Oropouche Virus: An Overview of the Current Status of Diagnostics
Abstract
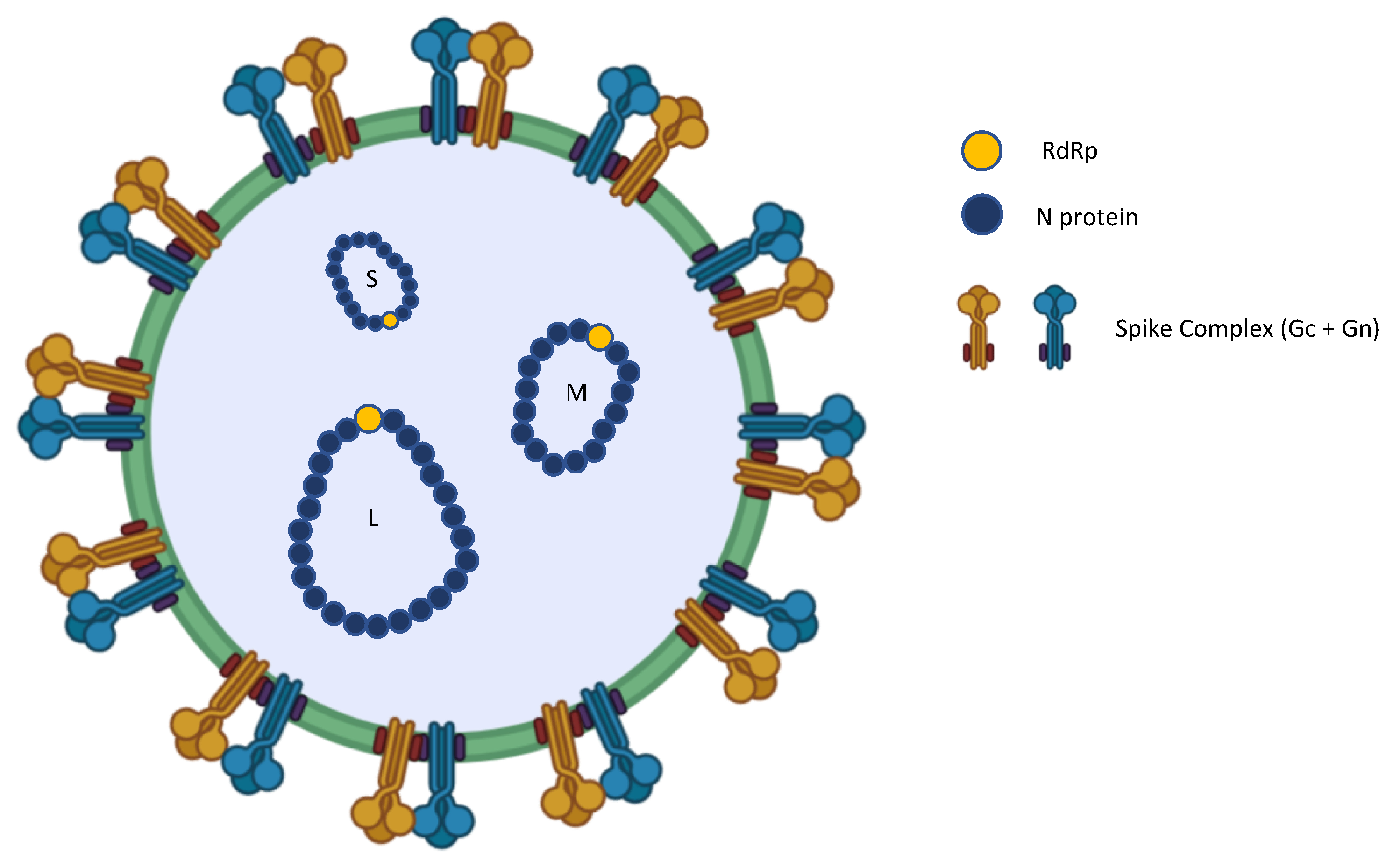
1. Introduction
2. Immunity
3. Clinical Aspects
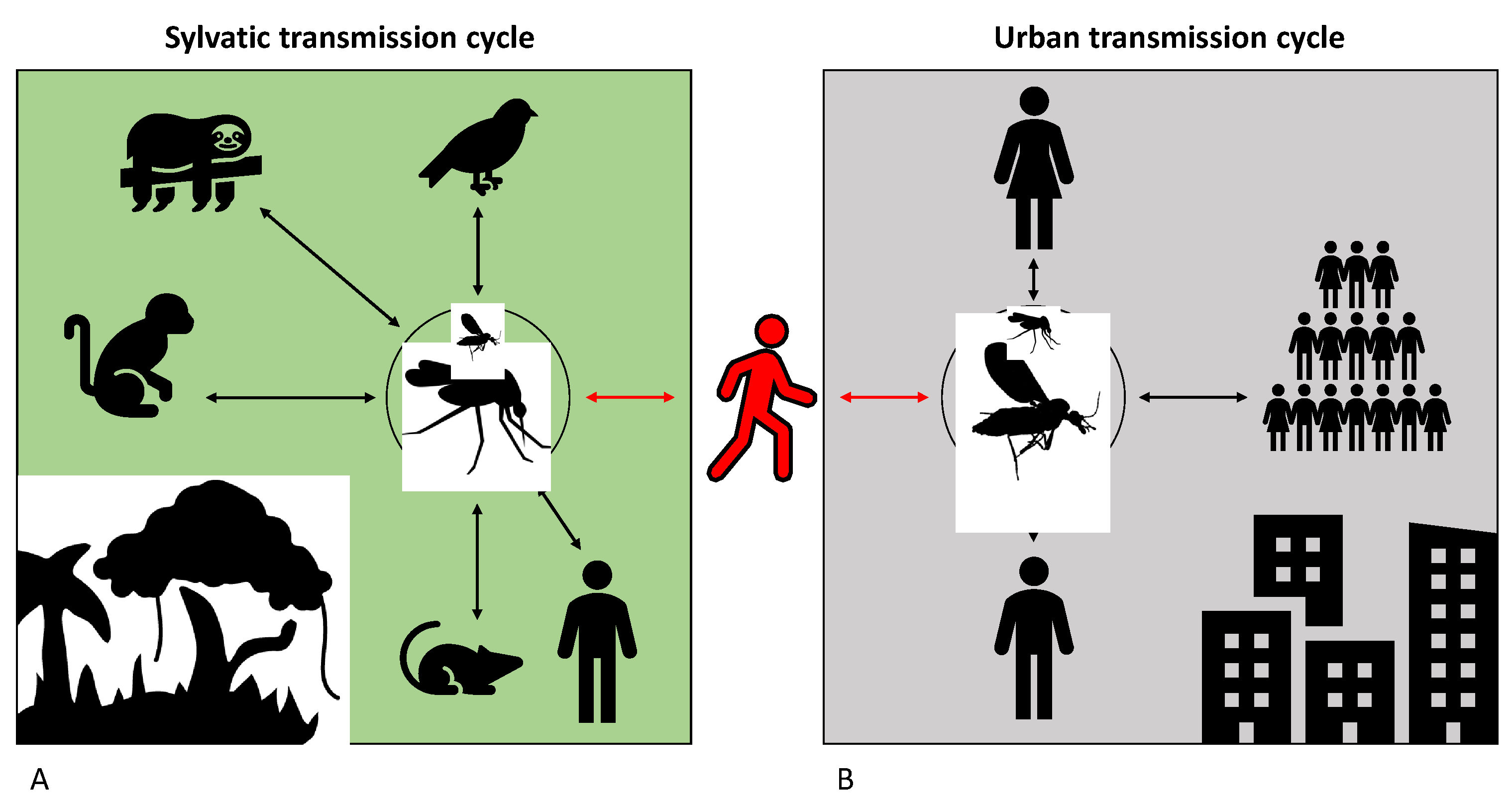
4. Reservoir Species
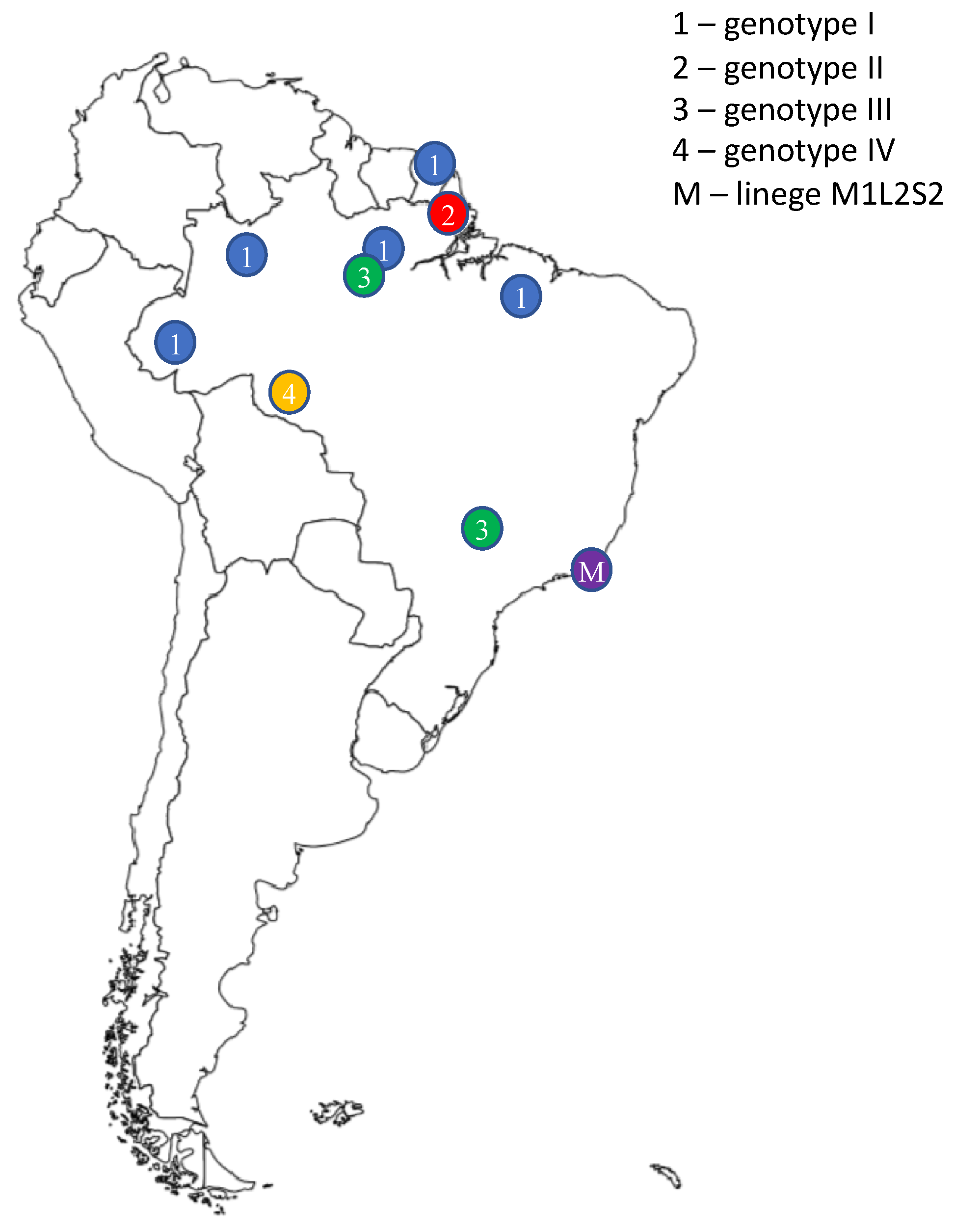
5. Epidemiology
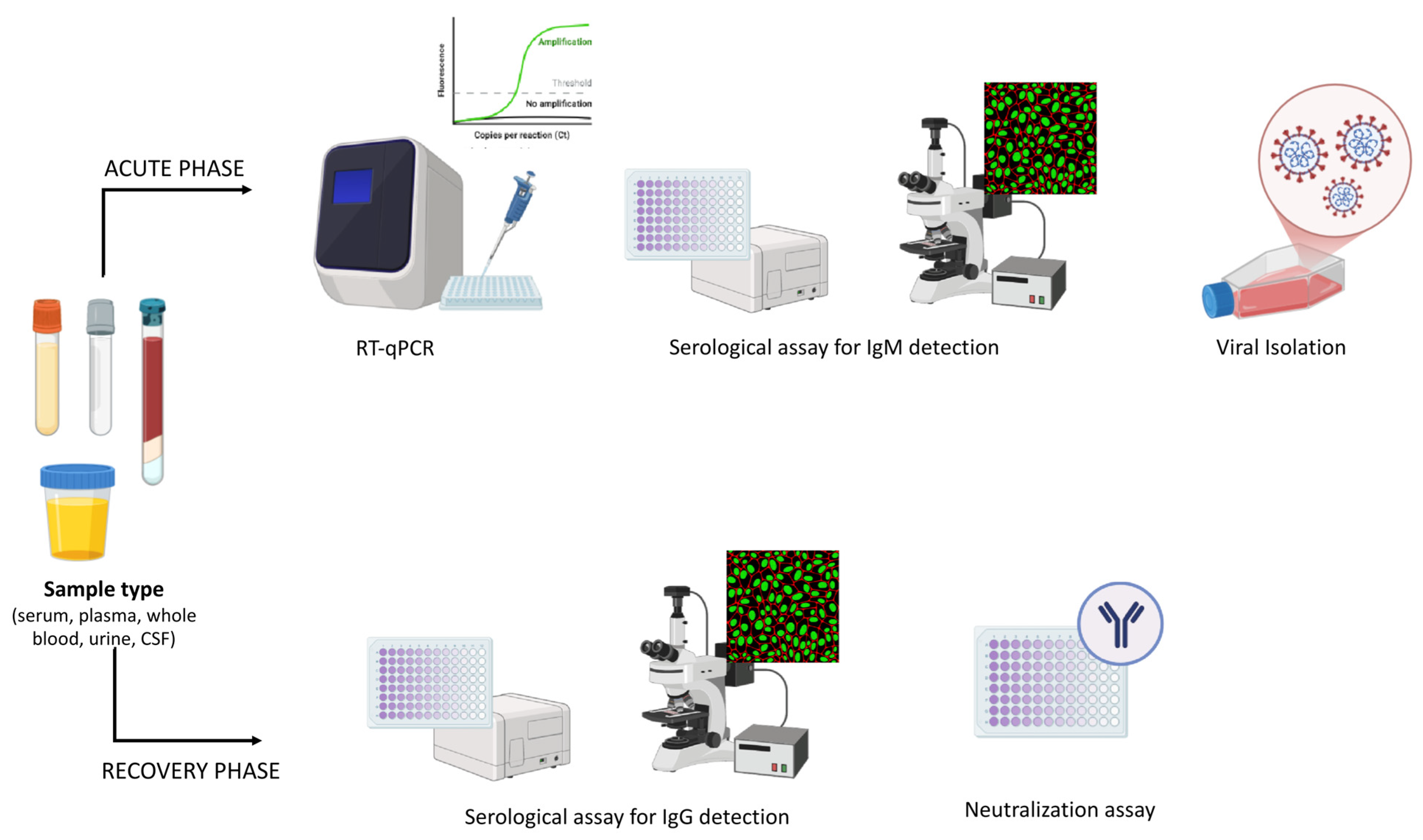
6. Diagnosis
6.1. Molecular Diagnosis
RT-qPCR
6.2. Isolation
6.3. Serological Diagnosis
6.4. Viral Sequencing
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| CF | Complement fixation test |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| ddPCR | Droplet digital PCR |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HI | Haemagglutination test |
| IFNs | Interferons |
| IQTV | Iquitos virus |
| ISGs | Interferon-stimulated genes |
| L | Large |
| LLOD | Lower limit of detection |
| LoD | Limit of detection |
| Lrp1 | Lipoprotein receptor-related protein 1 |
| M | Medium |
| MAYV | Mayaro virus |
| MDDV | Madre de Dios virus |
| N | Nucleocapsid protein |
| NGS | Next Generation Sequencing |
| NSM | Non-structural protein M |
| NSs | Non-structural protein S |
| NT | neutralisation test |
| OF | Oropouche fever |
| OROV | Oropouche virus |
| PAHO | Pan American Health Organization |
| PAMPs | Pathogen-associated molecular patterns |
| PERDV | Perdoes virus |
| PRRs | pattern recognition receptors |
| RACE | Rapid Amplification of cDNA Ends |
| S | Small |
| TLRs | Toll-like receptors |
| WHO | World Health Organization |
References
- Hellert, J.; Aebischer, A.; Wernike, K.; Haouz, A.; Brocchi, E.; Reiche, S.; Guardado-Calvo, P.; Beer, M.; Rey, F.A. Orthobunyavirus Spike Architecture and Recognition by Neutralizing Antibodies. Nat. Commun. 2019, 10, 879. [Google Scholar] [CrossRef]
- Barbosa, N.S.; Concha, J.O.; daSilva, L.L.P.; Crump, C.M.; Graham, S.C. Oropouche Virus Glycoprotein Topology and Cellular Requirements for Glycoprotein Secretion. J. Virol. 2023, 97, e01331-22. [Google Scholar] [CrossRef]
- Riccò, M. Epidemiology of Tick-Borne Encephalitis in North-Eastern Italy (2017–2020): International Insights from National Notification Reports. Acta Biomed. Atenei Parm. 2021, 92, e2021229. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche Virus: A New Human Disease Agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- CDC. Oropouche: Causes and How It Spreads. Available online: https://www.cdc.gov/oropouche/causes/index.html (accessed on 1 August 2025).
- Castilletti, C.; Huits, R.; Mantovani, R.P.; Accordini, S.; Alladio, F.; Gobbi, F. Replication-Competent Oropouche Virus in Semen of Traveler Returning to Italy from Cuba, 2024. Emerg. Infect. Dis. 2024, 30, 2684–2686. [Google Scholar] [CrossRef]
- Gräf, T.; Delatorre, E.; Do Nascimento Ferreira, C.; Rossi, A.; Santos, H.G.G.; Pizzato, B.R.; Nascimento, V.; Souza, V.; De Lima, G.B.; Dezordi, F.Z.; et al. Expansion of Oropouche Virus in Non-Endemic Brazilian Regions: Analysis of Genomic Characterisation and Ecological Drivers. Lancet Infect. Dis. 2025, 25, 379–389. [Google Scholar] [CrossRef]
- Oropouche—Ministério Da Saúde. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/o/oropouche (accessed on 2 August 2025).
- The Lancet Infectious Diseases. Oropouche Fever, the Mysterious Threat. Lancet Infect. Dis. 2024, 24, 935. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L. Oropouche Virus: An Emerging Orthobunyavirus. J. Gen. Virol. 2024, 105, 002027. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Azevedo, T.S.; Virginio, F.; Aguiar, B.S.; Chiaravalloti-Neto, F.; Suesdek, L. Impact of Environmental Factors on Neglected Emerging Arboviral Diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche Virus: A Neglected Global Arboviral Threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, F.B.; Cavalcanti, A.C.; Erbisti, R.S.; Dias, V.Z.; Moreira, C.G.D.C.; Grifo, M.M.; Vaz, M.C.D.S.; Camargo, A.C.; De Souza, L.M.; Dos Santos, F.B.; et al. Introduction and Spatial–Temporal Distribution of Oropouche Virus in Rio de Janeiro State, Brazil. Pathogens 2025, 14, 833. [Google Scholar] [CrossRef]
- Obijeski, J.F.; Bishop, D.H.; Murphy, F.A.; Palmer, E.L. Structural Proteins of La Crosse Virus. J. Virol. 1976, 19, 985–997. [Google Scholar] [CrossRef]
- Murillo, J.L.; Cabral, A.D.; Uehara, M.; Da Silva, V.M.; Dos Santos, J.V.; Muniz, J.R.C.; Estrozi, L.F.; Fenel, D.; Garcia, W.; Sperança, M.A. Nucleoprotein from the Unique Human Infecting Orthobunyavirus of Simbu Serogroup (Oropouche virus) Forms Higher Order Oligomers in Complex with Nucleic Acids in Vitro. Amino Acids 2018, 50, 711–721. [Google Scholar] [CrossRef]
- Elliott, R.M. Orthobunyaviruses: Recent Genetic and Structural Insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L.; Hughes, J.; Acrani, G.O.; Da Silva, D.E.A.; Azevedo, R.S.S.; Rodrigues, S.G.; Vasconcelos, P.F.C.; Nunes, M.R.T.; Elliott, R.M. Genetic Analysis of Members of the Species Oropouche Virus and Identification of a Novel M Segment Sequence. J. Gen. Virol. 2015, 96, 1636–1650. [Google Scholar] [CrossRef]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline Mapping of Oropouche Virology, Epidemiology, Therapeutics, and Vaccine Research and Development. Npj Vaccines 2022, 7, 38. [Google Scholar] [CrossRef]
- Schwarz, M.M.; Price, D.A.; Ganaie, S.S.; Feng, A.; Mishra, N.; Hoehl, R.M.; Fatma, F.; Stubbs, S.H.; Whelan, S.P.J.; Cui, X.; et al. Oropouche Orthobunyavirus Infection Is Mediated by the Cellular Host Factor Lrp1. Proc. Natl. Acad. Sci. USA 2022, 119, e2204706119. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, R.; De Toni Aquino Da Cruz, L.C.; De Souza, V.J.; Da Silva Neves, N.A.; De Souza, V.C.; Filho, L.C.F.; Da Silva Lemos, P.; De Lima, C.P.S.; Naveca, F.G.; Atanaka, M.; et al. Insect-Specific Viruses and Arboviruses in Adult Male Culicids from Midwestern Brazil. Infect. Genet. Evol. 2020, 85, 104561. [Google Scholar] [CrossRef]
- Moreli, M.L.; Aquino, V.H.; Figueiredo, L.T.M. Identification of Simbu, California and Bunyamwera Serogroup Bunyaviruses by Nested RT-PCR. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Travassos Da Rosa, J.F.; De Souza, W.M.; Pinheiro, F.D.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. Soc. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Santos, R.I.; Almeida, M.F.P.; Paula, F.E.; Rodrigues, A.H.; Saranzo, A.M.; Paula, A.E.; Silva, M.L.; Correa, V.M.A.; Acrani, G.O.; Neder, L.; et al. Experimental Infection of Suckling Mice by Subcutaneous Inoculation with Oropouche Virus. Virus Res. 2012, 170, 25–33. [Google Scholar] [CrossRef]
- Castro, F.L.D.; Brustolini, O.J.B.; Geddes, V.E.V.; Souza, J.P.B.M.D.; Alves-Leon, S.V.; Aguiar, R.S.; Vasconcelos, A.T.R. Modulation of HERV Expression by Four Different Encephalitic Arboviruses during Infection of Human Primary Astrocytes. Viruses 2022, 14, 2505. [Google Scholar] [CrossRef]
- Scachetti, G.C.; Forato, J.; Claro, I.M.; Hua, X.; Salgado, B.B.; Vieira, A.; Simeoni, C.L.; Barbosa, A.R.C.; Rosa, I.L.; De Souza, G.F.; et al. Re-Emergence of Oropouche Virus between 2023 and 2024 in Brazil: An Observational Epidemiological Study. Lancet Infect. Dis. 2025, 25, 166–175. [Google Scholar] [CrossRef]
- Liu, B.M. Epidemiological and Clinical Overview of the 2024 Oropouche Virus Disease Outbreaks, an Emerging/Re-emerging Neurotropic Arboviral Disease and Global Public Health Threat. J. Med. Virol. 2024, 96, e29897. [Google Scholar] [CrossRef]
- Schwartz, D.A. Novel Reassortants of Oropouche Virus (OROV) Are Causing Maternal–Fetal Infection During Pregnancy, Stillbirth, Congenital Microcephaly and Malformation Syndromes. Genes 2025, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.; Azevedo, R.D.S.S.; Coelho-dos-Reis, J.G.; Antonelli, L.R.D.V.; Ferreira, M.S.; Campi-Azevedo, A.C.; Costa-Silva, M.F.; Martins, L.C.; Chiang, J.O.; Teixeira-Carvalho, A.; et al. IFN-α as a Time-Sensitive Biomarker during Oropouche Virus Infection in Early and Late Seroconverters. Sci. Rep. 2019, 9, 17924. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Kochs, G.; Weber, F. The Interferon Response Circuit: Induction and Suppression by Pathogenic Viruses. Virology 2006, 344, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Sharpe, A.H. Dendritic Cells Giveth and Taketh Away. Nat. Immunol. 2005, 6, 227–228. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic Cells: Master Regulators of the Immune Response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Geddes, V.E.V.; De Oliveira, A.S.; Tanuri, A.; Arruda, E.; Ribeiro-Alves, M.; Aguiar, R.S. MicroRNA and Cellular Targets Profiling Reveal miR-217 and miR-576-3p as Proviral Factors during Oropouche Infection. PLoS Negl. Trop. Dis. 2018, 12, e0006508. [Google Scholar] [CrossRef] [PubMed]
- Có, A.C.G.; De Mendonça, G.C.; Gatti, F.D.; Sousa, T.D.J.; Tavares, E.A.; Nodari, J.Z.; De Moura, R.G.; Lopes, P.D.O.; Pereira, J.D.P.; Alves, L.N.R.; et al. Unravelling the Pathogenesis of Oropouche Virus. Lancet Infect. Dis. 2025, 25, e381–e382. [Google Scholar] [CrossRef]
- Vijukumar, A.; Kumar, A.; Kumar, H. Potential Therapeutics and Vaccines: Current Progress and Challenges in Developing Antiviral Treatments or Vaccines for Oropouche Virus. Diagn. Microbiol. Infect. Dis. 2025, 111, 116699. [Google Scholar] [CrossRef]
- Romero-Alvarez, D.; Escobar, L.E. Vegetation Loss and the 2016 Oropouche Fever Outbreak in Peru. Mem. Inst. Oswaldo Cruz 2017, 112, 292–298. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.C.; Calisher, C.H. Emergence of Human Arboviral Diseases in the Americas, 2000–2016. Vector-Borne Zoonotic Dis. 2016, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Gibrail, M.M.; Fiaccadori, F.S.; Souza, M.; Almeida, T.N.V.; Chiang, J.O.; Martins, L.C.; Ferreira, M.S.; Cardoso, D.D.D.D.P. Detection of Antibodies to Oropouche Virus in Non-Human Primates in Goiânia City, Goiás. Rev. Soc. Bras. Med. Trop. 2016, 49, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Ishak, R.; Freitas, R.B.; Gomes, M.L.; LeDuc, J.W.; Oliva, O.F. Oropouche Virus. I. A Review of Clinical, Epidemiological, and Ecological Findings. Am. J. Trop. Med. Hyg. 1981, 30, 149–160. [Google Scholar] [CrossRef]
- Bastos, M.D.S.; Figueiredo, L.T.M.; Naveca, F.G.; Monte, R.L.; Lessa, N.; Pinto De Figueiredo, R.M.; Gimaque, J.B.D.L.; Pivoto João, G.; Ramasawmy, R.; Mourão, M.P.G. Identification of Oropouche Orthobunyavirus in the Cerebrospinal Fluid of Three Patients in the Amazonas, Brazil. Am. Soc. Trop. Med. Hyg. 2012, 86, 732–735. [Google Scholar] [CrossRef]
- Gatti, F.D.; Nodari, J.Z.; Sousa, T.D.J.; Mendonça, G.C.D.; Tavares, E.A.; Pinto, I.A.; Pereira, J.D.P.; Filho, J.B.F.; Cabral, V.P.; Azevedo, S.S.D.D.; et al. Neuroinvasive Oropouche Virus in a Patient with HIV from Extra-Amazonian Brazil. Lancet Infect. Dis. 2025, 25, e441–e442. [Google Scholar] [CrossRef]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.D.S.; Zuchi, N.; Souza, V.C.D.; Naveca, F.G.; Santos, M.A.M.D.; Slhessarenko, R.D. Detection of Oropouche Virus Segment S in Patients and in Culex quinquefasciatus in the State of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Vita, S.; Colavita, F.; Maffongelli, G.; Carletti, F.; Scorzolini, L.; Matusali, G.; Tomassi, M.V.; Meschi, S.; D’Abramo, A.; Girardi, E.; et al. Viral Shedding in Saliva, Axillary, Rectal and Vaginal Swabs of an Imported Case of Dengue—Oropouche Virus Co-Infection. Travel Med. Infect. Dis. 2025, 66, 102854. [Google Scholar] [CrossRef]
- Azevedo, R.D.S.D.S.; Nunes, M.R.T.; Chiang, J.O.; Bensabath, G.; Vasconcelos, H.B.; Pinto, A.Y.D.N.; Martins, L.C.; Monteiro, H.A.D.O.; Rodrigues, S.G.; Vasconcelos, P.F.D.C. Reemergence of Oropouche Fever, Northern Brazil. Emerg. Infect. Dis. 2007, 13, 912–915. [Google Scholar] [CrossRef]
- Mercer, D.R.; Spinelli, G.R.; Watts, D.M.; Tesh, R.B. Biting Rates and Developmental Substrates for Biting Midges (Diptera: Ceratopogonidae) in Iquitos, Peru. J. Med. Entomol. 2003, 40, 807–812. [Google Scholar] [CrossRef]
- Borborema, C.A.; Pinheiro, F.P.; Albuquerque, B.C.; da Rosa, A.P.; da Rosa, J.F.; Dourado, H.V. Primeiro Registro de Epidemias Causadas Pelo Vírus Oropouche No Estado Do Amazonas. Rev. Inst. Med. Trop. São Paulo 1982, 24, 132–139. [Google Scholar]
- Ribeiro, B.D.F.R.; Barreto, A.R.F.; Pessoa, A.; Azevedo, R.D.S.D.S.; Rodrigues, F.D.F.; Borges, B.D.C.B.; Mantilla, N.P.M.; Muniz, D.D.; Chiang, J.O.; Fraga, L.R.; et al. Congenital Oropouche in Humans: Clinical Characterization of a Possible New Teratogenic Syndrome. Viruses 2025, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Cola, J.P.; Dos Santos, A.P.B.; Zanotti, R.L.; Dela Costa, A.E.D.S.; Del Carro, K.B.; Coelho, L.D.A.L.; Miranda, A.E.; Vicente, C.R. Maternal and Fetal Implications of Oropouche Fever, Espírito Santo State, Brazil, 2024. Emerg. Infect. Dis. 2025, 31, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Oropouche: Cases of Mother-to-Child Transmission Under Investigation in Brazil. Available online: https://www.paho.org/en/news/18-7-2024-oropouche-cases-mother-child-transmission-under-investigation-brazil (accessed on 2 July 2024).
- Epidemiological Alert Oropouche in the Region of the Americas: Vertical Transmission Event under Investigation in Brazil-17 July 2024. Available online: https://www.paho.org/en/documents/epidemiological-alert-oropouche-region-americas-vertical-transmission-event-under (accessed on 4 July 2024).
- Interim Clinical Considerations for Pregnant Women with Confirmed or Probable Oropouche Virus Disease 2025. Available online: https://www.cdc.gov/oropouche/hcp/clinical-care/pregnancy.html (accessed on 31 January 2025).
- Updated Interim Guidance for Health Departments on Testing and Reporting for Oropouche Virus Disease 2025. Available online: https://www.cdc.gov/oropouche/php/reporting/index.html (accessed on 10 April 2025).
- Update on Oropouche Virus and Potential Effects on Pregnancy 2024. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2024/08/update-on-oropouche-virus-and-potential-effects-on-pregnancy (accessed on 4 November 2024).
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Bensabath, G. An Outbreak of Oropouche Virus Diease in the Vicinity of Santarem, Para, Barzil. Tropenmed. Parasitol. 1976, 27, 213–223. [Google Scholar]
- LeDuc, J.W.; Hoch, A.L.; Pinheiro, F.P.; da Rosa, A.P. Epidemic Oropouche Virus Disease in Northern Brazil. Bull. Pan Am. Health Organ. 1981, 15, 97–103. [Google Scholar] [PubMed]
- Groot Liévano, H. Estudios Sobre Virus Transmitidos Por Artrópodos En Colombia. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2017, 41, 12–33. [Google Scholar] [CrossRef]
- Batista, P.M.; Andreotti, R.; Chiang, J.O.; Ferreira, M.S.; Vasconcelos, P.F. da C. Seroepidemiological Monitoring in Sentinel Animals and Vectors as Part of Arbovirus Surveillance in the State of Mato Grosso Do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 168–173. [Google Scholar] [CrossRef]
- Batista, P.M.; Andreotti, R.; Almeida, P.S.D.; Marques, A.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F.D.C. Detection of Arboviruses of Public Health Interest in Free-Living New World Primates (Sapajus spp.; Alouatta caraya) Captured in Mato Grosso Do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 684–690. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos Da Rosa, A.P.A.; Gomes, M.L.C.; LeDuc, J.W.; Hoch, A.L. Transmission of Oropouche Virus from Man to Hamster by the Midge Culicoides paraensis. Science 1982, 215, 1251–1253. [Google Scholar] [CrossRef]
- Santamaría, E.; Cabrera, O.L.; Zipa, Y.; Ferro, C.; Ahumada, M.L.; Pardo, R.H. Preliminary evaluation of the Culicoides biting nuisance (Diptera: Ceratopogonidae) in the province of Boyacá, Colombia. Biomedica 2008, 28, 497–509. [Google Scholar] [CrossRef]
- Hoch, A.L.; Pinheiro, F.P.; Roberts, D.R.; Gomes, M.L. Laboratory Transmission of Oropouche Virus by Culex quinquefasciatus Say. Bull. Pan Am. Health Organ. 1987, 21, 55–61. [Google Scholar]
- Walsh, C.E.S.; Robert, M.A.; Christofferson, R.C. Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides Paraenesis: Data Synthesis and Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.F.; Stout, J.; Dumoulin, P.; Locksmith, T.; Heberlein, L.A.; Mitchell, M.; Rodriguez-Hilario, A.; Dupuis, A.P.; Ciota, A.T. Lack of Competence of US Mosquito Species for Circulating Oropouche Virus. Emerg. Infect. Dis. 2025, 31, 619–621. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Connelly, C.R.; Kenney, J.L. Infection, Dissemination, and Transmission Potential of North American Culex quinquefasciatus, Culex tarsalis, and Culicoides sonorensis for Oropouche Virus. Viruses 2021, 13, 226. [Google Scholar] [CrossRef]
- Vasconcelos, P.F.D.C.; Travassos Da Rosa, J.F.S.; Guerreiro, S.C.; Dégallier, N.; Travassos Da Rosa, E.S.; Travassos Da Rosa, A.P.D.A. Primeiro Registro de Epidemias Causadas Pelo Vírus Oropouche Nos Estados Do Maranhão e Goiás, Brasil. Rev. Inst. Med. Trop. S. Paulo 1989, 31, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.; Pinheiro, F.P.; Santos, M.A.V. Epidemia de Vírus Oropouche No Leste Do Estado Da Pará 1979. Available online: https://patua.iec.gov.br/items/cc5ac320-39b1-4a9c-b0d5-40472caf0b9d (accessed on 5 August 2025).
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides Biting Midges: Their Role as Arbovirus Vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of Temperate and Tropical Culicoides Midges: Knowledge Gaps and Consequences for Transmission of Culicoides-Borne Viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Lassen, S.B.; Nielsen, S.A.; Kristensen, M. Identity and Diversity of Blood Meal Hosts of Biting Midges (Diptera: Ceratopogonidae: Culicoides latreille) in Denmark. Parasit. Vectors 2012, 5, 143. [Google Scholar] [CrossRef]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges-Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef]
- Feitoza, L.H.M.; de Carvalho, L.P.C.; da Silva, L.R.; Meireles, A.C.A.; Rios, F.G.F.; Silva, G.S.; de Paulo, P.F.M.; Pessoa, F.A.C.; de Medeiros, J.F.; Julião, G.R. Influence of Meteorological and Seasonal Parameters on the Activity of Culicoides Paraensis (Diptera: Ceratopogonidae), an Annoying Anthropophilic Biting Midge and Putative Vector of Oropouche Virus in Rondônia, Brazilian Amazon. Acta Trop. 2023, 243, 106928. [Google Scholar] [CrossRef]
- Wirth, W.W.; Felippe-Bauer, M.L. The Neotropical Biting Midges Related to Culicoides Paraensis (Diptera: Ceratopogonidae). Mem. Inst. Oswaldo Cruz 1989, 84, 551–565. [Google Scholar] [CrossRef]
- Aybar, C.A.V.; Juri, M.J.D.; De Grosso, M.S.L.; Spinelli, G.R. Species Diversity and Seasonal Abundance of Culicoides Biting Midges in Northwestern Argentina. Med. Vet. Entomol. 2010, 24, 95–98. [Google Scholar] [CrossRef]
- Aybar, C.A.V.; Juri, M.J.D.; Santana, M.; de Grosso, M.S.L.; Spinelli, G.R. The Spatio-Temporal Distribution Patterns of Biting Midges of the Genus Culicoides in Salta Province, Argentina. J. Insect Sci. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides Biting Midges, Arboviruses and Public Health in Europe. Antiviral Res. 2013, 100, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.L.; Roberts, D.R.; Pinheiro, F.P. Host-Seeking Behavior and Seasonal Abundance of Culicoides paraensis (Diptera: Ceratopogonidae) in Brazil. J. Am. Mosq. Control Assoc. 1990, 6, 110–114. [Google Scholar]
- Huerta, H.; Rodríguez-Martínez, L.M.; Benitez-Alva, J.I.; Dzul-Manzanilla, F.; Manrique-Saide, P. New Records of Biting Midges (Diptera: Ceratopogonidae) from Tabasco, Mexico. Rev. Mex. Biodiv. 2022, 93, e933605. [Google Scholar] [CrossRef]
- Felippe-Bauer, M.L.; Cáceres, A.G.; Silva, C.S.; Valderrama-Bazan, W.; Gonzales-Perez, A. Two New Culicoides of the Paraensis Species Group (Diptera: Ceratopogonidae) from the Amazonian Region of Peru. Mem. Inst. Oswaldo Cruz 2003, 98, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Perez-Restrepo, L.S.; Knuese, C.; Vargas, V.; Yepes, L.; Moreno, I.; Usuga, J.; Hernandez-Ortiz, J.P.; Osorio, J.E.; Limonta, D. Protocol for Detection of Oropouche Viruses from Human Serum. STAR Protoc. 2025, 6, 103805. [Google Scholar] [CrossRef]
- Benitez, A.J.; Alvarez, M.; Perez, L.; Gravier, R.; Serrano, S.; Hernandez, D.M.; Perez, M.M.; Gutierrez-Bugallo, G.; Martinez, Y.; Companioni, A.; et al. Oropouche Fever, Cuba, May 2024. Emerg. Infect. Dis. 2024, 30, 2155–2159. [Google Scholar] [CrossRef]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche Fever, an Emergent Disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef]
- de Lima, R.C.; Dias, H.G.; de Souza, T.M.A.; Familiar-Macedo, D.; Ribeiro, E.D.; Corrêa, V.C.E.; Pauvolid-Corrêa, A.; de Azeredo, E.L.; Dos Santos, F.B. Oropouche Virus Exposure in Febrile Patients during Chikungunya Virus Introduction in the State of Amapá, Amazon Region, Brazil. Pathogens 2024, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Yoosuf, B.T.; Gaidhane, A.M.; Vadia, N.; Menon, S.V.; Chennakesavulu, K.; Panigrahi, R.; Bushi, G.; Rani, A.; Sah, S.; Shabil, M.; et al. Epidemiology, Transmission Dynamics, Treatment Strategies, and Future Perspectives on Oropouche Virus. Diagn. Microbiol. Infect. Dis. 2025, 113, 116882. [Google Scholar] [CrossRef]
- Ciuoderis, K.A.; Berg, M.G.; Perez, L.J.; Hadji, A.; Perez-Restrepo, L.S.; Aristizabal, L.C.; Forberg, K.; Yamaguchi, J.; Cardona, A.; Weiss, S.; et al. Oropouche Virus as an Emerging Cause of Acute Febrile Illness in Colombia. Emerg. Microbes Infect. 2022, 11, 2645–2657. [Google Scholar] [CrossRef]
- Gaillet, M.; Pichard, C.; Restrepo, J.; Lavergne, A.; Perez, L.; Enfissi, A.; Abboud, P.; Lambert, Y.; Ma, L.; Monot, M.; et al. Outbreak of Oropouche Virus in French Guiana. Emerg. Infect. Dis. 2021, 27, 2711–2714. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo, R.D.S.D.S.; Travassos Da Rosa, A.P.A.; Vasconcelos, P.F.D.C. Oropouche Virus Isolation, Southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Epidemiological Alert—Oropouche in the Region of the Americas; PAHO: Washington, DC, USA, 2024. [Google Scholar]
- PAHO. Public Health Risk Assessment Related to Oropouche Virus (OROV) in the Region of the Americas; PAHO: Washington, DC, USA, 2024. [Google Scholar]
- ECDC. Communicable Disease Threats Report, 19−25 July 2025, Week 30; European Centre for Disease Prevention and Control: Solna, Sweden, 2025. [Google Scholar]
- ECDC. Communicable Disease Threats Report, 27 July–2 August 2024, Week 31; European Centre for Disease Prevention and Control: Solna, Sweden, 2024. [Google Scholar]
- PAHO. Epidemiological Update Oropouche in the Americas Region—11 February 2025; PAHO: Washington, DC, USA, 2025. [Google Scholar]
- Porwal, S.; Malviya, R.; Sridhar, S.B.; Shareef, J.; Wadhwa, T. Mysterious Oropouche Virus: Transmission, Symptoms, and Control. Infect. Med. 2025, 4, 100177. [Google Scholar] [CrossRef]
- Rossini, G.; Mola, B.; Rampini, A.; Ortalli, M.; Mattei, G.; Lazzarotto, T. Virological Findings in a Case of Travel-Associated Oropouche Virus (OROV) Infection Imported to Italy, June 2024. Travel Med. Infect. Dis. 2025, 66, 102874. [Google Scholar] [CrossRef]
- Focosi, D.; Colavita, F.; Meschi, S.; Lalle, E.; Franchini, M.; Maggi, F. Oropouche Virus: Implications for Transfusion Services. Rev. Med. Virol. 2025, 35, e70031. [Google Scholar] [CrossRef]
- Nunes, B.T.D.; De Mendonça, M.H.R.; Simith, D.D.B.; Moraes, A.F.; Cardoso, C.C.; Prazeres, I.T.E.; De Aquino, A.A.; Santos, A.D.C.M.; Queiroz, A.L.N.; Rodrigues, D.S.G.; et al. Development of RT-qPCR and Semi-Nested RT-PCR Assays for Molecular Diagnosis of Hantavirus Pulmonary Syndrome. PLoS Negl. Trop. Dis. 2019, 13, e0007884. [Google Scholar] [CrossRef]
- Aguilar, P.V.; Barrett, A.D.; Saeed, M.F.; Watts, D.M.; Russell, K.; Guevara, C.; Ampuero, J.S.; Suarez, L.; Cespedes, M.; Montgomery, J.M.; et al. Iquitos Virus: A Novel Reassortant Orthobunyavirus Associated with Human Illness in Peru. PLoS Negl. Trop. Dis. 2011, 5, e1315. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.B.; Nunes, M.R.T.; Casseb, L.M.N.; Carvalho, V.L.; Pinto Da Silva, E.V.; Silva, M.; Casseb, S.M.M.; Vasconcelos, P.F.C. Molecular Epidemiology of Oropouche Virus, Brazil. Emerg. Infect. Dis. 2011, 17, 800–806. [Google Scholar] [CrossRef]
- Weidmann, M.; Schmidt, P.; Vackova, M.; Krivanec, K.; Munclinger, P.; Hufert, F.T. Identification of Genetic Evidence for Dobrava Virus Spillover in Rodents by Nested Reverse Transcription (RT)-PCR and TaqMan RT-PCR. J. Clin. Microbiol. 2005, 43, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Stittleburg, V.; Cardozo, F.; Bopp, N.; Cantero, C.; López, S.; Bernal, C.; Mendoza, L.; Aguilar, P.; Pinsky, B.A.; et al. Real-Time RT-PCR for the Detection and Quantitation of Oropouche Virus. Diagn. Microbiol. Infect. Dis. 2020, 96, 114894. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Nascimento, V.A.D.; Souza, V.C.D.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P.F.D.C. Multiplexed Reverse Transcription Real-Time Polymerase Chain Reaction for Simultaneous Detection of Mayaro, Oropouche, and Oropouche-like Viruses. Mem. Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef]
- Srivastava, S.; Sah, R.; Babu, M.R.; Sharma, D.; Sharma, D.; Kumar, S.; Sridhar, S.B.; Wadhwa, T.; Shareef, J.; Rao, G.S.N.K.; et al. The Emergence of Oropouche Fever: A Potential New Threat? New Microbes New Infect. 2025, 65, 101596. [Google Scholar] [CrossRef]
- Pomari, E.; Matucci, A.; Accordini, S.; Mantovani, R.P.; Gianesini, N.; Mori, A.; Castilletti, C. ddPCR for the Detection and Absolute Quantification of Oropouche Virus. Viruses 2024, 16, 1426. [Google Scholar] [CrossRef]
- Silva, L.D.C.; Silva, D.M.F.D.; Calassa, I.M.C.; De Curcio, J.S.; Costa, L.H.A.; De Sousa, F.B.; Anunciação, C.E.; Silveira-Lacerda, E.D.P. Fast and Visual RT-LAMP Assay for Detection of Oropouche Virus. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 1987–1994. [Google Scholar] [CrossRef]
- Oropouche Virus Real Time PCR Kit (RUO). Creative Diagnostics, New York, USA. Available online: https://www.creative-diagnostics.com/ivd-materials/oropouche-virus-real-time-pcr-kit-ruo-item-pcr-wn-090-14124.html (accessed on 5 August 2025).
- Oropouche Virus Nucleic Acid Detection Kit. Creative Biogene, New York, USA. Available online: https://microbiosci.creative-biogene.com/oropouche-virus-nucleic-acid-detection-kit-item-cbmn-dg384-16008.html (accessed on 5 August 2025).
- OROPOUCHE-MAYARO VIRUS REALTIME PCR KIT. Vircell, Granada, Spain. Available online: https://www.vircell.com/en/products/oropouche-mayaro-virus-realtime-pcr-kit (accessed on 5 August 2025).
- RealStar® Oropouche Fever RT-PCR Kit 1.0 RUO. Altona Diagnostics, Milan, Italy. Available online: https://altona-diagnostics.com/product/realstar-oropouche-fever-rt-pcr-kit-1-0-ruo/ (accessed on 5 August 2025).
- Oropouche Virus Real Time PCR Kit (RUO). BioPerfectus, Taizhou City, China. Available online: https://www.bioperfectus.com/ProductDetail/OropoucheVirusRealTimePCRKitRUO (accessed on 5 August 2025).
- WHO. Risk Evaluation of Oropouche Virus and Its Reassortants; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Deiana, M.; Malagò, S.; Mori, A.; Accordini, S.; Matucci, A.; Passarelli Mantovani, R.; Gianesini, N.; Huits, R.; Piubelli, C.; Gobbi, F.G.; et al. Full Genome Characterization of the First Oropouche Virus Isolate Imported in Europe from Cuba. Viruses 2024, 16, 1586. [Google Scholar] [CrossRef]
- Hontz, R.D.; Guevara, C.; Halsey, E.S.; Silvas, J.; Santiago, F.W.; Widen, S.G.; Wood, T.G.; Casanova, W.; Vasilakis, N.; Watts, D.M.; et al. Itaya Virus, a Novel Orthobunyavirus Associated with Human Febrile Illness, Peru. Emerg. Infect. Dis. 2015, 21, 781–788. [Google Scholar] [CrossRef]
- Navarro, J.-C.; Giambalvo, D.; Hernandez, R.; Auguste, A.J.; Tesh, R.B.; Weaver, S.C.; Montañez, H.; Liria, J.; Lima, A.; Travassos Da Rosa, J.F.S.; et al. Isolation of Madre de Dios Virus (Orthobunyavirus; Bunyaviridae), an Oropouche Virus Species Reassortant, from a Monkey in Venezuela. Am. Soc. Trop. Med. Hyg. 2016, 95, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Watts, D.M.; Shope, R.E.; Barrett, A.D.T.; Tesh, R.B.; Nunes, M.; Wang, H.; Weaver, S.C.; Vasconcelos, P.F.C. Nucleotide Sequences and Phylogeny of the Nucleocapsid Gene of Oropouche Virus. J. Gen. Virol. 2000, 81, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.A.D.; Santos, J.H.A.; Monteiro, D.C.D.S.; Pessoa, K.P.; Cardoso, A.J.L.; Souza, V.C.D.; Abdalla, L.F.; Naveca, F.G. Oropouche Virus Detection in Saliva and Urine. Mem. Inst. Oswaldo Cruz 2020, 115, e190338. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, K.S.; Moreira, H.M.; Roca, T.P.; Pimentel, E.P.; Queiroz, J.A.D.S.; Ribeiro, J.R.; Passos-Silva, A.M.; Araújo, A.; Maia, K.I.D.S.; Naveca, F.G.; et al. Reemergence of Oropouche in the Brazilian Amazon: A Phylodynamic and Phylogenetic Analysis. Travel Med. Infect. Dis. 2025, 67, 102877. [Google Scholar] [CrossRef]
| Type of Test | Target Gene | Type of Sample | Performance Parameter | References |
|---|---|---|---|---|
| RT-LAMP | S segment | OROV strain | LoD: 24.54 copies/reaction | [102] |
| RT-qPCR | S segment | synthetic sequences, viral strains and serum samples | LoD: 5.6 copies/µL (H75) and 10.8 copies/µL (BeH) | [99] |
| RT-nested PCR | M segment | OROV isolates | LoD: 5.9 copies/mL | [96] |
| RT-qPCR | M segment | OROV isolates | LoD: 0.59 copies/mL | [96] |
| Multiplex RT-qPCR | S segment | cell supernatants and non-human samples | LoD: 2–20 copies/reaction | [100] |
| RT-qPCR | S segment | serum samples | 100 RNA molecules | [99] |
| Nested PCR | S segment | serum samples | NA | [24] |
| ddPCR | S segment | whole blood, serum, urine | LoD: 1 copies/mL | [103] |
| Test Name | Manufacturer | Test Type | RUO/IVD | LoD | Type of Samples | Internal Control | References |
|---|---|---|---|---|---|---|---|
| Oropouche Virus Real-Time PCR Kit | Creative Diagnostics (Shirley, NY, USA) | Real-Time PCR | RUO | LoD: 2.5 copies/reaction | serum | NA | [105] |
| Oropouche Virus Nucleic Acid Detection Kit | Creative Biogene (Shirley, NY, USA) | Real-Time PCR | RUO | NA | serum, plasma | Yes | [106] |
| Oropouche-Mayaro Virus Real-Time PCR Kit | Vircell (Granada, Spain) | RT PCR Multiplex | RUO | NA | serum, plasma, whole blood | Yes | [107] |
| RealStar® Oropouche Fever RT-qPCR Kit 1.0 RUO | Altona Diagnostics (Milan, Italy) | Real-Time PCR | RUO | NA | serum, plasma, whole blood | Yes | [108] |
| Oropouche Virus Real-Time PCR Kit (RUO) | Bioperfectus (Taizhou City, China) | Real-Time PCR | RUO | 2.5 copies/reaction | serum | NA | [109] |
| Type of Test | Target Gene | Type of Sample | Performance Parameter | References |
|---|---|---|---|---|
| Sanger sequencing + seminested PCR | S, M and L segments | plasma, saliva and urine | NA | [18] |
| NGS by random amplification | S, M and L segments | OROV isolates | NA | [96] |
| NGS and Sanger sequencing | S, M and L segments | clinical isolates and non-human samples | NA | [11] |
| Sanger sequencing | S, M and L segments | OROV isolates | NA | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapa, D.; Romeo, M.A.; Spina, A.; Specchiarello, E.; Maggi, F. Oropouche Virus: An Overview of the Current Status of Diagnostics. Viruses 2025, 17, 1382. https://doi.org/10.3390/v17101382
Lapa D, Romeo MA, Spina A, Specchiarello E, Maggi F. Oropouche Virus: An Overview of the Current Status of Diagnostics. Viruses. 2025; 17(10):1382. https://doi.org/10.3390/v17101382
Chicago/Turabian StyleLapa, Daniele, Maria Anele Romeo, Alessandra Spina, Eliana Specchiarello, and Fabrizio Maggi. 2025. "Oropouche Virus: An Overview of the Current Status of Diagnostics" Viruses 17, no. 10: 1382. https://doi.org/10.3390/v17101382
APA StyleLapa, D., Romeo, M. A., Spina, A., Specchiarello, E., & Maggi, F. (2025). Oropouche Virus: An Overview of the Current Status of Diagnostics. Viruses, 17(10), 1382. https://doi.org/10.3390/v17101382






