Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Ethics Statement
2.3. Case Reports
2.4. Serological Testing
2.5. Real-Time (RT)-PCR (RT-qPCR)
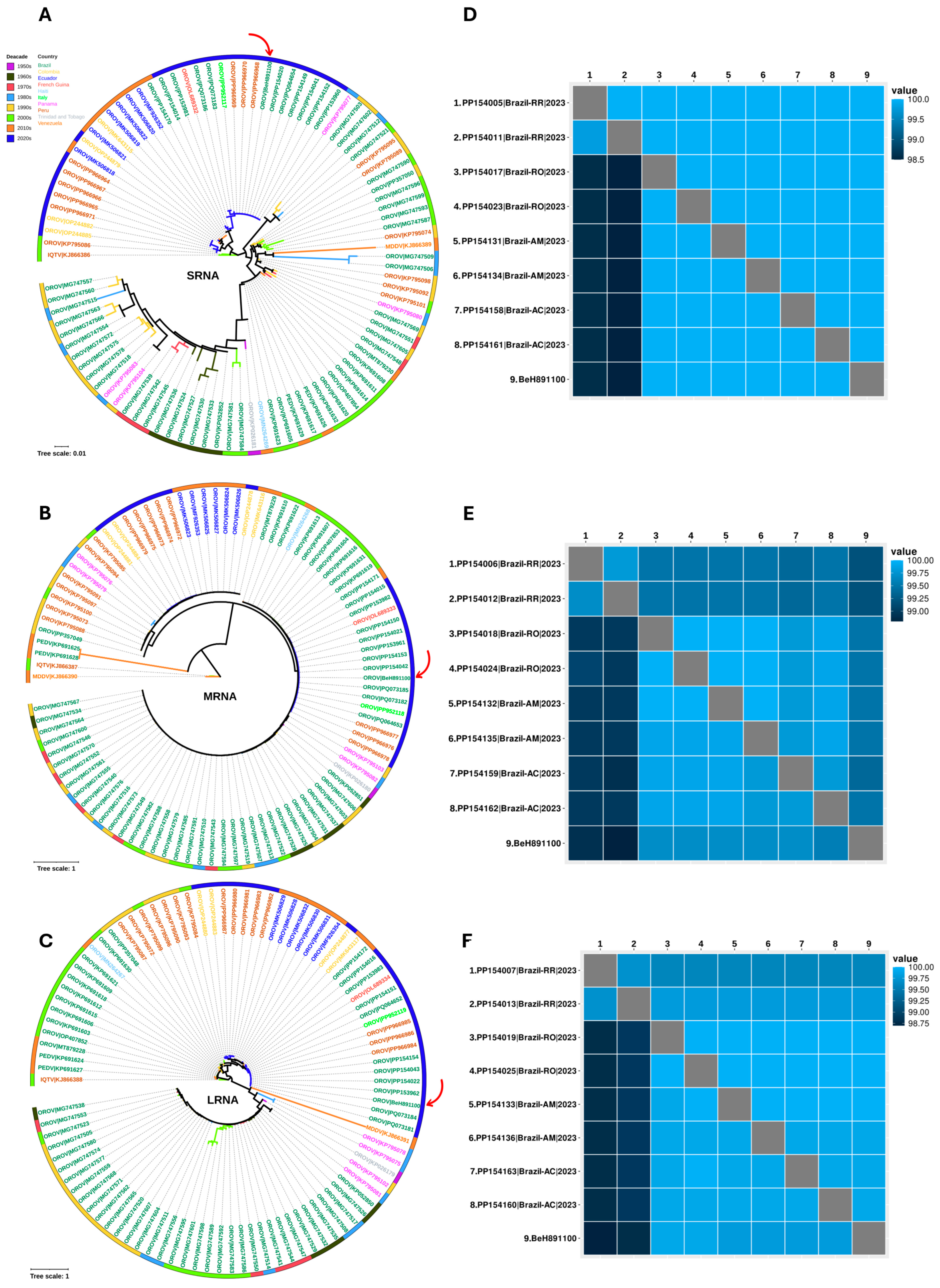
2.6. Next-Generation Sequencing
2.7. Bioinformatic Analysis
2.8. Necropsy, Histopathology, and Immunohistochemistry
3. Results
3.1. Investigation
3.2. Case Series
4. Discussion
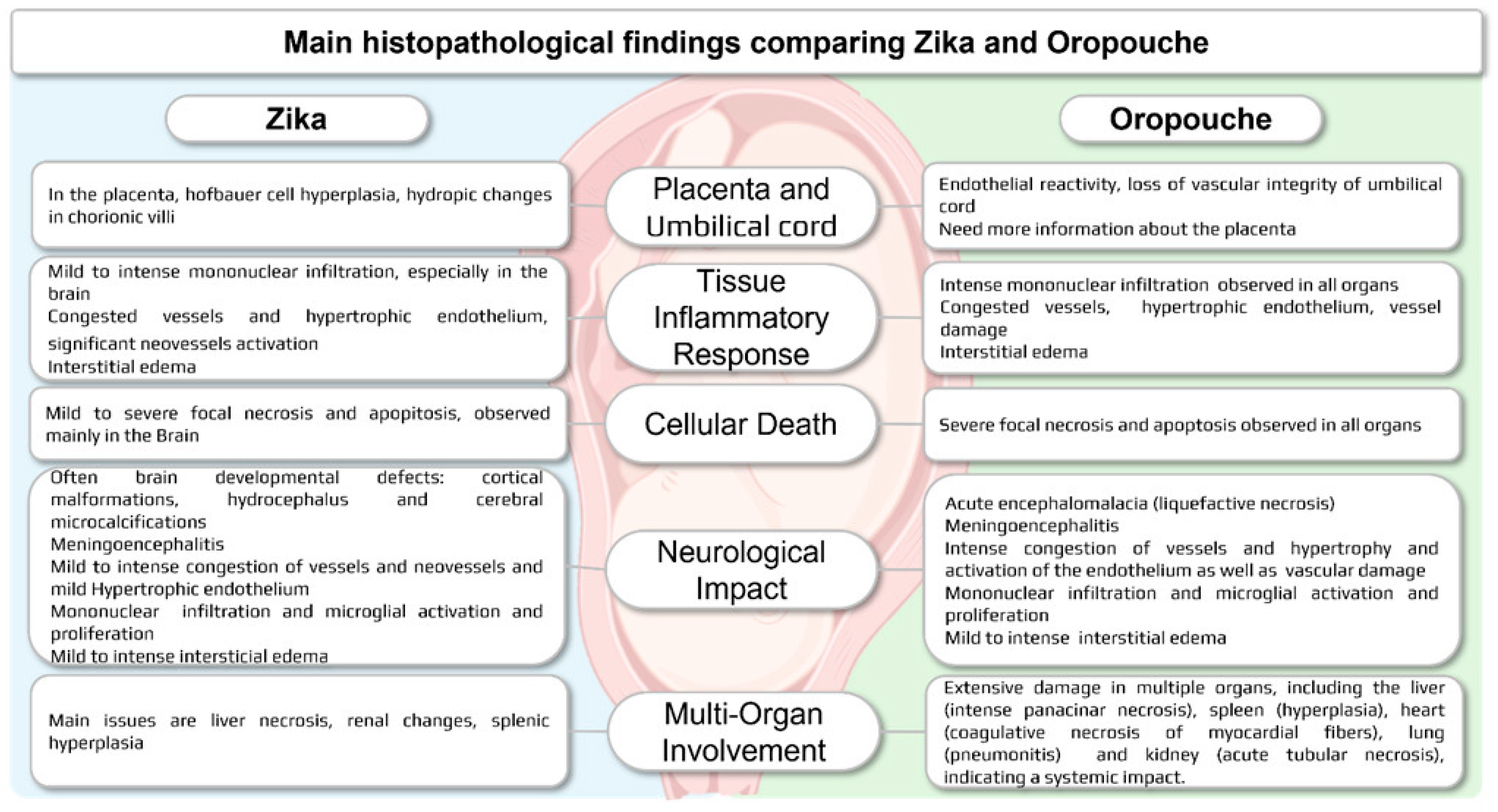
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, W.M.; Calisher, C.H.; Carrera, J.P.; Hughes, H.R.; Nunes, M.R.T.; Russell, B.; Tilson-Lunel, N.L.; Venter, M.; Xia, H. ICTV Virus Taxonomy Profile: Peribunyaviridae 2024. J. Gen. Virol. 2024, 105, 002034. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.O.; Azevedo, R.S.; Justino, M.C.A.; Matos, H.J.; Cabeça, H.L.S.; Silva, S.P.; Henriques, D.F.; Silva, E.V.; Andrade, G.S.; Vasconcelos, P.F.; et al. Neurological disease caused by Oropouche virus in northern Brazil: Should it be included in the scope of clinical neurological diseases? J. Neurovirol. 2021, 27, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.P.; Freitas, A.G.; Ohana, R.B.; Rosa, B.A.; Travassos da Rosa, A.P.A.; Juvenal, S.L. Meningitis associated with Oropouche virus infections. Rev. Inst. Med. Trop. São Paulo 1982, 24, 246–251. [Google Scholar]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.; Hoch, A.L.; Gomes, M.L.; Roberts, D.R. Oropouche virus. IV. Laboratory transmission by Culicoides paraensis. Am. J. Trop. Med. Hyg. 1981, 30, 172–176. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–678. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Pinheiro, M.; Bensabath, G.; Causey, O.R.; Shope, R.E. Epidemia de vírus Oropouche em Belém. Rev. Serv. Espec. Saude Publica 1962, 12, 15–23. [Google Scholar]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.; Zuchi, N.; Souza, V.C.; Naveca, F.G.; Santos, M.A.M.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and in Culex quinquefasciatus in the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Martins, F.E.N.; Chiang, J.O.; Nunes, B.T.D.; Ribeiro, B.F.R.; Martins, L.C.; Casseb, L.M.N.; Henriques, D.F.; Oliveira, C.S.; Maciel, E.L.N.; Azevedo, R.D.S.; et al. Newborns with microcephaly in Brazil and potential vertical transmission of Oropouche virus: A case series. Lancet Infect. Dis. 2025, 25, 155–165. [Google Scholar] [CrossRef]
- Vasconcelos, H.B.; Azevedo, R.S.; Casseb, S.M.; Nunes-Neto, J.P.; Chiang, J.O.; Cantuária, P.C.; Segura, M.N.; Martins, L.C.; Monteiro, H.A.; Rodrigues, S.G.; et al. Oropouche fever epidemic in Northern Brazil: Epidemiology and molecular characterization of isolates. J. Clin. Virol. 2009, 44, 129–133. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Souza, W.M.; Savji, N.; Figueiredo, M.L.; Cardoso, J.F.; Silva, S.P.; de Lima, C.P.D.S.; Vasconcelos, H.B.; Rodrigues, S.G.; Lipkin, W.I.; et al. Oropouche orthobunyavirus: Genetic characterization of full-length genomes and development of molecular methods to discriminate natural reassortments. Infect. Genet. Evol. 2019, 68, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Naveca, G.; Almeida, T.A.P.; Souza, V.; Nascimento, V.; Silva, D.; Nascimento, F.; Mejía, M.; Oliveira, Y.S.; Rocha, L.; Xavier, N.; et al. Emergence of a novel reassortant Oropouche virus drives persistent human outbreaks in the Brazilian Amazon region from 2022 to 2024. Nat. Med. 2024, 30, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Iani, F.C.M.; Pereira, F.M.; Oliveira, E.C.; Rodrigues, J.T.N.; Machado, M.H.; Fonseca, V.; Ribeiro Adelino, T.E.; Rocha Guimarães, N.; Ribeiro Tomé, L.M.; Astete Gómez, M.K. Rapid Viral Expansion Beyond the Amazon Basin: Increased Epidemic Activity of Oropouche Virus Across the Americas. medRxiv, 2024; Preprint. [Google Scholar]
- Brazilian Ministry of Health. Epimiological Panel for Oropouche. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/o/oropouche/painel-epidemiologico (accessed on 8 April 2025).
- Scachetti, G.C.; Forato, J.; Claro, I.M.; Hua, X.; Salgado, B.B.; Vieira, A.; Simeoni, C.L.; Barbosa, A.R.; Rosa, I.L.; de Souza, G.F.; et al. Reemergence of Oropouche Virus Between 2023 and 2024 in Brazil. medRxiv, 2024; Preprint. [Google Scholar] [CrossRef]
- WHO. Oropouche Virus Disease—Situation at a Glance. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON530 (accessed on 10 April 2025).
- Brazilian Ministry of Health. Saúde Confirma um Óbito Fetal por Oropouche em Pernambuco. 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/2024/agosto/saude-confirma-um-obito-fetal-por-oropouche-em-pernambuco (accessed on 10 April 2025).
- Pan American Health Organization (PAHO). Epidemiological Alert Oropouche in the Region of the Americas: Vertical Transmission Event Under Investigation in Brazil. 2024. Available online: https://www.paho.org/sites/default/files/2024-08/2024-aug-1-phe-epi-alert-oropoucheenfinal.pdf (accessed on 14 August 2024).
- Pan American Health Organization. Countries of the Americas Strengthen Preparedness for Oropouche Virus. 2024. Available online: https://www.paho.org/en/news/9-7-2024-countries-americas-strengthen-preparedness-oropouche-virus (accessed on 10 April 2025).
- Pan American Health Organization. Epidemiological Alert Oropouche in the Americas Region. 2024. Available online: https://www.paho.org/sites/default/files/2024-12/2024-dic-13-epialert-oropouche-eng-final.pdf (accessed on 8 April 2025).
- Beaty, B.J.; Calisher, C.H.; Shope, R.E. Arboviruses. In Diagnostic Procedures for Viral Rickettsial and Chlamydial Infections; Lennette, E.H., Lunette, D.A., Lenette, E.T., Eds.; American Public Health Association: Washington, DC, USA, 1995; pp. 189–212. Available online: https://scholar.google.com/scholar_lookup?title=Arboviruses&author=Beaty,+B.J.&author=Calisher,+C.H.&author=Shope,+R.E.&publication_year=1995&pages=189%E2%80%93212 (accessed on 8 April 2025).
- Henriques, D.F.; Nunes, J.A.; Anjos, M.V.; Melo, J.M.; Rosário, W.O.; Azevedo, R.S.; Chiang, J.O.; Martins, L.C.; dos Santos, F.B.; Casseb, L.M.; et al. Evaluation of immunoglobulin M-specific capture enzyme-linked immunosorbent assays and commercial tests for flaviviruses diagnosis by a National Reference Laboratory. J. Virol. Methods 2020, 286, 113976. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.A.D.; Souza, V.C.; Nunes, B.T.D.; Rodrigues, D.S.G.; Vasconcelos, P. Multiplexed reverse transcription real-time polymerase chain reaction for simultaneous detection of Mayaro, Oropouche, and Oropouche-like viruses. Memórias Inst. Oswaldo Cruz 2017, 112, 510–513. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and Clinical Performance of the CDC Real Time RT-PCR Assay for Detection and Typing of Dengue Virus. PLoS Neglected Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol. J. 2013, 10, 311. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2023, 2. [Google Scholar] [CrossRef]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Geneious Prime 2024. Auckland, New Zealand: Dotmatics. 2024. Available online: http://www.geneious.com/ (accessed on 11 November 2021).
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; von Haeseler, A. TREE-PUZZLE: Maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef]
- Myung, I.J. Tutorial on maximum likelihood estimation. J. Math. Psychol. 2003, 47, 90–100. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.4 [Software]. Edinburgh: Institute of Evolutionary Biology, University of Edinburgh. 2018. Available online: https://github.com/rambaut/figtree/releases/tag/v1.4.4 (accessed on 7 November 2024).
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Ribeiro, Y.P.; Falcão, L.F.M.; Smith, V.C.; de Sousa, J.R.; Pagliari, C.; Franco, E.C.S.; Cruz, A.C.R.; Chiang, J.O.; Martins, L.C.; Nunes, J.A.L.; et al. Comparative Analysis of Human Hepatic Lesions in Dengue, Yellow Fever, and Chikungunya: Revisiting Histopathological Changes in the Light of Modern Knowledge of Cell Pathology. Pathogens 2023, 12, 680. [Google Scholar] [CrossRef]
- Bollweg, B.C.; Silva-Flannery, L.; Spivey, P.; Hale, G.L. Optimization of commercially available Zika virus antibodies for use in a laboratory-developed immunohistochemical assay. J. Pathol. Clin. Res. 2017, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.H. Ancillary Testing in Brain Death. Semin. Neurol. 2015, 35, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Borborema, C.A.; Pinheiro, F.P.; Albuquerque, B.C.; Rosa, A.P.; Rosa, J.F.; Dourado, H.V. 1st occurrence of outbreaks caused by Oropouche virus in the State of Amazonas. Rev. Inst. Med. Trop. 1982, 24, 132–139. [Google Scholar] [PubMed]
- Schwartz, D.A.; Dashraath, P.; Baud, D. Oropouche Virus (OROV) in Pregnancy: An Emerging Cause of Placental and Fetal Infection Associated with Stillbirth and Microcephaly following Vertical Transmission. Viruses 2024, 16, 1435. [Google Scholar] [CrossRef]
- Samara, A.; Coutinho, C.M.; Veal, P.; Osborne, J.; Duarte, G.; Ladhani, S.; Khalil, A. Potential vertical transmission of Oropouche virus during the current outbreak. Lancet Infect. Dis. 2024, 24, e668–e669. [Google Scholar] [CrossRef]
- Filho, C.G.; Neto, A.S.L.; Maia, A.M.P.C.; Silva, L.O.R.; Cavalcante, R.D.C.; Monteiro, H.D.S.; Marques, K.C.A.; Oliveira, R.S.; Gadelha, S.A.C.; Melo, D.N.; et al. A Case of Vertical Transmission of Oropouche Virus in Brazil. N. Engl. J. Med. 2024, 391, 2055–2057. [Google Scholar] [CrossRef]
- Castilletti, C.; Huits, R.; Mantovani, R.P.; Accordini, S.; Alladio, F.; Gobbi, F. Replication-Competent Oropouche Virus in Semen of Traveler Returning to Italy from Cuba, 2024. Emerg. Infect. Dis. 2024, 30, 2684–2686. [Google Scholar] [CrossRef]
- Araújo, T.V.B.; Rodrigues, L.C.; Ximenes, R.A.A.; Miranda-Filho, D.B.; Montarroyos, U.R.; Melo, A.P.L.; Valongueiro, S.; Albuquerque, M.F.P.M.; Souza, W.V.; Braga, C.; et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 2016, 16, 1306–1313. [Google Scholar] [CrossRef]
- Halai, U.A.; Nielsen-Saines, K.; Moreira, M.L.; Sequeira, P.C.; Junior, J.P.P.; Zin, A.A.; Cherry, J.; Gabaglia, C.R.; Gaw, S.L.; Adachi, K.; et al. Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 877–883. [Google Scholar] [CrossRef]
- Teixeira, F.M.E.; Pietrobon, A.J.; Oliveira, L.M.; Oliveira, L.M.D.S.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11, 175. [Google Scholar] [CrossRef]
- Hcini, N.; Kugbe, Y.; Rafalimanana, Z.H.L.; Lambert, V.; Mathieu, M.; Carles, G.; Baud, D.; Panchaud, A.; Pomar, L. Association between confirmed congenital Zika infection at birth and outcomes up to 3 years of life. Nat. Commun. 2021, 12, 3270. [Google Scholar] [CrossRef] [PubMed]
- Marinho, P.S.; Cunha, A.J.; Amim Junior, J.; Prata-Barbosa, A. A review of selected Arboviruses during pregnancy. Matern. Health Neonatol. Perinatol. 2021, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.V.; Araújo, T.V.; Albuquerque, M.F.; Braga, M.C.; Ximenes, R.A.; Miranda-Filho, D.B.; Bezerra, L.C.; Dimech, G.S.; Carvalho, P.I.; Assunção, R.S.; et al. Microcephaly in Pernambuco State, Brazil: Epidemiological characteristics and evaluation of the diagnostic accuracy of cutoff points for reporting suspected cases. Rep. Public Health 2016, 32, e00017216. [Google Scholar]
- Engjom, H.M.; Ramakrishnan, R.; Vousden, N.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; et al. Perinatal outcomes after admission with COVID-19 in pregnancy: A UK national cohort study. Nat. Commun. 2024, 15, 3234. [Google Scholar] [CrossRef]
- Rosado, L.E.P.; Martelli, C.M.T.; Brickley, E.B.; Gomes, M.B.F.; Lima, T.T.; Costa, P.S.S.; Ávila, M.P.; Viggiano, M.B.; Amaral, W.N.; Feres, V.C.R.; et al. Risk of adverse pregnancy and infant outcomes associated with prenatal Zika virus infection: A post-epidemic cohort in Central-West Brazil. Sci. Rep. 2024, 13, 7335. [Google Scholar] [CrossRef]
- Ades, A.E.; Soriano-Arandes, A.; Alarcon, A.; Bonfante, F.; Thorne, C.; Peckham, C.S.; Giaquinto, C. Vertical transmission of Zika virus and its outcomes: A Bayesian synthesis of prospective studies. Lancet Infect. Dis. 2021, 21, 537–545. [Google Scholar] [CrossRef]
- Melo, A.S.; Aguiar, R.S.; Amorim, M.M.; Arruda, M.B.; Melo, F.O.; Ribeiro, S.T. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016, 73, 1407–1416. [Google Scholar] [CrossRef]
- Pérez-Padilla, J.; Paz-Bailey, G.; Meaney-Delman, D.; Doyle, K.; Gary, J.; Rodriguez, D.M.; Bhatnagar, J.; Pérez-Rodriguez, N.M.; Montalvo, S.; Alvarado, L.; et al. Persistent Zika Virus Infection Associated with Early Fetal Demise: A Case Report. Open J. Obstet. Gynecol. 2019, 9, 698–706. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Y.; Ma, L.; Li, X.; Schreiber, A. Maternal viral load and mother-to-child transmission of hepatitis B virus: A systematic review and meta-analysis. J. Viral Hepat. 2018, 25, 482–493. [Google Scholar]
- Garcia, P.M.; Kalish, L.A.; Pitt, J.; Minkoff, H.; Quinn, T.C.; Burchett, S.K.; Kornegay, J.; Jackson, B.; Moye, J.; Hanson, C.; et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 1999, 341, 394–402. [Google Scholar] [CrossRef]
- Lin, H.H.; Kao, J.H.; Hsu, H.Y.; Lee, C.N.; Tseng, Y.H.; Chen, D.S. Possible role of high-titer maternal viraemia in perinatal transmission of hepatitis C virus. J. Infect. Dis. 1994, 169, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Dickover, R.E.; Garratty, E.M.; Herman, S.A.; Sim, M.S.; Plaeger, S.; Boyer, P.J.; Keller, M.; Deveikis, A.; Stiehm, E.R.; Bryson, Y.J. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission. JAMA 1996, 275, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.S.; Araujo, M.T.; Oliveira, C.S.; Filho, A.J.M.; Nunes, B.T.D.; Henriques, D.F. Zika Virus Epidemic in Brazil. II. Post-Mortem Analyses of Neonates with Microcephaly, Stillbirths, and Miscarriage. J. Clin. Med. 2018, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Carbonnier, M.; Pasquet, M.; Bouhmani, B.; Ghazouani, J.; Noormahomed, J.; Beullier, G.; Attali, T.; Samperiz, S.; Fourmaintraux, A.; et al. Mother-to-Child Transmission of Chikungunya Virus Infection. Pediatr. Infect. Dis. J. 2007, 9, 811–815. [Google Scholar] [CrossRef]
- Martines, R.B.; Bhatnagar, J.; Keating, M.K.; Silva-Flannery, L.; Muehlenbachs, A.; Gary, J.; Goldsmith, C.; Hale, G.; Ritter, J.; Rollin, D.; et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 159–160. [Google Scholar] [CrossRef]
- Scachetti, G.C.; Forato, J.; Claro, I.M.; Hua, X.; Salgado, B.B.; Vieira, A.; Simeoni, C.L.; Barbosa, A.R.C.; Rosa, I.L.; de Souza, G.F.; et al. Re-emergence of Oropouche virus between 2023 and 2024 in Brazil: An observational epidemiological study. Lancet Infect. Dis. 2025, 25, 166–175. [Google Scholar] [CrossRef]
- Driggers, R.W.; Ho, C.Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef]
- Culjat, M.; Darling, S.E.; Nerurkar, V.R.; Ching, N.; Kumar, M.; Min, S.K.; Wong, R.; Grant, L.; Melish, M.E. Clinical and Imaging Findings in an Infant With Zika Embryopathy. Clin. Infect. Dis. 2016, 63, 805–811. [Google Scholar]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Rus, K.R.; Vipotnik, T.V.; Vodušek, V.F.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Santos, R.I.; Almeida, M.F.; Paula, F.E.; Rodrigues, A.H.; Saranzo, A.M.; Paula, A.E.; Silva, M.L.; Correa, V.M.; Acrani, G.O.; Neder, L.; et al. Experimental infection of suckling mice by subcutaneous inoculation with Oropouche virus. Virus Res. 2012, 170, 25–33. [Google Scholar] [CrossRef]
- Rodrigues, A.H.; Santos, R.I.; Arisi, G.M.; Bernardes, E.S.; Silva, M.L.; Rossi, M.A.; Lopes, M.B.; Arruda, E. Oropouche virus experimental infection in the golden hamster (Mesocrisetus auratus). Virus Res. 2011, 155, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Microcephaly Epidemic Research Group. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg. Infect. Dis. 2016, 22, 1090–1093. [Google Scholar] [CrossRef]
- Oliveira, W.K.; Cortez-Escalante, J.; Oliveira, W.T.; Carmo, G.M.; Henriques, C.M.; Coelho, G.E.; França, G.V.A. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
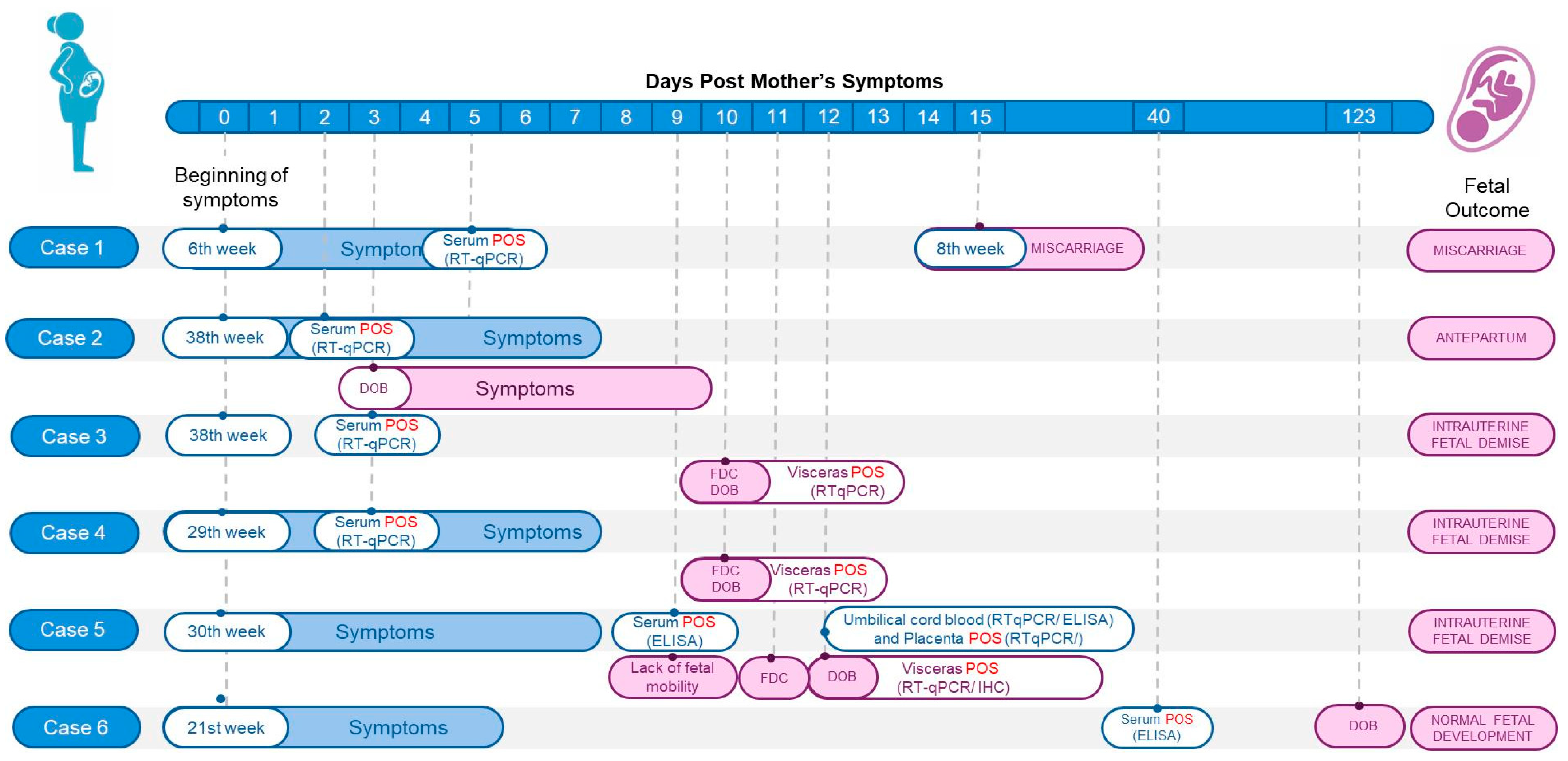
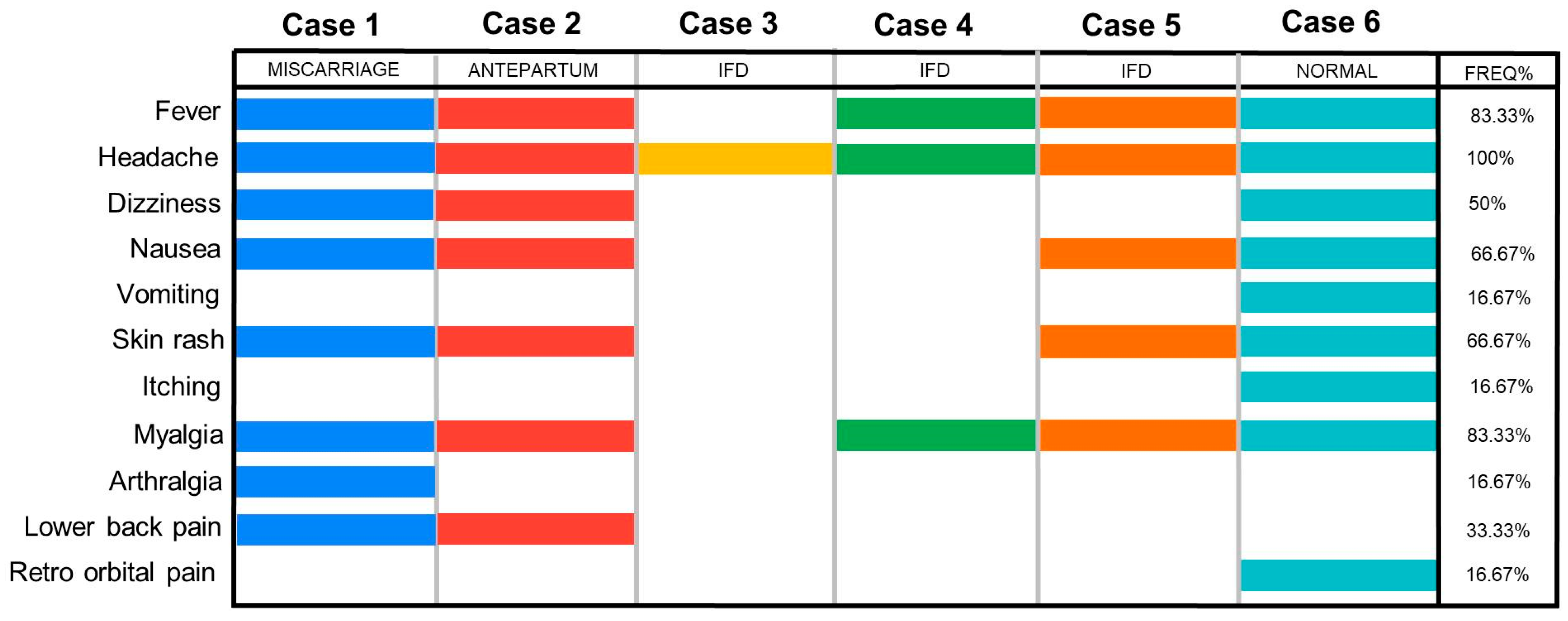

| Test | Samples | Case 1 | Case 2 | Case 2 Newborn | Case 3 | Case 3 Stillborn | Case 4 | Case 4 Stillborn | Case 5 | Case 5 Stillborn | Case 6 | Case 6 Newborn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miscarriage | Antepartum | Intrauterine Fetal Demise | Intrauterine Fetal Demise | Intrauterine Fetal Demise | Normal Fetal Development | |||||||
| RT-qPCR (Ct) | Serum 1 | 34.0 | 21.0 | - | Negative | - | 32.0 | - | Negative ¶ | - | - | - |
| Umbilical cord blood | - | - | - | - | - | - | 27.0 | - | - | - | - | |
| Umbilical cord | - | - | - | - | - | - | 37.0 | - | 25.5 | - | - | |
| Placenta | - | - | - | - | - | - | 24.0 | - | 32.5 | - | - | |
| Brain | - | - | - | - | 24.0 | - | 23.0 | - | 17.5 | - | - | |
| Liver | - | - | - | - | 32.0 | - | Negative | - | 12.5 | - | - | |
| Spleen | - | - | - | - | 30.0 | - | 24.0 | - | 12.9 | - | - | |
| Heart | - | - | - | - | 29.0 | - | 27.0 | - | 17.7 | - | - | |
| Lung | - | - | - | - | Negative | - | 28.0 | - | 18.8 | - | - | |
| Kidney | - | - | - | - | 29.0 | - | 26.0 | - | 20.5 | - | - | |
| ELISA (IgM) | Serum 2 | Positive | Positive | Positive | Positive | - | - | - | Positive (S1) Positive (S2) | - | Positive | - |
| Umbilical cord blood | - | - | - | - | - | - | - | - | Positive | - | - | |
| Date | Gestational Age | Mother | Fetus | Overview | ||||
|---|---|---|---|---|---|---|---|---|
| Health Condition | Weight | Blood Pressure (mmhg) | Fundal Height | Heartbeat | Fetal Movement | |||
| 26 June 2024 | 31 week, 3 days | Normal | 61.1 Kg | 100 × 70 | 32 cm | 137 bpm | Present | Normal fetal development |
| 5 August 2024 | 37 week, 5 days | Normal | 66.7 Kg | 110 × 70 | 37 cm | 155 bpm | Present | Normal fetal development |
| 9 August 2024 * | 38 week, 2 days | Mastitis, Weight loss | 63.8 Kg | 120 × 80 | 34 cm | 0 bpm | Absent | Confirmed fetal death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, D.B.d.A.; Quaresma, J.A.S.; Azevedo, R.d.S.d.S.; Cruz, A.C.R.; Silva, S.P.d.; Martins Filho, A.J.; Nunes, B.T.D.; Pinheiro, L.R.S.; Sousa, J.R.d.; Chiang, J.O.; et al. Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection. Viruses 2025, 17, 816. https://doi.org/10.3390/v17060816
Medeiros DBdA, Quaresma JAS, Azevedo RdSdS, Cruz ACR, Silva SPd, Martins Filho AJ, Nunes BTD, Pinheiro LRS, Sousa JRd, Chiang JO, et al. Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection. Viruses. 2025; 17(6):816. https://doi.org/10.3390/v17060816
Chicago/Turabian StyleMedeiros, Daniele Barbosa de Almeida, Juarez Antônio Simões Quaresma, Raimunda do Socorro da Silva Azevedo, Ana Cecilia Ribeiro Cruz, Sandro Patroca da Silva, Arnaldo Jorge Martins Filho, Bruno Tardelli Diniz Nunes, Lucas Rafael Santana Pinheiro, Jorge Rodrigues de Sousa, Jannifer Oliveira Chiang, and et al. 2025. "Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection" Viruses 17, no. 6: 816. https://doi.org/10.3390/v17060816
APA StyleMedeiros, D. B. d. A., Quaresma, J. A. S., Azevedo, R. d. S. d. S., Cruz, A. C. R., Silva, S. P. d., Martins Filho, A. J., Nunes, B. T. D., Pinheiro, L. R. S., Sousa, J. R. d., Chiang, J. O., Martins, L. C., Oliveira, C. S., Prazeres, I. T. E., Henriques, D. F., Oliveira, C. F., Carvalho, V. L., Morais, C. N. L., Acioli-Santos, B., Silva, K. M. P., ... Casseb, L. M. N. (2025). Case Series of Adverse Pregnancy Outcomes Associated with Oropouche Virus Infection. Viruses, 17(6), 816. https://doi.org/10.3390/v17060816












