were excluded. To reflect the natural conditions of environmental disease drivers, experimental studies performed on laboratory subjects or rodents were excluded. Finally, articles without English translation were excluded. Peer-reviewed, original articles which reported on primary data were included. Articles that performed secondary data collection and analysis, including modeling studies, were included. Papers that performed meta-analyses were not included. We qualitatively assessed bias using the Modified Downs and Black Checklist to guide our evaluation of bias in included studies, a commonly used tool for assessing the quality of observational studies. As summary statistics or meta-analyses were not conducted, full bias scores of all included studies were not performed.
2.2. Selection
After duplicate studies were removed, the titles and abstracts of retrieved studies were examined by two reviewers for relevancy to the research aims. Relevant studies were those that reported on LASV with relation to natural host reservoirs, environmental transmission, biodiversity, or land use. Differing opinions regarding study screening were discussed and brought to consensus between the two reviewers (n = 227). If consensus could not be reached, articles were sent to a third independent reviewer (n = 5). Full-text articles were obtained for studies that met the initial screening criteria (n = 208) and were reviewed by the same two authors. Discrepancies were again resolved through discussion and brought to a third reviewer when necessary. In total, 70 studies were retrieved for data extraction (Table S2).
2.3. Extraction and Data Synthesis
A standardized data extraction form was developed to systematically extract relevant data from the included studies (n = 70). The form was initially piloted by having the two reviewers extract data from the same five studies and then compare results to ensure cohesive data extraction. Studies were then divided and independently reviewed. The data extraction form retrieved study titles, first author, publication year, publication journal, inclusion criteria, and geographical information. Reviewers assigned a primary theme to each article to assist with data synthesis: virus host, seasonality, land use or biodiversity. The form was then further specified by theme to ensure all relevant data were extracted, including study aim, type of study, sampling methods, inclusion of serological and viral sampling, land-use change, and seasonal impacts.
3. Results
3.1. Search Results and Study Selection
A total of 9465 articles were identified by the search terms in electronic databases and 6427 duplicates were eliminated before screening (Figure 1). A pool of 2821 articles were eliminated after title and abstract screening, leaving 218 articles for full-text review. Of the 208 articles eligible for full-text review, 138 did not meet all the inclusion criteria, leaving 70 articles to be included in the review.
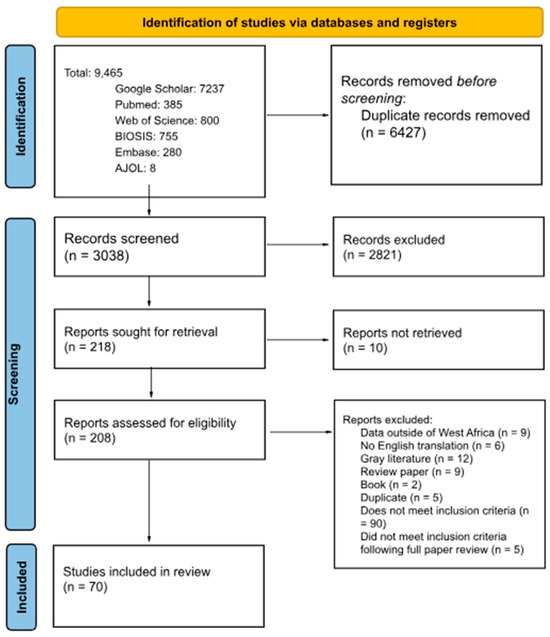
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of article selection. PRISMA Checklist can be found in Supplemental Table S3.
3.2. Characteristics of the Included Studies
Reviewed articles eligible for inclusion were published between 1969 and 2023. Of the included studies, 4.2% (n = 3) analyzed the entirety of continental Africa. Further, 1.4% (n = 1) analyzed sub-Saharan Africa and 7.1% (n = 5) analyzed all countries in West Africa and did not focus on a specific country. Within West Africa, 35.7% (n = 25) of studies reported findings from Nigeria, 35.7% (n = 25) from Guinea, 1.2% (n = 9) from Sierra Leone, 5.7% (n = 4) from Ghana and Mali, respectively, and 1.4% (n = 1) from Benin and Liberia, respectively (Figure 2).
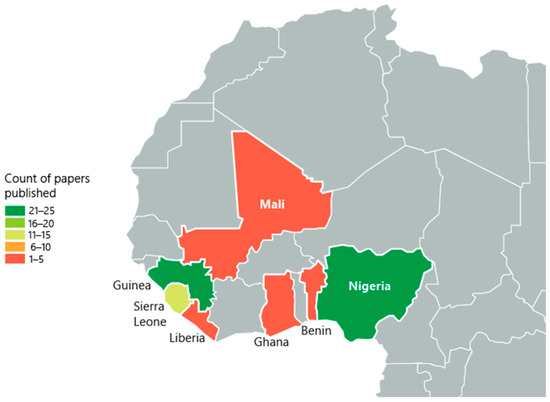
Figure 2.
The geographic distribution of included studies with published data in West Africa. The numbers on the map represent the quantity of studies conducted in each country, highlighting regional research focus areas. Included countries: Nigeria (n = 25), Guinea (n = 25), Sierra Leone (n = 4), Ghana (n = 1), Mali (n = 1, Benin (n = 1), Liberia (n = 1)).
The extraction form was utilized to separate included studies by environmental driver, including seasonality, land use, biodiversity and virus host species (Figure 3). The non-M. natalensis host category captures papers that analyzed LASV hosts, excluding the main reservoir species, M. natalensis. Studies that analyzed other potential rodent hosts, including M. erythroleucus, L. sikapusi, H. pamfi, R. rattus, and M. musculus, are represented in this category, as well as non-rodent hosts, such as mammals and avian species. Seasonality, land use, biodiversity impacts, and novel virus hosts were identified through the data extraction form as the most prominent environmental drivers of LASV spread in West Africa.
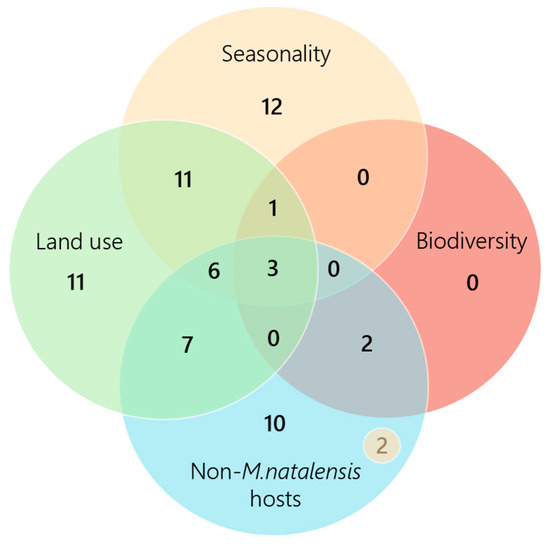
Figure 3.
Number of papers that discuss each environmental driver. Biodiversity impacts were the least often analyzed, with six papers in total discussing this theme. Five included studies were omitted from the figure for not explicitly discussing one of the drivers. N = 2 included in the “Non-M. natalensis hosts” category represents the two papers that discussed this theme and seasonality.
3.3. Seasonality
3.3.1. Seasonal Impacts on LASV Prevalence in Host Reservoirs
Seasonal precipitation patterns were the leading environmental driver associated with LASV infections in M. natalensis [2,10]. Several studies investigated the impact of seasonality on LASV and IgG prevalence in animal reservoirs and humans, with varying findings. LASV prevalence in M. natalensis was found to be 2–3 times higher in the rainy season than in the dry season in Guinea [4]. Further, mathematical models of LF epidemics in Nigeria have predicted that LASV infections in rodents occur in the first half of the year, approximately January–June. The number of infected rodents is predicted to peak in May, the rainy season, and decrease in December, the dry season [21,22].
Of the included studies, two longitudinal analyses found no significant differences in IgG antibody prevalence in M. natalensis between the sampling seasons. For one of the two studies, this result may have been influenced by a small sample size (n = 35) and longitudinal sampling across both endemic and non-endemic areas, as well as across multiple years [23]. In this same study, when seasonality was analyzed with a larger sample size (n = 553) strictly within Guinea, LASV prevalence in M. natalensis was significantly higher during the rainy season [23]. Therefore, seasonal differences in IgG antibodies may vary by species and location and should not be averaged across localities and years.
In the second of the two studies, failure to obtain a statistically significant result in the second longitudinal study may also have been due to longitudinal sampling across multiple localities, species and habitats [24]. This finding suggests that the phenomenon of seasonal viral fluctuations is best analyzed with species-specific, cross-sectional methods. This is also supported by the geographical disparity in M. natalensis seroprevalence being as high as 52% in Mayo Ranewo, Nigeria, 17–26% for other Nigerian localities, and as low as 1% in Abagboro [23].
3.3.2. Seasonal Impacts on Host Reproduction
M. natalensis breeding cycles have a strong seasonal trend and it has been hypothesized that fecundity may influence LASV infections in rodent populations, with reproduction being higher during the rainy season than in the dry season [25,26]. Only one included study investigated the relationship between fecundity and LASV prevalence in Mastomys and found no significant impact, leaving this to be a largely understudied topic [25].
After M. natalensis reproduce in the rainy season in Nigeria, there is a population boom in the dry season, introducing a large population of susceptible rodents to virus transmission [18]. When seasonal breeding is considered, mathematical modeling suggests that the number of infected rodents in Nigeria peaks in December, increasing the risk of LASV spillover to humans when rodents seek food inside homes during the dry season [18]. In contrast, another Nigerian study that also analyzed seasonal LASV trends predicted the number of infected rodents to be minimal in December; however, this model did not incorporate reproduction in their transmission model and predicted breeding to peak in the dry season, rather than the rainy season [21]. Incorporating M. natalensis reproduction into the LASV viral cycle has produced conflicting results. Further research would help to elucidate how the rodent breeding cycles influence viral spread, particularly in the context of the West African rainy and dry seasons.
Similarly to M. natalensis, M. erythroleucus was found to also primarily reproduce in the rainy season, creating a population increase and young age structure in the dry season [27]. However, M. erythroleucus have also been observed reproducing year round, while reproduction for M. natalensis is expected to almost completely halt from the late dry season (February) to the early rainy season (June) [26,27]. The relationship between secondary host breeding cycles, such as M. erythroleucus, and viral exposure has not been explored.
3.3.3. Rainfall and LF Incidence
Seasonal precipitation patterns were the leading environmental driver associated with LASV outbreaks in humans among articles screened. While precipitation may not affect the transmissibility of the virus directly, seasonal rainfall patterns influence the migration of host reservoirs and are significantly correlated with annual incidences of LF in humans [10,21,28]. M. natalensis populations are highly constrained by climatic variables and prefer areas of high, variable annual precipitation, with peak rainfall occurring in August and not exceeding 1500 mm annually [2,11,19,29,30]. Areas in West Africa that have experienced LF outbreaks in humans have had higher annual precipitation than those without, with epidemic peaks in the west of Africa and declining in the more arid northeast [12,19,21,31].
When this phenomenon was analyzed at the ward level within Nigeria, the smallest administrative level in Nigeria, wards with confirmed LF incidents had significantly lower average precipitation than those without LF incidents [32]. This finding suggests that precipitation may not influence epidemiological disease trends when analyzed at the community level, and may require larger expanses of spatial data to determine trends.
There is a significant, negative correlation between the timing of LF emergence events and rainfall, aligning with the established trend of increased LF cases in the dry season, particularly in Nigeria [21,29,33,34,35,36]. Mathematic epidemiological modeling demonstrates that epidemics in Nigeria between 2016 and 2020 consistently peaked in February, the height of the dry season in the region [37,38]. However, smaller outbreaks also occur year-round in Nigeria, due to endemic circulation [11,37,39,40,41].
3.4. Land Use
3.4.1. Impact of Households on LASV Transmission
Included studies determined that spatially autocorrelated clusters of seropositive Mastomys spp. are found more frequently in human houses in high-endemic villages in comparison to areas without LF endemics, indicating that household transmission of LASV constitutes a major disease pathway [42,43,44,45,46,47]. Additionally, LASV-infected rodents have also been shown to significantly cluster in refugee camps and student hostels, highlighting the risk that virus transmission extends beyond households to other types of anthropogenic settlements [48,49,50]. M. natalensis have a particularly dominant presence inside houses in West Africa, as evidenced by a study in Sierra Leone. Of 1490 small mammals captured, M. natalensis was the most commonly sampled species, with an overall prevalence of 23.8% (357 of 1490). Of the M. natalensis trapped inside homes, 28 of the 29 individuals tested positive for arenavirus IgG antibodies [24]. The transmission risk of LASV to humans was further confirmed in a 2021 study that isolated LASV RNA from M. natalensis feces, emphasizing the risk of transmission to humans within the household through rodent excrement [51].
Rodent infestations in homes in Guinea were also significantly correlated with high LASV antibodies in humans. In Gueckedou, study participants had a 14% antibody prevalence and 98.6% of respondents reported a rodent problem, whereas in Pita, Guinea, participants had a 2.6% antibody prevalence and 32.7% reported a rodent problem [52].
3.4.2. Rodent Abundance Inside Houses
Rodent abundance inside human houses is significantly influenced by seasonal precipitation patterns, regardless of infection status. Spatial analyses reveal that LASV-positive rodents cluster inside homes during the dry season, likely driven indoors in search of food during the agricultural off-season [44]. Rodent trapping success was found to be highest inside houses at the start of the rainy season, as the previous dry season concluded. As the rainy season progressed, rodent trapping success significantly decreased from 40% in April to 14% in July and then 24% in October, signifying the rodent migration from inside home during the dry season to outside homes during the rainy season as crops become plentiful again [23,26,29].
Two of the included studies also investigated the impact of seasonality on M. erythroleucus, a less prominent host of LASV. These studies determined that this species was less commonly found inside houses in comparison with M. natalensis, and had the highest overall abundance and trapping success indoors during the late rainy season [26,27].
When compared to other trapping locations, such as cultivations, forests, and savannah, included studies reported overall trapping success of Mastomys spp. to be highest inside human houses, particularly for M. natalensis [1,4,53,54,55,56]. A small mammal trapping study conducted in Sierra Leone determined that M. natalensis had an overall species prevalence of 23.8%, with 92% trapped inside homes [24]. The species abundance predominated indoors even when compared to surrounding agricultural land or bush area [42,45,57]. Specifically, having holes in the home, the presence of rodent burrows, and being in a multi-room square building were significantly associated with having increased rodent abundance [58]. When compared to other rodent species in West Africa, movements into or out of houses were mainly observed for M. natalensis [57]. In contrast, M. erthrythroleucus, had a low presence in human houses and can be predominately found in the savannah, fallow lands and cultivations, as defined by areas of land that are actively used for farming or agriculture [26].
3.4.3. Ecological Niche of LASV-Positive Rodents
Studies comparing the trapping success of seropositive M. natalensis outside of homes found that these rodents are most located in peridomestic settings, such as gardens and cultivated areas close to houses, and are less frequently found in cultivations and forests situated further from villages [1,13,19,43,53,59]. M. natalensis often move in between both locations and stay for extended periods of time indoors when food availability is limited outdoors [57]. For studies that did not include cultivations as trapping sites, or for areas where cultivations are not plentiful, the highest proportion of seropositive Mastomys spp. were found in savannahs, with forests being second, and coastal regions having the lowest percentage of seropositive rodents [19,53,57,60].
3.4.4. Habitat Fragmentation
Habitat fragmentation, as characterized by perimeter area ratios, was determined to be positively associated with LASV exposure risk in humans [61]. Additionally, high levels of vegetation are shown to be associated with lower levels of LASV [32,61]. LF occurrence is positively associated with agriculture land-use as the host species have shown a preference for recently cleared land and pastures, raising concerns that as agricultural and anthropogenic expansion in West Africa continues, the ecological niche for LASV may expand beyond current endemic areas [10,11,12,13,19].
Rice storage facilities and rice harvest yields were also shown to significantly characterize locations of human LF outbreaks [19,62,63].
3.4.5. Human LASV Differences in West Africa
The included studies determined that human LASV prevalence in endemic areas can vary across different landscapes. A study from Sierra Leone found notable disparities in human antibody prevalence, with 8% in a southern coastal village and 52% in an eastern province town characterized by tropical secondary forest and rich agricultural practices [45]. This trend was also determined in Guinea, where the difference in seropositivity was 11.9% in the coastal region and 59.6% in the forested region [61].
While Lassa fever is commonly regarded as a rural disease, there is conflicting evidence on this notion, suggesting a possible shift in epidemiologic transmission patterns. In Guinea, a difference in human LASV seroprevalence was observed between rural and urban areas, with higher prevalence in rural areas (12.9%) compared to urban (10.0%); however, it was also noted that sera from rural areas were less likely to be tested, revealing a possible sampling bias [64]. A small sample size (n = 11) from the 2016 outbreak in Nigeria revealed that eight (73%) of the confirmed cases resided in urban areas, while three (27%) were from rural areas [39]. This trend was further explored in a 2023 study that included data from the 2017–2021 outbreaks in Nigeria and 1057 laboratory confirmed cases. The average number of LF incidences in rural wards was significantly lower than in urban wards, where higher population densities may facilitate human-to-human transmission and improved access to healthcare [32].
3.5. Biodiversity Impacts on Lassa Virus
Two studies discussed the protective impacts of rodent species richness and biodiversity on LASV in West Africa [31,65]. Rodent species diversity was reported to be low in the LASV endemic areas of southern Mali, while species diversity was higher in northern Mali where the risk of LASV exposure is much lower [65]. While this study did not further analyze these findings, a 2021 modeling study concluded that rodent species richness had a significant negative association with LF emergence events, with areas of LF emergence having lower species richness than those without [31].
3.6. Other Hosts of Lassa Virus
3.6.1. Rodent Hosts
Mastomys spp. rodents, particularly M. natalensis, were the most common species found in the captured rodent populations across many studies [24,42,55,57,66,67,68,69,70]. LASV-positive M. natalensis adults had significantly smaller bodies, smaller brain volume, and smaller reproductive organs [71,72].
Of the included studies, five identified M. erythroleucus as a host species for LASV, with RNA LASV samples confirmed in Sierra Leone and Nigeria, and IgG antibodies detected in Guinea and Mali [14,23,43,50,73]. Phylogenetic analyses of the LASV sequences identified in M. erythroleucus in Nigeria revealed that these sequences emerged much more recently compared to those detected in M. natalensis, with emergence dates of 2005 and 1961, respectively [50,74]. It was also found that all M. erythroleucus derived variants always had an LASV sequence from M. natalensis as their closest relative, concluding that virus exchange between these species is possible and poses a threat of expansion into non-endemic areas through species exchange [50,75].
In addition to the identification of M. erythroleucus as a new host, PCR testing also identified RNA LASV sequences in L. sikapusi, H. pamfi, R. rattus, M. baoulei, Mus mattheyi and M. musculus, and IgG antibodies against LASV were detected in L. striatus, P. daltoni, M. minutoides and P. rostratus, P. misonnei [7,14,23,42,43,54,56,73,76,77,78]. Evidence of LASV RNA has also been identified in the Crocidura genus, also commonly referred to as white-toothed shrews, and the Tatera genus, also known as gerbils. However, these have not been classified to the species level and only a very small number of individuals have been detected with the virus [23,42].
Outbreaks in Ghana and Benin, where M. natalensis is not the predominant species, suggested that another rodent host may play a role in transmission. M. baoulei has been hypothesized as the fourth host species due to the presence of LASV-positive individuals in Benin, Togo, and Ghana. However, LASV has only been detected in a small number of individuals in two species of pygmy mice, including Mus baoulei and Mus mattheyi [7,54,78]. The role of pygmy mice as hosts of LASV should be further explored to elucidate the role that these species play in viral transfer to humans. To this end, included studies in this review identify M. natalensis, M. erythroleucus, and H. pamfi as the rodent species hosts for LASV.
While this review did not systematically search for rodent control measures, included studies concluded that the use of continuous control or rodent vaccination are more likely to significantly reduce LASV spillover [79,80].
3.6.2. Non-Rodent Hosts
Two included studies tested non-rodent animals to identify potential new hosts [81,82]. RNA LASV sequences were detected by PCR in multiple non-rodent, non-primate species; however, their involvement in LASV maintenance and transmission was not determined, requiring further research to analyze the infectivity and transmissibility amongst non-rodent hosts [81]. When primates were investigated, LF-specific antibodies were detected in a small number of Mona monkeys in Nigeria, suggesting that non-human primates are exposed to LASV, but the included study did not determine their role in virus transmission [82].
4. Discussion
4.1. Lassa Fever in a Changing Climate
4.1.1. Seasonality
Based on the included studies in this review, we identified seasonal precipitation as the primary driver of LASV in West Africa. Human LASV outbreaks typically occur during the dry seasons in West Africa, as particularly evidenced by the 2016–2020 epidemics in Nigeria, and are significantly dependent on the spatial occurrence of the host species [11,18,19,22]. Further, temperature and precipitation are significant contributing factors influencing ecological suitability for LASV, with precipitation being the main contributing factor to LASV in M. natalensis populations [10]. The cause of seasonal fluctuations of LASV prevalence in rodent populations and subsequent impact on human epidemics is still poorly understood and renders LASV transmission reliant on the environmental suitability for the host reservoirs. Climate models forecast an expansion of suitable habitat for M. natalensis in West Africa due to rising temperatures and increased precipitation, underscoring concerns that the global climate change may amplify disease transmission risks [19,83].
Global climate change is projected to impact the spread of the disease, with approximately twice as many spillover events predicted in West Africa by 2070 [19]. Historic climate data in West Africa have demonstrated a trend of increasing temperatures and precipitation from 1983 to 2010, with Ghana, Côte d’Ivoire, Guinea, and Senegal experiencing the most significant temperature changes, ranging from a 0.2 °C–0.5 °C increase per decade and increased precipitation of approximately 0.2–1.0 mm/day per decade across the entire region [83]. The present-day warming and increased variability of precipitation are projected to be exacerbated by future climate models, leading hypotheses that the endemic regions for LASV will expand. Ecological niche modeling predicts that by 2070 most of the region between Guinea and Nigeria will become suitable for LASV, as well as several non-endemic regions, including Cameroon, DRC, and areas in East Africa [10].
4.1.2. Growth of Agriculture
The prevalence of LASV host reservoirs is also influenced by land-use patterns. Habitat destruction and rapid human population growth in West Africa have led to the displacement of rodent species and forced them into closer proximity with humans. Included studies determined M. natalensis to have a higher abundance in human settlements and agriculture close to homes and found M. erythroleucus to prefer fallow lands and savannahs further from villages [26]. Rangeland and pasture coverage are a substantial factor influencing ecological suitability for LASV and projected deforestation and growth of agriculture threaten to expand the ecological niche of LASV [10]. Rapid population growth in West Africa is driving land conversion, particularly in areas suitable for rainfed agriculture, such as Sudanian and northern Guinean sub-regions, which constitute 93% of the transitions from natural to human-dominated land-cover types [84]. Substantial land conversion has also been observed in Nigeria and Sierra Leone. Nigeria’s savannahs have been cultivated by large-scale commercial farming, while the woodlands of Sierra Leone have been slash-and-burned to promote fallow and farmlands. The intensification of agriculture can be expected to increase as the population in West Africa continues to grow [84].
Human population growth in Africa is further marked by a distinct decrease in woody plant coverage due to agricultural expansion, with populations in Sub-Saharan Africa increasing by an average of 40 persons per km2 in areas where woody cover decreased due to agricultural expansion [85]. Areas of forest fragmentation have been shown to be hotspots for Ebola virus disease in Africa; however, the role of habitat fragmentation in LASV expansion is a critically understudied topic. One included study determined that fragmentation and the proportion of forests appear to affect exposure risk, but the role in virus circulation remains undetermined [61].
4.2. LF Host Expansion
Included studies identify M. erythroleucus as a secondary, less abundant host species for LASV. Though M. erythroleucus had a lower prevalence in trapping studies as compared to M. natalensis, the two Mastomys species occupy distinct ecological niches, suggesting that LASV has the potential to expand into new territories beyond the current range of M. natalensis [86]. While M. natalensis is considered a commensal species and dominates human-centric environments, M. erythroleucus is a generalist species and has greater abundance in natural habitats and fields [14,87]. The detection of viral RNA in the two Mastomys spp. species and LASV antibodies and RNA-isolates in several other rodent species provides evidence that the virus is capable of horizontal transmission amongst neighboring rodents. Further, a 2023 risk assessment ranked LASV the number one wildlife virus for risk of spillover based on a risk ranking system evaluating 31 host, virus and environmental risk factors [88]. While a few included studies discuss the inclusion of M. erythroleucus as a host, the role of this species in LASV transmission and spread is not yet known. The implications of host expansion and viral spread into non-endemic areas require deeper study to accurately target public health interventions. The promotion and implementation of vaccines for rodents and mammals as a prevention strategy should also be further explored [89].
4.3. Call to Action
Our review identified critical gaps in knowledge regarding the environmental drivers of LASV in West Africa. First, there is varying evidence on the seasonal fluctuations of LASV prevalence in host and human populations. Further research is required to comprehensively evaluate how seasonality impacts host ecology, food availability and reproduction and the subsequent influence on human epidemics. Second, climatic changes and agricultural growth in West Africa threaten to expand the ecological niche of LASV. Future climate projects are required to target areas of potential expansion and inform public health policy to prevent future epidemics. Finally, there is ample evidence to suggest that other rodent species are exposed to LASV and may be capable of transmitting the virus; however, current studies do not explore their role in the LASV transmission cycle. Additional research should explore how rodent reservoirs in West Africa are harboring and transmitting the virus between species, as viral adaptation and host switching suggest that LASV may be able to expand past its current range via new hosts.
Through extensive data extraction and literature searching, this review reveals the most critical gap in knowledge as the impact of biodiversity loss on LASV expansion and ecological dynamics in West Africa. West Africa has become a hub for agricultural development and human expansion in recent years, leading to a rise in deforestation and biodiversity loss [90]. As a result, understanding the potential implications of these environmental changes on viral transmission is critical for safeguarding human health. Despite the growing body of research on biodiversity loss and disease emergence, only two included studies investigated its effects explicitly on LASV. This phenomenon has been explored for West Nile encephalitis, Hantavirus pulmonary syndrome and Lyme disease, where biodiversity loss has been shown to increase rates of transmission [91]. The “dilution effect”, a commonly studied theory in ecology, suggests that a higher biodiversity often leads to lower infection prevalence in host species. Reservoir hosts, such as M. natalensis, tend to be generalist species and thrive in areas of human disturbance, while habitat specialists, such as predators, die out from a lack of complex habitat [92,93].
The “dilution effect” and the impact of biodiversity loss on viral transmission has been investigated for many infectious diseases. Notably, the dilution of Puumala hantavirus (PUUV) was tested in bank voles, the host of PUUV [92]. The field vole (Microtus agrestis) and the common shrew (Sorex anaeus) are competitors of the bank vole (Myodes glareolus) [92]. When the system of competitors and predators was altered, increasing the density of the competitors decreased infection probability [92].
The competitor and predator model requires further research for LASV hosts. There is growing evidence that the invasive black rat, Rattus rattus, is outcompeting M. natalensis, suggesting a possible impact on zoonotic LASV spillover. Capture sites in Sierra Leone and Guinea that had high R. rattus captures had lower M. natalensis catches per trap [94]. Zoonotic disease spillover risk was also negatively related to the presence of R. rattus, while no other rodent species had a similar impact on catch per trap of LASV-positive M. natalensis. Further, the mean R. rattus presence effect was larger in magnitude when compared to seasonality effect within the fitted models, suggesting that R. rattus presence may be even more influential on M. natalensis abundance than seasonality. Further research is needed to elucidate the exact mechanisms by which R. rattus may influence M. natalensis populations and subsequently impact LASV spillover; however, these preliminary data exemplify the potential impact of biodiversity, as well as competitor ecology, on zoonotic disease spillover.
4.4. Study Limitations
This systematic review had several important limitations. First, due to journal accessibility and language restrictions, our literature search only included one database search of African origin. African Journals Online (AJOL) was chosen due to its global impact, commitment to the amplification of African research and diverse inclusion of many open-access, African journals. However, as a result, we may have missed papers not published in the journals included through AJOL.
Second, our search strategy only included articles with an English translation and may have missed relevant papers published in other languages. We chose to include the term “host” and not “reservoir” in our search strategy given that hosts include reservoirs as well as secondary hosts; however, it is possible that this decision affected the number of studies included in the systematic review. Third, we limited our Google Scholar extractions to the first 400 articles returned. This quantity was chosen to encapsulate search returns of all sizes; however, this strategy may have missed important articles that were not captured in this amount.
Fourth, many included studies focused on specific countries or regions in West Africa, particularly those with a higher socioeconomic standing and access to research facilities. This geographical bias may not be representative of the entire region. Further, our review focused on environmental drivers of LASV and may have missed other contextual factors that influence viral transmission, including socioeconomic conditions, human behavior, and healthcare systems. While these variables can have crucial impacts on viral expansion, it was beyond the scope of this review to include in the search strategies and analyses.
Finally, biases were considered in the interpretation of results from all included papers. Included papers were evaluated for inherent biases, including confounding, exposure/case selection, classification of interventions, missing data, measurement of outcomes, and selection of reported results. Papers with identified biases were discussed by the study team and our protocol was to remove inclusion eligibility for any paper that included biases that may have impacted reported results.
5. Conclusions
This systematic review provides a comprehensive overview of the environmental drivers of LASV in West Africa. Climate models predict an expansion of suitable environmental conditions for M. natalensis due to rising temperatures and increased precipitation, while rapid population growth in West Africa is influencing the conversion of natural habitats to agricultural lands and threatening to expand the ecological niche of LASV host reservoirs. The mechanisms by which seasonal precipitation, land-use change, and host dynamics drive human LASV epidemics in West Africa are not fully understood, highlighting a need for further research to guide future public health efforts and targeted research. Ultimately, this review underscores the urgent need for interdisciplinary research and proactive preventative strategies to mitigate the impacts of environmental change on LASV transmission and protect vulnerable populations in West Africa.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/v17040504/s1, (Table S1) Search strategy tables, (Table S2) List of included articles, (Table S3) Excel file with all studies from PRISMA diagram.
Author Contributions
Conceptualization, N.A.D. and J.D.G.J.; methodology, N.A.D., J.D.G.J., N.A.D., J.L.D.S. and J.D.G.J.; formal analysis, N.A.D., J.L.D.S. and J.D.G.J.; investigation, N.A.D. and M.A.K.; resources, J.D.G.J.; data curation, N.A.D. and M.A.K.; writing—original draft preparation, N.A.D.; writing—review and editing, N.A.D., M.A.K., B.M.G., J.L.D.S. and J.D.G.J.; visualization, N.A.D. and M.A.K.; supervision, J.D.G.J.; project administration, J.D.G.J.; funding acquisition, J.D.G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
The authors would like to thank John Bourgeois at Tufts Veterinary School and Page Scudder at Tufts School of Medicine for their guidance and support in conducting the literature searches for this systematic review. Funding for this systematic review was provided through internal funds of The Gass Lab of Tufts University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa Virus Isolation from Mastomys natalensis Rodents during an Epidemic in Sierra Leone. Science 1974, 185, 263–265. [Google Scholar] [PubMed]
- Basinski, A.J.; Fichet-Calvet, E.; Sjodin, A.R.; Varrelman, T.J.; Remien, C.H.; Layman, N.C.; Bird, B.H.; Wolking, D.J.; Monagin, C.; Ghersi, B.M.; et al. Bridging the gap: Using reservoir ecology and human serosurveys to estimate Lassa virus spillover in West Africa. PLoS Comput. Biol. 2021, 17, e1008811. [Google Scholar]
- Agbonlahor, D.E.; Akpede, G.O.; Happi, C.T.; Tomori, O. 52 Years of Lassa Fever Outbreaks in Nigeria, 1969–2020: An Epidemiologic Analysis of the Temporal and Spatial Trends. Am. J. Trop. Med. Hyg. 2021, 105, 974–985. [Google Scholar]
- Fichet-Calvet, E.; Lecompte, E.; Koivogui, L.; Soropogui, B.; Doré, A.; Kourouma, F.; Sylla, O.; Daffis, S.; Koulemou, K.; Ter Meulen, J. Fluctuation of Abundance and Lassa Virus Prevalence in Mastomys natalensis in Guinea, West Africa. Vector-Borne Zoonotic Dis. 2007, 7, 119–128. [Google Scholar] [PubMed]
- Frame, J.D.; Gocke, D.J.; Baldwin, J.M.; Troup, J.M. Lassa Fever, a New Virus Disease of Man from West Africa: I. Clinical Description and Pathological Findings. Am. J. Trop. Med. Hyg. 1970, 19, 670–676. [Google Scholar]
- Garry, R.F. Lassa fever—The road ahead. Nat. Rev. Microbiol. 2023, 21, 87–96. [Google Scholar]
- Yadouleton, A.; Picard, C.; Rieger, T.; Loko, F.; Cadar, D.; Kouthon, E.C.; Job, E.O.; Bankolé, H.; Oestereich, L.; Gbaguidi, F.; et al. Lassa fever in Benin: Description of the 2014 and 2016 epidemics and genetic characterization of a new Lassa virus. Emerg. Microbes Infect. 2020, 9, 1761–1770. [Google Scholar]
- Gibb, R.; Moses, L.M.; Redding, D.W.; Jones, K.E. Understanding the cryptic nature of Lassa fever in West Africa. Pathog. Glob. Health 2017, 111, 276–288. [Google Scholar] [PubMed]
- Mylne, A.Q.N.; Pigott, D.M.; Longbottom, J.; Shearer, F.; Duda, K.A.; Messina, J.P.; Weiss, D.J.; Moyes, C.L.; Golding, N.; Hay, S.I. Mapping the zoonotic niche of Lassa fever in Africa. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 483–492. [Google Scholar]
- Klitting, R.; Kafetzopoulou, L.E.; Thiery, W.; Dudas, G.; Gryseels, S.; Kotamarthi, A.; Vrancken, B.; Gangavarapu, K.; Momoh, M.; Sandi, J.D.; et al. Predicting the evolution of the Lassa virus endemic area and population at risk over the next decades. Nat. Commun. 2022, 13, 5596. [Google Scholar]
- Redding, D.W.; Gibb, R.; Dan-Nwafor, C.C.; Ilori, E.A.; Yashe, R.U.; Oladele, S.H.; Amedu, M.O.; Iniobong, A.; Attfield, L.A.; Donnelly, C.A.; et al. Geographical drivers and climate-linked dynamics of Lassa fever in Nigeria. Nat. Commun. 2021, 12, 5759. [Google Scholar] [CrossRef]
- Nimo-Paintsil, S.C.; Fichet-Calvet, E.; Borremans, B.; Letizia, A.G.; Mohareb, E.; Bonney, J.H.K.; Obiri-Danso, K.; Ampofo, W.K.; Schoepp, R.J.; Kronmann, K.C. Rodent-borne infections in rural Ghanaian farming communities. PLoS ONE 2019, 14, e0215224. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.; Bett, B.; Said, M.; Bukachi, S.; Sang, R.; Anderson, N.; Machila, N.; Kuleszo, J.; Schaten, K.; Dzingirai, V.; et al. Local disease–ecosystem–livelihood dynamics: Reflections from comparative case studies in Africa. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160163. [Google Scholar]
- Olayemi, A.; Cadar, D.; Magassouba, N.; Obadare, A.; Kourouma, F.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Rieger, T.; Bockholt, S.; et al. New Hosts of The Lassa Virus. Sci. Rep. 2016, 6, 25280. [Google Scholar]
- Centers for Disease Control and Prevention. Bioterrorism Agents/Diseases [Internet]. CDC|Bioterrorism Agents/Diseases (by Category)|Emergency Preparedness & Response. 2018. Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 16 August 2024).
- World Health Organization. Prioritizing Diseases for Research and Development in Emergency Contexts [Internet]. 2019. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts (accessed on 24 August 2024).
- Fischer, W.A.; Wohl, D.A. Moving Lassa Fever Research and Care into the 21st Century. J. Infect. Dis. 2017, 215, 1779–1781. [Google Scholar] [PubMed]
- McKendrick, J.Q.; Tennant, W.S.D.; Tildesley, M.J. Modelling seasonality of Lassa fever incidences and vector dynamics in Nigeria. PLoS Negl. Trop. Dis. 2023, 17, e0011543. [Google Scholar]
- Redding, D.W.; Moses, L.M.; Cunningham, A.A.; Wood, J.; Jones, K.E. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: A case study of Lassa fever. Methods Ecol. Evol. 2016, 7, 646–655. [Google Scholar]
- García-Peña, G.E.; Rubio, A.V.; Mendoza, H.; Fernández, M.; Milholland, M.T.; Aguirre, A.A.; Suzán, G.; Zambrana-Torrelio, C. Land-use change and rodent-borne diseases: Hazards on the shared socioeconomic pathways. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200362. [Google Scholar] [CrossRef]
- Akhmetzhanov, A.R.; Asai, Y.; Nishiura, H. Quantifying the seasonal drivers of transmission for Lassa fever in Nigeria. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180268. [Google Scholar]
- Musa, S.S.; Zhao, S.; Gao, D.; Lin, Q.; Chowell, G.; He, D. Mechanistic modelling of the large-scale Lassa fever epidemics in Nigeria from 2016 to 2019. J. Theor. Biol. 2020, 493, 110209. [Google Scholar]
- Olayemi, A.; Oyeyiola, A.; Obadare, A.; Igbokwe, J.; Adesina, A.S.; Onwe, F.; Ukwaja, K.N.; Ajayi, N.A.; Rieger, T.; Günther, S.; et al. Widespread arenavirus occurrence and seroprevalence in small mammals, Nigeria. Parasites Vectors 2018, 11, 416. [Google Scholar]
- Bangura, U.; Buanie, J.; Lamin, J.; Davis, C.; Bongo, G.N.; Dawson, M.; Ansumana, R.; Sondufu, D.; Thomson, E.C.; Sahr, F.; et al. Lassa Virus Circulation in Small Mammal Populations in Bo District, Sierra Leone. Biology 2021, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; LeCompte, E.; Koivogui, L.; Daffis, S.; Meulen, J.T. Reproductive Characteristics of Mastomys natalensis and Lassa Virus Prevalence in Guinea, West Africa. Vector-Borne Zoonotic Dis. 2008, 8, 41–48. [Google Scholar]
- Olayemi, A.; Obadare, A.; Oyeyiola, A.; Fasogbon, S.; Igbokwe, J.; Igbahenah, F.; Ortsega, D.; Günther, S.; Verheyen, E.; Fichet-Calvet, E. Small mammal diversity and dynamics within Nigeria, with emphasis on reservoirs of the lassa virus. Syst. Biodivers. 2018, 16, 118–127. [Google Scholar]
- Fichet-Calvet, E.; Lecompte, E.; Veyrunes, F.; Barrière, P.; Nicolas, V.; Koulémou, K. Diversity and dynamics in a community of small mammals in coastal Guinea, West Africa. Belg. J. Zool. 2009, 139, 93–102. [Google Scholar]
- Zhao, S.; Musa, S.S.; Fu, H.; He, D.; Qin, J. Large-scale Lassa fever outbreaks in Nigeria: Quantifying the association between disease reproduction number and local rainfall. Epidemiol. Infect. 2020, 148, e4. [Google Scholar] [PubMed]
- Ifejube, O.J.; Babalola, S.O.; Mukaila, I.O.; Badewa, A.O. A GIS-based approach to risk mapping of lassa fever outbreak in akure south local government area, Nigeria. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2022, 46, 147–153. [Google Scholar]
- Fichet-Calvet, E.; Rogers, D.J. Risk Maps of Lassa Fever in West Africa. PLoS Negl. Trop. Dis. 2009, 3, e388. [Google Scholar]
- Min, K.D.; Hwang, J.; Schneider, M.C.; So, Y.; Lee, J.Y.; Cho, S.-I. An exploration of the protective effect of rodent species richness on the geographical expansion of Lassa fever in West Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009108. [Google Scholar]
- Cadmus, S.; Taiwo, O.J.; Akinseye, V.; Cadmus, E.; Famokun, G.; Fagbemi, S.; Ansumana, R.; Omoluabi, A.; Ayinmode, A.; Oluwayelu, D.; et al. Ecological correlates and predictors of Lassa fever incidence in Ondo State, Nigeria 2017–2021: An emerging urban trend. Sci. Rep. 2023, 13, 20855. [Google Scholar]
- Shaffer, J.G.; Grant, D.S.; Schieffelin, J.S.; Boisen, M.L.; Goba, A.; Hartnett, J.N.; Levy, D.C.; Yenni, R.E.; Moses, L.M.; Fullah, M.; et al. Lassa Fever in Post-Conflict Sierra Leone. PLoS Negl. Trop. Dis. 2014, 8, e2748. [Google Scholar]
- Barua, S.; Dénes, A.; Ibrahim, M.A. A seasonal model to assess intervention strategies for preventing periodic recurrence of Lassa fever. Heliyon 2021, 7, e07760. [Google Scholar] [PubMed]
- Nchom, J.I.; Abubakar, A.S.; Arimoro, F.O.; Mohammed, B.Y. The Role of Weather in the Spread of Lassa Fever in Parts of Northern Nigeria. Int. J. Trop. Dis. Health 2021, 42, 33–40. [Google Scholar]
- OYaa, P.N.; Oluseyi, O.B.; Chukwuyem, A.; Muyiwa, O.J. Mapping of Lassa Fever Epidemics in Owo, Ondo State, Nigeria, 2018–2020: A Descriptive and Categorical Analysis of Age, Gender and Seasonal Pattern. J. Public Allied Health Sci. 2021, 3, 22. [Google Scholar]
- Ibrahim, M.A.; Dénes, A. A mathematical model for Lassa fever transmission dynamics in a seasonal environment with a view to the 2017–20 epidemic in Nigeria. Nonlinear Anal. Real. World Appl. 2021, 60, 103310. [Google Scholar]
- Yaro, C.A.; Kogi, E.; Opara, K.N.; Batiha, G.E.-S.; Baty, R.S.; Albrakati, A.; Altalbawy, F.M.A.; Etuh, I.U.; Oni, J.P. Infection pattern, case fatality rate and spread of Lassa virus in Nigeria. BMC Infect. Dis. 2021, 21, 149. [Google Scholar]
- Shehu, N.Y.; Gomerep, S.S.; Isa, S.E.; Iraoyah, K.O.; Mafuka, J.; Bitrus, N.; Dachom, M.; Ogwuche, J.; Onukak, A.; Onyedibe, K.; et al. Lassa Fever 2016 Outbreak in Plateau State, Nigeria—The Changing Epidemiology and Clinical Presentation. Front. Public Health 2018, 6, 232. [Google Scholar]
- Fasipe, O.J.; Osho, P.O.; Osho, E.S.; Adu, B.S.; Akinrotimi, O.J.; Folayan, W.A.; Adebimpe, W.O. The observed seasonal variation pattern and changing epidemiology of lassa viral hemorrhagic fever disease in Ondo State, Nigeria. Med. J. Dr. Patil. Vidyapeeth 2020, 13, 22. [Google Scholar]
- Okoro, O.A.; Bamgboye, E.; Dan-Nwafor, C.; Umeokonkwo, C.; Ilori, E.; Yashe, R.; Balogun, M.; Nguku, P.; Ihekweazu, C. Descriptive epidemiology of Lassa fever in Nigeria, 2012–2017. Pan Afr. Med. J. 2020, 37, 15. [Google Scholar]
- Happi, A.N.; Olumade, T.J.; Ogunsanya, O.A.; Sijuwola, A.E.; Ogunleye, S.C.; Oguzie, J.U.; Nwofoke, C.; Ugwu, C.A.; Okoro, S.J.; Otuh, P.I.; et al. Increased Prevalence of Lassa Fever Virus-Positive Rodents and Diversity of Infected Species Found during Human Lassa Fever Epidemics in Nigeria. Microbiol. Spectr. 2022, 10, e00366-22. [Google Scholar]
- Fichet-Calvet, E.; Becker-Ziaja, B.; Koivogui, L.; Günther, S. Lassa Serology in Natural Populations of Rodents and Horizontal Transmission. Vector-Borne Zoonotic Dis. 2014, 14, 665–674. [Google Scholar] [PubMed]
- Mariën, J.; Lo Iacono, G.; Rieger, T.; Magassouba, N.; Günther, S.; Fichet-Calvet, E. Households as hotspots of Lassa fever? Assessing the spatial distribution of Lassa virus-infected rodents in rural villages of Guinea. Emerg. Microbes Infect. 2020, 9, 1055–1064. [Google Scholar] [PubMed]
- McCormick, J.B.; Webb, P.A.; Krebs, J.W.; Johnson, K.M.; Smith, E.S. A Prospective Study of the Epidemiology and Ecology of Lassa Fever. J. Infect. Dis. 1987, 155, 437–444. [Google Scholar]
- Bonwitt, J.; Saez, A.M.; Lamin, J.; Ansumana, R.; Dawson, M.; Buanie, J.; Lamin, J.; Sondufu, D.; Borchert, M.; Sahr, F.; et al. At Home with Mastomys and Rattus: Human–Rodent Interactions and Potential for Primary Transmission of Lassa Virus in Domestic Spaces. Am. J. Trop. Med. Hyg. 2017, 96, 935. [Google Scholar]
- Fichet-Calvet, E.; Ölschläger, S.; Strecker, T.; Koivogui, L.; Becker-Ziaja, B.; Camara, A.B.; Soropogui, B.; Magassouba, N.; Günther, S. Spatial and temporal evolution of Lassa virus in the natural host population in Upper Guinea. Sci. Rep. 2016, 6, 21977. [Google Scholar]
- Fair, J.; Jentes, E.; Inapogui, A.; Kourouma, K.; Goba, A.; Bah, A.; Tounkara, M.; Coulibaly, M.; Garry, R.F.; Bausch, D.G. Lassa Virus-Infected Rodents in Refugee Camps in Guinea: A Looming Threat to Public Health in a Politically Unstable Region. Vector-Borne Zoonotic Dis. 2007, 7, 167–171. [Google Scholar]
- Lalis, A.; Leblois, R.; Lecompte, E.; Denys, C.; Ter Meulen, J.; Wirth, T. The Impact of Human Conflict on the Genetics of Mastomys natalensis and Lassa Virus in West Africa. PLoS ONE 2012, 7, e37068. [Google Scholar]
- Adesina, A.S.; Oyeyiola, A.; Obadare, A.; Igbokwe, J.; Abejegah, C.; Akhilomen, P.; Bangura, U.; Asogun, D.; Tobin, E.; Ayodeji, O.; et al. Circulation of Lassa virus across the endemic Edo-Ondo axis, Nigeria, with cross-species transmission between multimammate mice. Emerg. Microbes Infect. 2023, 12, 2219350. [Google Scholar]
- Wood, R.; Bangura, U.; Mariën, J.; Douno, M.; Fichet-Calvet, E. Detection of Lassa virus in wild rodent feces: Implications for Lassa fever burden within households in the endemic region of Faranah, Guinea. One Health 2021, 13, 100317. [Google Scholar]
- Ter Meulen, J.; Lukashevich, I.; Sidibe, K.; Inapogui, A.; Marx, M.; Dorlemann, A.; Yansane, M.L.; Koulemou, K.; Chang-Claude, J.; Schmitz, H. Hunting of Peridomestic Rodents and Consumption of Their Meat as Possible Risk Factors for Rodent-to-Human Transmission of Lassa Virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 1996, 55, 661–666. [Google Scholar]
- Demby, A.H.; Inapogui, A.; Kargbo, K.; Koninga, J.; Kourouma, K.; Kanu, J.; Coulibaly, M.; Wagoner, K.D.; Ksiazek, T.G.; Peters, C.; et al. Lassa Fever in Guinea: II. Distribution and Prevalence of Lassa Virus Infection in Small Mammals. Vector-Borne Zoonotic Dis. 2001, 1, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Kronmann, K.C.; Nimo-Paintsil, S.; Guirguis, F.; Kronmann, L.C.; Bonney, K.; Obiri-Danso, K.; Ampofo, W.; Fichet-Calvet, E. Two Novel Arenaviruses Detected in Pygmy Mice, Ghana. Emerg. Infect. Dis. 2013, 19, 1832–1835. [Google Scholar] [CrossRef]
- Johnson, K.M.; Webb, P.A.; Smith, E.; Keenlyside, R.A.; Elliott, L.; McCormick, J.B. Case-Control Study of Mastomys natalensis and Humans in Lassa Virus-Infected Households in Sierra Leone. Am. J. Trop. Med. Hyg. 1983, 32, 829–837. [Google Scholar]
- Gonzalez, J.P.; McCormick, J.B.; Saluzzo, J.F.; Herve, J.P.; Georges, A.J.; Johnson, K.M. An arenavirus isolated from wild-caught rodents (Pramys species) in the Central African Republic. Intervirology 1983, 19, 105–112. [Google Scholar] [CrossRef]
- Mariën, J.; Kourouma, F.; Magassouba, N.; Leirs, H.; Fichet-Calvet, E. Movement Patterns of Small Rodents in Lassa Fever-Endemic Villages in Guinea. EcoHealth 2018, 15, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Yakob, L.; Douno, M.; Lamine, J.; Magassouba, N.; Fichet-Calvet, E.; Mari-Saez, A. Domestic risk factors for increased rodent abundance in a Lassa fever endemic region of rural Upper Guinea. Sci. Rep. 2021, 11, 20698. [Google Scholar] [CrossRef] [PubMed]
- Layman, N.C.; Basinski, A.J.; Zhang, B.; Eskew, E.A.; Bird, B.H.; Ghersi, B.M.; Bangura, J.; Fichet-Calvet, E.; Remien, C.H.; Vandi, M.; et al. Predicting the fine-scale spatial distribution of zoonotic reservoirs using computer vision. Ecol. Lett. 2023, 26, 1974–1986. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Koulemou, K.; Koivogui, L.; Soropogui, B.; Sylla, O.; Lecompte, E.; Daffis, S.; Bernard, A.; Kouassi, K.; Akoua-Koffi, C.; et al. Spatial distribution of commensal rodents in regions with high and low Lassa fever prevalence in Guinea. Belg. J. Zool. 2005, 135, 63–67. [Google Scholar]
- Longet, S.; Leggio, C.; Bore, J.A.; Key, S.; Tipton, T.; Hall, Y.; Koundouno, F.R.; Bower, H.; Bhattacharyya, T.; Magassouba, N.; et al. Influence of Landscape Patterns on Exposure to Lassa Fever Virus, Guinea. Emerg. Infect. Dis. 2023, 29, 304–313. [Google Scholar] [CrossRef]
- Olugasa, B.O.; Dogba, J.B.; Ogunro, B.; Odigie, E.A.; Nykoi, J.; Ojo, J.F.; Taiwo, O.; Kamara, A.; Mulbah, C.K.; Fasunla, A.J. The rubber plantation environment and Lassa fever epidemics in Liberia, 2008–2012: A spatial regression. Spat. Spatio-Temporal Epidemiol. 2014, 11, 163–174. [Google Scholar] [CrossRef]
- Amifofum, O.S.; Fadugba, D.; Awosanya, E.; Icomiare, A.; Evbuomwan, K.; Balogun, M.S.; Nguku, P.; Ajisegiri, W.S.; Eugene, O.; Olugasa, B. Spatial analysis of confirmed Lassa fever cases in Edo State, Nigeria, 2008–2014. PAMJ—One Health 2021, 5, 11. [Google Scholar] [CrossRef]
- Kernéis, S.; Koivogui, L.; Magassouba, N.; Koulemou, K.; Lewis, R.; Aplogan, A.; Grais, R.F.; Guerin, P.J.; Fichet-Calvet, E. Prevalence and Risk Factors of Lassa Seropositivity in Inhabitants of the Forest Region of Guinea: A Cross-Sectional Study. PLoS Negl. Trop. Dis. 2009, 3, e548. [Google Scholar] [CrossRef] [PubMed]
- Safronetz, D.; Sogoba, N.; Lopez, J.E.; Maiga, O.; Dahlstrom, E.; Zivcec, M.; Feldmann, F.; Haddock, E.; Fischer, R.; Anderson, J.; et al. Geographic Distribution and Genetic Characterization of Lassa Virus in Sub-Saharan Mali. PLoS Negl. Trop. Dis. 2013, 7, e2582. [Google Scholar]
- Saez, A.M.; Haidara, M.C.; Camara, A.; Kourouma, F.; Sage, M.; Magassouba, N.; Fichet-Calvet, E. Rodent control to fight Lassa fever: Evaluation and lessons learned from a 4-year study in Upper Guinea. PLoS Negl. Trop. Dis. 2018, 12, e0006829. [Google Scholar]
- Leski, T.A.; Stockelman, M.G.; Moses, L.M.; Park, M.; Stenger, D.A.; Ansumana, R.; Bausch, D.G.; Lin, B. Sequence Variability and Geographic Distribution of Lassa Virus, Sierra Leone. Emerg. Infect. Dis. 2015, 21, 609–618. [Google Scholar]
- Safronetz, D.; Lopez, J.E.; Sogoba, N.; Traore’, S.F.; Raffel, S.J.; Fischer, E.R.; Ebihara, H.; Branco, L.; Garry, R.F.; Schwan, T.G.; et al. Detection of Lassa Virus, Mali. Emerg. Infect. Dis. 2010, 16, 1123–1126. [Google Scholar] [CrossRef]
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulemou, K.; Sylla, O.; Kourouma, F.; Dore, A.; Soropogui, B.; Aniskin, V.; Bernard, A.K.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [PubMed]
- Simons, D.; Attfield, L.A.; Jones, K.E.; Watson-Jones, D.; Kock, R. Rodent trapping studies as an overlooked information source for understanding endemic and novel zoonotic spillover. PLoS Negl. Trop. Dis. 2023, 17, e0010772. [Google Scholar]
- Lalis, A.; Evin, A.; Janier, M.; Koivogui, L.; Denys, C. Host evolution in Mastomys natalensis (Rodentia: Muridae): An integrative approach using geometric morphometrics and genetics. Integr. Zool. 2015, 10, 505–514. [Google Scholar] [CrossRef]
- Demartini, J.C.; Green, D.E.; Monath, T.P. Lassa virus infection in Mastomys natalensis in Sierra Leone. Gross and microscopic findings in infected and uninfected animals. Bull. World Health Organ. 1975, 52, 651–663. [Google Scholar]
- Bangura, U.; Davis, C.; Lamin, J.; Bangura, J.; Soropogui, B.; Davison, A.J.; Nichols, J.; Vucak, M.; Dawson, M.; Ansumana, R.; et al. Spatio-temporal spread of Lassa virus and a new rodent host in the Mano River Union area, West Africa. Emerg. Microbes Infect. 2024, 13, 2290834. [Google Scholar] [CrossRef]
- Olayemi, A.; Adesina, A.S.; Strecker, T.; Magassouba, N.; Fichet-Calvet, E. Determining Ancestry between Rodent- and Human-Derived Virus Sequences in Endemic Foci: Towards a More Integral Molecular Epidemiology of Lassa Fever within West Africa. Biology 2020, 9, 26. [Google Scholar] [CrossRef]
- Mariën, J.; Borremans, B.; Gryseels, S.; Soropogui, B.; De Bruyn, L.; Bongo, G.N.; Becker-Ziaja, B.; de Bellocq, J.G.; Günther, S.; Magassouba, N.; et al. No measurable adverse effects of Lassa, Morogoro and Gairo arenaviruses on their rodent reservoir host in natural conditions. Parasit. Vectors 2017, 10, 210. [Google Scholar] [PubMed]
- Agbonlahor, D.; Erah, A.; Agba, I.; Oviasogie, F.; Ehiaghe, A.; Wankasi, M.; Eremwanarue, O.; Ehiaghe, I.; Ogbu, E.; Iyen, R.; et al. Prevalence of Lassa virus among rodents trapped in three South-South States of Nigeria. J. Vector Borne Dis. 2017, 54, 146. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Fabiyi, A.; Monath, T.P. Recent isolations of Lassa virus from Nigerian rodents. Bull. World Health Organ. 1975, 52, 609–613. [Google Scholar]
- Yadouleton, A.; Agolinou, A.; Kourouma, F.; Saizonou, R.; Pahlmann, M.; Bedié, S.K.; Bankolé, H.; Becker-Ziaja, B.; Gbaguidi, F.; Thielebein, A.; et al. Lassa Virus in Pygmy Mice, Benin, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1977–1979. [Google Scholar] [CrossRef]
- Mariën, J.; Borremans, B.; Kourouma, F.; Baforday, J.; Rieger, T.; Günther, S.; Magassouba, N.; Leirs, H.; Fichet-Calvet, E. Evaluation of rodent control to fight Lassa fever based on field data and mathematical modelling. Emerg. Microbes Infect. 2019, 8, 640–649. [Google Scholar] [CrossRef]
- Nuismer, S.L.; Remien, C.H.; Basinski, A.J.; Varrelman, T.; Layman, N.; Rosenke, K.; Bird, B.; Jarvis, M.; Barry, P.; Hanley, P.W.; et al. Bayesian estimation of Lassa virus epidemiological parameters: Implications for spillover prevention using wildlife vaccination. PLoS Negl. Trop. Dis. 2020, 14, e0007920. [Google Scholar] [CrossRef]
- Happi, A.N.; Ogunsanya, O.A.; Ayinla, A.O.; Sijuwola, A.E.; Saibu, F.M.; Akano, K.; Nwofoke, C.; Elias, O.T.; Achonduh-Atijegbe, O.; Daodu, R.O.; et al. Lassa virus in novel hosts: Insights into the epidemiology of lassa virus infections in southern Nigeria. Emerg. Microbes Infect. 2024, 13, 2294859. [Google Scholar] [CrossRef]
- Ogunro, B.N.; Olugasa, B.O.; Kayode, A.; Ishola, O.O.; Kolawole, O.N.; Odigie, E.A.; Happi, C. Detection of Antibody and Antigen for Lassa Virus Nucleoprotein in Monkeys from Southern Nigeria. J. Epidemiol. Glob. Health 2019, 9, 125–127. [Google Scholar] [CrossRef]
- Sylla, M.B.; Nikiema, P.M.; Gibba, P.; Kebe, I.; Klutse, N.A.B. Climate Change over West Africa: Recent Trends and Future Projections. In Adaptation to Climate Change and Variability in Rural West Africa [Internet]; Yaro, J.A., Hesselberg, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–40. Available online: http://link.springer.com/10.1007/978-3-319-31499-0_3 (accessed on 6 August 2024).
- Herrmann, S.M.; Brandt, M.; Rasmussen, K.; Fensholt, R. Accelerating land cover change in West Africa over four decades as population pressure increased. Commun. Earth Environ. 2020, 1, 53. [Google Scholar]
- Brandt, M.; Rasmussen, K.; Peñuelas, J.; Tian, F.; Schurgers, G.; Verger, A.; Mertz, O.; Palmer, J.R.B.; Fensholt, R. Human population growth offsets climate-driven increase in woody vegetation in sub-Saharan Africa. Nat. Ecol. Evol. 2017, 1, 0081. [Google Scholar]
- Granjon, L.; Artige, E.; Bâ, K.; Brouat, C.; Dalecky, A.; Diagne, C.; Diallo, M.; Fossati-Gaschignard, O.; Gauthier, P.; Kane, M.; et al. Sharing space between native and invasive small mammals: Study of commensal communities in Senegal. Ecol. Evol. 2023, 13, e10539. [Google Scholar]
- Fichet-Calvet, E.; Audenaert, L.; Barrière, P.; Verheyen, E. Diversity, dynamics and reproduction in a community of small mammals in Upper Guinea, with emphasis on pygmy mice ecology. Afr. J. Ecol. 2010, 48, 600–614. [Google Scholar]
- Grange, Z.L.; Goldstein, T.; Johnson, C.K.; Anthony, S.; Gilardi, K.; Daszak, P.; Olival, K.J.; O’rourke, T.; Murray, S.; Olson, S.H.; et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118. [Google Scholar]
- Kenmoe, S.; Tchatchouang, S.; Ebogo-Belobo, J.T.; Ka’E, A.C.; Mahamat, G.; Simo, R.E.G.; Bowo-Ngandji, A.; Emoh, C.P.D.; Che, E.; Ngongang, D.T.; et al. Systematic review and meta-analysis of the epidemiology of Lassa virus in humans, rodents and other mammals in sub-Saharan Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008589. [Google Scholar]
- USAID Environment—Biodiversity and Climate Change [Internet]. United States Agency for International Development. Available online: https://www.usaid.gov/west-africa-regional/environment-biodiversty-and-climate-change (accessed on 14 August 2024).
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.E.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar]
- Khalil, H.; Ecke, F.; Evander, M.; Magnusson, M.; Hörnfeldt, B. Declining ecosystem health and the dilution effect. Sci. Rep. 2016, 6, 31314. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Effects of Host Diversity on Infectious Disease. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 157–182. [Google Scholar]
- Eskew, E.A.; Bird, B.H.; Ghersi, B.M.; Bangura, J.; Basinski, A.J.; Amara, E.; Bah, M.A.; Kanu, M.C.; Kanu, O.T.; Lavalie, E.G.; et al. Reservoir displacement by an invasive rodent reduces Lassa virus zoonotic spillover risk. Nat. Commun. 2024, 15, 3589. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).