Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and Sample Preparation
2.3. RNA Extraction and cDNA Synthesis
2.4. Standard Plasmid Construction
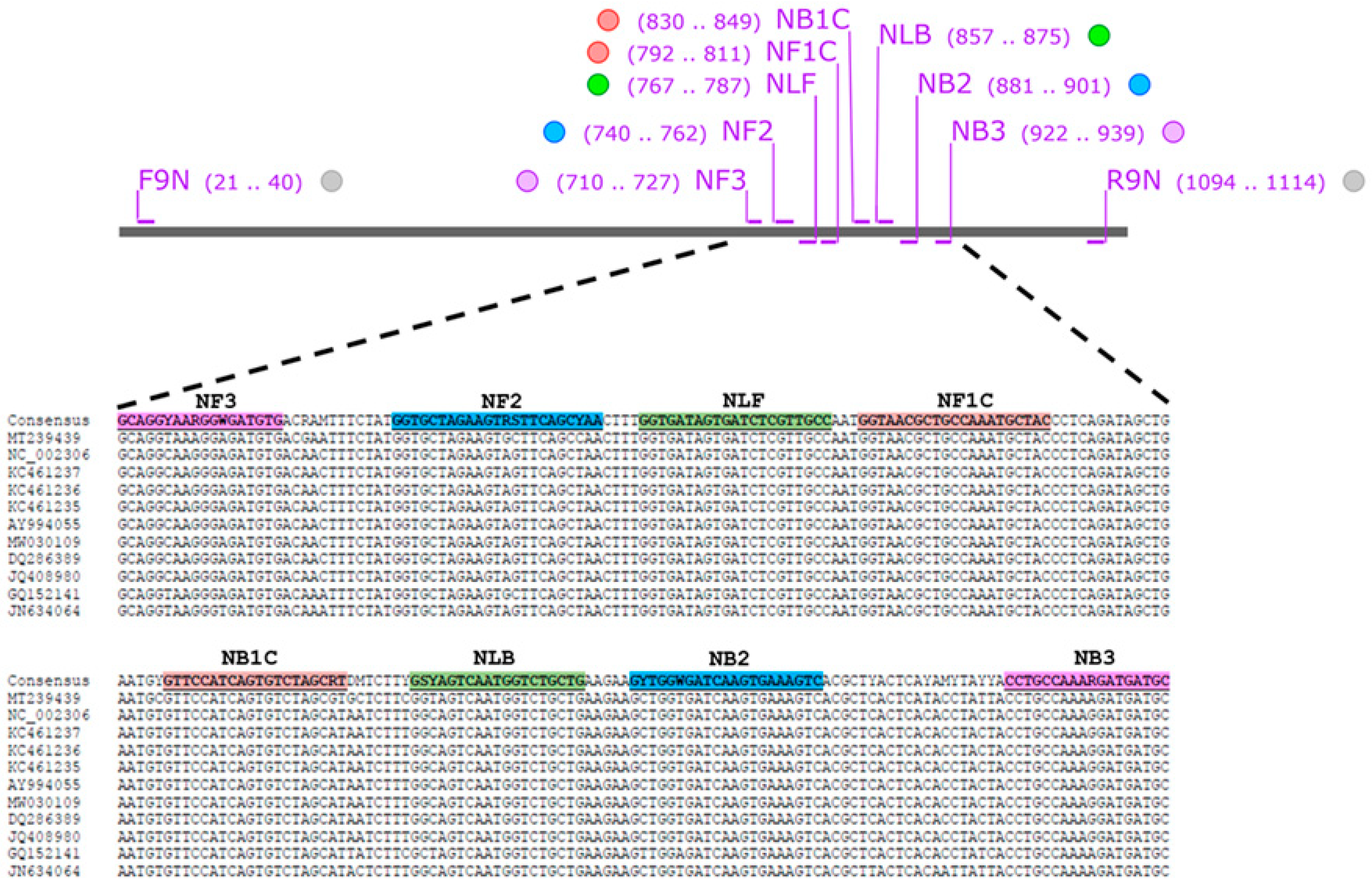
2.5. LAMP Primer Design
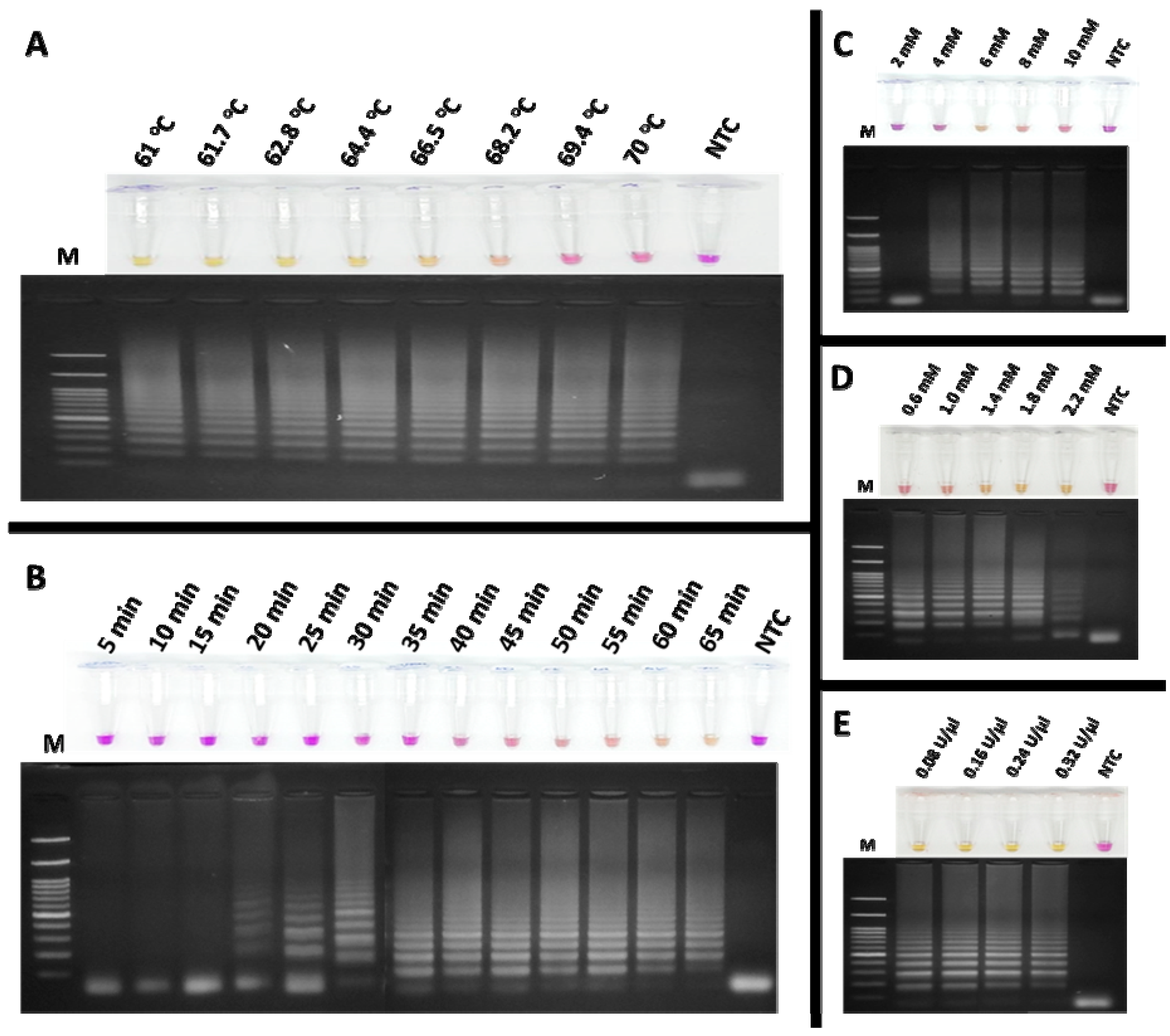
2.6. RT-LAMP Reaction and Optimization
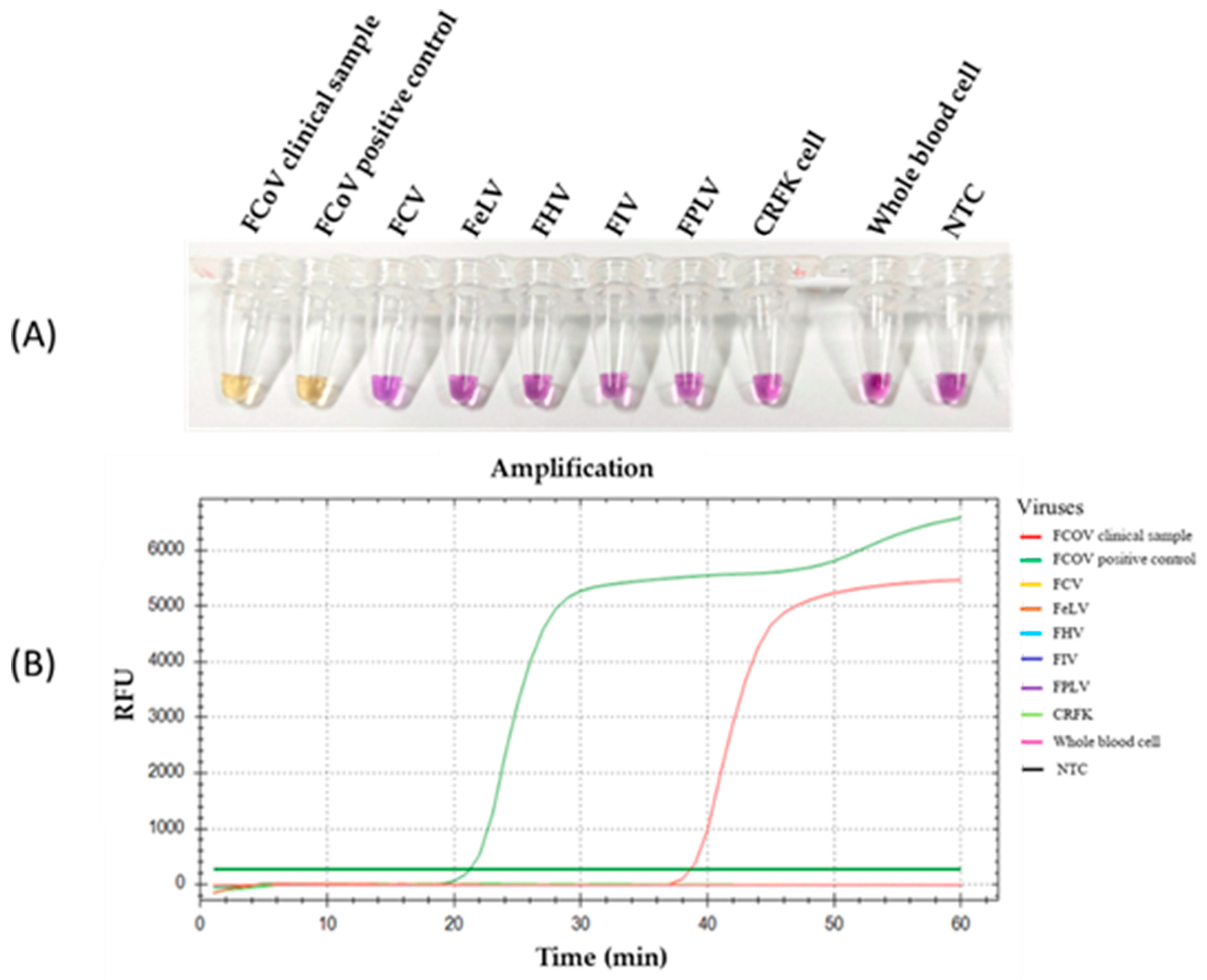
2.7. Analytical Specificity of RT-LAMP-XO
2.8. Quantitative PCR (qPCR) Reaction Condition
2.9. Comparative Sensitivity of RT-LAMP-XO and qPCR Assay
2.10. Diagnosis and Statistical Analysis of FCoV Infected Clinical Sample
3. Results
3.1. Design and Verification of FCoV LAMP Primer
3.2. Optimization of RT-LAMP Conditions for FCoV Detection
3.3. Specificity of RT-LAMP-XO for FCoV Detection
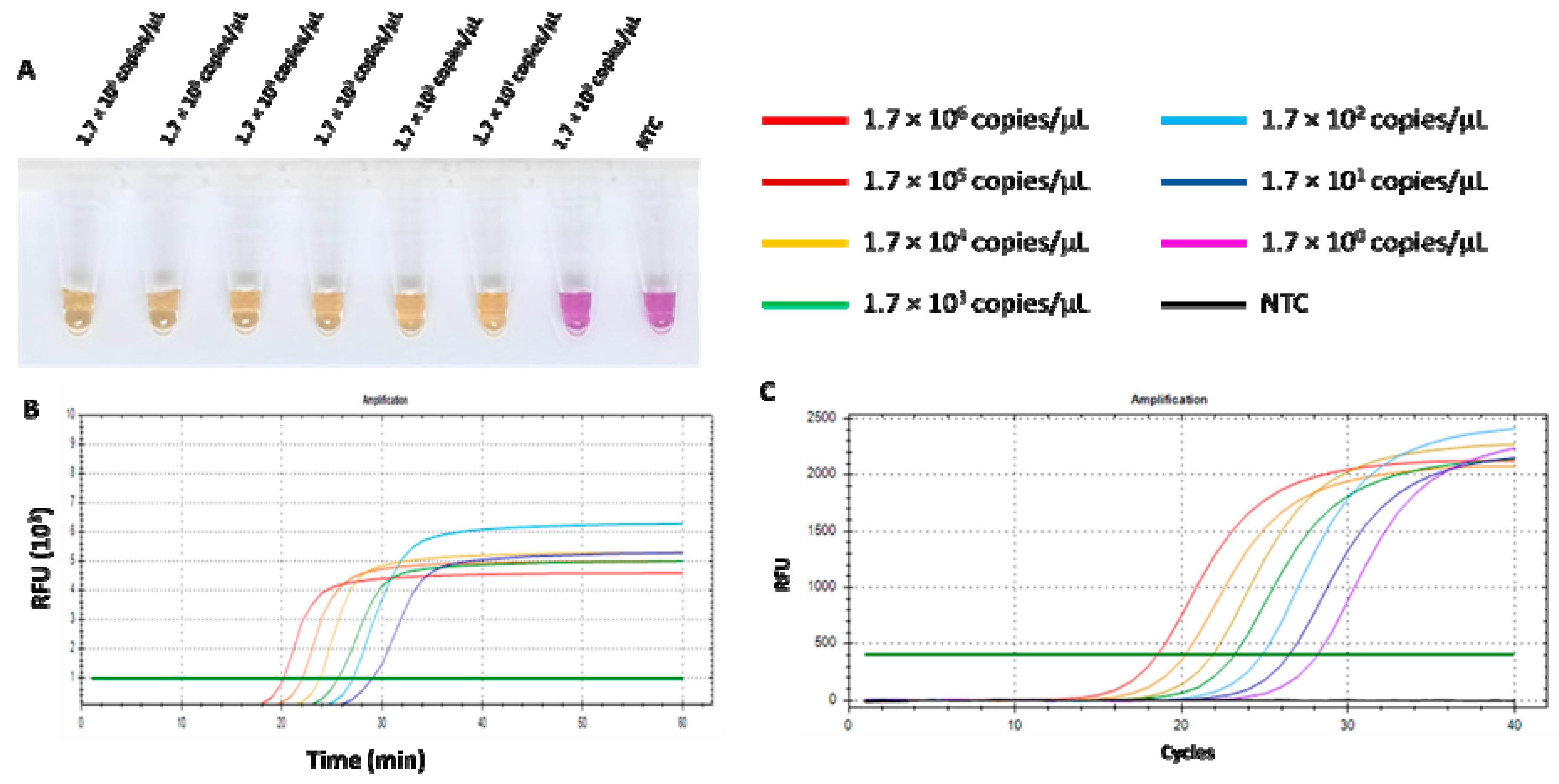
3.4. Comparison of RT-LAMP-XO and qPCR on Sensitivity for FCoV Detection
3.5. RT-LAMP-XO Assay Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, N.C. An Update on Feline Infectious Peritonitis: Diagnostics and Therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef]
- Kipar, A.; Meli, M.L. Feline Infectious Peritonitis: Still an Enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef]
- Masters, P.S.; Perlman, S. Coronaviridae. Fields Virol. 2013, 1, 825–858. [Google Scholar]
- Ehmann, R.; Kristen-Burmann, C.; Bank-Wolf, B.; König, M.; Herden, C.; Hain, T.; Thiel, H.J.; Ziebuhr, J.; Tekes, G. Reverse Genetics for Type I Feline Coronavirus Field Isolate to Study the Molecular Pathogenesis of Feline Infectious Peritonitis. mBio 2018, 9, e01422-18. [Google Scholar] [CrossRef]
- Poder, S. Le Feline and Canine Coronaviruses: Common Genetic and Pathobiological Features. Adv. Virol. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Fish, E.J.; Diniz, P.P.V.P.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-Sectional Quantitative RT-PCR Study of Feline Coronavirus Viremia and Replication in Peripheral Blood of Healthy Shelter Cats in Southern California. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef]
- Bank-Wolf, B.R.; Stallkamp, I.; Wiese, S.; Moritz, A.; Tekes, G.; Thiel, H.J. Mutations of 3c and Spike Protein Genes Correlate with the Occurrence of Feline Infectious Peritonitis. Vet. Microbiol. 2014, 173, 177–188. [Google Scholar] [CrossRef]
- Jaimes, J.A.; Millet, J.K.; Stout, A.E.; André, N.M.; Whittaker, G.R. A Tale of Two Viruses: The Distinct Spike Glycoproteins of Feline Coronaviruses. Viruses 2020, 12, 83. [Google Scholar] [CrossRef]
- Herrewegh, A.A.P.M.; De Groot, R.J.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J.M. Detection of Feline Coronavirus RNA in Feces, Tissues, and Body Fluids of Naturally Infected Cats by Reverse Transcriptase PCR. J. Clin. Microbiol. 1995, 33, 684. [Google Scholar] [CrossRef]
- Terada, Y.; Matsui, N.; Noguchi, K.; Kuwata, R.; Shimoda, H.; Soma, T.; Mochizuki, M.; Maeda, K. Emergence of Pathogenic Coronaviruses in Cats by Homologous Recombination between Feline and Canine Coronaviruses. PLoS ONE 2014, 9, e106534. [Google Scholar] [CrossRef]
- Haijema, B.J.; Volders, H.; Rottier, P.J.M. Switching Species Tropism: An Effective Way to Manipulate the Feline Coronavirus Genome. J. Virol. 2003, 77, 4528–4538. [Google Scholar] [CrossRef] [PubMed]
- Rottier, P.J.M.; Nakamura, K.; Schellen, P.; Volders, H.; Haijema, B.J. Acquisition of Macrophage Tropism during the Pathogenesis of Feline Infectious Peritonitis Is Determined by Mutations in the Feline Coronavirus Spike Protein. J. Virol. 2005, 79, 14122. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, J.; Zhang, M.; Deng, X.; Song, J.; Zhu, J.; Yu, L.; Li, G.; Liu, G. An Adenovirus-Vectored Vaccine Based on the N Protein of Feline Coronavirus Elicit Robust Protective Immune Responses. Antivir. Res. 2024, 223, 105825. [Google Scholar] [CrossRef]
- Battilani, M.; Foschi, A.; Scagliarini, A.; Ciulli, S.; Prosperi, S.; Morganti, L. Analysis of the N Protein in Feline Coronavirus Strains in Italy. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2006; Volume 581, pp. 403–406. [Google Scholar]
- Poncelet, L.; Coppens, A.; Peeters, D.; Bianchi, E.; Grant, C.K.; Kadhim, H. Detection of Antigenic Heterogeneity in Feline Coronavirus Nucleocapsid in Feline Pyogranulomatous Meningoencephalitis. Vet. Pathol. 2008, 45, 140–153. [Google Scholar] [CrossRef]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of Different Diagnostic Tests for Feline Infectious Peritonitis in Challenging Clinical Cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef]
- Wilkes, R.P.; Anis, E.; Dunbar, D.; Lee, P.Y.A.; Tsai, Y.L.; Lee, F.C.; Chang, H.F.G.; Wang, H.T.T.; Graham, E.M. Rapid and Sensitive Insulated Isothermal PCR for Point-of-Need Feline Leukaemia Virus Detection. J. Feline Med. Surg. 2018, 20, 362–369. [Google Scholar] [CrossRef]
- Günther, S.; Felten, S.; Wess, G.; Hartmann, K.; Weber, K. Detection of Feline Coronavirus in Effusions of Cats with and without Feline Infectious Peritonitis Using Loop-Mediated Isothermal Amplification. J. Virol. Methods 2018, 256, 32. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Nagamine, K.; Watanabe, K.; Ohtsuka, K.; Hase, T.; Notomi, T. Loop-Mediated Isothermal Amplification Reaction Using a Nondenatured Template. Clin. Chem. 2001, 47, 1742–1743. [Google Scholar] [CrossRef]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.V.L.; Morita, K. Loop Mediated Isothermal Amplification (LAMP): A New Generation of Innovative Gene Amplification Technique; Perspectives in Clinical Diagnosis of Infectious Diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP) of Gene Sequences and Simple Visual Detection of Products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, X.; Wang, X.; Yang, J.; Zhang, L.; Deng, Q.; Zhang, X.; Wang, Z.; Hou, T.; Li, S. A Novel One-Pot Rapid Diagnostic Technology for COVID-19. Anal. Chim. Acta 2021, 1154, 338310. [Google Scholar] [CrossRef] [PubMed]
- Saharan, P.; Khatri, P.; Dingolia, S.; Duhan, J.S.; Gahlawat, S.K.; Khatri, P.; Dingolia, S.; Gahlawat, S.K. Rapid Detection of Viruses Using Loop-Mediated Isothermal Amplification (LAMP): A Review. In Biotechnology: Prospects and Applications; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Rapichai, W.; Saejung, W.; Khumtong, K.; Boonkaewwan, C.; Tuanthap, S.; Lieberzeit, P.A.; Choowongkomon, K.; Rattanasrisomporn, J. Development of Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Detecting Feline Coronavirus. Animals 2022, 12, 2075. [Google Scholar] [CrossRef]
- Jaroenram, W.; Cecere, P.; Pompa, P.P. Xylenol Orange-Based Loop-Mediated DNA Isothermal Amplification for Sensitive Naked-Eye Detection of Escherichia coli. J. Microbiol. Methods 2019, 156, 9–14. [Google Scholar] [CrossRef]
- Tuanthap, S.; Chiteafea, N.; Rattanasrisomporn, J.; Choowongkomon, K. Comparative Sequence Analysis of the Accessory and Nucleocapsid Genes of Feline Coronavirus Strains Isolated from Cats Diagnosed with Effusive Feline Infectious Peritonitis. Arch. Virol. 2021, 166, 2779–2787. [Google Scholar] [CrossRef]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A Method for the Absolute Quantification of CDNA Using Real-Time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Moyadee, W.; Jaroensong, T.; Roytrakul, S.; Boonkaewwan, C.; Rattanasrisomporn, J. Characteristic Clinical Signs and Blood Parameters in Cats with Feline Infectious Peritonitis. Agr. Nat. Resour. 2019, 53, 433–438. [Google Scholar]
- Moyadee, W.; Sunpongsri, S.; Choowongkomon, K.; Roytrakul, S.; Rattanasrisomporn, A.; Tansakul, N.; Rattanasrisomporn, J. Feline Infectious Peritonitis: A Comprehensive Evaluation of Clinical Manifestations, Laboratory Diagnosis, and Therapeutic Approaches. J. Adv. Vet. Anim. Res. 2024, 11, 19–26. [Google Scholar] [CrossRef]
- Moyadee, W.; Chiteafea, N.; Tuanthap, S.; Choowongkomon, K.; Roytrakul, S.; Rungsuriyawiboon, O.; Boonkaewwan, C.; Tansakul, N.; Rattanasrisomporn, A.; Rattanasrisomporn, J. The First Study on Clinicopathological Changes in Cats with Feline Infectious Peritonitis with and without Retrovirus Coinfection. Vet. World 2023, 16, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Inaba, M.; Higashimoto, Y.; Toyama, Y.; Horiguchi, T.; Hibino, M.; Iwata, M.; Imaizumi, K.; Doi, Y. Diagnostic Accuracy of LAMP versus PCR over the Course of SARS-CoV-2 Infection. Int. J. Infect. Dis. 2021, 107, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.H.; Drummond, R.S.M.; Jelley, L.; Baker, L.; Smit, E.; Fleming, R.; Billington, C. Optimization and Benchmarking of RT-LAMP-CRISPR-Cas12a for the Detection of SARS-CoV-2 in Saliva. Int. J. Mol. Sci. 2025, 26, 1806. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S. Diagnosis of Feline Infectious Peritonitis: Update on Evidence Supporting Available Tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef]
- Nemoto, M.; Imagawa, H.; Tsujimura, K.; Yamanaka, T.; Kondo, T.; Matsumura, T. Detection of Equine Rotavirus by Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP). J. Vet. Med. Sci. 2010, 72, 823–826. [Google Scholar] [CrossRef]
- Banoo, S.; Bell, D.; Bossuyt, P.; Herring, A.; Mabey, D.; Poole, F.; Smith, P.G.; Sriram, N.; Wongsrichanalai, C.; Linke, R.; et al. Evaluation of Diagnostic Tests for Infectious Diseases: General Principles. Nat. Rev. Microbiol. 2006, 4, S20–S32. [Google Scholar] [CrossRef]
- Sheikhi, F.; Zeinoddini, M.; Jalili, S. Optimization for Rapid Detection of Staphylococcus Aureus Using Real-Time LAMP. J. Appl. Biotechnol. Rep. 2023, 10, 984–991. [Google Scholar] [CrossRef]
- Tanner, N.A.; Evans, T.C. Loop-Mediated Isothermal Amplification for Detection of Nucleic Acids. Curr. Protoc. Mol. Biol. 2014, 105, 15.14.1–15.14.14. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of Loop-Mediated Isothermal Amplification to a Culture Medium and Biological Substances. J. Biochem. Biophys Methods 2007, 70, 499–501. [Google Scholar] [CrossRef]
- Behler, J.; Hess, W.R. Approaches to Study CRISPR RNA Biogenesis and the Key Players Involved. Methods 2020, 172, 12–26. [Google Scholar] [CrossRef]
- Poole, C.B.; Tanner, N.A.; Zhang, Y.; Evans, T.C.; Carlow, C.K.S. Diagnosis of Brugian Filariasis by Loop-Mediated Isothermal Amplification. PLoS Negl. Trop. Dis. 2012, 6, e1948. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.; Guo, J.; Chen, R.; Grisham, M.P.; Que, Y. Development of Loop-Mediated Isothermal Amplification for Detection of Leifsonia xyli subsp. xyli in Sugarcane. Biomed. Res. Int. 2013, 2013, 357692. [Google Scholar] [CrossRef]
- Nie, X. Reverse Transcription Loop-Mediated Isothermal Amplification of DNA for Detection of Potato Virus Y. Plant Dis. 2005, 89, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex Polymerase Chain Reaction: A Practical Approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef]
- Chandra, A.; Keizerweerd, A.T.; Que, Y.; Grisham, M.P. Loop-Mediated Isothermal Amplification (LAMP) Based Detection of Colletotrichum Falcatum Causing Red Rot in Sugarcane. Mol. Biol. Rep. 2015, 42, 1309–1316. [Google Scholar] [CrossRef]
- Tanner, N.A.; Zhang, Y.; Evans, T.C. Visual Detection of Isothermal Nucleic Acid Amplification Using PH-Sensitive Dyes. Biotechniques 2015, 58, 59–68. [Google Scholar] [CrossRef]
- Park, G.-S.; Kim, S.-J.; Maeng, J.-S. Development and Evaluation of RT-LAMP Assays to Identify Variants of SARS-CoV-2. bioRxiv 2022, 2022, 496383. [Google Scholar] [CrossRef]
- Bálint, Á.; Farsang, A.; Zádori, Z.; Hornyák, Á.; Dencső, L.; Almazán, F.; Enjuanes, L.; Belák, S. Molecular Characterization of Feline Infectious Peritonitis Virus Strain DF-2 and Studies of the Role of ORF3abc in Viral Cell Tropism. J. Virol. 2012, 86, 6258–6267. [Google Scholar] [CrossRef]
- Khamsingnok, P.; Rapichai, W.; Rattanasrisomporn, A.; Rungsuriyawiboon, O.; Choowongkomon, K.; Rattanasrisomporn, J. Comparison of PCR, Nested PCR, and RT-LAMP for Rapid Detection of Feline Calicivirus Infection in Clinical Samples. Animals 2024, 14, 2432. [Google Scholar] [CrossRef]
- Bhat, A.I.; Aman, R.; Mahfouz, M. Onsite Detection of Plant Viruses Using Isothermal Amplification Assays. Plant Biotechnol. J. 2022, 20, 1859–1873. [Google Scholar] [CrossRef]
- Nie, K.; Qi, S.-X.; Zhang, Y.; Luo, L.; Xie, Y.; Yang, M.-J.; Zhang, Y.; Li, J.; Shen, H.; Li, Q.; et al. Evaluation of a Direct Reverse Transcription Loop-Mediated Isothermal Amplification Method without RNA Extraction for the Detection of Human Enterovirus 71 Subgenotype C4 in Nasopharyngeal Swab Specimens. PLoS ONE 2012, 7, e52486. [Google Scholar] [CrossRef]
- Mahmud, S.; Paul, G.K.; Afroze, M.; Islam, S.; Gupt, S.B.R.; Razu, M.H.; Biswas, S.; Zaman, S.; Uddin, M.S.; Khan, M.; et al. Efficacy of Phytochemicals Derived from Avicennia Officinalis for the Management of COVID-19: A Combined in Silico and Biochemical Study. Molecules 2021, 26, 2210. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Rashid, S.; Iralu, N.; Ullah, P.; Nabi, S.U.; Hamid, A. Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) for Plant Viral Detection. In Detection of Plant Viruses: Advanced Techniques; Springer: New York, NY, USA, 2025; pp. 187–192. [Google Scholar]
- Mehdi Aghapour-ojaghkandi, M.A.A. Visual Detection of Curly Top Virus by the Colorimetric Loop-Mediated Isothermal Amplification. J. Plant Pathol. Microbiol. 2013, 4, 198. [Google Scholar] [CrossRef]
- De Biase, I.; Yuzyuk, T.; Hernandez, A.; Basinger, A. An Unusually High Excretion of Ethylmalonic Acid in a Patient with Multiple Acyl-CoA Dehydrogenase Deficiency. Clin. Chem. 2021, 67, 1290–1292. [Google Scholar] [CrossRef]
- Moehling, T.J.; Choi, G.; Dugan, L.C.; Salit, M.; Meagher, R.J. LAMP Diagnostics at the Point-of-Care: Emerging Trends and Perspectives for the Developer Community. Expert. Rev. Mol. Diagn. 2021, 21, 43–61. [Google Scholar] [CrossRef]
- Fellner, M.D.; Bonaventura, R.; Basiletti, J.; Avaro, M.; Benedetti, E.; Campos, A.; Dattero, M.E.; Russo, M.; Vladmirsky, S.; Molina, V.; et al. Evaluation of Rt-Qpcr and Loop-Mediated Isothermal Amplification (Lamp) Assays for the Detection of Sars-Cov-2 in Argentina. Genes 2021, 12, 659. [Google Scholar] [CrossRef]
- Brotons, P.; De Paz, H.D.; Esteva, C.; Latorre, I.; Muñoz-Almagro, C. Validation of a Loop-Mediated Isothermal Amplification Assay for Rapid Diagnosis of Pertussis Infection in Nasopharyngeal Samples. Expert. Rev. Mol. Diagn. 2016, 16, 125–130. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Saejung, W.; Khumtong, K.; Rapichai, W.; Ratanabunyong, S.; Rattanasrisomporn, A.; Choowongkomon, K.; Rungsuriyawiboon, O.; Rattanasrisomporn, J. Detection of Feline Immunodeficiency Virus by Neutral Red-Based Loop-Mediated Isothermal Amplification Assay. Vet. World 2024, 17, 72–81. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; et al. OpvCRISPR: One-Pot Visual RT-LAMP-CRISPR Platform for SARS-Cov-2 Detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef]
- Craw, P.; Balachandran, W. Isothermal Nucleic Acid Amplification Technologies for Point-of-Care Diagnostics: A Critical Review. Lab. Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef] [PubMed]
- Özay, B.; McCalla, S.E. A Review of Reaction Enhancement Strategies for Isothermal Nucleic Acid Amplification Reactions. Sens. Actuators Rep. 2021, 3, 100033. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors—Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Wilson, I.G. Inhibition and Facilitation of Nucleic Acid Amplification. Appl. Environ. Microbiol. 1997, 63, 3741–3751. [Google Scholar] [CrossRef]
- Helfer-Hungerbuehler, A.K.; Spiri, A.M.; Meili, T.; Riond, B.; Krentz, D.; Zwicklbauer, K.; Buchta, K.; Zuzzi-Krebitz, A.M.; Hartmann, K.; Hofmann-Lehmann, R.; et al. Alpha-1-Acid Glycoprotein Quantification via Spatial Proximity Analyte Reagent Capture Luminescence Assay: Application as Diagnostic and Prognostic Marker in Serum and Effusions of Cats with Feline Infectious Peritonitis Undergoing GS-441524 Therapy. Viruses 2024, 16, 791. [Google Scholar] [CrossRef]
- Rodriguez-Manzano, J.; Malpartida-Cardenas, K.; Moser, N.; Pennisi, I.; Cavuto, M.; Miglietta, L.; Moniri, A.; Penn, R.; Satta, G.; Randell, P.; et al. Handheld Point-of-Care System for Rapid Detection of SARS-CoV-2 Extracted RNA in under 20 Min. ACS Cent. Sci. 2021, 7, 307–317. [Google Scholar] [CrossRef]
- Seetaha, S.; Khamplong, P.; Wanaragthai, P.; Aiebchun, T.; Ratanabunyong, S.; Krobthong, S.; Yingchutrakul, Y.; Rattanasrisomporn, J.; Choowongkomon, K. KERRA, Mixed Medicinal Plant Extracts, Inhibits SARS-CoV-2 Targets Enzymes and Feline Coronavirus. Covid 2022, 2, 621–632. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine Coronavirus: Not Only an Enteric Pathogen. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1121–1132. [Google Scholar] [CrossRef]
- Pourakbari, R.; Gholami, M.; Shakerimoghaddam, A.; Khiavi, F.M.; Mohammadimehr, M.; Khomartash, M.S. Comparison of RT-LAMP and RT-QPCR Assays for Detecting SARS-CoV-2 in the Extracted RNA and Direct Swab Samples. J. Virol. Methods 2024, 324, 114871. [Google Scholar] [CrossRef]
- Amer, A.; Siti Suri, A.; Abdul Rahman, O.; Mohd, H.B.; Faruku, B.; Saeed, S.; Tengku Azmi, T.I. Isolation and Molecular Characterization of Type i and Type II Feline Coronavirus in Malaysia. Virol. J. 2012, 9, 278. [Google Scholar] [CrossRef]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.-H.; Kim, Y.; Oh, S.; Kim, Y.-I.; Choi, W.-S.; Kim, S.G.; Jeong, J.H.; et al. Development of a Reverse Transcription-Loop-Mediated Isothermal Amplification as a Rapid Early-Detection Method for Novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Feline Infectious Peritonitis. ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11, 594–604. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Sequence (5′ → 3′) | Ref. |
|---|---|---|
| NF3 | GCAGGYAARGGWGATGTG | This study |
| NB3 | GCATCATCYTTTGGCAGG | |
| NFIP (NF1c-NF2) | GTAGCRTTTGGCAGCGWTAYGG-TGCTAGAWGTRSTTCAGCYAA | |
| NBIP(NB1c-NB2) | GTTCCATCAGTGTCTAGCRTG-ACTTTCACYTGRTCWCCARC | |
| NLF | RGYAACGAGATCACTATCACC | |
| NLB | GSYAGTCAATGGTCTGCTG | |
| F9N | CGTCAACTGGGGAGATGAAC | [28] |
| R9N | CATCTCAACCTGTGTGTCATC |
| qPCR+ | qPCR− | Total | |
|---|---|---|---|
| RT-LAMP-XO + | 17 | 0 | 17 |
| RT-LAMP-XO − | 0 | 60 | 60 |
| Total | 17 | 60 | |
| (%) Sensitivity (95% CI) | 100 (80.49% to 100%) | ||
| (%) Specificity (95% CI) | 100 (94.04% to 100%) | ||
| FCoV prevalence | 22.08 (13.42% to 32.98%) | ||
| (%) Positive Predictive Value (95% CI) | 100 (80.49% to 100%) | ||
| (%) Negative Predictive Value (95% CI) | 100 (94.04% to 100%) | ||
| (%) Accuracy (95% CI) | 100 (95.32% to 100%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumtong, K.; Rapichai, W.; Saejung, W.; Khamsingnok, P.; Meecharoen, N.; Ratanabunyong, S.; Dong, H.V.; Tuanthap, S.; Rattanasrisomporn, A.; Choowongkomon, K.; et al. Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection. Viruses 2025, 17, 418. https://doi.org/10.3390/v17030418
Khumtong K, Rapichai W, Saejung W, Khamsingnok P, Meecharoen N, Ratanabunyong S, Dong HV, Tuanthap S, Rattanasrisomporn A, Choowongkomon K, et al. Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection. Viruses. 2025; 17(3):418. https://doi.org/10.3390/v17030418
Chicago/Turabian StyleKhumtong, Kotchaporn, Witsanu Rapichai, Wichayet Saejung, Piyamat Khamsingnok, Nianrawan Meecharoen, Siriluk Ratanabunyong, Hieu Van Dong, Supansa Tuanthap, Amonpun Rattanasrisomporn, Kiattawee Choowongkomon, and et al. 2025. "Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection" Viruses 17, no. 3: 418. https://doi.org/10.3390/v17030418
APA StyleKhumtong, K., Rapichai, W., Saejung, W., Khamsingnok, P., Meecharoen, N., Ratanabunyong, S., Dong, H. V., Tuanthap, S., Rattanasrisomporn, A., Choowongkomon, K., Rungsuriyawiboon, O., & Rattanasrisomporn, J. (2025). Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification with Xylenol Orange Targeting Nucleocapsid Gene for Detection of Feline Coronavirus Infection. Viruses, 17(3), 418. https://doi.org/10.3390/v17030418








