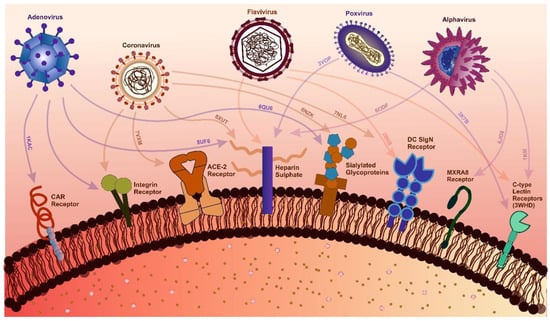
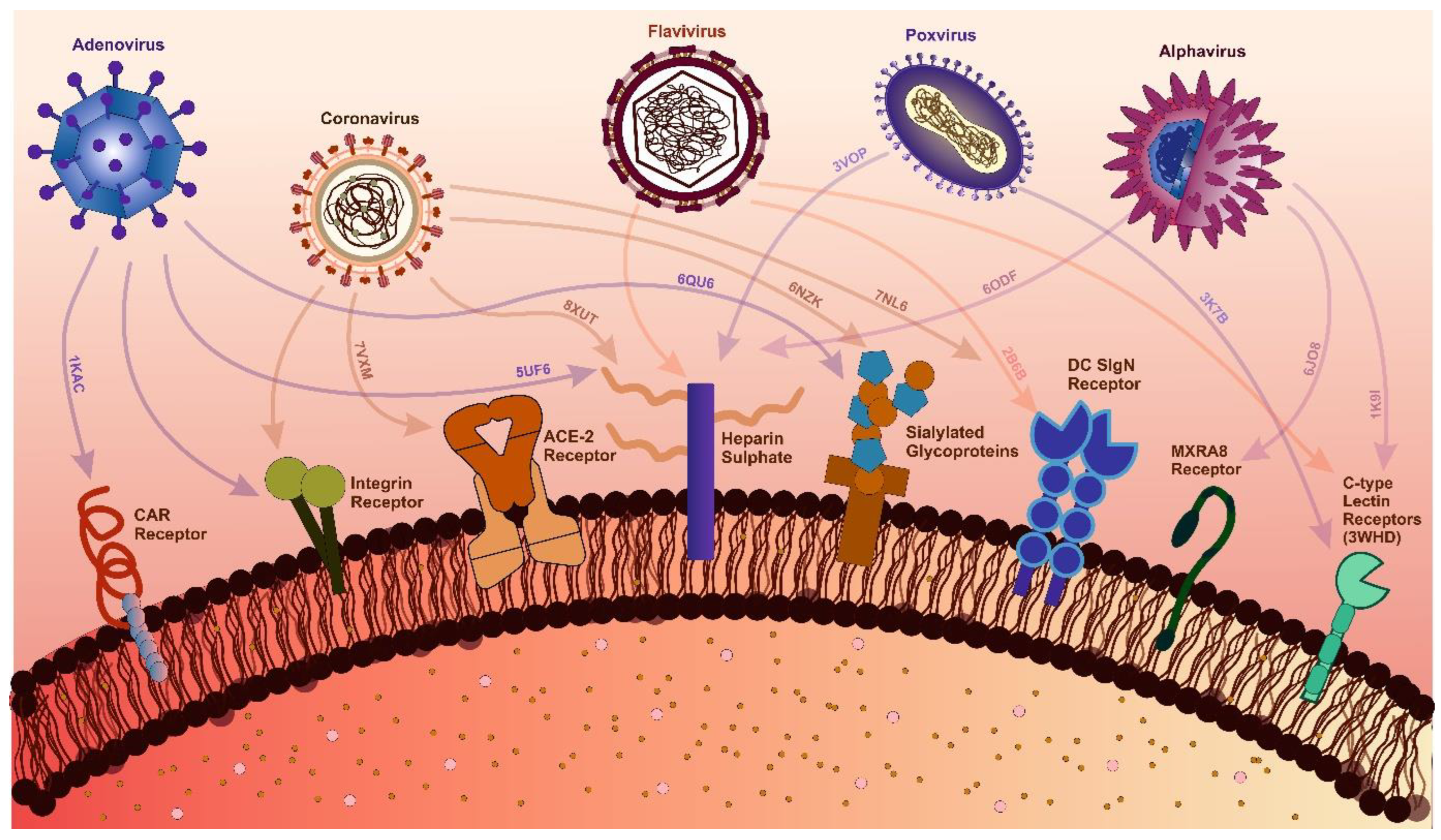
Abstract
Structural virology has emerged as the foundation for the development of effective antiviral therapeutics. It is pivotal in providing crucial insights into the three-dimensional frame of viruses and viral proteins at atomic-level or near-atomic-level resolution. Structure-based assessment of viral components, including capsids, envelope proteins, replication machinery, and host interaction interfaces, is instrumental in unraveling the multiplex mechanisms of viral infection, replication, and pathogenesis. The structural elucidation of viral enzymes, including proteases, polymerases, and integrases, has been essential in combating viruses like HIV-1 and HIV-2, SARS-CoV-2, and influenza. Techniques including X-ray crystallography, Nuclear Magnetic Resonance spectroscopy, Cryo-electron Microscopy, and Cryo-electron Tomography have revolutionized the field of virology and significantly aided in the discovery of antiviral therapeutics. The ubiquity of chronic viral infections, along with the emergence and reemergence of new viral threats necessitate the development of novel antiviral strategies and agents, while the extensive structural diversity of viruses and their high mutation rates further underscore the critical need for structural analysis of viral proteins to aid antiviral development. This review highlights the significance of structure-based investigations for bridging the gap between structure and function, thus facilitating the development of effective antiviral therapeutics, vaccines, and antibodies for tackling emerging viral threats.
1. Introduction
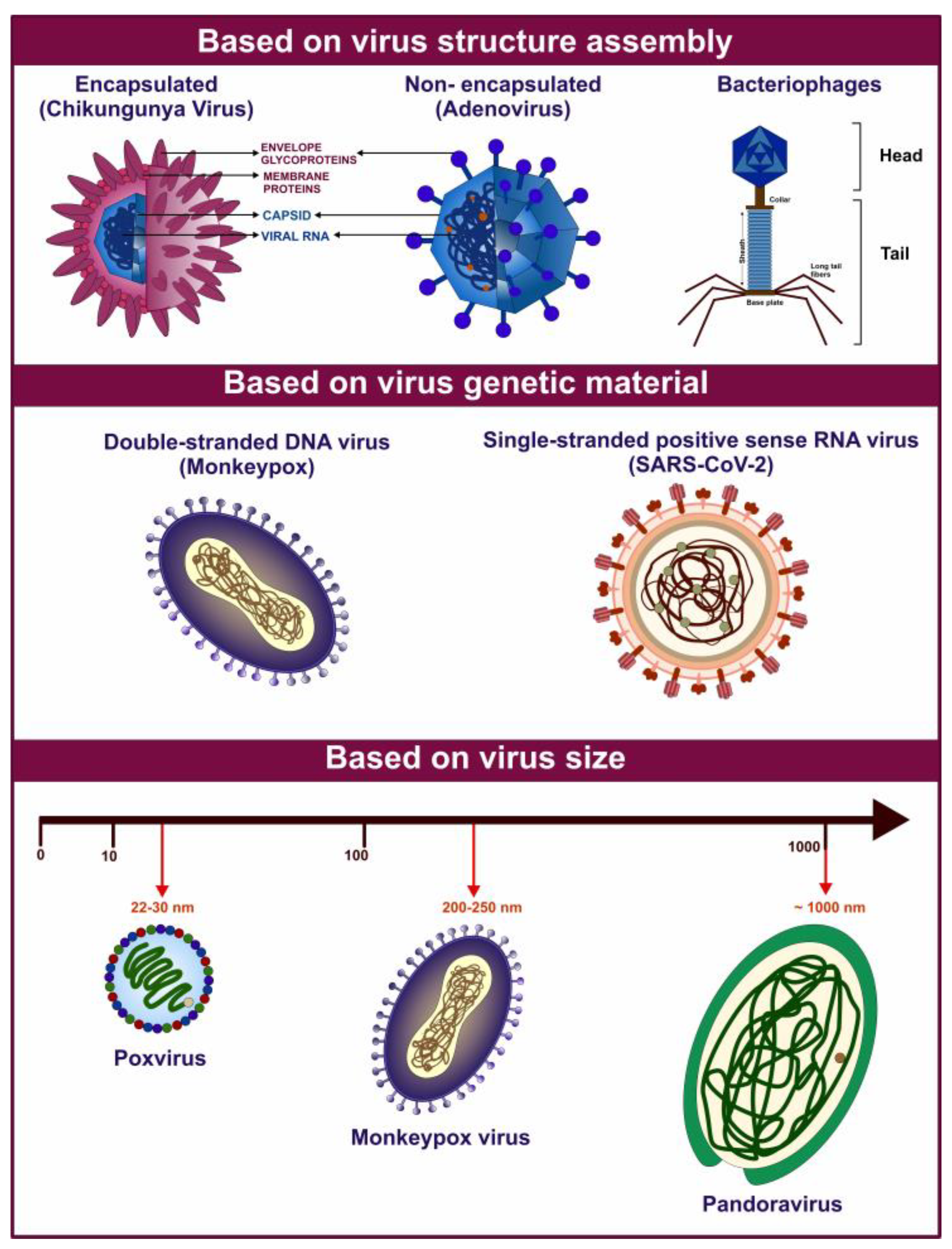
Viruses constitute a diverse group of sub-microscopic infectious agents that are reliant on the host cells’ metabolism to replicate. They lack cellular structures and possess a genome composed of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), which can be single-stranded or double-stranded. The genome can range from 3000 to over 1,000,000 nucleotides, and virus size can vary from 10 to 1000 nm in diameter [1] (Figure 1). According to the International Committee on Taxonomy of Viruses (ICTV), there are 368 families of viruses as of 2024 [2]. Viruses replicate via a series of complex steps, including initial attachment to the host cell, followed by entry, subsequent uncoating, genome replication, protein synthesis, virion assembly, and release of viral particles. Viral infection can thereby hijack the cellular machinery and disrupt cellular metabolism. These perturbations can manifest as a broad range of pathological outcomes for the host organism and even death [1]. Owing to the wide variety of viruses and the diversity of hosts, including bacteria, blue-green algae, fungi, plants, insects, and vertebrates, viruses pose a threat of infection across the three cellular domains of life- Archaea, Bacteria, and Eukarya.
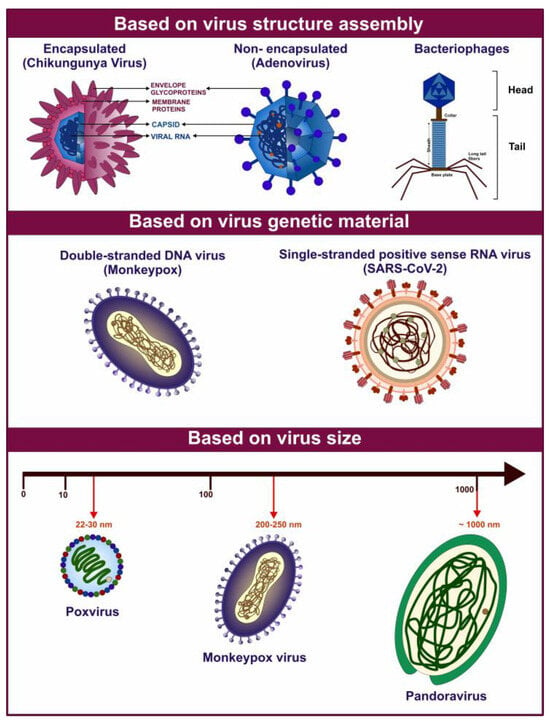
Figure 1.
Graphical representation of various viruses exhibiting diverse characteristics based on their structural composition and genetic organization. The figure above highlights examples of various virus types based on their diverse features.
Archaeal viruses can be broadly divided into archaea-specific viruses and cosmopolitan archaeal viruses, classified into 12 and 5 families, respectively. Most known archaeal viruses have been isolated from extreme environments, from hyperthermophiles or hyperhalophiles. These viruses are known to encode anti-clustered regularly interspaced short palindromic repeats (anti-CRISPR) proteins. It is suggested that the viruses play a significant role in ocean biogeochemical cycling [3,4]. Bacteriophages infect bacteria and exhibit ubiquitous distribution in the environment and diverse genomes. They exhibit lytic or lysogenic life cycles and can facilitate horizontal gene transfer, playing a crucial role in microbial ecology and evolutionary dynamics. Temperate phages can form a mutually beneficial relationship with their host. Phages have played a significant role in developing several molecular biology techniques, including CRISPR-Cas (CRISPR-associated protein) system for genome editing. They have recently been utilized in phage display technology and as phage therapy to combat antimicrobial resistance [5,6]. Amongst eukaryotes, protists, including amoebae, ciliates, and flagellates, can be infected by protist-infecting viruses. Giant viruses (GVs) such as Mimivirus belonging to phylum Nucleocytoviricota have genome and particle sizes comparable to prokaryotes and small eukaryotes [7,8]. Algal viruses, such as chloroviruses and phaeoviruses infecting Chlorella and brown algae, respectively, influence host evolution via predator-prey selection and genetic exchange, thereby affecting host fitness and microbial community composition. The infections can lead to aquatic “viral shunt”, i.e., alteration of organic matter composition and distribution [9]. Viruses that infect fungi are known as mycoviruses and are classified into 23 families and the genus Botybirnavirus. Mycoviruses infecting plant pathogenic fungi are the primary research focus due to their potential to act as biocontrol agents against the host fungi. They are reliant on hyphal anastomosis for intracellular spread and lack an extracellular transmission mechanism, limiting cross-strain spread [10]. Plant viruses pose a significant threat to agriculture and food security and can potentially cause pandemics and epidemics globally [11,12]. They are predominantly RNA viruses transmitted via vectors such as aphids, nematodes, whiteflies, fungi, or mechanical injury. Inside the plant host, they employ plasmodesmata and vasculature to spread internally, exhibiting symptoms such as mosaics, chlorosis, stunting, and wilting. Notable examples include Tobacco mosaic virus (TMV), Potato virus Y (PVY), and Cucumber mosaic virus (CMV) [11,12].
Animal viruses infect an extensive range of hosts, including vertebrates and invertebrates such as insects [1]. They display significant structural and genetic diversity and are categorized into several families. Insect viruses include families such as Baculoviridae and Iridoviridae, which are known to infect hosts such as lepidopteran larvae and other insects [13]. These viruses can be vital for pest management and agriculture. Moreover, arthropods serve as vectors for the transmission of arboviruses, such as members of Flaviviridae, Togaviridae, and Nairoviridae, to other animals, including humans [14,15,16]. Infection with animal viruses can manifest into numerous disease pathologies, including localized and systemic infection in wild and domestic animals. Members of Orthomyxoviridae (e.g., influenza viruses) [17,18] and Rhabdoviridae (e.g., rabies virus- RABV) [19] have caused substantial disease burden and mortality. A subset of animal viruses is represented by human viruses, which include notable pathogens, such as members of Coronaviridae (e.g., Severe Acute Respiratory Syndrome Coronavirus 2- SARS-CoV-2) [20,21], Orthomyxoviridae (e.g., influenza viruses) [17,18], Herpesviridae (e.g., herpes simplex virus- HSV) [22,23,24,25], Papillomaviridae (e.g., human papillomavirus- HPV) [26,27,28], Retroviridae (e.g., human immunodeficiency virus- HIV-1 and HIV-2) [29,30], and Picornaviridae (e.g., poliovirus) families [31,32]. Seven identified human oncoviruses, including Epstein-Barr virus (EBV), human T-cell leukemia virus type 1 (HTLV-1), hepatitis B virus (HBV), HPV, hepatitis C virus (HCV), Kaposi’s sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (HHV-8), and Merkel cell polyomavirus (MCV or MCPyV) account for causing an estimated 12–15% of cancers globally [33]. Additionally, the zoonotic spillover from animals to humans or reverse zoonosis from humans to animals, potentially facilitated by wildlife farming and trade, is of great concern [34,35]. Examples of zoonoses include rabies and avian influenza. All known human coronaviruses (HCoVs) are believed to have originated in animals, with five of the seven HCoVs originating in bats [36]. Most recently, the Coronavirus disease 2019 (COVID-19) outbreak is believed to have transmitted from bats to humans. Therefore, adopting a One Health approach, considering human, animal, and environmental health, for disease prevention and control is imperative.
Throughout history, viral infections have periodically emerged as epidemics and pandemics, resulting in significant loss of life. It has been estimated that there have been at least 14 influenza pandemics since 1500, including the Russian flu (1889–1893), Spanish flu (1918–1920), Asian flu (1957–1959), Hong Kong flu (1968–1970), and the first influenza pandemic of the 21st century, Swine flu (2009–2010) [37,38]. The ongoing HIV/AIDS pandemic (1981-present) has claimed millions of lives [39,40]. Severe acute respiratory syndrome coronavirus (SARS-CoV) [41], Middle East respiratory syndrome coronavirus (MERS-CoV) [42], and SARS-CoV-2 [20,21,43] are distinct coronaviruses that emerged in 2002, 2012, and 2019, respectively. On 11 March 2020, the World Health Organization (WHO) declared COVID-19, caused by SARS-CoV-2, a pandemic, which raised an alarming situation and caused ~7 million fatalities worldwide [44]. Other significant outbreaks include Smallpox epidemics in the 17th century [45], polio epidemics in the 20th century [46], frequent outbreaks of Ebola [47], Dengue fever [48], yellow fever [49], Zika [50], Measles [51], Chikungunya [52], Japanese encephalitis [53], West Nile fever [54] and rabies [19,55]. As many RNA viruses are emerging and reemerging viruses, they can evolve and reappear in the future with mutations [56,57]. The frequent viral outbreaks and lack of effective treatment and vaccination strategies underscore the urgent need to identify and develop antiviral therapeutics and advanced drug discovery for preparedness against future viral pandemics.
The study of three-dimensional (3D) structures of proteins has been recognized as crucial to expediting drug discovery. It offers insights into the shape of targets, hydrophobic and hydrophilic behaviors of macromolecules, and their interactions with substrates. Structural biology techniques are employed to study the key components of viruses, including structural proteins, replication proteins, and host interaction sites, thereby bridging the gap between viral structure and function, playing a pivotal role in shaping the development of antiviral therapies. The present review provides a comprehensive summary of structure-based investigations in the field of virology that lead to the identification and development of antiviral therapeutics and advanced drug discovery and explores the potential of structural virology in addressing emerging viral threats.
2. Exploring the 3D Protein Landscape: Structural Biology Techniques
Structural biology aims to understand the 3D structure of biological macromolecules, including proteins. These techniques have been employed in the study of viruses for close to a century. It not only furthers our understanding of life’s molecular machinery but also enhances our ability to design targeted therapeutic interventions against disease. Researchers can gain valuable insights into biochemical activities and mechanisms by solving protein complex structures, which can be instrumental in drug design, and biotechnology. These methods have helped us visualize the molecular world and expose dynamic and transient stages of proteins [58]. The evolution of structural biology from basic chemical analysis to advanced imaging techniques mirrors scientific inquiry and technology. Chemical degradation and conventional optical microscopy initially provided limited information regarding molecular composition and structure. However, the discovery of X-ray crystallography by the pioneering work of Max von Laue and William Henry Bragg in the early 20th century provided atomic-level resolution and paved the way for structure-guided molecular biology [59,60].
2.1. X-Ray Crystallography
X-ray crystallography has been instrumental in antiviral research by enabling the analysis of high-resolution atomic details of crystallized proteins and complexes by interpreting the diffraction patterns [61,62,63]. TMV was the first virus to be crystallized by Wendell Stanley in 1935. It was demonstrated that the infectivity of the virus was retained in crystalline form. He was awarded the Nobel Prize in Chemistry in 1946 [64,65]. The virus structure was described in detail for the first time by Bernal and Fankuchen, who examined TMV suspension via X-ray diffraction [66]. The first atomic resolution structure of a virus was provided in a pioneering study by Harrison et al., revealing an icosahedral arrangement of 180 capsid protein subunits of the Tomato bushy stunt virus (TBSV) at 2.9 Å resolution [67]. Parallelly, Aaron Klug and colleagues determined the structure of the TMV protein disk at a resolution of 2.8 Å [68] and revealed the structure of nucleosome core particle at 7 Å resolution [69]. Aaron Klug was awarded the Nobel Prize in Chemistry in 1982 for his development of crystallographic electron microscopy and his structural elucidation of biologically important nucleic acid-protein complexes [65]. With the development of therapeutics against HIV-1, HIV-2, and HCV, X-ray crystallography became pivotal in antiviral and vaccine research in the late 20th century. Protein crystallization faces challenges such as limited solubility, unresolved protein dynamics, and chemical heterogeneity, complicating structural determination and drug discovery. Additionally, X-ray crystallography offers only static snapshots of molecules, potentially missing important dynamic interactions. The limitations of X-ray crystallography, especially its reliance on crystalline crystals, spurred further advancements [63,70]. While size is not the limitation here, many proteins tend to have flexible regions which makes it difficult to form crystals [71]. Despite these limitations, the impact of X-ray crystallography on fields such as biochemistry, pharmacology, and virology has been profound. The primary advantage of the technique over others is its ability to provide highly detailed atomic resolution structures, essential for understanding the precise molecular mechanics of biological processes. It has enabled the detailed mapping of the interaction sites for drug molecules, providing a foundation for rational drug design and a deeper understanding of fundamental biological processes. Overall, X-ray crystallography remains a vital method in structural biology, complemented by newer techniques that provide insights into the structures of non-crystallizable molecules [63].
2.2. Nuclear Magnetic Resonance (NMR)
NMR spectroscopy was developed as a complement to study chemicals, including biomolecules in solution, revealing insights into their conformational flexibility. NMR spectroscopy has evolved from a chemical analysis tool to a fundamental technique in structural biology since the mid-20th century. Advances in technology, higher magnetic field strengths, and computational methods during the 1970s and 1980s allowed NMR to determine the structures of proteins in solution, providing dynamic molecular insights and study of dynamics and interactions within hosts [72]. The technique’s ability to reveal protein dynamics and atomic interactions opened new avenues for the study of protein folding, enzyme activity, and ligand interaction critical to viral pathogenesis and lifecycle, and eventually identifying antivirals to target the viral antigen proteins. NMR serves as a distinctive investigative tool for obtaining atom-resolved information regarding the structural and dynamic characteristics of highly flexible and disordered proteins, such as intrinsically disordered proteins (IDPs). In contrast to the more compact structures of globular protein domains, IDPs significantly influence NMR observables, necessitating the customization of NMR experiments for their study. In this context, 13C direct detection NMR has emerged as a valuable instrument for the characterization of IDPs/IDRs at an atomic resolution [73]. NMR is, therefore, uniquely suited for examining physiological states and complex biological processes. However, it is limited by its applicability mainly to smaller proteins (up to about 35 kDa), the requirement of large sample amounts, and extensive time [73]. Its spectral complexity demands high expertise for data interpretation, presenting challenges in high-throughput environments. Despite its limitations, NMR has profoundly impacted structural biology. The conjugation of X-ray crystallography and NMR aids in a deeper understanding of the protein structures [70]. NMR spectroscopy aids antiviral drug discovery by identifying ligand-protein interactions, optimizing drug properties, detecting false positives, and supporting multidisciplinary approaches. NMR spectroscopy can help accelerate the design of antiviral drugs. It has been instrumental in studying the HCV non-structural proteins, including protease, helicase, and polymerase, optimizing drug properties, and validating hits from screening and identifying peptidomimetics against HCV non-structural protein (NS3) serine protease [74].
2.3. Transmission Electron Microscopy (TEM)
Electron microscopy is used to visualize the ultrastructure of specimens using focused electron beams. Helmut Ruska made significant contributions to the field of virology by visualizing viruses in the 1930s. In the next decade, he detailed the sub-microscopic structures of various viruses, including poxviruses, TMV, varicella-zoster virus (VZV), and bacteriophages primarily employing TEM [75]. During the 1940s, TEM was employed for the diagnosis of smallpox and chickenpox [76]. In 1959, a negative staining method for high-resolution electron microscopy of viruses was developed [77]. TEM has since been used to understand viral structures, virus-host interaction studies, vaccine development, mutation monitoring, nanomedicine imaging, and diagnostics [78]. The challenges of TEM, such as uneven specimen staining and staining-induced distortions, were overcome when the first successful implementation of cryo-electron microscopy (cryo-EM) was reported.
2.4. Cryo-Electron Microscopy (Cryo-EM)
Cryo-EM methodology acquires images of specimens cooled at cryogenic temperatures, aiding in the visualization of proteins, viruses, and complexes in their native state [79]. Its advent revolutionized the field of structural biology as it allowed the study of large protein complexes and fleeting protein states that are hard to crystalize. The technique gained popularity as it facilitates the visualization of biomolecules in their native, hydrated conformations and can achieve near-atomic resolutions comparable to X-ray crystallography without crystallization. With more sensitive detectors and better image processing tools, cryo-EM has become the standard for structural studies of viruses and components such as viral capsids, membrane proteins, and protein complexes [80]. This has provided invaluable insights into viral assembly, infection mechanisms, and interactions with host cells, directly impacting the development of antiviral drugs and vaccines [81]. One of the key advantages of Cryo-EM over other techniques is its ability to study complex and large biomolecular assemblies at near-atomic resolutions, enabling the study of membrane proteins, large protein complexes, and viruses. Additionally, Cryo-EM can capture snapshots of multiple conformational states of a molecule, providing a dynamic perspective on the functional mechanisms. For instance, the structure of RNA polymerase determined using Cryo-EM has advanced the understanding of the dynamic behavior of the enzyme [82]. The technique is not without challenges, including the need for expensive, high-maintenance equipment and the requirement for significant computational resources to process large datasets. Protein size limitations arise as it is almost impossible to obtain images of protein with a size of less than 100 kDa. Limitations can be overcome by targeting the proteins with nanobodies to capture the protein–protein complexes [83]. Moreover, achieving the highest resolutions often necessitates many images and averaging to obtain the 3D structures, which can be time-consuming to collect and analyze. Nevertheless, it enabled unprecedented molecular insights, facilitating structure-guided therapeutic design and driving continual advancements in deciphering intricate biological processes [84].
2.5. Small Angle X-Ray Scattering (SAXS)
Building upon the principles of X-ray crystallography, SAXS offers a complementary approach by allowing the study of macromolecules in solution, providing insights into their size, shape, and conformational changes. Unlike X-ray crystallography, which requires the formation of crystals and primarily gives high-resolution static structures, SAXS can analyze samples that are difficult to crystalize and provides low-resolution data on flexible and dynamic assemblies in near-native conditions [85]. SAXS is particularly advantageous for examining large complexes and conducting rapid screenings of samples under various conditions, making it a valuable tool in cases where X-ray crystallography is not feasible. SAXS has been essential in studying IDPs, revealing 3D structures of aggregates, and identifying different stages of protein aggregation due to their flexible domains and smaller size [86]. Conversely, for detailed atomic resolution structures necessary for precise molecular interactions, X-ray crystallography remains the superior technique [87].
2.6. Cryo-Electron Tomography (Cryo-ET)
Leaning on the capabilities of cryo-EM, cryo-ET helps study structural biology further by providing detailed 3D visualizations of cells and viruses in their native environment. While cryo-EM offers revolutionary insights into individual proteins and complexes, cryo-ET extends this by allowing scientists to examine the spatial organization and interactions within entire cells or tissues at near-atomic resolutions. In the early 2000s, cryo-ET provided the first visualization of HIV-1 envelope glycoproteins (Env) on the virion surface, revealing their unique tripod-like structure [88]. Subsequent studies have further elucidated Env’s conformational dynamics, aiding in the design of broadly neutralizing antibodies and vaccines [81]. This makes Cryo-ET a superior technique for understanding complex viral infection mechanisms and cellular architecture dynamics, as it captures biological processes in situ without the need for sample sectioning or markers, providing a more comprehensive and realistic view of molecular biology [89].
2.7. Emerging Techniques
Together, these techniques complement each other, and ongoing and future advances in technologies such as X-ray Free Electron Laser (XFEL) imaging are broadening the horizons of structural biology as these may disclose new biomolecular behavior, especially in cells and in reaction to inhibitors [90]. Advancements in computational biology have enabled simulations that predict protein folding and dynamics based on known sequences. Techniques such as molecular dynamics (MD) simulations complement experimental data by providing insights into conformational changes over time. During the COVID-19 pandemic, in silico screening and MD simulations of FDA-approved drugs available in the market played a huge role in drug repurposing [43,91,92]. Moreover, AlphaFold 3, the latest iteration of Google DeepMind’s artificial intelligence (AI) tool, offers unparalleled accuracy in predicting 3D protein structures and complex biomolecular assemblies, including protein-nucleic acid and protein-small molecule complexes [93]. This has also helped researchers in modeling protein-based therapeutics against viral infections [93]. In 2024, the Nobel Prize for Chemistry was awarded to David Baker for computational protein design, and to Demis Hassabis and John M. Jumper (Google DeepMind) for protein structure prediction [94].
3. Exploring Viral Structural Proteins
Structural proteins are the first to engage with the host receptors during an infection. Structural proteins form the virus’s architecture, comprise a protective outer shell for the genetic material (nucleocapsid), a lipid bilayer containing embedded viroporins (membrane proteins) encasing the capsid, and external proteins facilitating interactions between the virus and host cells (envelope proteins). These proteins are essential for the virus as they depend on these to bind to host receptors, take over host cells, and establish their replication machinery [95]. Adherence to host cells is an essential preliminary phase in the infection process for numerous viruses. Proteins facilitating these interactions have been identified as critical therapeutic targets. For example, The H3 protein in Monkeypox virus (MPXV) is crucial for viral adherence through its interaction with cell-surface heparan sulfate (HS) [96]. Likewise, the E2 protein is crucial for attachment in alphaviruses. Hence, it becomes an essential target for virus entry inhibition [97]. Following attachment, viral infiltration via endocytosis is frequently aided by structural proteins. This process is crucial for virus entry and genome release [98]. In alphaviruses, the E1 protein is pivotal for membrane fusion, facilitating virus entry. As the viral infection progresses, the virus hijacks the host system for the assembly and budding of viruses [99]. Structural proteins are also essential for forming new viral particles within infected cells and their subsequent egress (budding) to infect more cells. For instance, the 6 K protein in alphaviruses, while its function is not entirely elucidated, is believed to contribute to viral budding and enhance membrane permeability [100]. Additionally, numerous viruses possess an internal nucleocapsid core that encases the viral genome. The capsid protein (Cp) constitutes the primary element of the nucleocapsid in many viruses; it engages with the viral RNA and establishes the core structure [101]. Encapsulating the viral genome within the protective protein shell necessitates precise interactions between structural proteins and the viral genetic material. In Coronaviridae, the replication and transcription of the viral genome are primarily conducted by the replicase; however, other factors, including viral structural proteins and host proteins, have also been implicated. The coronavirus nucleocapsid protein functions as an RNA chaperone, facilitating template switching in the synthesis of sgRNA [102]. Via attachment, these viruses make critical interactions with the host receptors, which eventually hijack the host cells and develop the virus assembly line. Hence, blocking these interactions of viral structural proteins and host receptors is a crucial step for developing antivirals as entry inhibitors [103]. A structure-guided approach for identifying host receptors that interact with viral proteins, as well as identifying critical residues for viral antigen and host receptor interaction, can act as a therapeutic target covering a range of viruses [104]. Many therapeutics in the form of antivirals and antibodies have been identified against viral structural proteins based on a structure-guided approach and have been in use (Table 1).

Table 1.
List of FDA-approved therapeutics against viral structural proteins.
3.1. Envelope Glycoproteins
Virus envelope glycoproteins are critical structural components that facilitate viral entry into host cells. These proteins play a pivotal role in the infection process by interacting with host cell receptors and enabling viral fusion with the host membrane [105,106]. Envelope glycoproteins can be classified based on their functions into categories such as Spike (S), Envelope (E), or Membrane (M) glycoproteins. S proteins, which protrude from the virus’s surface, are particularly important for attachment to host cells. For example, the S protein of coronaviruses like SARS-CoV-2 is essential for receptor-binding and membrane fusion. Each glycoprotein variant has specific structural features that determine its unique functions. For instance, S proteins often contain receptor-binding domains that enable them to interact with host cell receptors, while some envelope proteins facilitate membrane fusion [102]. Influenza viruses feature hemagglutinin (HA) and neuraminidase (NA) proteins, which regulate the processes of viral entry and exit from host cells [107]. In contrast, HIV-1 and HIV-2 employ the gp160 membrane protein, which splits into gp120, responsible for receptor-binding, and gp41, which aids in membrane fusion [108]. Similarly, SARS-CoV-2 uses its spike (S) proteins to attach to the Angiotensin-converting enzyme 2 (ACE2) receptor in human cells, leading to infection [109]. Viral fusion peptides are essential for viruses to interact and perform endocytosis in the host cell [110]. To further blend with the host system, glycosylation helps enveloped viruses evade immune detection by masking viral epitopes with host-derived glycans, making it challenging for antibodies to identify and neutralize the virus [111]. In other cases, viral envelope proteins are reported to undergo conformational changes that assist in virulence, as observed in SARS-CoV-2 [112] and Dengue virus (DENV) [113].
When viral envelope proteins are detected, the immune system initiates a defense response. While envelope proteins may successfully trigger an immune response, this response is sometimes insufficient to neutralize the virus. For instance, vaccine development for HCV has shown limited effectiveness in generating a robust antibody response [114]. Viruses have developed sophisticated mechanisms to evade these defenses. They suppress interferon (IFN) signaling, inhibit the actions of IFN-stimulated gene products, and disrupt the communication between IFNs and other cellular pathways. These strategies allow viruses to avoid immune detection and maintain their infectivity [115]. Interleukin 10 (IL-10) plays an essential role in supporting Coxsackie B4 virus (CVB4) infection by modulating the immune response to favor viral survival. Interestingly, the antiviral agent Umifenovir has been shown to downregulate IL-10 expression, thereby disrupting the virus’s ability to exploit this pathway. This interaction underscores the importance of IL-10 in viral pathogenesis and highlights the potential of targeting its regulation as an effective strategy for treating CVB4 infections [116] (Table 1).
To escape antiviral treatments, viruses undergo mutations that allow them to target host receptors while avoiding detection by antivirals [117]. Most of these mutations occur in envelope glycoproteins, e.g., the Omicron variant of concern exhibits 37 mutations in its spike protein, which facilitates entry into host cells. Many of these mutations are in two key domains targeted by neutralizing antibodies: the receptor-binding domain (RBD) and the N-terminal domain (NTD). Despite these changes, some therapeutic antibodies, such as 309 (PDB: 7TLY), retain neutralizing activity against Omicron [118]. The conserved region in antigenic site IV in the flank of RBD is conserved due to which the antibody can retain its neutralizing activity [119]. In other cases, envelope proteins can evade immune defenses by binding to antibodies without being neutralized. This phenomenon is observed in the DENV, where antibody binding does not neutralize the virus, leading to a condition called antibody-dependent enhancement (ADE) of infection. ADE occurs during secondary infections and exacerbates disease severity [120,121]. Due to structural similarity between the serotypes, the host responds to viruses with antibodies generated based on previous infection. These antibodies may not bind to neutralizing sites such as fusion loops in DENV or receptor recognition domains which are conserved across virus serotypes [122]. Displaying antigens in a non-infectious manner can help in conferring immunity without compromising the host system with infection. Currently, Dengvaxia is the only vaccine developed against the DENV, and it is effective primarily during secondary infections, as attenuated viruses display the envelope proteins (membrane and envelope glycoproteins) using a different viral vector (yellow fever virus) for the host to generate an immune response but may not lead to infection [123]. Limitations arise as this vaccine cannot be used to immunize prior to primary infection and is only recommended in the case of secondary infection [124]. To combat infections with high mutation rates, such as SARS-CoV-2, targeting highly conserved epitope regions within the antigen is essential. This approach minimizes the risk of immune evasion through mutations [125].
To combat viral infections, entry inhibitors play a critical role by targeting viral envelope proteins and preventing infection [126]. These inhibitors have shown significant efficacy against influenza, one of the most studied viruses in this context. Several potent, Food and Drug Administration (FDA)-approved inhibitors, such as Zanamivir [127], Oseltamivir [128], and Laninamivir octanoate [129], effectively target envelope glycoproteins of influenza virus and block its entry into the host [130] (Table 1). Glycoproteins also have significant potential in vaccine development. When applied appropriately, they can act as antigens and stimulate immune responses, as seen in respiratory syncytial virus (RSV) subunit vaccines for pregnant women and the elderly [131]. Virus-like particles (VLPs) encoding envelope glycoproteins have been effective for multiple purposes, including as diagnostic tools and vaccines [132,133]. With emerging applications, it has been possible to identify antivirals based on entry sites and inhibit virus entry into the host.
3.2. Viroporins
Viroporins are small, hydrophobic proteins encoded by viruses. They oligomerize within host cell membranes, forming hydrophilic pores that disrupt cellular processes [134]. Many viruses incorporate membrane proteins into their lipid bilayer envelopes, which are essential for viral entry, assembly, release, and structural integrity. These proteins act as scaffolds, supporting other viral proteins and encapsulating viral DNA [135]. Viroporins facilitate viral budding by interacting with viral proteins and host cell membranes. They also influence membrane fluidity and fusion processes critical for viral entry, although they do not directly bind to receptors like spike or envelope proteins [136].
Viroporins were first identified in 1992 with the discovery of the ion channel activity of the M2 protein of the influenza A virus [137]. Since then, numerous viroporins with diverse structural and functional characteristics have been discovered in various viruses. These proteins are classified into two main groups, class I and class II, based on the number of transmembrane domains they possess. Subclasses are further defined by their position within the membrane [134]. Despite their importance, the study of viroporins as potential targets for antiviral drugs remains challenging. This is due to the lack of reliable 3D structures, difficulties in functional characterization, and the absence of direct, verifiable binding between inhibitors and viroporins [134]. Nevertheless, while the presence of viroporins may not always be critical for viral survival, their absence significantly weakens the virus [138].
Viroporins perform diverse functions across different virus families, reflecting adaptations to specific hosts and biological environments. Membrane proteins contribute to viral envelope stabilization and may also participate in cell signaling and immune evasion. Currently, Amantadine [139], and Rimantadine [140] are the only FDA-approved antivirals targeting the influenza M2 protein. Amantadine binds with the N-terminal of the M2 channel protein for inhibition [141]. It is also reported to have antiviral efficacy against the chikungunya virus ion channel [142], showcasing potential as broad-spectrum viroporins inhibitor. Although Rimantadine has been shown to have antiviral activity against Influenza A, it is reported to show little activity against the influenza B virus and drug-resistant variants arise within a few days after dosage [143]. These drugs elicit antiviral responses, although many other viroporin-targeting therapies remain in pre-clinical and clinical development [144] (Table 1). Advances in cryo-EM have enabled high-resolution imaging of viroporins in their native, membrane-bound conformations. Structural studies reveal the dynamic properties of viroporins, including structural changes during viral entry and membrane fusion [134].
3.3. Capsid
Capsids are protective structures in viruses that shield viral genomic material until it enters a host cell [145]. Their primary function is to protect the viral genome from enzymatic degradation by host enzymes and enable its transfer into host cells [146]. Upon entry, the capsid may either disassemble to release the genome for replication or remain intact, allowing transcription within the capsid, depending on the architecture of different virus types [147]. Capsid proteins vary in shape among virus families, containing both major and minor structural proteins. These geometries, studied through models such as Caspar-Klug nomenclature [148] and Alpha shape theory [149], explain assembly patterns in large molecular systems. Computational methods play a vital role in analyzing capsid dynamics, assembly, and interactions with lipid membranes [148]. Capsids are promising antiviral targets due to their essential role in viral infectivity. Disrupting capsid formation or stability can hinder replication [145,150,151,152,153]. Virus-specific non-structural proteins involved in capsid assembly provide opportunities for selective antiviral therapies [154,155]. The nucleocapsid (N) protein of SARS-CoV-2 constitutes a pivotal structural component of the virion, facilitating the encapsulation of the viral RNA into a ribonucleoprotein (RNP) complex and mediating key processes in viral replication and propagation. The C-terminal domain of the N-protein (N-CTD) is indispensable for genome packaging, serving a critical role in the stabilization of the RNP assembly, whereas the RNA-binding site is located in the N-terminal domain (NTD) [156,157]. The first capsid structure identified was that of the TMV [158]. In the 1950s, Rosalind Franklin visualized TMV’s rod-shaped capsid using X-ray crystallography, revealing its protein and RNA organization [159]. Early structural studies employed techniques such as electron microscopy and X-ray crystallography [160,161]. Modern advances, including cryo-EM, have enabled high-resolution imaging of capsids in near-native states, providing insights into structural changes during viral life cycles. Capsid inhibitors are designed to disrupt capsid assembly or disassembly, blocking viral replication. For example, Lenacapavir (marketed as Sunlenca) targets HIV-1 capsids, interfering with multiple replication stages [152]. Immunotherapy strategies also target capsid proteins to elicit immune responses that neutralize viruses. Vaccines often incorporate capsid proteins to stimulate protective immunity [162]. Antivirals based on a structural approach, such as pleconaril, inhibit picornavirus (enteroviruses and rhinoviruses) even with differences in the amino acid sequence of the capsid proteins [163] (Table 1).
4. Exploring Viral Non-Structural Proteins
Non-structural proteins are encoded by viral genomes but are not part of the structural components of the virus. These proteins may function to facilitate viral replication or partake in the regulation of replication and assembly. Viral replication enzymes are a subset of viral non-structural proteins encoded by the virus to facilitate the replication and transcription of the viral genome within host cells. Viral replication enzymes, including proteases, polymerases, integrases, and helicases, play crucial roles in synthesizing viral RNA or DNA, enabling genome amplification and the production of new viral progeny. Antiviral drug discovery and repurposing majorly focus on these enzymes (Table 2).

Table 2.
List of FDA-approved drugs targeting viral replication enzymes.
4.1. Protease
Virus-encoded proteases catalyze the cleavage of specific peptide bonds in viral polyprotein precursors or in cellular proteins, allowing them to function. Over the years, pre-clinical investigations have focused on these proteases due to their essential role in virus replication. The determination of 3D crystal structures of retroviral proteases began in 1989 with the elucidation of the RSV protease structure, followed by the HIV-1 protease structure [164]. Additionally, the structure of HIV-1 protease was modeled using known eukaryotic aspartic protease structures as templates. The breakthrough in structure-based drug design for viral proteases was enabled when the enzyme and substrate-binding sites of HIV-1 protease (aspartyl protease) were analyzed via X-ray crystallography [165,166,167]. A number of peptide inhibitors (PIs) were designed based on the transition state mimetic concept [168]. The Hoffmann–La Roche drug Saquinavir (Ro 31-8959, Invirase) was the first FDA-approved drug (1995) of HIV-1 protease. The interaction of the drug with the protease was studied by X-ray crystallography [169]. This was followed by Ritonavir (Norvir)’s approval. X-ray structures guided the development, and computational design was incorporated to augment binding and pharmacokinetics [170,171]. Indinavir (Crixivan) was the next HIV protease drug to be approved by the FDA in 1996. Its development was driven by molecular modeling and X-ray crystallographic studies [172,173,174]. Nelfinavir (Viracept) design employed iterative co-crystallographic analyses of protease-bound peptidic inhibitors followed by substitution of parts of the inhibitors with non-peptidic functional groups. It was the first protease inhibitor authorized for pediatric use (1997) [174,175,176]. The other approved HIV antivirals, discovered predominantly employing X-ray diffraction (XRD), include Atazanavir (Reyataz), Darunavir (Prezista), Fosamprenavir (Lexiva), Lopinavir-Ritonavir (Kaletra), and Tipranavir (Aptivus) [62]. In 2013, a joint X-ray/neutron structure HIV-1 protease in complex with now discontinued drug, Amprenavir was determined. The structural data collected by neutron diffraction revealed the precise localization of hydrogen atoms within the active site, which disclosed that some hydrogen bonds may be weaker than previously inferred from non-hydrogen interatomic distances. This insight could prove useful for the development of enhanced protease inhibitors [177] (Table 2). NMR has significantly contributed to the development and optimization of many HIV protease inhibitors. Of these, one notable drug is Ritonavir. Two years post-market launch, certain batches failed to meet dissolution standards. Investigations revealed the presence of an additional crystal Form II other than Form I. Solid-state characterization techniques, NMR, and Infrared spectroscopy (IR) confirmed the existence of two distinct crystalline forms (polymorphs) of ritonavir. However, solution-state analysis demonstrated that both forms dissolve to yield identical molecular structures. Therefore, either form could be employed for manufacture provided complete dissolution [178].
The NS3 protein of HCV comprises two distinct functional domains: an N-terminal serine protease domain and a C-terminal RNA helicase domain. NS4a peptide binds to NS3 and serves as a cofactor for polyprotein maturation. The NS3/4a complex imparts proteolysis of the HCV polyprotein. HCV PIs are designed to inhibit this proteolytic activity [179]. The crystal structure of NS3 was published in 1996, revealing a trypsin-like fold and a structural zinc binding site [180], and the crystal structure of NS3/4a complex revealed NS4A peptide intercalates within a β sheet of the enzyme core [181]. The solution NMR structure of N-terminal protease of NS3 published in 1998 revealed insights into its activation and catalytic mechanism [182]. Initial investigations into the suppression of NS3/4A protease concentrated on the identification and optimization of peptide-based inhibitors [179,183]. Boceprevir and Telaprevir (Table 2) were discovered via structure-guided drug design, both of which belong to the ketoamide class of molecules. They feature a ketoamide group that forms a reversible covalent bond with the enzyme’s catalytic serine residue, thereby inhibiting the enzymatic activity [179]. This was followed by the discovery of several inhibitors via structure-guided drug design. High-resolution crystallographic data served as the basis for discovering small molecule inhibitors (Table 2). In 2012, a highly conserved new allosteric pocket at the HCV protease and helicase domain interface was identified via crystallographic fragment-based screening and proposed as a drug target [184].
The main proteases (Mpro), also termed 3-chymotrypsin-like proteases (3CL pro) and Papain-like protease (PLpro), are cysteine proteases encoded by SARS-CoV-2. Mpro (nsP5) and PLpro (nsP3) co-translationally process pp1a and pp1ab polyproteins into mature non-structural proteins (nsPs), making them attractive drug targets [185,186]. Each protomer of Mpro, a homodimer, consists of three domains (I, II, and III). The substrate-binding pocket is located in the interdomain cleft between domains I and II, where a non-canonical catalytic dyad, Cys145-His41, mediates proteolytic cleavage at 11 distinct sites on pp1a and pp1ab. The substrate-binding sites of SARS-CoV-1 and SARS-CoV-2 Mpro exhibit 100% sequence homology; inhibitors of the former were also screened for the latter [187]. X-ray structures of the unliganded SARS-CoV-2 Mpro and its complex with an α-ketoamide inhibitor were reported, which provided the basis for development of improved inhibitors [188]. These two studies resulted in the development of the inhibitor Nirmatrelvir (PF-07321332) [187]. The FDA-approved Paxlovid (a dual-therapy of Nirmatrelvir and Ritonavir) on 25 May 2023 as an oral antiviral pill [189,190] (Table 2). Ensitrelvir (S-217622), the first oral noncovalent and nonpeptidic SARS-CoV-2 Mpro PI clinical candidate, was discovered via virtual screening and optimization of the hit compound using a structure-based drug design strategy (SBDD). X-ray constructure of the enzyme and PI provided insights into binding and interaction [191] (Table 2). In another study, Cryo-EM structure of polyprotein bound and apo form of Mpro highlighted the flexible nature of the active site [192].
4.2. Polymerase
Virus-encoded polymerases are enzymes that catalyze the synthesis of nucleic acid, either DNA or RNA for the replication of viruses, making them key targets for antiviral research [193,194,195]. Different types of polymerases include RNA-Dependent RNA Polymerase (RdRp), RNA-Dependent DNA Polymerase or Reverse Transcriptase (RT), DNA-Dependent DNA Polymerase (DdDp), and DNA-Dependent RNA Polymerase (DdRp) [196].
HSV DNA polymerase (UL30) possesses polymerase activity, intrinsic 3′-5′ exonuclease activity, and ribonuclease (RNase) H activity. The first structure of HSV DNA polymerase was published in 2006. It has five conserved structural domains including an NH2-terminal, 3′-5′ exonuclease, thumb, fingers, and palm domains, and an additional pre-NH2-terminal domain at the N-terminal end [197,198]. Cryo-EM structures published in 2024 revealed the dynamics of the UL30 during DNA synthesis and proof-reading [199]. Viral DNA polymerase cryo-EM structures elucidated how Pol and UL42 bind DNA for processive synthesis, with Pol adopting multiple closed-state conformations in the absence of nucleotides. Structures were not elucidated at the time of approval, but recently drug-bound (Foscarnet and Acyclovir) and drug-resistant mutant analyses indicated that resistance mutations alter conformational dynamics rather than drug binding, clarifying selectivity mechanisms [200].
The mature HIV-1 RT is a heterodimer comprising two subunits: the larger p66 subunit (560 amino acids) and the smaller p51 subunit, derived from the first 440 residues of p66. While the p66 subunit harbors the active polymerase and RNase H domains, the p51 subunit is believed to adopt a structural role with analogous subdomains (fingers, palm, thumb, and connection) differing in relative arrangement to support the heterodimer’s functional integrity. Stavudine, Lamivudine, and Tenofovir disoproxil fumarate are nucleoside reverse transcriptase inhibitors (NRTIs) that bind to the active site of polymerase on activation. These drugs were developed based on the mechanism of action; however, the crystal and cryo-EM structures were elucidated later [201,202] (Table 2). Discovered in the late 1980s, non-nucleoside reverse transcriptase inhibitors (NNRTIs) include five approved anti-HIV drugs: nevirapine, delavirdine, efavirenz, etravirine, and rilpivirine. Dapivirine is approved in several countries. They function as noncompetitive inhibitors by engaging the allosteric site of HIV-1 reverse transcriptase (RT), approximately 15 Å from its catalytic domain. This interaction induces conformational alterations that disrupt the enzyme’s catalytic function, thereby inhibiting viral replication. The hydrophobic binding sites of HIV-1 RT and NNRTIs were identified through compound library screening and structural biology analysis. NNRTIs target HIV-1, while HIV-2’s structural features confer innate resistance [201,202,203,204] (Table 2).
The influenza virus RdRp is a heterotrimeric enzyme composed of PA, PB1, and PB2 subunits. Transcription involves a “cap-snatching” process, wherein nascent capped host RNA transcripts are bound by the PB2 subunit, cleaved by the cap-dependent endonuclease (CEN) of the PA subunit, and utilized as primers by the PB1 subunit for viral mRNA synthesis [205]. The reported cryo-EM structure provides a basis for understanding the enzyme’s activity [206]. FDA-approved baloxavir acid and its prodrug Baloxavir Marboxil, which target the conserved active site of the PA proteins in influenza A and B viruses, were developed by rational drug design leveraging the two-metal pharmacophore framework initially established for Dolutegravir. Recognizing that both HIV integrase and CEN utilize divalent metal ions as cofactors for endonuclease activity, DTG’s metal-chelating scaffold was adapted for CEN inhibition. Crystal structures have been elucidated to study the interaction [205] (Table 1).
SARS-CoV-2’s multi-subunit RdRp complex is composed of a catalytic subunit nsP12 and the accessory subunits, nsP7 and nsP8 [207]. The nsP12 subunit is composed of a nidovirus RdRp-associated nucleotidyltransferase (NiRAN) domain, an interface domain, and a catalytic domain at the C-terminus. These domains collectively mediate key functions, including the catalysis of phosphodiester bond formation between nucleoside triphosphates (NTPs), as well as template binding, entry, primer-template release, and polymerization. The drug remdesivir was approved by FDA in 2020 [208]. While docking and molecular dynamics simulations revealed the dynamic interactions and binding pockets, the cryo-EM structures of SARS-CoV-2 RdRP complex with Remdesivir revealed its incorporation into the nascent RNA strand, stalling elongation and provided a rational template for drug design [209,210,211]. In 2021, the FDA issued an emergency use authorization (EUA) for Molnupiravir, a broad-spectrum ribonucleoside analog, originally developed to treat influenza, and discovered via screening a library of compounds [212,213]. Favipiravir, another inhibitor of influenza RdRP [211] was approved for SARS-CoV-2 treatment in several countries [214]. Cryo-EM structures of the three inhibitors bound to RdRp provide crucial data like the mechanism of enzyme catalysis and base-pairing pattern with inhibitors (Table 2). These drugs are designed to mimic natural nucleotides and incorporate themselves into the growing viral DNA or RNA chain, thereby terminating viral replication.
4.3. Integrase
HIV integrase (IN) mediates the insertion of viral DNA into host chromosomal DNA, employing two consecutive reactions named 3′-processing (3-P) and strand transfer (ST). The cleavage of Pol polyprotein by HIV protease leads to the generation of IN, a 32-kDa enzyme composed of three domains: the amino-terminal (NTD), catalytic core (CCD), and carboxy-terminal (CTD). The atomic structures of these domains were resolved using X-ray crystallography or solution NMR spectroscopy [215,216,217]. The IN active site at the CCD domain contains the highly conserved DDE motif, responsible for coordinating two Mg (II) ions. The substantial spatial distance between the enzyme active site in the CCD domain and both the NTD and CTD regions suggests a high degree of multimeric organization within the IN enzyme. Within infected cells, integrase (IN) is predominantly localized in the cytoplasm as a component of the viral pre-integration complex (PIC). The minimal functional subunit of the PIC, the Intasome (INT), catalyzes the integration, a process specific to retroviruses [217]. Combination antiretroviral therapy (cART) comprises four pharmacological classes: (i) NRTIs, (ii) NNRTIs, (iii) PIs, and (iv) integrase strand transfer inhibitors (INSTIs) [217,218]. Since approval, INSTIs have assumed a pivotal role in HIV antiretroviral therapy (ART) due to favorable clinical attributes like high antiviral potency with expedited reductions in HIV RNA levels, absence of significant drug–drug interactions, and cross-resistance to other drugs [219].
All INSTIs share structural similarity to experimental compound 1-(5-chloroindol-3-yl)-3-hydroxy-3-(2H-tetrazol-5-yl)propenone (5-CITEP) characterized by the pivotal diketoacid (DKA) moiety. It represents the first integrase inhibitor successfully co-crystallized with the CCD, with electron density confirming interactions with residues D64, D116, and E152, as well as additional contacts involved in host DNA docking [217,220,221]. Further studies revealed that PICs are predominantly assembled via coordination with two Mg(II) ions during viral DNA integration, one of these metal ions is coordinated by residues D64 and D116, whereas the other is coordinated by residues D116 and E152. DKA-containing compounds maintain antiviral activity provided their divalent metal ion chelation capacity is retained. Raltegravir and Elvitegravir are first-generation INSTIs. The widespread use of Raltegravir since its approval in 2007 resulted in the rapid emergence of viral variants harboring mutations within the IN-CCD domain [217]. Second-generation INSTIs include Dolutegravir [222], Bictegravir, and Cabotegravir. Elucidation of crystal structure revealed enhanced structural flexibility of Dolutegravir improved the drug’s integration into the enzyme active site, increasing efficacy against mutations triggered by first-generation INSTIs [217,221] (Table 2). Since 2018, WHO has recommended the use of Dolutegravir as the preferred first- and second-line HIV treatment [223]. Bictegravir and Cabotegravir exhibited superior genetic barriers to resistance [217]. Cryo-EM structures of second-generation INSTIs bound to INTs and drug-resistant INTs further revealed mechanisms of inhibition and resistance and may aid in the development of better drugs [224,225].
X-ray and cryo-EM structures demonstrated that allosteric IN inhibitors (ALLINIs) disrupt the catalytic activity of the enzyme by binding to allosteric sites [217,226]. Lens Epithelium-Derived Growth Factor/p75 (LEDGF/p75) is a cellular factor that enhances host DNA interaction with functionally active PICs by binding to the integrase binding domain (IBD) at the C-terminus IN, via coordination with residues D366, V408, I365, and F406. LEDGINs (coined after Lens epithelium-derived growth factor/p75 cofactor binding pocket on IN) target this interaction and affect IN multimerization and catalytic activity. These can be developed via rational drug design and show no cross-resistance with INSTIs [217,218,227]. Multimerization-selective integrase inhibitors (MINIs) act by promoting aberrant IN multimerization. IN-RT RNase H inhibitors and INI-LEDGF/p75-IN interaction disruptors are examples of dual-acting inhibitors that simultaneously act on different and/or multiple targets in an additive or synergistic manner to confer antiviral effect [217].
4.4. Thymidine Kinase (TK)
Thymidine kinase (TK) genes of alpha-herpesviruses are virulence-related genes encoding key kinases in the nucleoside salvage pathway. The HSV-1 TK enzyme phosphorylates four nucleosides and various nucleoside analogs. Hence, this enzyme is a pivotal target in antiviral therapeutic strategies [228]. The crystallographic structures of HSV-1 TK bound to its endogenous substrate deoxythymidine (dT) and the guanosine analog Ganciclovir were elucidated and published in 1995 [229,230]. Three 5-substituted 2′-deoxyuridine analogs (Idoxuridine, Trifluridine, and Brivudine [BVDU]) have been approved as antiviral drugs. These analogs mimic natural substrates, facilitating phosphorylation. These phosphorylated antiviral agents interfere with viral DNA replication (Table 2). Acyclic guanosine analogs like Acyclovir and Penciclovir are phosphorylated by TK to monophosphate form. Cellular enzymes convert the compound to its active triphosphate form, which is then incorporated into viral DNA, halting replication [230] (Table 2). Mutations in TK gene can result in resistance to these inhibitors [231].
4.5. Methyltransferase (MTase)
In viral systems, MTases facilitate the synthesis of the 5′ cap-0 structure, thereby enhancing the virus’s ability to evade the host’s innate immune defenses [232]. nsP1 of alphaviruses exhibits S-adenosyl-l-methionine (SAM)-dependent methyltransferase (MTase) and m7GTP transferase (GTase) activities that are membrane-binding dependent and are essential for viral capping [233]. Solution NMR and cryo-EM structures provide insights into the membrane-binding and enzyme capping mechanism [234,235]. Berbamine hydrochloride (BH), ABT199/venetoclax (ABT), ponatinib (PT), and selective estrogen receptor modulators (SERMs) are reported to inhibit nsP1 of Chikungunya Virus (CHIKV) and Sindbis Virus (SINV) [236,237,238].
The plus-strand RNA genome of flaviviruses is capped with a 5′ terminal Cap 1 structure (m7GpppAmG). The flaviviruses encode one methyltransferase, located at the N-terminal portion of the NS5 protein, responsible for catalyzing both guanine N-7 and ribose 2′-OH methylations [239]. X-ray structures aided in the elucidation of the catalytic site and drug-binding pockets [239,240,241,242,243]. Ribavirin, aurintricarboxylic acid, sinefungin, S-adenosyl homocysteine (SAH), Compound 10, herbacetin (HC), and caffeic acid phenethyl ester (CAPE) are reported as DENV MTase inhibitors [244,245,246,247]
SARS-CoV-2 MTases, nsp10/nsp16 and nsp14, facilitate viral RNA capping. Nsp14 catalyzes the 7-methylation of the 5′-cap guanosine, while nsp16 with nsp10, mediates the 2′-O-methylation of the ribose, thereby finalizing the cap structure. X-ray and cryo-EM structures were solved for both enzymes [248]. Several adenosine-like inhibitors and non-nucleoside inhibitors have been reported against nsp14 and nsp16 [249].
4.6. Helicases
Virus-encoded helicases are exploited by certain viruses to unwind DNA or RNA duplexes, a process driven by the hydrolysis of ATP [250]. Prominent examples include a domain of HCV-NS3 [251], vaccinia nucleoside triphosphate phosphohydrolase-II (NPH-II) [252], SARS-CoV-2 Nsp13 helicase [253], Simian virus 40 (SV40) TAg protein [254], HPV E1 protein [255], DENV NS3 helicase [256], MPXV E5 helicase [257], and N-terminal helicase of CHIKV nsP2 [258]. Inhibitors are reported to target the helicase-mediated ATP hydrolysis and nucleic acid binding [259]. X-ray [256,260,261,262,263,264,265], cryo-EM [257,266], NMR [267], and structure prediction [266] data have been reported for several viruses. Helicase enzymes are essential during multiple stages of genome replication, rendering them compelling antiviral targets, particularly given the extensive structural data available. Despite significant efforts to develop helicase-targeting antivirals, clinical advancement has been hindered by challenges related to cytotoxicity, bioavailability, and pharmacokinetics [259,268]. As of now, no virus-encoded helicase inhibitors have received FDA approval.
6. Rational Drug Design
Viral outbreaks, such as COVID-19 and Monkeypox, highlight the challenges posed by viral mutations that enhance immune escape and virulence, potentially leading to pandemics. Predicting the next mutation remains difficult [289]. Therefore, global preparedness is essential to counter future pandemics [290]. The pursuit of advanced, structure-guided treatments through AI-driven technologies is crucial for addressing viral mutations that could reduce therapeutic efficacy [291]. For instance, after the COVID-19 outbreak, it became evident that a strategic pipeline is required to identify antigenic sites, design non-cytotoxic ligands, and enable mass production and distribution of drugs [292]. Similarly, the Monkeypox outbreak reinforced the need to identify viral targets and develop specific drugs against them [293].
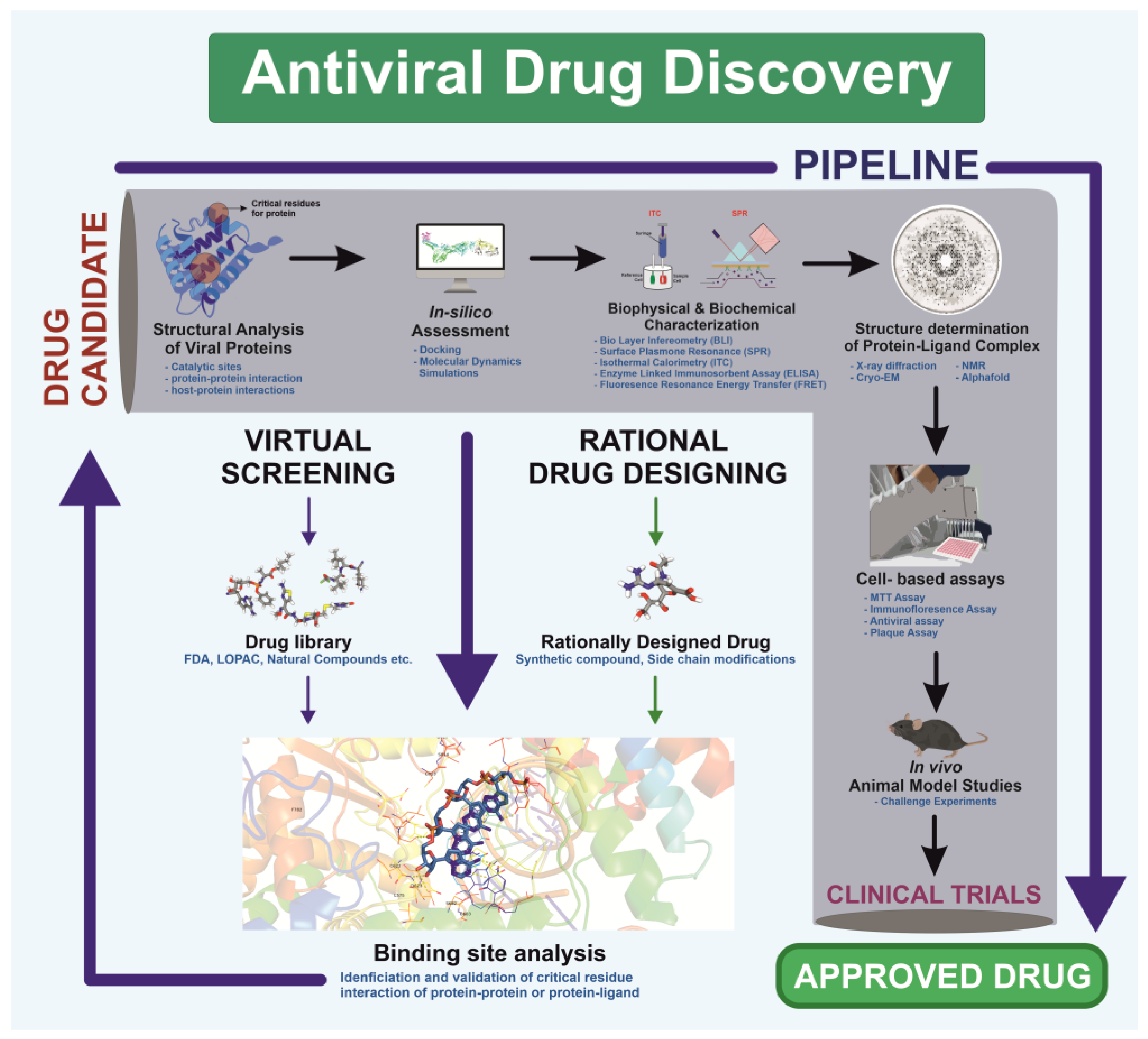
Visualization forms the foundation of rational drug design, enabling researchers to analyze viral structures [294]. This structural understanding facilitates precise drug design based on viral protein architecture, as demonstrated during the COVID-19 pandemic [295]. Unlike traditional methods, which relied on screening drugs for antiviral activity without prior molecular insight, rational drug design employs high-resolution structural techniques—X-ray crystallography, NMR spectroscopy, and cryo-EM—to study viral proteins and processes essential for replication [296]. The process begins by identifying a target viral protein critical to replication or host entry. Structural analyses then reveal active sites and binding pockets where small molecules can inhibit protein function [297]. Using this data, scientists design molecules tailored to fit these pockets, thereby blocking protein activity. This targeted strategy improves efficacy and minimizes side effects by avoiding interactions with non-target proteins (Figure 4).
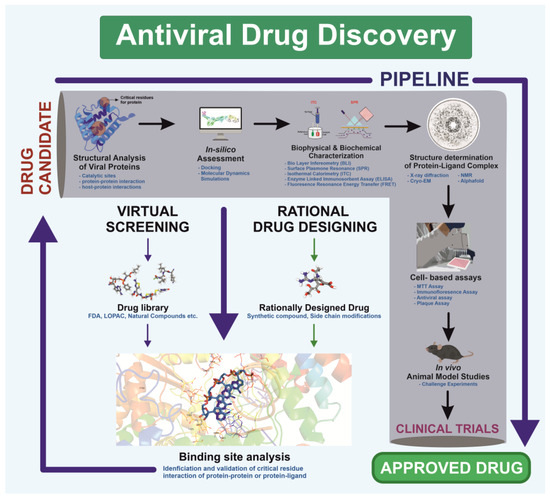
Figure 4.
Schematic illustration of the antiviral drug discovery pipeline targeting a viral protein, integrating screening and rational design approaches. The workflow encompasses virtual screening, hit identification, in vitro validation, co-crystallization, cell-based assays, and in vivo efficacy evaluation, ensuring a systematic progression from compound identification to drug development.
Rational drug design combines structural and biochemical insights to create highly specific therapies, transforming antiviral treatment approaches. For example, the small molecule Nirmatrelvir targets SARS-CoV-2’s main protease, showing high antiviral efficacy [298]. Similarly, analogs like spirolactam, derived from zamnair, improve antiviral potency, while peptides such as VIR250 selectively inhibit the papain-like protease of SARS-CoV-2 [297]. Nanoparticles, like the ICO-RBD nanovaccine, mimic virus-like structures to boost immunogenicity [299]. Future developments in rational drug design promise faster and more precise solutions for emerging pathogens, leveraging computational tools and structural biology advancements.
Rational drug design has transformed structural biology by providing a direct pathway from understanding a virus’s atomic structure to developing effective antiviral therapies [300]. This approach has led to the creation of antiviral drugs for diseases such as HIV/AIDS and influenza, where structural studies of viral enzymes and surface proteins play a key role in designing inhibitors. Additionally, this method is vital for quickly developing treatments against emerging viral threats, as demonstrated by the rapid creation of SARS-CoV-2 spike protein inhibitors during the COVID-19 pandemic. AI has further accelerated this process by reducing screening times for antivirals, improving predictive models for binding affinity, and enabling data mapping to track trends in viral mutations and forecast future changes [301,302]. AI technologies have enhanced drug discovery by optimizing, generating, and identifying molecules with drug-like properties [303]. The need for such advancements in rational drug design is clear, as they support the rapid development of therapeutics to combat future pandemics. Ultimately, rational drug design not only deepens our understanding of drug mechanisms but also advances public health by enabling the swift creation of targeted treatments for both existing and emerging viral diseases [304] (Figure 4).
De Novo Designing—A Targeted Approach with Improved Features
De novo protein design has emerged as a promising strategy for creating molecules from scratch, contributing significantly to combating viral infections [305]. Computational pipelines can be developed and deployed for the streamlined development of antibody-based therapeutic interventions against emerging pathogens [306]. Applications include an in silico affinity maturation pipeline developed and employed to successfully bioengineer nanobodies with enhanced affinity [307]. With the integration of generative AI, it is now possible to engineer proteins targeting specific protein structures. This advancement also enables personalized therapeutic research tailored to individual patients [308,309]. Large language models, such as PALM-H3, further enhance these capabilities by generating antibodies using pre-trained models, allowing for the de novo synthesis of antibodies [310]. Beyond drug design, vaccines targeting viral proteins can also be developed to present surface glycoproteins and elicit immunization responses. Modeling tools such as SabPred assist further in modeling antibodies and in silico validation of structures [311]. For instance, HIV vaccines displaying envelope glycoproteins have demonstrated strong neutralizing titers, emphasizing the effectiveness of nanoparticle-based strategies [312,313]. Moreover, generative AI can be utilized to produce antibodies specific to target antigens, enhancing therapeutic applications [314].
Breakthroughs such as AlphaFold have revolutionized protein modeling, enabling the accurate prediction of protein structures [315]. These tools facilitate the identification of protein functions and their interactions with other molecules [93]. Further advancements, like RFdiffusion, simplify the design of protein binders that target specific antigen sites, eliciting neutralizing responses against viruses [316]. Various approaches taken for designing these antivirals are mentioned in Table 3. A rational approach has not only been evident in designing antivirals but also antibodies and vaccines designing too.

Table 3.
List of therapeutics and the rational approaches used in their designing.
7. Identifying the Threat of Future—A Structural Approach
Viruses pose a significant global health threat due to their potential to cause epidemics and pandemics. The WHO R&D Blueprint for Epidemics prioritizes identifying high-risk viruses for early detection, targeted research, efficient resource allocation, and global collaboration (WHO Pathogen Prioritization Report, 2024) [329] (Table 4). “Pathogen X”, an unknown future threat, underscores the need for preparedness. Identifying new pathogens involves robust surveillance, genomic sequencing, and epidemiological studies. Targeting Pathogen X requires focusing on pathogen families, studying prototype pathogens, fostering international collaboration, and investing in R&D [330]. Prioritizing high-risk viruses and preparing for Pathogen X enhances global response capabilities, ultimately saving lives and protecting public health (WHO Pathogen Prioritization Report, 2024) [329]. Knowing the threat, their structures, and a rational approach to designing therapeutics, there is hope of fighting the upcoming pandemics with advanced knowledge of structural virology.

Table 4.
Virus families and pathogens identified as high-priority Public Health Emergencies of International Concern (PHEICs) for 2024. The corresponding PDB IDs of structural and non-structural proteins for these “high-risk” viruses are provided, highlighting potential antiviral therapeutic targets. (Source: WHO Pathogen Prioritization Report, 2024).
8. Conclusions and Future Direction
Structural virology has played an instrumental role in shaping our understanding of viral mechanisms and enabling the development of targeted therapeutics. Key techniques like X-ray crystallography, cryo-EM, and NMR spectroscopy have yielded high-resolution images of viral structures, revealing key molecular targets on enzymes, receptors, and structural proteins. With the ever-increasing data and research on virus-encoded proteins, structural biology has proven to be an indispensable tool for rational design and optimization of antiviral drugs. Additionally, computational methods have become integral to the early stages of drug discovery and development. The review delves into existing and new technological advancements to further the depth of structural understanding of viral proteins, interactions, and the strategies for antiviral therapeutic development.
Although notable strides have been made in the field, the structural data of many of the key proteins are yet to be elucidated. Therefore, it is imperative to develop strategies for obtaining structural data and establishing a link between structure and function. COVID-19 demonstrated the significance of pandemic preparedness for the world. Based on historical precedents of pandemics and epidemics that have plagued the world, debilitated global healthcare systems, and caused substantial mortality and lasting health impact. COVID-19 is unlikely to be the final pandemic. Therefore, continuous efforts from the scientific community to develop antiviral therapeutics against emerging pathogens and pathogens with pandemic potential is the need of the hour. Further investigations are crucial for identifying druggable host factors for the development of broad-spectrum therapeutics.
Due to rapid rate of mutation, many viruses are emerging and reemerging pathogens. Mutation prediction, leveraging sequence and structural data along with surveillance data are now critical in forecasting evolution, aiding to guide the design of adaptive therapeutic strategies that can keep up with the rapid rate of viral evolution. As witnessed during the SARS-CoV-2 pandemic, available and ongoing research to elucidate the protein structures expedited vaccine development, whereas the rapid identification of variants underscored the importance of anticipating viral evolution in real-time. In the future, real-time structural surveillance, coupled with AI-powered tools, will be pivotal in swiftly identifying mutations and new pathogens. It will be essential to assess the impact of viral mutations on transmissibility and immune escape. Considering the rise of emerging and reemerging viruses, it is crucial to identify the viruses that may pose a threat to our health in the future and to develop strategies to address them before they escalate into a severe pandemic. Consequently, numerous global organizations, such as the WHO, have recognized several of these viruses and have initiated efforts to address them. Having a strategic plan in place to address the threat will provide greater safety for dealing with future viral challenges.
Author Contributions
Conceptualization, T.H., A.S., S.T., A.N., E.R., P.K. and J.S.; writing—original draft preparation T.H., A.S. and S.T.; writing—review and editing, T.H., A.S., S.T., A.N., E.R., P.K. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors P.K. and S.T. duly acknowledge the financial support from the Scheme for Transformational and Advanced Research in Sciences (STARS), Ministry of Education (MoE) (project reference no. STARS2/2023–0209) for supporting this study.
Acknowledgments
A.S. thanks the Prime Minister’s Research Fellows (PMRF) scheme, Ministry of Education (MOE), India for the research fellowship. T.H. acknowledges Ministry of Human Resource Development (MHRD), India for research fellowship.
Conflicts of Interest
The authors declare that they have no competing interest concerning the publication of this review.
Abbreviations
The following abbreviations are used in this manuscript:
| 3CLpro | 3-chymotrypsin-like proteases |
| 3D | Three-dimensional |
| 3-P | 3′-processing |
| 3TC | Lamivudine |
| 5-CITEP | 1-(5-chloroindol-3-yl)-3-hydroxy-3-(2H-tetrazol-5-yl)propenone |
| ABT | ABT199/venetoclax |
| ACE2 | Angiotensin-converting enzyme 2 |
| ACV | Acyclovir |
| ACV | Acyclovir |
| ADE | Antibody-dependent enhancement |
| AI | Artificial Intelligence |
| ALLINI | Allosteric IN inhibitors |
| APV | Amprenavir |
| ART | Antiretroviral therapy |
| ASV | Asunaprevir |
| ATV | Atazanavir |
| BH | Berbamine hydrochloride |
| BIC | Bictegravir |
| BOC | Boceprevir |
| BVDU | Brivudine |
| BXA | Baloxavir |
| CAPE | Caffeic acid phenethyl ester |
| CAR | Coxsackievirus and Adenovirus Receptor |
| cART | Combination antiretroviral therapy |
| CCR5 | C-C chemokine receptor type 5 |
| CD4 | Cluster of differentiation 4 |
| CEN | Cap-dependent endonuclease |
| CHIKV | Chikungunya Virus |
| CLR | C-type lectin receptor |
| CMV | Cucumber Mosaic Virus |
| COVID-19 | Coronavirus disease 2019 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| cryo-EM | Cryo-electron microscopy |
| Cryo-ET | Cryo-electron tomography |
| CsA | Cyclosporin A |
| CVB4 | Coxsackie B4 virus |
| CXCR4 | C-X-C chemokine receptor type 4 |
| Cyp | Cyclophilin |
| d4T | Stavudine |
| DC-SIGN | Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin |
| DdDp | DNA-Dependent DNA Polymerase |
| DdRp | DNA-Dependent RNA Polymerase |
| DENV | Dengue Virus |
| DKA | Diketoacid |
| DLV | Delavirdine |
| DNA | Deoxyribonucleic Acid |
| DOR | Doravirine |
| DPV | Dapivirine |
| DRV | Darunavir |
| dT | Deoxythymidine |
| DTG | Dolutegravir |
| EFV | Efavirenz |
| ENS | Ensitrelvir |
| ETR | Etravirine |
| EUA | Emergency use authorization |
| FDA | Food and Drug Administration |
| FVP | Favipiravir |
| G3BP1 | Ras GTPase-activating protein SH3-domain-binding protein 1 |
| GLE | Glecaprevir |
| GTase | m7GTP transferase |
| GV | Giant viruses |
| GZR | Grazoprevir |
| HA | hemagglutinin |
| HBV | Hepatitis B Virus |
| HC | Herbacetin |
| HCoV | Human Coronavirus- |
| HCV | Hepatitis C Virus |
| HHV | Human Herpesvirus |
| HIV | Human Immunodeficiency Virus |
| HPV | Human Papillomavirus |
| HS | heparan sulfate |
| HTLV | Human T-Cell Leukemia Virus |
| IBD | Integrase binding domain |
| ICTV | International Committee on Taxonomy of Viruses |
| IDP | Intrinsically disordered proteins |
| IDR | Intrinsically disordered regions |
| IDU | Idoxuridine |
| IDV | Indinavir |
| IFN | Interferon |
| IFN | Interferon |
| IL-10 | Interleukin 10 |
| IN | Integrase |
| INSTI | Integrase strand transfer inhibitors |
| INT | Intasome |
| IQM | Imiquimod |
| IR | Infrared |
| ISG | Interferon-Stimulated Genes |
| KSHV | Kaposi‘s Sarcoma-Associated Herpesvirus |
| LEDGF/p75 | Lens Epithelium-Derived Growth Factor/p75 |
| LPV | Lopinavir |
| MCV or MCPyV | Merkel Cell Polyomavirus |
| MD | Molecular Dynamics |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MINI | Multimerization-selective integrase inhibitor |
| MOV | Molnupiravir |
| Mpro | Main protease |
| MPXV | Monkeypox virus |
| MTase | Methyltransferase |
| MXRA8 | Matrix remodeling-associated protein 8 |
| NA | Neuraminidase |
| NFV | Nelfinavir |
| NiRAN | RdRp-associated nucleotidyltransferase |
| NMR | Nuclear Magnetic Resonance |
| NMV | Nirmatrelvir |
| NNRTI | Non-nucleoside reverse transcriptase inhibitors |
| NRTI | Nucleoside reverse transcriptase inhibitors |
| NTD | N-terminal domain |
| NTD | N-terminal domain |
| NTP | Nucleoside triphosphate |
| NVP | Nevirapine |
| PCV | Penciclovir |
| PDX | Podofilox |
| PFA | Foscarnet |
| PI | Peptide inhibitors |
| PIC | Pre-integration complex |
| PLpro | Papain-like protease |
| PPI | Protein–protein interactions |
| PRF | Programmed ribosomal frameshifting |
| PT | Ponatinib |
| PVY | Potato Virus Y |
| RABV | Rabies Virus |
| RBD | Receptor-binding domain |
| RdRp | RNA-Dependent RNA Polymerase |
| RDV | Remdesivir |
| RNA | Ribonucleic Acid |
| RNase | Ribonuclease |
| RNP | Ribonucleoprotein |
| RPV | Rilpivirine |
| RSV | Respiratory syncytial virus |
| RT | Reverse Transcriptase |
| RTV | Ritonavir |
| RV | Rotavirus |
| SAH | S-adenosyl homocysteine |
| SAM | S-adenosyl-l-methionine |
| SARS-CoV | Severe Acute Respiratory Syndrome Coronavirus |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SAXS | Small Angle X-ray Scattering |
| SBDD | Structure-based drug design |
| SERM | Selective estrogen receptor modulators |
| SG | Stress granule |
| SINE | Sinecatechins |
| SINV | Sindbis Virus |
| SMV | Simeprevir |
| SQV | Saquinavir |
| ST | Strand transfer |
| TBSV | Tomato bushy stunt virus |
| TDF | Tenofovir disoproxil fumarate |
| TEM | Transmission electron microscopy |
| TK | Thymidine kinase |
| TMV | Tobacco Mosaic Virus |
| TPV | Tipranavir |
| TVR | Telaprevir |
| VEEV | Venezuelan Equine Encephalitis virus |
| VLP | Virus-like particle |
| VOX | Voxilaprevir |
| VPV | Vaniprevir |
| VZV | Varicella-zoster virus |
| WHO | World Health Organization |
| XFEL | X-ray Free Electron Laser |
| XRD | X-ray diffraction |
References
- Payne, S. Introduction to Animal Viruses. In Viruses; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–11. [Google Scholar]
- Current ICTV Taxonomy Release|ICTV. Available online: https://ictv.global/taxonomy (accessed on 27 December 2024).
- Wirth, J.; Young, M. The Intriguing World of Archaeal Viruses. PLoS Pathog. 2020, 16, e1008574. [Google Scholar] [CrossRef]
- Krupovic, M.; Cvirkaite-Krupovic, V.; Iranzo, J.; Prangishvili, D.; Koonin, E.V. Viruses of Archaea: Structural, Functional, Environmental and Evolutionary Genomics. Virus Res. 2018, 244, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Costa, A.R.; van Beljouw, S.P.B.; Fineran, P.C.; Brouns, S.J.J. Approaches for Bacteriophage Genome Engineering. Trends Biotechnol. 2023, 41, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, V.F.; Tatara, J.M.; Botelho, B.B.; Rodrigues, R.A.L.; Almeida, G.M.d.F.; Abrahao, J.S. The Consequences of Viral Infection on Protists. Commun. Biol. 2024, 7, 306. [Google Scholar] [CrossRef]
- Kalafati, E.; Papanikolaou, E.; Marinos, E.; Anagnou, N.P.; Pappa, K.I. Mimiviruses: Giant viruses with novel and intriguing features. Mol. Med. Rep. 2022, 25, 207. [Google Scholar] [CrossRef]
- Coy, S.R.; Gann, E.R.; Pound, H.L.; Short, S.M.; Wilhelm, S.W. Viruses of Eukaryotic Algae: Diversity, Methods for Detection, and Future Directions. Viruses 2018, 10, 487. [Google Scholar] [CrossRef]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Janssen, D. Global Plant Virus Disease Pandemics and Epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant Viruses of Agricultural Importance: Current and Future Perspectives of Virus Disease Management Strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-reared Edible Insect Species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.R.; Alkhovsky, S.V.; Avšič-Županc, T.; Bente, D.A.; Bergeron, É.; Burt, F.; Paola, N.D.; Ergünay, K.; Hewson, R.; Kuhn, J.H.; et al. ICTV Virus Taxonomy Profile: Nairoviridae. J. Gen. Virol. 2020, 101, 798–799. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.K.R.; Ebrahimpour, S. A Brief Review of Influenza Virus Infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef]
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef]
- Brunker, K.; Mollentze, N. Rabies Virus. Trends Microbiol. 2018, 26, 886–887. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 365–388. [Google Scholar] [CrossRef]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef]
- Rechenchoski, D.Z.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Herpesvirus: An Underestimated Virus. Folia Microbiol. 2017, 62, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The Structural Basis of Herpesvirus Entry. Nat. Rev. Microbiol. 2020, 19, 110–121. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Laboratory and Clinical Aspects of Human Herpesvirus 6 Infections. Clin. Microbiol. Rev. 2015, 28, 313–335. [Google Scholar] [CrossRef] [PubMed]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The Natural History of Human Papillomavirus Infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M.; Dean, C.L. Human Papillomavirus. In Diagnostic Microbiology of the Immunocompromised Host; ASM Press: Washington, DC, USA, 2016; pp. 177–195. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Primers 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Yoshimura, K. Current Status of HIV/AIDS in the ART Era. J. Infect. Chemother. 2017, 23, 12–16. [Google Scholar] [CrossRef]
- Bekker, L.G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV Infection. Nat. Rev. Dis. Primers 2023, 9, 1–21. [Google Scholar] [CrossRef]
- Lévêque, N.; Semler, B.L. A 21st Century Perspective of Poliovirus Replication. PLoS Pathog. 2015, 11, e1004825. [Google Scholar] [CrossRef]
- Marzi, A.; Blanco, J.R.; Gibellini, D.; Mbani, C.J.; Pandoua Nekoua, M.; Moukassa, D.; Hober, D. The Fight against Poliovirus Is Not Over. Microorganisms 2023, 11, 1323. [Google Scholar] [CrossRef]
- Cao, J.; Li, D. Searching for Human Oncoviruses: Histories, Challenges, and Opportunities. J. Cell Biochem. 2018, 119, 4897–4906. [Google Scholar] [CrossRef]
- Noguera, Z.L.P.; Charypkhan, D.; Hartnack, S.; Torgerson, P.R.; Rüegg, S.R. The Dual Burden of Animal and Human Zoonoses: A Systematic Review. PLoS Negl. Trop. Dis. 2022, 16, e0010540. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.A.; Carnevale de Almeida Moraes, D.; Ciacci Zanella, G.; Souza, C.K.; Anderson, T.K.; Baker, A.L.; Gauger, P.C. Reverse Zoonosis of the 2022–2023 Human Seasonal H3N2 Detected in Swine. npj Viruses 2024, 2, 1–12. [Google Scholar] [CrossRef]
- Lv, J.X.; Liu, X.; Pei, Y.Y.; Song, Z.G.; Chen, X.; Hu, S.J.; She, J.L.; Liu, Y.; Chen, Y.M.; Zhang, Y.Z. Evolutionary Trajectory of Diverse SARS-CoV-2 Variants at the Beginning of COVID-19 Outbreak. Virus Evol. 2024, 10, veae020. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. Influenza: The Once and Future Pandemic. Public Health Rep. 2010, 125, 15–26. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Fauci, A.S. Ending the HIV/AIDS Pandemic. Emerg. Infect. Dis. 2018, 24, 413. [Google Scholar] [CrossRef]
- De Cock, K.M.; Jaffe, H.W.; Curran, J.W. Reflections on 40 Years of AIDS. In Advances in Clinical Immunology, Medical Microbiology, COVID-19, and Big Data; Jenny Stanford Publishing: Singapore, 2021; pp. 231–245. [Google Scholar] [CrossRef]
- Chan-Yeung, E.M.; Xu, R.; Chan-Yeung, M.; Chan-yeung, M. SARS: Epidemiology. Respirology 2003, 8, S9–S14. [Google Scholar] [CrossRef]
- Raj, V.S.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; Haagmans, B.L. MERS: Emergence of a Novel Human Coronavirus. Curr. Opin. Virol. 2014, 5, 58–62. [Google Scholar] [CrossRef]
- Saha, A.; Choudhary, S.; Walia, P.; Kumar, P.; Tomar, S. Transformative Approaches in SARS-CoV-2 Management: Vaccines, Therapeutics and Future Direction. Virology 2025, 604, 110394. [Google Scholar] [CrossRef]
- Listings of WHO’s Response to COVID-19. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 27 December 2024).
- Duggan, A.T.; Perdomo, M.F.; Piombino-Mascali, D.; Marciniak, S.; Poinar, D.; Emery, M.V.; Buchmann, J.P.; Duchêne, S.; Jankauskas, R.; Humphreys, M.; et al. 17th Century Variola Virus Reveals the Recent History of Smallpox. Curr. Biol. 2016, 26, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio Vaccination: Past, Present and Future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; de La Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola Virus Disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Z.; Wen, Z.; Liu, Y.; Zeng, C.; Xiao, D.; Ou, M.; Han, Y.; Huang, S.; Liu, D.; et al. Global Epidemiology of Dengue Outbreaks in 1990–2015: A Systematic Review and Meta-Analysis. Front. Cell Infect. Microbiol. 2017, 7, 275966. [Google Scholar] [CrossRef]
- Douam, F.; Ploss, A. Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends Microbiol. 2018, 26, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An Update on Zika Virus Infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Perry, R.T.; Halsey, N.A. The Clinical Significance of Measles: A Review. J. Infect. Dis. 2004, 189, S4–S16. [Google Scholar] [CrossRef]
- Weibel Galluzzo, C.; Kaiser, L.; Chappuis, F. Reemergence of Chikungunya Virus. Rev. Med. Suisse 2015, 11, 1012–1016. [Google Scholar] [CrossRef]
- Turtle, L.; Solomon, T. Japanese Encephalitis—The Prospects for New Treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile Virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Baer, G.M. History of Rabies and Global Aspects; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Sperk, M.; Van Domselaar, R.; Rodriguez, J.E.; Mikaeloff, F.; Sá Vinhas, B.; Saccon, E.; Sönnerborg, A.; Singh, K.; Gupta, S.; Végvári, Á.; et al. Utility of Proteomics in Emerging and Re-Emerging Infectious Diseases Caused by RNA Viruses. J. Proteome Res. 2020, 19, 4259–4274. [Google Scholar] [CrossRef] [PubMed]
- Çelik, İ.; Saatçi, E.; Eyüboğlu, F.Ö. Emerging and Reemerging Respiratory Viral Infections up to COVID-19. Turk. J. Med. Sci. 2020, 50, 557–562. [Google Scholar] [CrossRef]
- Curry, S. Structural Biology: A Century-Long Journey into an Unseen World. Interdiscip. Sci. Rev. 2015, 40, 308–328. [Google Scholar] [CrossRef]
- Brooks-Bartlett, J.C.; Garman, E.F. The Nobel Science: One Hundred Years of Crystallography. Interdiscip. Sci. Rev. 2015, 40, 244–264. [Google Scholar] [CrossRef]
- Thomas, J.M. Centenary: The Birth of X-Ray Crystallography. Nature 2012, 491, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. A Glimpse of Structural Biology through X-Ray Crystallography. Cell 2014, 159, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Zheng, H.; Handing, K.B.; Zimmerman, M.D.; Shabalin, I.G.; Almo, S.C.; Minor, W. X-Ray Crystallography over the Past Decade for Novel Drug Discovery—Where Are We Heading Next? Expert Opin. Drug Discov. 2015, 10, 975–989. [Google Scholar] [CrossRef]
- Stanley, W.M. Isolation of a crystalline protein possessing the properties of tobacco-mosaic virus. Science 1935, 81, 644–645. [Google Scholar] [CrossRef]
- Norrby, E. Nobel Prizes and the Emerging Virus Concept. Arch. Virol. 2008, 153, 1109–1123. [Google Scholar] [CrossRef]
- Bernal, J.D.; Fankuchen, I. X-Ray and crystallographic studies of plant virus preparations: I. introduction and preparation of specimens II. modes of aggregation of the virus particles. J. Gen. Physiol. 1941, 25, 111–146. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C.; Olson, A.J.; Schutt, C.E.; Winkler, F.K.; Bricogne, G. Tomato Bushy Stunt Virus at 2.9 A Resolution. Nature 1978, 276, 368–373. [Google Scholar] [CrossRef]
- Bloomer, A.C.; Champness, J.N.; Bricogne, G.; Staden, R.; Klug, A. Protein Disk of Tobacco Mosaic Virus at 2.8 A Resolution Showing the Interactions within and between Subunits. Nature 1978, 276, 362–368. [Google Scholar] [CrossRef]
- Richmond, T.J.; Finch, J.T.; Rushton, B.; Rhodes, D.; Klug, A. Structure of the Nucleosome Core Particle at 7 A Resolution. Nature 1984, 311, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Schirò, A.; Carlon, A.; Parigi, G.; Murshudov, G.; Calderone, V.; Ravera, E.; Luchinat, C. On the Complementarity of X-Ray and NMR Data. J. Struct. Biol. X 2020, 4, 100019. [Google Scholar] [CrossRef]
- Holcomb, J.; Spellmon, N.; Zhang, Y.; Doughan, M.; Li, C.; Yang, Z. Protein Crystallization: Eluding the Bottleneck of X-Ray Crystallography. AIMS Biophys. 2017, 4, 557–575. [Google Scholar] [CrossRef]
- Vincenzi, M.; Leone, M. The Fight against Human Viruses: How NMR Can Help? Curr. Med. Chem. 2021, 28, 4380–4453. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. Extending the Size Limit of Protein Nuclear Magnetic Resonance. Proc. Natl. Acad. Sci. USA 1999, 96, 332–334. [Google Scholar] [CrossRef]
- LaPlante, S.R.; Coric, P.; Bouaziz, S.; França, T.C.C. NMR Spectroscopy Can Help Accelerate Antiviral Drug Discovery Programs. Microbes Infect. 2024, 26, 105297. [Google Scholar] [CrossRef]
- Kruger, D.H.; Schneck, P.; Gelderblom, H.R. Helmut Ruska and the Visualisation of Viruses. Lancet 2000, 355, 1713–1717. [Google Scholar] [CrossRef]
- Nagler, F.P.; Rake, G. The Use of the Electron Microscope in Diagnosis of Variola, Vaccinia, and Varicella. J. Bacteriol. 1948, 55, 45–51. [Google Scholar] [CrossRef]
- Brenner, S.; Horne, R.W. A Negative Staining Method for High Resolution Electron Microscopy of Viruses. Biochim. Biophys. Acta 1959, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.A.J.; Almeida, J.D. Direct Electron-Microscopy of Organ Cultures for the Detection and Characterization of Viruses. Arch. Gesamte Virusforsch. 1967, 22, 417–425. [Google Scholar] [CrossRef]
- Adrian, M.; Dubochet, J.; Lepault, J.; McDowall, A.W. Cryo-Electron Microscopy of Viruses. Nature 1984, 308, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Schoehn, G.; Chenavier, F.; Crépin, T. Advances in Structural Virology via Cryo-EM in 2022. Viruses 2023, 15, 1315. [Google Scholar] [CrossRef]
- Dutta, M.; Acharya, P. Cryo-Electron Microscopy in the Study of Virus Entry and Infection. Front. Mol. Biosci. 2024, 11, 1429180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Zhang, K. Cryo-EM: A Window into the Dynamic World of RNA Molecules. Curr. Opin. Struct. Biol. 2024, 88, 102916. [Google Scholar] [CrossRef]
- Wu, X.; Rapoport, T.A. Cryo-EM Structure Determination of Small Proteins by Nanobody-Binding Scaffolds (Legobodies). Proc. Natl. Acad. Sci. USA 2021, 118, e2115001118. [Google Scholar] [CrossRef]
- Renaud, J.P.; Chari, A.; Ciferri, C.; Liu, W.T.; Rémigy, H.W.; Stark, H.; Wiesmann, C. Cryo-EM in Drug Discovery: Achievements, Limitations and Prospects. Nat. Rev. Drug Discov. 2018, 17, 471–492. [Google Scholar] [CrossRef]
- Boldon, L.; Laliberte, F.; Liu, L. Review of the Fundamental Theories behind Small Angle X-Ray Scattering, Molecular Dynamics Simulations, and Relevant Integrated Application. Nano Rev. 2015, 6, 25661. [Google Scholar] [CrossRef]
- Handa, T.; Kundu, D.; Dubey, V.K. Perspectives on Evolutionary and Functional Importance of Intrinsically Disordered Proteins. Int. J. Biol. Macromol. 2023, 224, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Barradas-Bautista, D.; Rosell, M.; Pallara, C.; Fernández-Recio, J. Structural Prediction of Protein–Protein Interactions by Docking: Application to Biomedical Problems. Adv. Protein Chem. Struct. Biol. 2018, 110, 203–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Winkler, H.; Chertova, E.; Taylor, K.A.; Roux, K.H. Cryoelectron Tomography of HIV-1 Envelope Spikes: Further Evidence for Tripod-like Legs. PLoS Pathog. 2008, 4, e1000203. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, W. Cryo-Electron Tomography: The Power of Seeing the Whole Picture. Biochem. Biophys. Res. Commun. 2022, 633, 26–28. [Google Scholar] [CrossRef]
- Meents, A.; Wiedorn, M.O. Virus Structures by X-Ray Free-Electron Lasers. Annu. Rev. Virol. 2019, 6, 161–176. [Google Scholar] [CrossRef]
- Wang, J. Fast Identification of Possible Drug Treatment of Coronavirus Disease-19 (COVID-19) through Computational Drug Repurposing Study. J. Chem. Inf. Model 2020, 60, 3277–3286. [Google Scholar] [CrossRef]
- Singh, A.; Dhaka, P.; Kumar, P.; Tomar, S.; Singla, J. Bioinformatics Databases and Tools Available for the Development of Antiviral Drugs. In Advances in Antiviral Research; Springer: Singapore, 2024; pp. 41–71. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- O’Leary, K. AlphaFold Gets an Upgrade (and a Nobel). Nat. Med. 2024, 30, 3393. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F.; Bachmann, P.A.; Gibbs, E.P.J.; Murphy, F.A.; Studdert, M.J.; White, D.O. Structure and Composition of Viruses. In Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 3–19. [Google Scholar]
- Zheng, B.; Duan, M.; Huang, Y.; Wang, S.; Qiu, J.; Lu, Z.; Liu, L.; Tang, G.; Cheng, L.; Zheng, P. Discovery of a Heparan Sulfate Binding Domain in Monkeypox Virus H3 as an Anti-Poxviral Drug Target Combining AI and MD Simulations. Elife 2024, 13, RP100545. [Google Scholar] [CrossRef]
- Delogu, I.; Pastorino, B.; Baronti, C.; Nougairède, A.; Bonnet, E.; de Lamballerie, X. In Vitro Antiviral Activity of Arbidol against Chikungunya Virus and Characteristics of a Selected Resistant Mutant. Antivir. Res. 2011, 90, 99–107. [Google Scholar] [CrossRef]
- Barrow, E.; Nicola, A.V.; Liu, J. Multiscale Perspectives of Virus Entry via Endocytosis. Virol. J. 2013, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Diamond, M.S. A Molecular Understanding of Alphavirus Entry and Antibody Protection. Nat. Rev. Microbiol. 2022, 21, 396–407. [Google Scholar] [CrossRef]
- Melton, J.V.; Ewart, G.D.; Weir, R.C.; Board, P.G.; Lee, E.; Gage, P.W. Alphavirus 6K Proteins Form Ion Channels. J. Biol. Chem. 2002, 277, 46923–46931. [Google Scholar] [CrossRef] [PubMed]
- Button, J.M.; Mukhopadhyay, S. Capsid-E2 Interactions Rescue Core Assembly in Viruses That Cannot Form Cytoplasmic Nucleocapsid Cores. J. Virol. 2021, 95, e01062-21. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). In Encyclopedia of Virology: Volume 1–5, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2021; Volume 1–5, pp. 428–440. [Google Scholar] [CrossRef]
- Schlicksup, C.J.; Zlotnick, A. Viral Structural Proteins as Targets for Antivirals. Curr. Opin. Virol. 2020, 45, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, B.; Deng, L.; Liang, B.; Ping, J. Virus-Host Interaction Networks as New Antiviral Drug Targets for IAV and SARS-CoV-2. Emerg. Microbes Infect. 2022, 11, 1371–1389. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Virion Structure and Composition. Fenner White’s Med. Virol. 2017, 27–37. [Google Scholar] [CrossRef]
- Kaur, R.; Neetu; Mudgal, R.; Jose, J.; Kumar, P.; Tomar, S. Glycan-Dependent Chikungunya Viral Infection Divulged by Antiviral Activity of NAG Specific Chi-like Lectin. Virology 2019, 526, 91–98. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin–Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 Envelope Glycoprotein Biosynthesis, Trafficking, and Incorporation. J. Mol. Biol. 2011, 410, 582–608. [Google Scholar] [CrossRef]
- Wrobel, A.G. Mechanism and Evolution of Human ACE2 Binding by SARS-CoV-2 Spike. Curr. Opin. Struct. Biol. 2023, 81, 102619. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Fusion Peptides and the Mechanism of Viral Fusion. Biochim. Biophys. Acta (BBA) Biomembr. 2003, 1614, 116–121. [Google Scholar] [CrossRef]
- Zhai, X.; Yuan, Y.; He, W.T.; Wu, Y.; Shi, Y.; Su, S.; Du, Q.; Mao, Y. Evolving Roles of Glycosylation in the Tug-of-War between Virus and Host. Natl. Sci. Rev. 2024, 11, nwae086. [Google Scholar] [CrossRef]
- Matsuyama, S.; Taguchi, F. Two-Step Conformational Changes in a Coronavirus Envelope Glycoprotein Mediated by Receptor Binding and Proteolysis. J. Virol. 2009, 83, 11133. [Google Scholar] [CrossRef] [PubMed]
- Marzinek, J.K.; Raghuvamsi Palur, V.; Salem, G.; Chen, F.-C.; Wu, S.-R.; Bond, P.J.; Chao, D.-Y. Uncovering the Conformational Dynamics of Dengue Virus and Its Virus-like Particles as Novel Vaccine Candidates. Biophys. J. 2023, 122, 508a–509a. [Google Scholar] [CrossRef]
- Chen, F.; Nagy, K.; Chavez, D.; Willis, S.; McBride, R.; Giang, E.; Honda, A.; Bukh, J.; Ordoukhanian, P.; Zhu, J.; et al. Antibody Responses to Immunization With HCV Envelope Glycoproteins as a Baseline for B-Cell-Based Vaccine Development. Gastroenterology 2020, 158, 1058–1071.e6. [Google Scholar] [CrossRef]
- Katze, M.G.; He, Y.; Gale, M. Viruses and Interferon: A Fight for Supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, X.; Qiao, S.; Jia, L.; Wen, X.; Wang, Y.; Wang, C.; Li, H.; Cui, J. Umifenovir Epigenetically Targets the IL-10 Pathway in Therapy against Coxsackievirus B4 Infection. Microbiol. Spectr. 2023, 11, e04248-22. [Google Scholar] [CrossRef]
- Sargsyan, K.; Mazmanian, K.; Lim, C. A Strategy for Evaluating Potential Antiviral Resistance to Small Molecule Drugs and Application to SARS-CoV-2. Sci. Rep. 2023, 13, 502. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural Basis of SARS-CoV-2 Omicron Immune Evasion and Receptor Engagement. Science 2022, 375, 894–898. [Google Scholar] [CrossRef]
- Tang, H.; Ke, Y.; Liao, Y.; Bian, Y.; Yuan, Y.; Wang, Z.; Yang, L.; Ma, H.; Sun, T.; Zhang, B.; et al. Mutational Escape Prevention by Combination of Four Neutralizing Antibodies That Target RBD Conserved Regions and Stem Helix. Virol. Sin. 2022, 37, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.I.; Wang, Y.C.; Wang, Y.P.; Chi, C.Y.; Chien, Y.W. Risk of Severe Dengue during Secondary Infection: A Population-Based Cohort Study in Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 730–738. [Google Scholar] [CrossRef]
- Wells, T.J.; Esposito, T.; Henderson, I.R.; Labzin, L.I. Mechanisms of Antibody-Dependent Enhancement of Infectious Disease. Nat. Rev. Immunol. 2024, 25, 6–21. [Google Scholar] [CrossRef]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue Virus Neutralizing Antibody: A Review of Targets, Cross-Reactivity, and Antibody-Dependent Enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef]
- Malik, S.; Ahsan, O.; Mumtaz, H.; Tahir Khan, M.; Sah, R.; Waheed, Y. Tracing down the Updates on Dengue Virus—Molecular Biology, Antivirals, and Vaccine Strategies. Vaccines 2023, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Dengvaxia|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dengvaxia (accessed on 10 January 2025).
- Ragonnet-Cronin, M.; Nutalai, R.; Huo, J.; Dijokaite-Guraliuc, A.; Das, R.; Tuekprakhon, A.; Supasa, P.; Liu, C.; Selvaraj, M.; Groves, N.; et al. Generation of SARS-CoV-2 Escape Mutations by Monoclonal Antibody Therapy. Nat. Commun. 2023, 14, 3334. [Google Scholar] [CrossRef]
- Choudhary, S.; Malik, Y.S.; Tomar, S. Identification of SARS-CoV-2 Cell Entry Inhibitors by Drug Repurposing Using in Silico Structure-Based Virtual Screening Approach. Front. Immunol. 2020, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Osterhaus, A.D.M.E.; Treanor, J.J.; Fleming, D.M.; Aoki, F.Y.; Nicholson, K.G.; Bohnen, A.M.; Hirst, H.M.; Keene, O.; Wightman, K. Efficacy and Safety of the Neuraminidase Inhibitor Zanamivir in the Treatment of Influenzavirus Infections. N. Engl. J. Med. 1997, 337, 874–880. [Google Scholar] [CrossRef]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Russell, R.J.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S.J. Crystal Structures of Oseltamivir-Resistant Influenza Virus Neuraminidase Mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Li, Q.; Wu, Y.; Qi, J.; Wang, M.; Liu, Y.; Gao, F.; Liu, J.; Feng, E.; He, J.; et al. Structural and Functional Analysis of Laninamivir and Its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition. PLoS Pathog. 2011, 7, e1002249. [Google Scholar] [CrossRef]
- Pattnaik, G.P.; Chakraborty, H. Entry Inhibitors: Efficient Means to Block Viral Infection. J. Membr. Biol. 2020, 253, 425–444. [Google Scholar] [CrossRef]
- Beugeling, M.; De Zee, J.; Woerdenbag, H.J.; Frijlink, H.W.; Wilschut, J.C.; Hinrichs, W.L.J. Respiratory Syncytial Virus Subunit Vaccines Based on the Viral Envelope Glycoproteins Intended for Pregnant Women and the Elderly. Expert Rev. Vaccines 2019, 18, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.A.; Nehul, S.; Kumar, C.S.; Banerjee, M.; Kumar, P.; Sharma, G.; Tomar, S. Chimeric Chikungunya Virus-like Particles with Surface Exposed SARS-CoV-2 RBD Elicits Potent Immunogenic Responses in Mice. bioRxiv 2023. bioRxiv:2023.01.29.526074. [Google Scholar] [CrossRef]
- Singh, V.A.; Kumar, C.S.; Khare, B.; Kuhn, R.J.; Banerjee, M.; Tomar, S. Surface Decorated Reporter-Tagged Chikungunya Virus-like Particles for Clinical Diagnostics and Identification of Virus Entry Inhibitors. Virology 2023, 578, 92–102. [Google Scholar] [CrossRef]
- Nieva, J.L.; Madan, V.; Carrasco, L. Viroporins: Structure and Biological Functions. Nat. Rev. Microbiol. 2012, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Cheng, A.; Wang, M.; Ou, X.; Sun, D.; Mao, S.; Huang, J.; Yang, Q.; Wu, Y.; Chen, S.; et al. Functions of Viroporins in the Viral Life Cycle and Their Regulation of Host Cell Responses. Front. Immunol. 2022, 13, 890549. [Google Scholar] [CrossRef] [PubMed]
- Devantier, K.; Kjær, V.M.S.; Griffin, S.; Kragelund, B.B.; Rosenkilde, M.M. Advancing the Field of Viroporins—Structure, Function and Pharmacology: IUPHAR Review 39. Br. J. Pharmacol. 2024, 181, 4450–4490. [Google Scholar] [CrossRef]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza Virus M2 Protein Has Ion Channel Activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef]
- Surya, W.; Samsó, M.; Torres, J. Structural and Functional Aspects of Viroporins in Human Respiratory Viruses: Respiratory Syncytial Virus and Coronaviruses. In Respiratory Disease and Infection-A New Insight; TechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Das, K. Antivirals Targeting Influenza a Virus. J. Med. Chem. 2012, 55, 6263–6277. [Google Scholar] [CrossRef]
- Thomaston, J.L.; Polizzi, N.F.; Konstantinidi, A.; Wang, J.; Kolocouris, A.; Degrado, W.F. Inhibitors of the M2 Proton Channel Engage and Disrupt Transmembrane Networks of Hydrogen-Bonded Waters. J. Am. Chem. Soc. 2018, 140, 15219–15226. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Castaño-Rodriguez, C.; Aguilella, V.M.; Enjuanes, L. Relevance of Viroporin Ion Channel Activity on Viral Replication and Pathogenesis. Viruses 2015, 7, 3552–3573. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Siddiqui, S.I.; Mamidi, P.; Ghosh, S.; Kumar, C.S.; Chattopadhyay, S.; Ghosh, S.; Banerjee, M. The Effect of Amantadine on an Ion Channel Protein from Chikungunya Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007548. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A. Influenza. Encycl. Virol. 2008, 1–5, 95–104. [Google Scholar] [CrossRef]
- Scott, C.; Griffin, S. Viroporins: Structure, Function and Potential as Antiviral Targets. J. Gen. Virol. 2015, 96, 2000–2027. [Google Scholar] [CrossRef] [PubMed]
- Fatma, B.; Kumar, R.; Singh, V.A.; Nehul, S.; Sharma, R.; Kesari, P.; Kuhn, R.J.; Tomar, S. Alphavirus Capsid Protease Inhibitors as Potential Antiviral Agents for Chikungunya Infection. Antivir. Res. 2020, 179, 104808. [Google Scholar] [CrossRef]
- Chakravarty, A.; Rao, A.L. The Interplay between Capsid Dynamics and Pathogenesis in Tripartite Bromoviruses. Curr. Opin. Virol. 2021, 47, 45–51. [Google Scholar] [CrossRef]
- Ghaemi, Z.; Gruebele, M.; Tajkhorshid, E. Molecular Mechanism of Capsid Disassembly in Hepatitis B Virus. Proc. Natl. Acad. Sci. USA 2021, 118, e2102530118. [Google Scholar] [CrossRef]
- Mohajerani, F.; Tyukodi, B.; Schlicksup, C.J.; Hadden-Perilla, J.A.; Zlotnick, A.; Hagan, M.F. Multiscale Modeling of Hepatitis B Virus Capsid Assembly and Its Dimorphism. ACS Nano 2022, 16, 13845–13859. [Google Scholar] [CrossRef]
- Koehl, P.; Akopyan, A.; Edelsbrunner, H. Computing the Volume, Surface Area, Mean, and Gaussian Curvatures of Molecules and Their Derivatives. J. Chem. Inf. Model 2023, 63, 973–985. [Google Scholar] [CrossRef]
- Aggarwal, M.; Kaur, R.; Saha, A.; Mudgal, R.; Yadav, R.; Dash, P.K.; Parida, M.; Kumar, P.; Tomar, S. Evaluation of Antiviral Activity of Piperazine against Chikungunya Virus Targeting Hydrophobic Pocket of Alphavirus Capsid Protein. Antivir. Res. 2017, 146, 102–111. [Google Scholar] [CrossRef]
- Thenin-Houssier, S.; T Valente, S. HIV-1 Capsid Inhibitors as Antiretroviral Agents. Curr. HIV Res. 2016, 14, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Segal-Maurer, S.; DeJesus, E.; Stellbrink, H.-J.; Castagna, A.; Richmond, G.J.; Sinclair, G.I.; Siripassorn, K.; Ruane, P.J.; Berhe, M.; Wang, H.; et al. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N. Engl. J. Med. 2022, 386, 1793–1803. [Google Scholar] [CrossRef]
- Klumpp, K.; Crépin, T. Capsid Proteins of Enveloped Viruses as Antiviral Drug Targets. Curr. Opin. Virol. 2014, 5, 63–71. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Jia, R.; Wang, M.; Yin, Z.; Cheng, A. Structure and Function of Capsid Protein in Flavivirus Infection and Its Applications in the Development of Vaccines and Therapeutics. Vet. Res. 2021, 52, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, R.; Zhou, J.; Wang, M.; Yin, Z.; Cheng, A. Capsid-Targeted Viral Inactivation: A Novel Tactic for Inhibiting Replication in Viral Infections. Viruses 2016, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, P.; Mahto, J.K.; Singh, A.; Kumar, P.; Tomar, S. Structural Insights into the RNA Binding Inhibitors of the C-Terminal Domain of the SARS-CoV-2 Nucleocapsid. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dhaka, P.; Singh, A.; Choudhary, S.; Peddinti, R.K.; Kumar, P.; Sharma, G.K.; Tomar, S. Mechanistic and Thermodynamic Characterization of Antiviral Inhibitors Targeting Nucleocapsid N-Terminal Domain of SARS-CoV-2. Arch. Biochem. Biophys. 2023, 750, 109820. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.M. The Isolation and Properties of Crystalline Tobacco Mosaic Virus. Nobel Lect. 1946, 12, 1942–1962. [Google Scholar]
- Namba, K.; Pattanayek, R.; Stubbs, G. Visualization of Protein-Nucleic Acid Interactions in a Virus: Refined Structure of Intact Tobacco Mosaic Virus at 2.9 Å Resolution by X-Ray Fiber Diffraction. J. Mol. Biol. 1989, 208, 307–325. [Google Scholar] [CrossRef]
- Aggarwal, M.; Tapas, S.; Preeti; Siwach, A.; Kumar, P.; Kuhn, R.J.; Tomar, S. Crystal Structure of Aura Virus Capsid Protease and Its Complex with Dioxane: New Insights into Capsid-Glycoprotein Molecular Contacts. PLoS ONE 2012, 7, e51288. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Dhindwal, S.; Kumar, P.; Kuhn, R.J.; Tomar, S. Trans -Protease Activity and Structural Insights into the Active Form of the Alphavirus Capsid Protease. J. Virol. 2014, 88, 12242–12253. [Google Scholar] [CrossRef] [PubMed]
- Kanodia, S.; Da Silva, D.M.; Kast, W.M. Recent Advances in Strategies for Immunotherapy of Human Papillomavirus-Induced Lesions. Int. J. Cancer 2008, 122, 247–259. [Google Scholar] [CrossRef]
- Demmler-Harrison, G.J. Antiviral Agents. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 6th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2009; pp. 3245–3271. [Google Scholar] [CrossRef]
- Wlodawer, A.; Vondrasek, J. Inhibitors of HIV-1 Protease: A Major Success of Structure-Assisted Drug Design. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 249–284. [Google Scholar] [CrossRef] [PubMed]
- FDA-Approved HIV Medicines|NIH. Available online: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines (accessed on 27 December 2024).
- Weber, I.T.; Miller, M.; Jaskólski, M.; Leis, J.; Skalka, A.M.; Wlodawer, A. Molecular Modeling of the HIV-1 Protease and Its Substrate Binding Site. Science 1989, 243, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Lapatto, R.; Blundell, T.; Hemmings, A.; Overington, J.; Wilderspin, A.; Wood, S.; Merson, J.R.; Whittle, P.J.; Danley, D.E.; Geoghegan, K.F.; et al. X-Ray Analysis of HIV-1 Proteinase at 2.7 Å Resolution Confirms Structural Homology among Retroviral Enzymes. Nature 1989, 342, 299–302. [Google Scholar] [CrossRef]
- Roberts, N.A.; Martin, J.A.; Kinchington, D.; Broadhurst, A.V.; Craig, J.C.; Duncan, I.B.; Galpin, S.A.; Handa, B.K.; Kay, J.; Kröhn, A.; et al. Rational Design of Peptide-Based HIV Proteinase Inhibitors. Science (1979) 1990, 248, 358–361. [Google Scholar] [CrossRef]
- Craig, J.C.; Duncan, I.B.; Hockley, D.; Grief, C.; Roberts, N.A.; Mills, J.S. Antiviral Properties of Ro 31-8959, an Inhibitor of Human Immunodeficiency Virus (HIV) Proteinase. Antivir. Res. 1991, 16, 295–305. [Google Scholar] [CrossRef]
- Kempf, D.J.; Marsh, K.C.; Denissen, J.F.; McDonald, E.; Vasavanonda, S.; Flentge, C.A.; Green, B.E.; Fino, L.; Park, C.H.; Kong, X.P.; et al. ABT-538 Is a Potent Inhibitor of Human Immunodeficiency Virus Protease and Has High Oral Bioavailability in Humans. Proc. Natl. Acad. Sci. USA 1995, 92, 2484–2488. [Google Scholar] [CrossRef]
- Erickson, J.W. Design and Structure of Symmetry-Based Inhibitors of HIV-1 Protease. Perspect. Drug Discov. Des. 1993, 1, 109–128. [Google Scholar] [CrossRef]
- Dorsey, B.D.; Levin, R.B.; McDaniel, S.L.; Vacca, J.P.; Guare, J.P.; Anderson, P.S.; Huff, J.R.; Darke, P.L.; Zugay, J.A.; Emini, E.A.; et al. L-735,524: The Design of a Potent and Orally Bioavailable HIV Protease Inhibitor. J. Med. Chem. 1994, 37, 3443–3451. [Google Scholar] [CrossRef]
- Vacca, J.P.; Dorsey, B.D.; Schleif, W.A.; Levin, R.B.; Mcdaniel, S.L.; Darke, P.L.; Zugay, J.; Quintero, J.C.; Blahy, O.M.; Roth, E.; et al. L-735,524: An Orally Bioavailable Human Immunodeficiency Virus Type 1 Protease Inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Wlodawer, A. Rational Approach to AIDS Drug Design through Structural Biology. Annu. Rev. Med. 2002, 53, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.D.; Appelt, K.; Kalish, V.; Reddy, M.R.; Tatlock, J.; Palmer, C.L.; Romines, W.H.; Wu, B.W.; Musick, L. Crystal-Structure-Based Design and Synthesis of Novel C-Terminal Inhibitors of HIV Protease. J. Med. Chem. 1994, 37, 2274–2284. [Google Scholar] [CrossRef]
- Kaldor, S.W.; Kalish, V.J.; Davies, J.F.; Shetty, B.V.; Fritz, J.E.; Appelt, K.; Burgess, J.A.; Campanale, K.M.; Chirgadze, N.Y.; Clawson, D.K.; et al. Viracept (Nelfinavir Mesylate, AG1343): A Potent, Orally Bioavailable Inhibitor of HIV-1 Protease. J. Med. Chem. 1997, 40, 3979–3985. [Google Scholar] [CrossRef]
- Weber, I.T.; Waltman, M.J.; Mustyakimov, M.; Blakeley, M.P.; Keen, D.A.; Ghosh, A.K.; Langan, P.; Kovalevsky, A.Y. Joint X-Ray/Neutron Crystallographic Study of HIV-1 Protease with Clinical Inhibitor Amprenavir: Insights for Drug Design. J. Med. Chem. 2013, 56, 5631–5635. [Google Scholar] [CrossRef] [PubMed]
- Chemburkar, S.R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.; Morris, J.; Henry, R.; Spanton, S.; Dziki, W.; Porter, W.; et al. Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development. Org. Process. Res. Dev. 2000, 4, 413–417. [Google Scholar] [CrossRef]
- McCauley, J.A.; Rudd, M.T. Hepatitis C Virus NS3/4a Protease Inhibitors. Curr. Opin. Pharmacol. 2016, 30, 84–92. [Google Scholar] [CrossRef]
- Love, R.A.; Parge, H.E.; Wickersham, J.A.; Hostomsky, Z.; Habuka, N.; Moomaw, E.W.; Adachi, T.; Hostomska, Z. The Crystal Structure of Hepatitis C Virus NS3 Proteinase Reveals a Trypsin-like Fold and a Structural Zinc Binding Site. Cell 1996, 87, 331–342. [Google Scholar] [CrossRef]
- Kim, J.L.; Morgenstern, K.A.; Lin, C.; Fox, T.; Dwyer, M.D.; Landro, J.A.; Chambers, S.P.; Markland, W.; Lepre, C.A.; O’Malley, E.T.; et al. Crystal Structure of the Hepatitis C Virus NS3 Protease Domain Complexed with a Synthetic NS4A Cofactor Peptide. Cell 1996, 87, 343–355. [Google Scholar] [CrossRef]
- Barbato, G.; Cicero, D.O.; Nardi, M.C.; Steinkühler, C.; Cortese, R.; De Francesco, R.; Bazzo, R. The Solution Structure of the N-Terminal Proteinase Domain of the Hepatitis C Virus (HCV) NS3 Protein Provides New Insights into Its Activation and Catalytic Mechanism. J. Mol. Biol. 1999, 289, 371–384. [Google Scholar] [CrossRef]
- Llinàs-Brunet, M.; Bailey, M.; Fazal, G.; Goulet, S.; Halmos, T.; Laplante, S.; Maurice, R.; Poirier, M.; Poupart, M.A.; Thibeault, D.; et al. Peptide-Based Inhibitors of the Hepatitis C Virus Serine Protease. Bioorg. Med. Chem. Lett. 1998, 8, 1713–1718. [Google Scholar] [CrossRef]
- Saalau-Bethell, S.M.; Woodhead, A.J.; Chessari, G.; Carr, M.G.; Coyle, J.; Graham, B.; Hiscock, S.D.; Murray, C.W.; Pathuri, P.; Rich, S.J.; et al. Discovery of an Allosteric Mechanism for the Regulation of HcV Ns3 Protein Function. Nat. Chem. Biol. 2012, 8, 920–925. [Google Scholar] [CrossRef]
- Choudhary, S.; Nehul, S.; Singh, A.; Panda, P.K.; Kumar, P.; Sharma, G.K.; Tomar, S. Unraveling Antiviral Efficacy of Multifunctional Immunomodulatory Triterpenoids against SARS-COV-2 Targeting Main Protease and Papain-like Protease. IUBMB Life 2024, 76, 228–241. [Google Scholar] [CrossRef]
- Choudhary, S.; Nehul, S.; Nagaraj, S.K.; Narayan, R.; Verma, S.; Sharma, S.; Kumari, A.; Rani, R.; Saha, A.; Sircar, D.; et al. Activity Profiling of Deubiquitinating Inhibitors-Bound to SARS-CoV-2 Papain like Protease with Antiviral Efficacy in Murine Infection Model. bioRxiv 2022. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved a-Ketoamide Inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed]
- FDA. Approves First Oral Antiviral for Treatment of COVID-19 in Adults|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults (accessed on 30 December 2024).
- Pagliano, P.; Spera, A.; Sellitto, C.; Scarpati, G.; Folliero, V.; Piazza, O.; Franci, G.; Conti, V.; Ascione, T. Preclinical Discovery and Development of Nirmatrelvir/Ritonavir Combinational Therapy for the Treatment of COVID-19 and the Lessons Learned from SARS-COV-2 Variants. Expert Opin. Drug Discov. 2023, 18, 1301–1311. [Google Scholar] [CrossRef]
- Unoh, Y.; Uehara, S.; Nakahara, K.; Nobori, H.; Yamatsu, Y.; Yamamoto, S.; Maruyama, Y.; Taoda, Y.; Kasamatsu, K.; Suto, T.; et al. Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19. J. Med. Chem. 2022, 65, 6499–6512. [Google Scholar] [CrossRef]
- Narwal, M.; Armache, J.P.; Edwards, T.J.; Murakami, K.S. SARS-CoV-2 Polyprotein Substrate Regulates the Stepwise Mpro Cleavage Reaction. J. Biol. Chem. 2023, 299, 104697. [Google Scholar] [CrossRef]
- Pareek, A.; Kumar, R.; Mudgal, R.; Neetu, N.; Sharma, M.; Kumar, P.; Tomar, S. Alphavirus Antivirals Targeting RNA-Dependent RNA Polymerase Domain of NsP4 Divulged Using Surface Plasmon Resonance. FEBS J. 2022, 289, 4901–4924. [Google Scholar] [CrossRef]
- Rani, R.; Long, S.; Pareek, A.; Dhaka, P.; Singh, A.; Kumar, P.; McInerney, G.; Tomar, S. Multi-Target Direct-Acting SARS-CoV-2 Antivirals against the Nucleotide-Binding Pockets of Virus-Specific Proteins. Virology 2022, 577, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Nehul, S.; Choudhary, S.; Upadhyay, A.; Kumar Sharma, G.; Kumar, P.; Tomar, S.; scientist, S. Revealing and Evaluation of Antivirals Targeting Multiple Druggable Sites of RdRp Complex in SARS-CoV-2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Choi, K.H. Viral Polymerases. Adv. Exp. Med. Biol. 2012, 726, 267–304. [Google Scholar] [CrossRef]
- Liu, S.; Knafels, J.D.; Chang, J.S.; Waszak, G.A.; Baldwin, E.T.; Deibel, M.R.; Thomsen, D.R.; Homa, F.L.; Wells, P.A.; Tory, M.C.; et al. Crystal Structure of the Herpes Simplex Virus 1 DNA Polymerase. J. Biol. Chem. 2006, 281, 18193–18200. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA Polymerases: Structures, Functions and Inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, E.; Grünewald, K.; Elias, P.; Hällberg, B.M. Dynamics of the Herpes Simplex Virus DNA Polymerase Holoenzyme during DNA Synthesis and Proof-Reading Revealed by Cryo-EM. Nucleic Acids Res. 2024, 52, 7292–7304. [Google Scholar] [CrossRef]
- Shankar, S.; Pan, J.; Yang, P.; Bian, Y.; Oroszlán, G.; Yu, Z.; Mukherjee, P.; Filman, D.J.; Hogle, J.M.; Shekhar, M.; et al. Viral DNA Polymerase Structures Reveal Mechanisms of Antiviral Drug Resistance. Cell 2024, 187, 5572–5586. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Uckun, F.M. Dawn of Non-Nucleoside Inhibitor-Based Anti-HIV Microbicides. J. Antimicrob. Chemother. 2006, 57, 411–423. [Google Scholar] [CrossRef]
- Tuaillon, E.; Gueudin, M.; Lemée, V.; Gueit, I.; Roques, P.; Corrigan, G.E.; Plantier, J.C.; Simon, F.; Braun, J. Phenotypic susceptibility to nonnucleoside inhibitors of virion-associated reverse transcriptase from different HIV types and groups. JAIDS J. Acquir. Immune Defic. Syndr. 2024, 37, 1543–1549. [Google Scholar] [CrossRef]
- Smerdon, S.J.; Jäger, J.; Wang, J.; Kohlstaedt, L.A.; Chirino, A.J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Structure of the Binding Site for Nonnucleoside Inhibitors of the Reverse Transcriptase of Human Immunodeficiency Virus Type 1. Proc. Natl. Acad. Sci. USA 1994, 91, 3911–3915. [Google Scholar] [CrossRef]
- Merluzzi, V.J.; Hargrave, K.D.; Labadia, M.; Grozinger, K.; Skoog, M.; Wu, J.C.; Shih, C.-K.; Eckner, K.; Hattox, S.; Adams, J.; et al. Inhibition of HIV-1 Replication by a Nonnucleoside Reverse Transcriptase Inhibitor. Science 1990, 250, 1411–1413. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Speranzini, V.; Hashimoto, T.; Noshi, T.; Yamaguchi, H.; Kawai, M.; Kawaguchi, K.; Uehara, T.; Shishido, T.; Naito, A.; et al. Characterization of Influenza Virus Variants Induced by Treatment with the Endonuclease Inhibitor Baloxavir Marboxil. Sci. Rep. 2018, 8, 9633. [Google Scholar] [CrossRef]
- Chang, S.; Sun, D.; Liang, H.; Wang, J.; Li, J.; Guo, L.; Wang, X.; Guan, C.; Boruah, B.M.; Yuan, L.; et al. Cryo-EM Structure of Influenza Virus RNA Polymerase Complex at 4.3Å Resolution. Mol. Cell 2015, 57, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Kirchdoerfer, R.N.; Ward, A.B. Structure of the SARS-CoV Nsp12 Polymerase Bound to Nsp7 and Nsp8 Co-Factors. Nat. Commun. 2019, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- FDA. Approves First Treatment for COVID-19|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 1 January 2025).
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural Basis for Inhibition of the RNA-Dependent RNA Polymerase from SARS-CoV-2 by Remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp): A Molecular Docking Study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef]
- Sangawa, H.; Komeno, T.; Nishikawa, H.; Yoshida, A.; Takahashi, K.; Nomura, N.; Furuta, Y. Mechanism of Action of T-705 Ribosyl Triphosphate against Influenza Virus RNA Polymerase. Antimicrob. Agents Chemother. 2013, 57, 5202–5208. [Google Scholar] [CrossRef]
- Painter, G.R.; Natchus, M.G.; Cohen, O.; Holman, W.; Painter, W.P. Developing a Direct Acting, Orally Available Antiviral Agent in a Pandemic: The Evolution of Molnupiravir as a Potential Treatment for COVID-19. Curr. Opin. Virol. 2021, 50, 17–22. [Google Scholar] [CrossRef]
- Yoon, J.J.; Toots, M.; Lee, S.; Lee, M.E.; Ludeke, B.; Luczo, J.M.; Ganti, K.; Cox, R.M.; Sticher, Z.M.; Edpuganti, V.; et al. Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses. Antimicrob. Agents Chemother. 2018, 62, e01062-21. [Google Scholar] [CrossRef]
- Peng, Q.; Peng, R.; Yuan, B.; Wang, M.; Zhao, J.; Fu, L.; Qi, J.; Shi, Y. Structural Basis of SARS-CoV-2 Polymerase Inhibition by Favipiravir. Innovation 2021, 2, 100080. [Google Scholar] [CrossRef]
- Pommier, Y.; Johnson, A.A.; Marchand, C. Integrase Inhibitors to Treat HIV/AIDS. Nat. Rev. Drug Discov. 2005, 4, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zheng, R.; Caffrey, M.; Craigie, R.; Marius Clore, G.; Gronenborn, A.M. Solution Structure of the N-Terminal Zinc Binding Domain of HIV-1 Integrase. Nat. Struct. Bio.l 1997, 4, 567–577. [Google Scholar] [CrossRef]
- Renzi, G.; Carta, F.; Supuran, C.T. The Integrase: An Overview of a Key Player Enzyme in the Antiviral Scenario. Int. J. Mol. Sci. 2023, 24, 12187. [Google Scholar] [CrossRef] [PubMed]
- Maertens, G.N.; Engelman, A.N.; Cherepanov, P. Structure and Function of Retroviral Integrase. Nat Rev Microbiol. 2022, 20, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.L.; Whitlock, G.; Milinkovic, A.; Moyle, G. HIV Integrase Inhibitors: A New Era in the Treatment of HIV. Expert Opin. Pharmacother. 2015, 16, 1313–1324. [Google Scholar] [CrossRef]
- Goldgur, Y.; Craigie, R.; Cohen, G.H.; Fujiwara, T.; Yoshinaga, T.; Fujishita, T.; Sugimoto, H.; Endo, T.; Murai, H.; Davies, D.R. Structure of the HIV-1 Integrase Catalytic Domain Complexed with an Inhibitor: A Platform for Antiviral Drug Design. Proc. Natl. Acad. Sci. USA 1999, 96, 13040–13043. [Google Scholar] [CrossRef]
- Hare, S.; Smith, S.J.; Métifiot, M.; Jaxa-Chamiec, A.; Pommier, Y.; Hughes, S.H.; Cherepanov, P. Structural and Functional Analyses of the Second-Generation Integrase Strand Transfer Inhibitor Dolutegravir (S/GSK1349572). Mol. Pharmacol. 2011, 80, 565–572. [Google Scholar] [CrossRef]
- Ballantyne, A.D.; Perry, C.M. Dolutegravir: First Global Approval. Drugs 2013, 73, 1627–1637. [Google Scholar] [CrossRef]
- New report documents increase in HIV drug resistance to dolutegravir|WHO. Available online: https://www.who.int/news/item/05-03-2024-new-report-documents-increase-in-hiv-drug-resistance-to-dolutegravir (accessed on 1 January 2025).
- Li, M.; Passos, D.O.; Shan, Z.; Smith, S.J.; Sun, Q.; Biswas, A.; Choudhuri, I.; Strutzenberg, T.S.; Haldane, A.; Deng, N.; et al. Mechanisms of HIV-1 Integrase Resistance to Dolutegravir and Potent Inhibition of Drug-Resistant Variants. Sci. Adv. 2023, 9, eadg5953. [Google Scholar] [CrossRef]
- Passos, D.O.; Li, M.; Jóźwik, I.K.; Zhao, X.Z.; Santos-Martins, D.; Yang, R.; Smith, S.J.; Jeon, Y.; Forli, S.; Hughes, S.H.; et al. Structural Basis for Strand-Transfer Inhibitor Binding to HIV Intasomes. Science 2020, 367, 810–814. [Google Scholar] [CrossRef]
- Bonnard, D.; Le Rouzic, E.; Eiler, S.; Amadori, C.; Orlov, I.; Bruneau, J.M.; Brias, J.; Barbion, J.; Chevreuil, F.; Spehner, D.; et al. Structure-Function Analyses Unravel Distinct Effects of Allosteric Inhibitors of HIV-1 Integrase on Viral Maturation and Integration. J. Biol. Chem. 2018, 293, 6172–6186. [Google Scholar] [CrossRef] [PubMed]
- Christ, F.; Voet, A.; Marchand, A.; Nicolet, S.; Desimmie, B.A.; Marchand, D.; Bardiot, D.; Van Der Veken, N.J.; Van Remoortel, B.; Strelkov, S.V.; et al. Rational Design of Small-Molecule Inhibitors of the LEDGF/P75-Integrase Interaction and HIV Replication. Nat. Chem. Biol. 2010, 6, 442–448. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Visse, R.; Sandhu, G.; Davies, A.; Rizkallah, P.J.; Melitz, C.; Summers, W.C.; Sanderson, M.R. Crystal Structures of the Thymidine Kinase from Herpes Simplex Virus Type-I in Complex with Deoxythymidine and Ganciclovir. Nat. Struct. Biol. 1995, 2, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Wild, K.; Bohner, T.; Aubry, A.; Folkers, G.; Schulz, G.E. The Three-Dimensional Structure of Thymidine Kinase from Herpes Simplex Virus Type 1. FEBS Lett. 1995, 368, 289–292. [Google Scholar] [CrossRef]
- Frobert, E.; Ooka, T.; Cortay, J.C.; Lina, B.; Thouvenot, D.; Morfin, F. Herpes Simplex Virus Thymidine Kinase Mutations Associated with Resistance to Acyclovir: A Site-Directed Mutagenesis Study. Antimicrob. Agents Chemother. 2005, 49, 1055–1059. [Google Scholar] [CrossRef]
- Ramdhan, P.; Li, C. Targeting Viral Methyltransferases: An Approach to Antiviral Treatment for SsRNA Viruses. Viruses 2022, 14, 379. [Google Scholar] [CrossRef]
- Jones, R.; Bragagnolo, G.; Arranz, R.; Reguera, J. Capping Pores of Alphavirus NsP1 Gate Membranous Viral Replication Factories. Nature 2021, 589, 615–619. [Google Scholar] [CrossRef]
- Lampio, A.; Kilpeläinen, I.; Pesonen, S.; Karhi, K.; Auvinen, P.; Somerharju, P.; Kääriäinen, L. Membrane Binding Mechanism of an RNA Virus-Capping Enzyme. J. Biol. Chem. 2000, 275, 37853–37859. [Google Scholar] [CrossRef]
- Jones, R.; Hons, M.; Rabah, N.; Zamarreño, N.; Arranz, R.; Reguera, J. Structural Basis and Dynamics of Chikungunya Alphavirus RNA Capping by NsP1 Capping Pores. Proc. Natl. Acad. Sci. USA 2023, 120, e2213934120. [Google Scholar] [CrossRef]
- Bhutkar, M.; Saha, A.; Tomar, S. Viral Methyltransferase Inhibitors: Berbamine, Venetoclax, and Ponatinib as Efficacious Antivirals against Chikungunya Virus. Arch. Biochem. Biophys. 2024, 759, 110111. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, R.; Bharadwaj, C.; Dubey, A.; Choudhary, S.; Nagarajan, P.; Aggarwal, M.; Ratra, Y.; Basak, S.; Tomar, S. Selective Estrogen Receptor Modulators Limit Alphavirus Infection by Targeting the Viral Capping Enzyme NsP1. Antimicrob. Agents Chemother. 2022, 66, e01943-21. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, R.; Mahajan, S.; Tomar, S. Inhibition of Chikungunya Virus by an Adenosine Analog Targeting the SAM-Dependent NsP1 Methyltransferase. FEBS Lett. 2020, 594, 678–694. [Google Scholar] [CrossRef]
- Zhou, Y.; Ray, D.; Zhao, Y.; Dong, H.; Ren, S.; Li, Z.; Guo, Y.; Bernard, K.A.; Shi, P.-Y.; Li, H. Structure and Function of Flavivirus NS5 Methyltransferase. J. Virol. 2007, 81, 3891–3903. [Google Scholar] [CrossRef]
- Lim, S.P.; Sonntag, L.S.; Noble, C.; Nilar, S.H.; Ng, R.H.; Zou, G.; Monaghan, P.; Chung, K.Y.; Dong, H.; Liu, B.; et al. Small Molecule Inhibitors That Selectively Block Dengue Virus Methyltransferase. J. Biol. Chem. 2011, 286, 6233–6240. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Cyr, M.; Longenecker, K.; Tripathi, R.; Sun, C.; Kempf, D.J. Crystal Structure of Full-Length Zika Virus NS5 Protein Reveals a Conformation Similar to Japanese Encephalitis Virus NS5. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 116–122. [Google Scholar] [CrossRef]
- Jia, H.; Zhong, Y.; Peng, C.; Gong, P. Crystal Structures of Flavivirus NS5 Guanylyltransferase Reveal a GMP-Arginine Adduct. J. Virol. 2022, 96, e00418-22. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, S.; Yang, F.; Chen, Z.; Guo, L.; Yang, J.; Lin, X.; Wang, L.; Duan, Y.; Wen, A.; et al. Structural and Functional Basis of Low-Affinity SAM/SAH-Binding in the Conserved MTase of the Multi-Segmented Alongshan Virus Distantly Related to Canonical Unsegmented Flaviviruses. PLoS Pathog. 2023, 19, e1011694. [Google Scholar] [CrossRef]
- Bhutkar, M.; Kumar, A.; Rani, R.; Singh, V.; Pathak, A.; Kothiala, A.; Mahajan, S.; Waghmode, B.; Kumar, R.; Mudgal, R.; et al. SAM-Dependent Viral MTase Inhibitors: Herbacetin and Caffeic Acid Phenethyl Ester, Structural Insights into Dengue MTase. bioRxiv 2024. bioRxiv:2022.05.31.494145. [Google Scholar] [CrossRef]
- García, L.L.; Padilla, L.; Castaño, J.C. Inhibitors Compounds of the Flavivirus Replication Process. Virol. J. 2017, 14, 95. [Google Scholar] [CrossRef]
- Benarroch, D.; Egloff, M.P.; Mulard, L.; Guerreiro, C.; Romette, J.L.; Canard, B. A Structural Basis for the Inhibition of the NS5 Dengue Virus MRNA 2′-O-Methyltransferase Domain by Ribavirin 5′-Triphosphate. J. Biol. Chem. 2004, 279, 35638–35643. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Mastrangelo, E.; Bollati, M.; Selisko, B.; Decroly, E.; Bouvet, M.; Canard, B.; Bolognesi, M. Flaviviral Methyltransferase/RNA Interaction: Structural Basis for Enzyme Inhibition. Antivir. Res. 2009, 83, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nencka, R.; Silhan, J.; Klima, M.; Otava, T.; Kocek, H.; Krafcikova, P.; Boura, E. Coronaviral RNA-Methyltransferases: Function, Structure and Inhibition. Nucleic Acids Res. 2022, 50, 635–650. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Perspective for Drug Discovery Targeting SARS Coronavirus Methyltransferases: Function, Structure and Inhibition. J. Med. Chem. 2024, 67, 18642–18655. [Google Scholar] [CrossRef]
- Pyle, A.M. Translocation and Unwinding Mechanisms of RNA and DNA Helicases. Annu. Rev. Biophys. 2008, 37, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Kolykhalov, A.A.; Mihalik, K.; Feinstone, S.M.; Rice, C.M. Hepatitis C Virus-Encoded Enzymatic Activities and Conserved RNA Elements in the 3′ Nontranslated Region Are Essential for Virus Replication In Vivo. J. Virol. 2000, 74, 2046–2051. [Google Scholar] [CrossRef]
- Jankowsky, E.; Gross, C.H.; Shuman, S.; Pyle, A.M. The DExH Protein NPH-II Is a Processive and Directional Motor for Unwinding RNA. Nature 2000, 403, 447–451. [Google Scholar] [CrossRef]
- Newman, J.A.; Douangamath, A.; Yadzani, S.; Yosaatmadja, Y.; Aimon, A.; Brandão-Neto, J.; Dunnett, L.; Gorrie-stone, T.; Skyner, R.; Fearon, D.; et al. Structure, Mechanism and Crystallographic Fragment Screening of the SARS-CoV-2 NSP13 Helicase. Nat. Commun. 2021, 12, 4848. [Google Scholar] [CrossRef]
- Smelkova, N.V.; Borowiec, J.A. Dimerization of Simian Virus 40 T-Antigen Hexamers Activates T-Antigen DNA Helicase Activity. J. Virol. 1997, 71, 8766–8773. [Google Scholar] [CrossRef]
- Hughes, F.J.; Romanos, M.A. E1 Protein of Human Papillomavirus Is a DNA Helicase/ATPase. Nucleic Acids Res. 1993, 21, 5817–5823. [Google Scholar] [CrossRef]
- Xu, T.; Sampath, A.; Chao, A.; Wen, D.; Nanao, M.; Chene, P.; Vasudevan, S.G.; Lescar, J. Structure of the Dengue Virus Helicase/Nucleoside Triphosphatase Catalytic Domain at a Resolution of 2.4 Å. J. Virol. 2005, 79, 10278–10288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Yang, M.; Yang, J.; Shao, Z.; Gao, Y.; Jiang, X.; Cui, R.; Zhang, Y.; Zhao, X.; et al. Structural and Functional Insights into the Helicase Protein E5 of Mpox Virus. Cell Discov. 2024, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.-S.; Wang, S.; Tan, Y.B.; Shih, O.; Utt, A.; Goh, W.Y.; Lian, B.-J.; Chen, M.W.; Jeng, U.-S.; Merits, A.; et al. Interdomain Flexibility of Chikungunya Virus NsP2 Helicase-Protease Differentially Influences Viral RNA Replication and Infectivity. J. Virol. 2021, 95, e01470-20. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Rao, Z. Current Progress in Antiviral Strategies. Trends Pharmacol. Sci. 2014, 35, 86–102. [Google Scholar] [CrossRef]
- Wu, J.; Bera, A.K.; Kuhn, R.J.; Smith, J.L. Structure of the Flavivirus Helicase: Implications for Catalytic Activity, Protein Interactions, and Proteolytic Processing. J. Virol. 2005, 79, 10268–10277. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Duquerroy, S.; Kwok, J.; Vonrhein, C.; Perez, J.; Lamp, B.; Bricogne, G.; Rümenapf, T.; Vachette, P.; Rey, F.A. X-Ray Structure of the Pestivirus NS3 Helicase and Its Conformation in Solution. J. Virol. 2015, 89, 4356–4371. [Google Scholar] [CrossRef]
- Fang, X.; Lu, G.; Deng, Y.; Yang, S.; Hou, C.; Gong, P. Unusual Substructure Conformations Observed in Crystal Structures of a Dicistrovirus RNA-Dependent RNA Polymerase Suggest Contribution of the N-Terminal Extension in Proper Folding. Virol. Sin. 2023, 38, 531–540. [Google Scholar] [CrossRef]
- Anindita, P.D.; Halbeisen, M.; Řeha, D.; Tuma, R.; Franta, Z. Mechanistic Insight into the RNA-Stimulated ATPase Activity of Tick-Borne Encephalitis Virus Helicase. J. Biol. Chem. 2022, 298, 102383. [Google Scholar] [CrossRef]
- Shao, Z.; Su, S.; Yang, J.; Zhang, W.; Gao, Y.; Zhao, X.; Zhang, Y.; Shao, Q.; Cao, C.; Li, H.; et al. Structures and Implications of the C962R Protein of African Swine Fever Virus. Nucleic Acids Res. 2023, 51, 9475–9490. [Google Scholar] [CrossRef]
- Gu, M.; Rice, C.M. Three Conformational Snapshots of the Hepatitis C Virus NS3 Helicase Reveal a Ratchet Translocation Mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 521–528. [Google Scholar] [CrossRef]
- Hutin, S.; Ling, W.L.; Tarbouriech, N.; Schoehn, G.; Grimm, C.; Fischer, U.; Burmeister, W.P. The Vaccinia Virus DNA Helicase Structure from Combined Single-Particle Cryo-Electron Microscopy and AlphaFold2 Prediction. Viruses 2022, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Y.S.; Gesell, J.J.; Wyss, D.F. Solution Structure and Backbone Dynamics of an Engineered Arginine-Rich Subdomain 2 of the Hepatitis C Virus NS3 RNA Helicase. J. Mol. Biol. 2001, 314, 543–561. [Google Scholar] [CrossRef]
- Frick, D.; Lam, A. Understanding Helicases as a Means of Virus Control. Curr. Pharm. Des. 2006, 12, 1315–1338. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhu, Y.; Li, J.; Chen, Z.; Han, G.W.; Kufareva, I.; Li, T.; Ma, L.; Fenalti, G.; Li, J.; et al. Structure of the CCR5 Chemokine Receptor-HIV Entry Inhibitor Maraviroc Complex. Science 2013, 341, 1387–1390. [Google Scholar] [CrossRef]
- Wu, B.; Chien, E.Y.T.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef]
- Haqqani, A.A.; Tilton, J.C. Entry Inhibitors and Their Use in the Treatment of HIV-1 Infection. Antivir. Res. 2013, 98, 158–170. [Google Scholar] [CrossRef]
- Blair, H.A. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs 2020, 80, 189–196. [Google Scholar] [CrossRef]
- Freeman, M.M.; Seaman, M.S.; Rits-Volloch, S.; Hong, X.; Kao, C.Y.; Ho, D.D.; Chen, B. Crystal Structure of HIV-1 Primary Receptor CD4 in Complex with a Potent Antiviral Antibody. Structure 2010, 18, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Bhutkar, M.; Singh, V.; Dhaka, P.; Tomar, S. Virus-Host Protein-Protein Interactions as Molecular Drug Targets for Arboviral Infections. Front. Virol. 2022, 2, 959586. [Google Scholar] [CrossRef]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral Strategies Targeting Host Factors and Mechanisms Obliging +ssRNA Viral Pathogens. Bioorg. Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef]
- Dhaka, P.; Singh, A.; Nehul, S.; Choudhary, S.; Panda, P.K.; Sharma, G.K.; Kumar, P.; Tomar, S. Disruption of Molecular Interactions between the G3BP1 Stress Granule Host Protein and the Nucleocapsid (NTD-N) Protein Impedes SARS-CoV-2 Virus Replication. Biochemistry 2024, 64, 823–840. [Google Scholar] [CrossRef]
- Mahajan, S.; Kumar, R.; Singh, A.; Pareek, A.; Kumar, P.; Tomar, S. Targeting the Host Protein G3BP1 for the Discovery of Novel Antiviral Inhibitors against Chikungunya Virus. bioRxiv 2024. bioRxiv:2022.11.11.516135. [Google Scholar] [CrossRef]
- De Chassey, B.; Meyniel-Schicklin, L.; Aublin-Gex, A.; André, P.; Lotteau, V. New Horizons for Antiviral Drug Discovery from Virus–Host Protein Interaction Networks. Curr. Opin. Virol. 2012, 2, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Idrees, S.; Chen, H.; Panth, N.; Paudel, K.R.; Hansbro, P.M. Exploring Viral–Host Protein Interactions as Antiviral Therapies: A Computational Perspective. Microorganisms 2024, 12, 630. [Google Scholar] [CrossRef]
- Idrees, S.; Paudel, K.R.; Sadaf, T.; Hansbro, P.M. How Different Viruses Perturb Host Cellular Machinery via Short Linear Motifs. EXCLI J. 2023, 22, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- de Chassey, B.; Meyniel-Schicklin, L.; Vonderscher, J.; André, P.; Lotteau, V. Virus-Host Interactomics: New Insights and Opportunities for Antiviral Drug Discovery. Genome Med. 2014, 6, 115. [Google Scholar] [CrossRef]
- Brito, A.F.; Pinney, J.W. Protein-Protein Interactions in Virus-Host Systems. Front. Microbiol. 2017, 8, 01557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-Directed Antiviral Therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Eslami, N.; Aghbash, P.S.; Shamekh, A.; Entezari-Maleki, T.; Nahand, J.S.; Sales, A.J.; Baghi, H.B. SARS-CoV-2: Receptor and Co-Receptor Tropism Probability. Curr. Microbiol. 2022, 79, 133. [Google Scholar] [CrossRef]
- Baggen, J.; Vanstreels, E.; Jansen, S.; Daelemans, D. Cellular Host Factors for SARS-CoV-2 Infection. Nat. Microbiol. 2021, 6, 1219–1232. [Google Scholar] [CrossRef]
- Lee, M.F.; Wu, Y.S.; Poh, C.L. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses 2023, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.N.; Akhtar, R.; Abid, M.; Khan, S.A.; Rehman, Z.U.; Tayyub, M.; Malik, M.I.; Shahzad, M.K.; Mubeen, H.; Qadir, M.S.; et al. The Interactions of Flaviviruses with Cellular Receptors: Implications for Virus Entry. Virology 2022, 568, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Q.Z.; Zhang, H.; Liu, Z.X.; Chen, X.H.; Ye, L.L.; Luo, Y. The Land-Scape of Immune Response to Monkeypox Virus. EBioMedicine 2023, 87, 104424. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front. Immunol. 2022, 13, 801522. [Google Scholar] [CrossRef]
- Lemaitre, J.C.; Grantz, K.H.; Kaminsky, J.; Meredith, H.R.; Truelove, S.A.; Lauer, S.A.; Keegan, L.T.; Shah, S.; Wills, J.; Kaminsky, K.; et al. A Scenario Modeling Pipeline for COVID-19 Emergency Planning. Sci. Rep. 2021, 11, 7534. [Google Scholar] [CrossRef]
- Crucitti, D.; Pérez Míguez, C.; Ángel, J.; Arias, D.; Beltrá, D.; Prada, F.; Orgueira, A.M. De Novo Drug Design through Artificial Intelligence: An Introduction. Front. Hematol. 2024, 3, 1305741. [Google Scholar] [CrossRef]
- Floresta, G.; Zagni, C.; Patamia, V.; Rescifina, A. How Can Artificial Intelligence Be Utilized for de Novo Drug Design against COVID-19 (SARS-CoV-2)? Expert Opin. Drug Discov. 2023, 18, 1061–1064. [Google Scholar] [CrossRef]
- Patel, C.N.; Mall, R.; Bensmail, H. AI-Driven Drug Repurposing and Binding Pose Meta Dynamics Identifies Novel Targets for Monkeypox Virus. J. Infect. Public Health 2023, 16, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.; Nair, N.; Suétt, R.; Mac Donnchadha, R.; Bamford, C.; Jasim, S.; Livingstone, D.; Hutchinson, E. Visualising Viruses. J. Gen. Virol. 2022, 103, 001730. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Q.; Ran, T.; Zhang, G.; Li, W.; Zhou, P.; Tang, J.; Dai, M.; Zhong, J.; Chen, H.; et al. Discovery of Orally Bioavailable SARS-CoV-2 Papain-like Protease Inhibitor as a Potential Treatment for COVID-19. Nat. Commun. 2024, 15, 10169. [Google Scholar] [CrossRef]
- Turzynski, V.; Monsees, I.; Moraru, C.; Probst, A.J. Imaging Techniques for Detecting Prokaryotic Viruses in Environmental Samples. Viruses 2021, 13, 2126. [Google Scholar] [CrossRef]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M.; et al. Activity Profiling and Crystal Structures of Inhibitor-Bound SARS-CoV-2 Papain-like Protease: A Framework for Anti–COVID-19 Drug Design. Sci. Adv. 2020, 6, 4596–4612. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.E.; Noell, S.; Plotnikova, O.; Ferre, R.A.; Liu, W.; Bolanos, B.; Fennell, K.; Nicki, J.; Craig, T.; Zhu, Y.; et al. Structural Basis for the in Vitro Efficacy of Nirmatrelvir against SARS-CoV-2 Variants. J. Biol. Chem. 2022, 298, 101972. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Cheng, K.; Zhang, L.; Wang, D.; Gao, X.; Liang, J.; Liu, G.; Ma, N.; Xu, C.; Tang, M.; et al. Rationally Designed Multimeric Nanovaccines Using Icosahedral DNA Origami for Display of SARS-CoV-2 Receptor Binding Domain. Nat. Commun. 2024, 15, 9581. [Google Scholar] [CrossRef] [PubMed]
- Dokhale, S.; Garse, S.; Devarajan, S.; Thakur, V.; Kolhapure, S. Rational Design of Antiviral Therapeutics. Comput. Methods Ration. Drug Des. 2025, 423–443. [Google Scholar] [CrossRef]
- Al-Amran, F.G.; Hezam, A.M.; Rawaf, S.; Yousif, M.G. Genomic Analysis and Artificial Intelligence: Predicting Viral Mutations and Future Pandemics. arXiv 2023, arXiv:2309.15936. [Google Scholar] [CrossRef]
- Parvatikar, P.P.; Patil, S.; Khaparkhuntikar, K.; Patil, S.; Singh, P.K.; Sahana, R.; Kulkarni, R.V.; Raghu, A.V. Artificial Intelligence: Machine Learning Approach for Screening Large Database and Drug Discovery. Antivir. Res 2023, 220, 105740. [Google Scholar] [CrossRef]
- KP Jayatunga, M.; Ayers, M.; Bruens, L.; Jayanth, D.; Meier, C. How Successful Are AI-Discovered Drugs in Clinical Trials? A First Analysis and Emerging Lessons. Drug. Discov. Today 2024, 29, 104009. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Afantitis, A.; Serra, A.; Fratello, M.; Papadiamantis, A.G.; Aidinis, V.; Lynch, I.; Greco, D.; Melagraki, G. Advances in De Novo Drug Design: From Conventional to Machine Learning Methods. Int. J. Mol. Sci. 2021, 22, 1676. [Google Scholar] [CrossRef]
- Atz, K.; Cotos, L.; Isert, C.; Håkansson, M.; Focht, D.; Hilleke, M.; Nippa, D.F.; Iff, M.; Ledergerber, J.; Schiebroek, C.C.G.; et al. Prospective de Novo Drug Design with Deep Interactome Learning. Nat. Commun. 2024, 15, 3408. [Google Scholar] [CrossRef]
- Singh, V.; Bhutkar, M.; Choudhary, S.; Nehul, S.; Kumar, R.; Singla, J.; Kumar, P.; Tomar, S. Structure-Guided Mutations in CDRs for Enhancing the Affinity of Neutralizing SARS-CoV-2 Nanobody. Biochem. Biophys. Res. Commun. 2024, 734, 150746. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Choudhary, S.; Bhutkar, M.; Nehul, S.; Ali, S.; Singla, J.; Kumar, P.; Tomar, S. Designing and Bioengineering of CDRs with Higher Affinity against Receptor-Binding Domain (RBD) of SARS-CoV-2 Omicron Variant 2024. Int. J. Biol. Macromol. 2025, 290, 138751. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Vegesna, R.; Mukherjee, S.; Kammula, A.V.; Dhruba, S.R.; Wu, W.; Kerr, D.L.; Nair, N.U.; Jones, M.G.; Yosef, N.; et al. PERCEPTION Predicts Patient Response and Resistance to Treatment Using Single-Cell Transcriptomics of Their Tumors. Nature Cancer 2024, 5, 938–952. [Google Scholar] [CrossRef]
- Mak, K.-K.; Wong, Y.-H.; Pichika, M.R. Artificial Intelligence in Drug Discovery and Development. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Springer International: Cham, Switzerland, 2024; pp. 1461–1498. [Google Scholar] [CrossRef]
- He, H.; He, B.; Guan, L.; Zhao, Y.; Jiang, F.; Chen, G.; Zhu, Q.; Chen, C.Y.C.; Li, T.; Yao, J. De Novo Generation of SARS-CoV-2 Antibody CDRH3 with a Pre-Trained Generative Large Language Model. Nat. Commun. 2024, 15, 6867. [Google Scholar] [CrossRef]
- Dunbar, J.; Krawczyk, K.; Leem, J.; Marks, C.; Nowak, J.; Regep, C.; Georges, G.; Kelm, S.; Popovic, B.; Deane, C.M. SAbPred: A Structure-Based Antibody Prediction Server. Nucleic Acids Res. 2016, 44, W474–W478. [Google Scholar] [CrossRef]
- Caradonna, T.M.; Schmidt, A.G. Protein Engineering Strategies for Rational Immunogen Design. npj Vaccines 2021, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Marcandalli, J.; Fiala, B.; Ols, S.; Perotti, M.; de van der Schueren, W.; Snijder, J.; Hodge, E.; Benhaim, M.; Ravichandran, R.; Carter, L.; et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus. Cell 2019, 176, 1420–1431.e17. [Google Scholar] [CrossRef]
- Shanehsazzadeh, A.; McPartlon, M.; Kasun, G.; Steiger, A.K.; Sutton, J.M.; Yassine, E.; McCloskey, C.; Haile, R.; Shuai, R.; Alverio, J.; et al. Unlocking de Novo Antibody Design with Generative Artificial Intelligence. bioRxiv 2024. bioRxiv:2023.01.08.523187. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. De Novo Design of Protein Structure and Function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef]
- Mohan, S.; Kerry, P.S.; Bance, N.; Niikura, M.; Pinto, B.M. Serendipitous Discovery of a Potent Influenza Virus A Neuraminidase Inhibitor. Angew. Chem. Int. Ed. 2014, 53, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhu, X.; He, W.T.; Zhou, P.; Kaku, C.I.; Capozzola, T.; Zhu, C.Y.; Yu, X.; Liu, H.; Yu, W.; et al. A Broad and Potent Neutralization Epitope in SARS-Related Coronaviruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2205784119. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Fiala, B.; Schäfer, A.; Wrenn, S.; Pham, M.N.; Murphy, M.; Tse, L.V.; Shehata, L.; O’Connor, M.A.; Chen, C.; et al. Elicitation of Potent Neutralizing Antibody Responses by Designed Protein Nanoparticle Vaccines for SARS-CoV-2. Cell 2020, 183, 1367–1382.e17. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Antanasijevic, A.; Ronk, A.J.; Müller-Kräuter, H.; Watanabe, Y.; Claireaux, M.; Perrett, H.R.; Bijl, T.P.L.; Grobben, M.; Umotoy, J.C.; et al. Lassa Virus Glycoprotein Nanoparticles Elicit Neutralizing Antibody Responses and Protection. Cell Host Microbe 2022, 30, 1759–1772.e12. [Google Scholar] [CrossRef]
- Sesterhenn, F.; Yang, C.; Bonet, J.; Cramer, J.T.; Wen, X.; Wang, Y.; Chiang, C.I.; Abriata, L.A.; Kucharska, I.; Castoro, G.; et al. De Novo Protein Design Enables the Precise Induction of RSV-Neutralizing Antibodies. Science 2020, 368, eaay5051. [Google Scholar] [CrossRef] [PubMed]
- Mousa, J.J.; Sauer, M.F.; Sevy, A.M.; Finn, J.A.; Bates, J.T.; Alvarado, G.; King, H.G.; Loerinc, L.B.; Fong, R.H.; Doranz, B.J.; et al. Structural Basis for Nonneutralizing Antibody Competition at Antigenic Site II of the Respiratory Syncytial Virus Fusion Protein. Proc. Natl. Acad. Sci. USA 2016, 113, E6849–E6858. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody Cocktail to SARS-CoV-2 Spike Protein Prevents Rapid Mutational Escape Seen with Individual Antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Behre, G.; Hebert, C.; Kumar, P.; Farmer MacPherson, L.; Graham-Clarke, P.L.; De La Torre, I.; Nichols, R.M.; Hufford, M.M.; Patel, D.R.; et al. Bamlanivimab and Etesevimab Improve Symptoms and Associated Outcomes in Ambulatory Patients at Increased Risk for Severe Coronavirus Disease 2019: Results From the Placebo-Controlled Double-Blind Phase 3 BLAZE-1 Trial. Open Forum Infect. Dis. 2022, 9, ofac172. [Google Scholar] [CrossRef]
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and Structural Basis for SARS-CoV-2 Variant Neutralization by a Two-Antibody Cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A.; Franchini, M.; Maggi, F. Sotrovimab: A Review of Its Efficacy against SARS-CoV-2 Variants. Viruses 2024, 16, 217. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Hnath, B.; Rackley, B.; Wang, J.; Gontu, A.; Chandler, M.; Afonin, K.A.; Kuchipudi, S.V.; Christensen, N.; Yennawar, N.H.; et al. Adaptation-Proof SARS-CoV-2 Vaccine Design. Adv. Funct. Mater. 2022, 32, 2206055. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, Y.; Cai, C.; Huang, Y.; Zhou, L.; Guan, Y.; Fu, S.; Lin, Y.; Yan, H.; Zhang, Z.; et al. A Broad Neutralizing Nanobody against SARS-CoV-2 Engineered from an Approved Drug. Cell Death Dis. 2024, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Bardhan, M.; Ray, I.; Roy, S.; Roy, P.; Thanneeru, P.; Twayana, A.R.; Prasad, S.; Bardhan, M.; Anand, A. Disease X and COVID-19: Turning Lessons from India and the World into Policy Recommendations. Ann. Med. Surg. 2024, 86, 5914–5921. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).