Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cells
2.2. Chickens
2.3. Animal Experiments for Pathogenicity Study
2.4. Vaccination and Virus Challenge Experiments
2.5. Determination of Body and Immune Organ Weights
2.6. Statistical Analysis
3. Results
3.1. Growth Rates and Immunosuppression of Birds Infected with Seven MDV Isolates
3.2. Variable Pathogenicities and Oncogenicities of Seven MDV Strains
3.3. Growth Rates and Lymphoid Organ Weights of Distinct MD-Vaccinated Birds Challenged by the SDCW01 Strain
3.4. Comparison of the Immune Protection Efficacy of Distinct MD Vaccines against the SDCW01 Strain
3.5. Survival Curves of MD-Vaccinated Birds Challenged by the SDCW01 Strain
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, V.; Gimeno, I.; Dunn, J. Marek’s Disease. In Disease of Poultry; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 550–587. [Google Scholar]
- Kennedy, D.A.; Cairns, C.; Jones, M.J.; Bell, A.S.; Salathe, R.M.; Baigent, S.J.; Nair, V.K.; Dunn, P.A.; Read, A.F. Industry-wide surveillance of Marek’s disease virus on commercial poultry farms. Avian Dis. 2017, 61, 153–164. [Google Scholar] [CrossRef]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benko, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 1673. [Google Scholar] [CrossRef]
- Nair, V. Latency and Tumorigenesis in Marek’s Disease. Avian Dis. 2013, 57, 360–365. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Conradie, A.M.; You, Y.; Kaufer, B.B. Latest insights into Marek’s disease virus pathogenesis and tumorigenesis. Cancers 2020, 12, 647. [Google Scholar] [CrossRef]
- Teng, M.; Zhu, Z.J.; Yao, Y.; Nair, V.; Zhang, G.P.; Luo, J. Critical roles of non-coding RNAs in lifecycle and biology of Marek’s disease herpesvirus. Sci. China Life Sci. 2023, 66, 251–268. [Google Scholar] [CrossRef]
- Witter, R.L. Increased virulence of Marek’s disease virus field isolates. Avian Dis. 1997, 41, 149–163. [Google Scholar] [CrossRef]
- Witter, R.L.; Calnek, B.W.; Buscaglia, C.; Gimeno, I.M.; Schat, K.A. Classification of Marek’s disease viruses according to pathotype: Philosophy and methodology. Avian Pathol. J. WVPA 2005, 34, 75–90. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, Z.J.; Bao, K.Y.; Lv, H.C.; Gao, Y.L.; Gao, H.L.; Qi, X.L.; Cui, H.Y.; Wang, Y.Q.; Ren, X.G.; et al. Pathogenic characteristics of Marek’s disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Vet. Microbiol. 2015, 177, 62–68. [Google Scholar] [CrossRef]
- Teng, M.; Zheng, L.P.; Li, H.Z.; Ma, S.M.; Zhu, Z.J.; Chai, S.J.; Yao, Y.; Nair, V.; Zhang, G.P.; Luo, J. Pathogenicity and pathotype analysis of Henan isolates of Marek’s disease virus reveal long-term circulation of highly virulent MDV variant in China. Viruses 2022, 14, 1651. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Liu, C.J.; Zhang, F.; Shi, W.; Li, J. Sequence analysis of the Meq gene in the predominant Marek’s disease virus strains isolated in China during 2006–2008. Virus Genes 2011, 43, 353–357. [Google Scholar] [CrossRef]
- Yu, Z.H.; Teng, M.; Luo, J.; Wang, X.W.; Ding, K.; Yu, L.L.; Su, J.W.; Chi, J.Q.; Zhao, P.; Hu, B.; et al. Molecular characteristics and evolutionary analysis of field Marek’s disease virus prevalent in vaccinated chicken flocks in recent years in China. Virus Genes 2013, 47, 282–291. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Lv, H.-C.; Bao, K.-Y.; Gao, Y.-L.; Gao, H.-L.; Qi, X.-L.; Cui, H.-Y.; Wang, Y.-Q.; Li, K.; Gao, L.; et al. Molecular and pathogenicity characterization of Gallid herpesvirus 2 newly isolated in China from 2009 to 2013. Virus Genes 2016, 52, 51–60. [Google Scholar] [CrossRef]
- He, L.; Li, J.; Zhang, Y.; Luo, J.; Cao, Y.; Xue, C. Phylogenetic and molecular epidemiological studies reveal evidence of recombination among Marek’s disease viruses. Virology 2018, 516, 202–209. [Google Scholar] [CrossRef]
- Okazaki, W.; Purchase, H.G.; Burmester, B.R. Protection against Marek’s disease by vaccination with a herpesvirus of Turkeys. Avian Dis. 1970, 14, 413–429. [Google Scholar] [CrossRef]
- Schat, K.A.; Calnek, B.W. Protection against Marek’s disease-derived tumor transplants by the nononcogenic SB-1 strain of Marek’s disease virus. Infect. Immun. 1978, 22, 225–232. [Google Scholar] [CrossRef]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, J.L.; Schat, K.A. Control of Marek’s disease in the Netherlands. I. Isolation of an avirulent Marek’s disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Diseases. 1972, 16, 108–125. [Google Scholar] [CrossRef]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, J.L.; Schat, K.A. Control of Marek’s disease in the Netherlands. II. Field trials on vaccination with an avirulent strain (CVI 988) of Marek’s disease virus. Avian Dis. 1972, 16, 126–138. [Google Scholar] [CrossRef]
- Tong, K.Z.; Lin, Y.H.; Xu, Y.W.; Fu, D.Z.; Li, C.F.; Liu, C.X.; Pu, Z.Z.; Dai, K.Z. Study on the immunization of chicken against Marek’s disease: Report on a naturally avirulent vaccine strain of Marek’s disease herpesvirus. Acta Vet. Zootech. Sin. 1984, 15, 107–114. [Google Scholar]
- Witter, R.L. Protective efficacy of Marek’s disease vaccines. Curr. Top Microbiol. Immunol. 2001, 255, 57–90. [Google Scholar]
- Nair, V. Successful control of Marek’s disease by vaccination. Dev. Biol. 2004, 119, 147–154. [Google Scholar]
- Dunn, J.R.; Gimeno, I.M. Current status of Marek’s disease in the United States and worldwide based on a questionnaire survey. Avian Dis. 2013, 57 (Suppl. S2), 483–490. [Google Scholar] [CrossRef]
- Read, A.F.; Baigent, S.J.; Powers, C.; Kgosana, L.B.; Blackwell, L.; Smith, L.P.; Kennedy, D.A.; Walkden-Brown, S.W.; Nair, V.K. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 2015, 13, 2198–2215. [Google Scholar] [CrossRef]
- Song, B.; Zeb, J.; Hussain, S.; Aziz, M.U.; Circella, E.; Casalino, G.; Camarda, A.; Yang, G.; Buchon, N.; Sparagano, O. A review on the Marek’s disease outbreak and its virulence-related meq genovariation in Asia between 2011 and 2021. Animals 2022, 12, 540. [Google Scholar] [CrossRef]
- Zhuang, X.; Zou, H.; Shi, H.; Shao, H.; Ye, J.; Miao, J.; Wu, G.; Qin, A. Outbreak of Marek’s disease in a vaccinated broiler breeding flock during its peak egg-laying period in China. BMC Vet. Res. 2015, 11, 157–162. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, Y.; Sun, G.; Bao, K.; Gao, Y.; Qi, X.; Cui, H.; Wang, Y.; Li, K.; Gao, L.; et al. Genetic evolution of Gallid herpesvirus 2 isolated in China. Infect. Genet. Evol. 2017, 51, 263–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, X.; Wang, Y.; Lin, Y.; Yu, Z.; Guo, R.; Li, K.; Cui, H.; Qi, X.; Wang, Y.; et al. Emerging natural recombinant Marek’s disease virus between vaccine and virulence strains and their pathogenicity. Transbound. Emerg. Dis. 2022, 69, e1702–e1709. [Google Scholar] [CrossRef]
- Zheng, L.P.; Teng, M.; Li, G.X.; Zhang, W.K.; Wang, W.D.; Liu, J.L.; Li, L.Y.; Yao, Y.; Nair, V.; Luo, J. Current epidemiology and co-infections of avian immunosuppressive and neoplastic diseases in chicken flocks in central China. Viruses 2022, 14, 2599. [Google Scholar] [CrossRef]
- Luo, J.; Sun, A.J.; Teng, M.; Zhou, H.; Cui, Z.Z.; Qu, L.H.; Zhang, G.P. Expression profiles of microRNAs encoded by the oncogenic Marek’s disease virus reveal two distinct expression patterns in vivo during different phases of disease. J. Gen. Virol. 2011, 92, 608–620. [Google Scholar] [CrossRef]
- Teng, M.; Zhou, Z.Y.; Yao, Y.; Nair, V.; Zhang, G.P.; Luo, J. A new strategy for efficient screening and identification of monoclonal antibodies against oncogenic avian herpesvirus utilizing CRISPR/Cas9-based gene-editing technology. Viruses 2022, 14, 2045. [Google Scholar] [CrossRef]
- Teng, M.; Liu, J.-L.; Luo, Q.; Zheng, L.-P.; Yao, Y.; Nair, V.; Zhang, G.-P.; Luo, J. Efficient cross-screening and characterization of monoclonal antibodies against Marek’s disease specific Meq oncoprotein using CRISPR/Cas9-gene-edited viruses. Viruses 2023, 15, 817. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, L.; Wang, J.; Chen, L.; Shan, H.; Wang, Z.; Ma, H. Isolation and analysis of a very virulent Marek’s disease virus strain in China. Virol. J. 2013, 10, 155–162. [Google Scholar] [CrossRef]
- Cui, N.; Su, S.; Sun, P.; Zhang, Y.; Han, N.; Cui, Z. Isolation and pathogenic analysis of virulent Marek’s disease virus field strain in China. Poult. Sci. 2016, 95, 1521–1528. [Google Scholar] [CrossRef]
- Sun, G.R.; Zhang, Y.P.; Lv, H.C.; Zhou, L.Y.; Cui, H.Y.; Gao, Y.L.; Qi, X.L.; Wang, Y.Q.; Li, K.; Gao, L.; et al. A Chinese variant Marek’s disease virus strain with divergence between virulence and vaccine resistance. Viruses 2017, 9, 71. [Google Scholar] [CrossRef]
- Shi, M.Y.; Li, M.; Wang, W.W.; Deng, Q.M.; Li, Q.H.; Gao, Y.L.; Wang, P.K.; Huang, T.; Wei, P. The emergence of a vv + MDV can break through the protections provided by the current vaccines. Viruses 2020, 12, 1048. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, M.; Li, Q.; Wang, P.; Li, M.; Wang, W.; Gao, Y.; Li, H.; Lin, L.; Huang, T.; et al. Analysis of the evolution and transmission dynamics of the field MDV in China during the years 1995–2020, indicating the emergence of a unique cluster with the molecular characteristics of vv+MDV that has become endemic in southern China. Transbound. Emerg. Dis. 2021, 68, 3574–3587. [Google Scholar] [CrossRef]
- Conradie, A.M.; Bertzbach, L.D.; Trimpert, J.; Patria, J.N.; Murata, S.; Parcells, M.S.; Kaufer, B.B. Distinct polymorphisms in a single herpesvirus gene are capable of enhancing virulence and mediating vaccinal resistance. PLoS Pathog. 2020, 16, e1009104. [Google Scholar] [CrossRef]
- Lee, L.F.; Kreager, K.; Heidari, M.; Zhang, H.; Lupiani, B.; Reddy, S.M.; Fadly, A. Properties of a meq-deleted rmd5 Marek’s disease vaccine: Protection against virulent MDV challenge and induction of lymphoid organ atrophy are simultaneously attenuated by serial passage in vitro. Avian Dis. 2013, 57, 491–497. [Google Scholar] [CrossRef]
- Li, Y.; Sun, A.; Su, S.; Zhao, P.; Cui, Z.; Zhu, H. Deletion of the Meq gene significantly decreases immunosuppression in chickens caused by pathogenic Marek’s disease virus. Virol. J. 2011, 8, 2–9. [Google Scholar] [CrossRef]
- Su, S.; Cui, N.; Zhou, Y.; Chen, Z.; Li, Y.; Ding, J.; Wang, Y.; Duan, L.; Cui, Z. A recombinant field strain of Marek’s disease (MD) virus with reticuloendotheliosis virus long terminal repeat insert lacking the meq gene as a vaccine against MD. Vaccine 2015, 33, 596–603. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Yan, F.; Liu, A.; Cheng, Y.; Li, Z.; Sun, G.; Lv, H.; Wang, X. Recombinant Gallid herpesvirus 2 with interrupted meq genes confers safe and efficacious protection against virulent field strains. Vaccine 2017, 35, 4695–4701. [Google Scholar] [CrossRef]
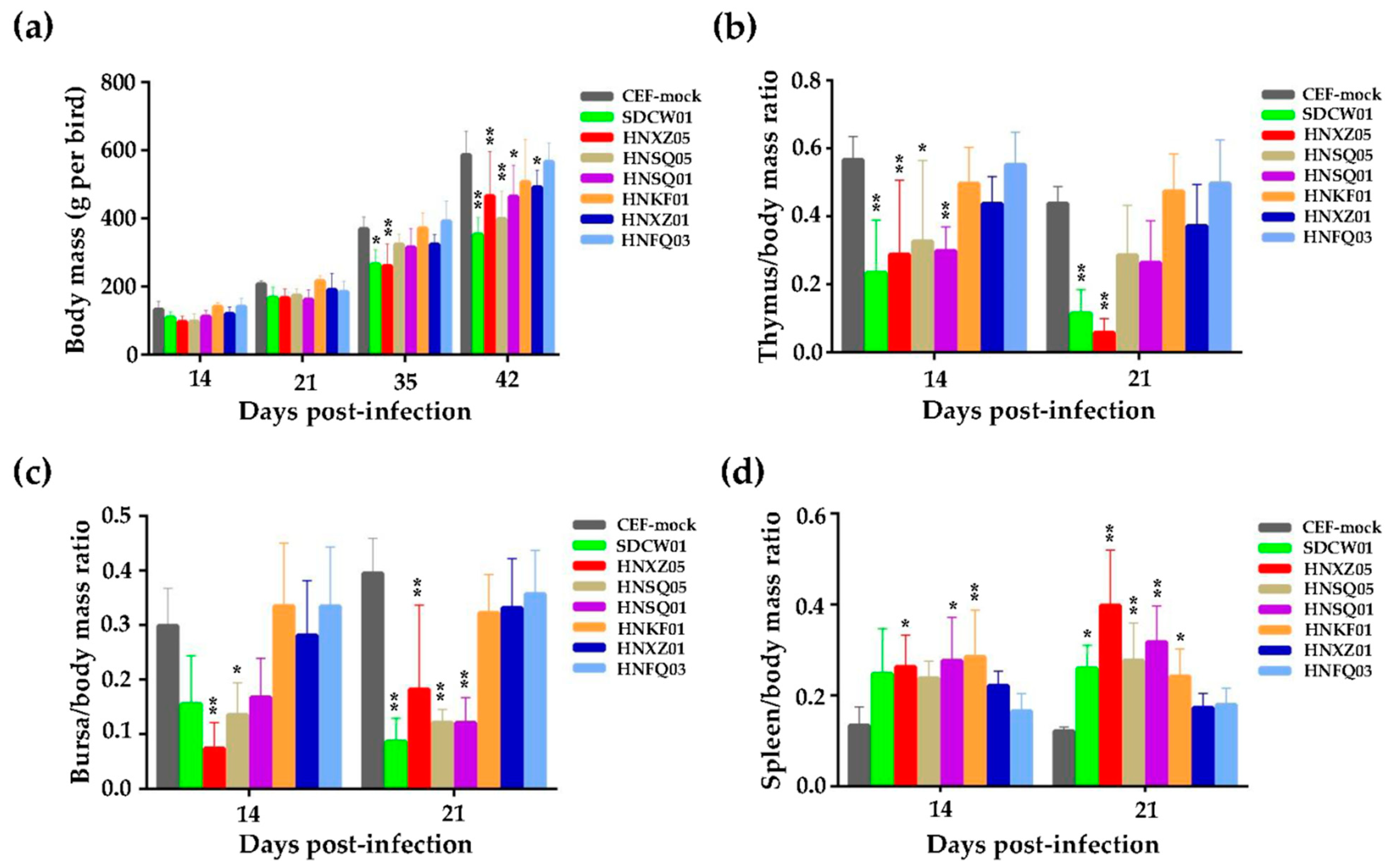
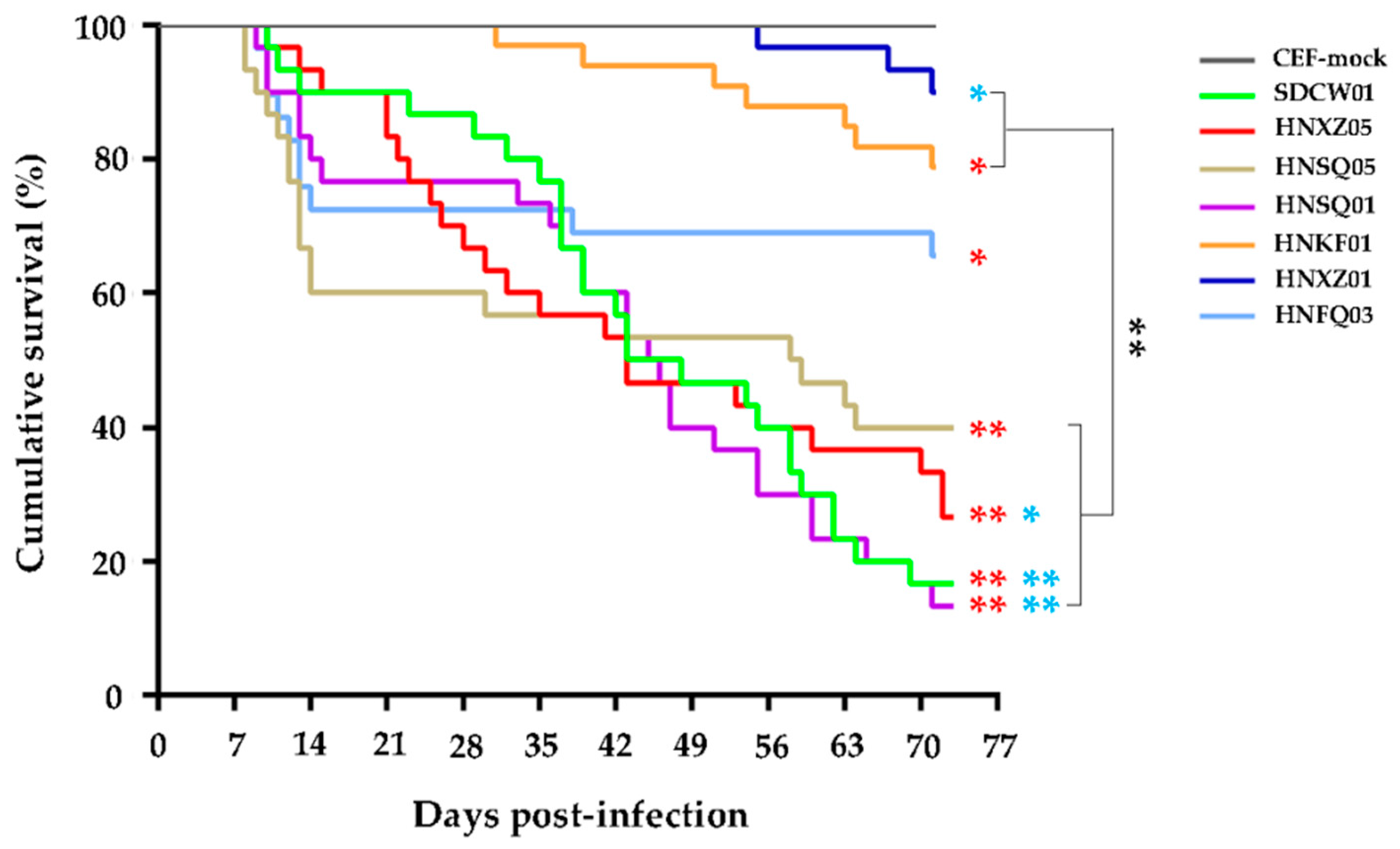

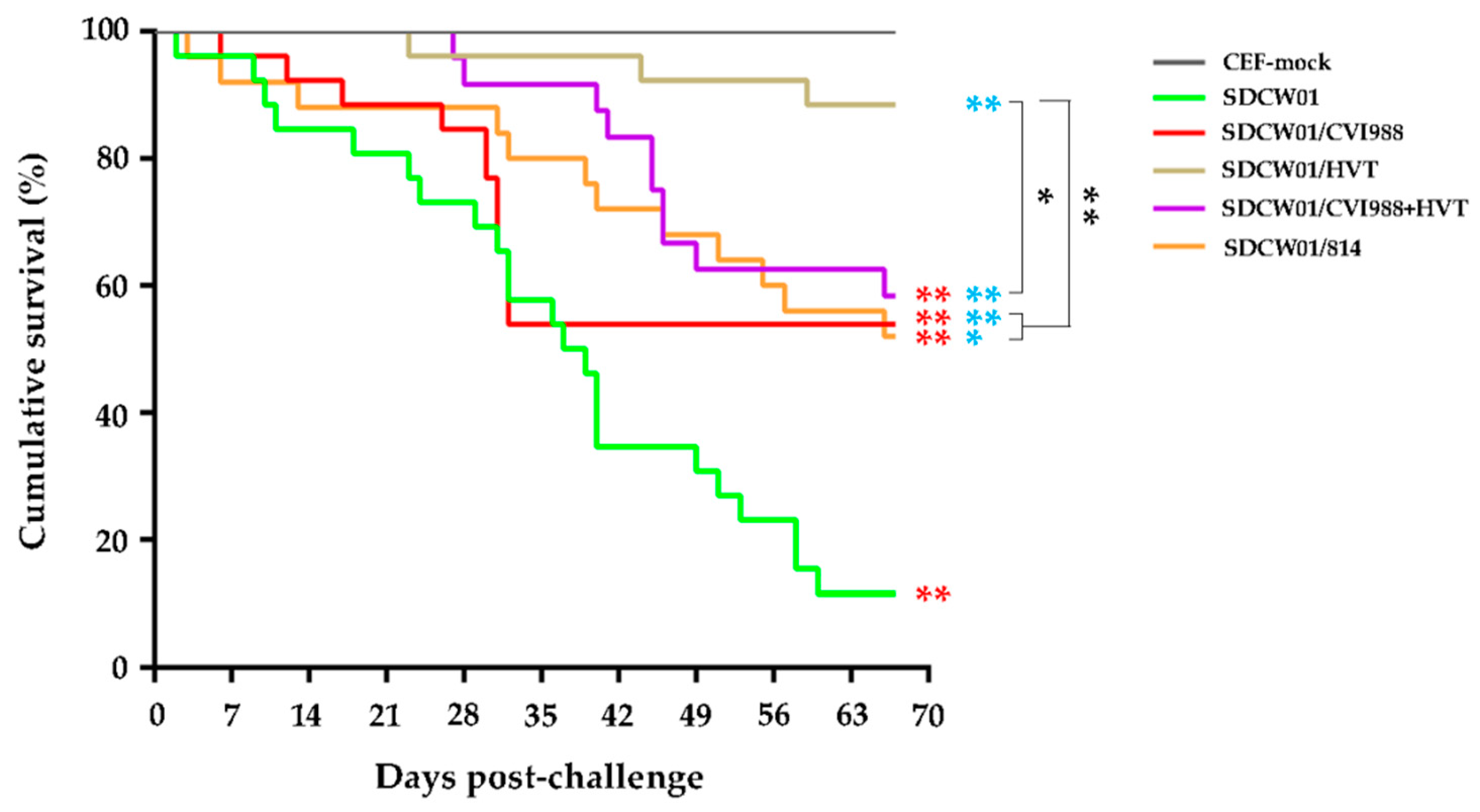
| No. | Strain Abbreviation | Original Signature Used for MDV Isolates a | Year and Month | Source b | Host | Ages for Virus Isolation (Days) | Passages on CEF |
|---|---|---|---|---|---|---|---|
| 1 | SDCW01 | SD-CW-01-C3 | 2021, May | SDCW | Hy-Line Brown | 70 | 6 |
| 2 | HNXZ05 | HN-XZ-XZZ-05-C1 | 2021, May | HNXZ | Partridge chicken | 80 | 5 |
| 3 | HNSQ05 | HN-SQ-YC-ZL-05-C2 | 2021, May | HNYC | Jinghong | 65 | 6 |
| 4 | HNSQ01 | HN-SQ-ZC-DW-01 | 2021, May | HNZC | Jinghong | 70 | 6 |
| 5 | HNKF01 | HN-KF-WS-01-C2 | 2021, September | HNWS | Muyuan Red | 70 | 5 |
| 6 | HNXZ01 | HN-XZ-XZZ-01-C4 | 2021, May | HNXZ | Partridge chicken | 80 | 5 |
| 7 | HNFQ03 | HN-FQ-YJ-03-C1 | 2021, September | HNFQ | Hy-Line Brown | 70 | 5 |
| No. | Strains | Total Numbers A | Diseased Birds | Morbidity (%) B | Deaths | Mortality (%) C | Gross Tumors | Tumor Incidence (%) D |
|---|---|---|---|---|---|---|---|---|
| 1 | SDCW01 | 30 | 30 | 100.0 a b c d | 25 | 83.3 a b c d | 15 | 50.0 a b c |
| 2 | HNSQ01 | 30 | 30 | 100.0 a b c d | 26 | 86.7 a b c d e | 19 | 63.3 a b c d e f |
| 3 | HNXZ05 | 30 | 28 | 93.3 a b c d | 22 | 73.3 a b c d | 10 | 33.3 a b c |
| 4 | HNSQ05 | 30 | 27 | 90.0 a b c d | 18 | 60.0 a b d | 9 | 30.0 a b c |
| 5 | HNKF01 | 33 | 15 | 45.5 a b | 7 | 21.2 a | 8 | 24.2 a b c |
| 6 | HNFQ03 | 29 | 10 | 34.5 a | 10 | 34.5 a | 0 | 0 |
| 7 | HNXZ01 | 30 | 3 | 10.0 | 3 | 10.0 | 0 | 0 |
| 8 | CEF mock | 26 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vaccines * | Strains | Total Numbers | Deaths | Mortality (%) | Diseased Birds # | Morbidity (%) | PI (%) |
|---|---|---|---|---|---|---|---|
| CEF-mock | CEF-mock | 22 | 0 | 0 | 0 | 0 | / |
| CEF-mock | SDCW01 | 26 | 23 | 88.5 a | 26 | 100.0 a | / |
| CVI988 | SDCW01 | 26 | 12 | 46.2 a b | 14 | 53.9 a b | 46.2 |
| HVT | SDCW01 | 26 | 3 | 11.5 b c | 16 | 61.5 a b | 38.5 |
| CVI988+HVT | SDCW01 | 24 | 10 | 41.7 a b d | 12 | 50.0 a b | 50.0 |
| 814 | SDCW01 | 25 | 12 | 48.0 a b d | 18 | 72.0 a b | 28.0 |
| Vaccines * | MDV Strains * | Total Birds | Deaths | Survivals | Total | |||
|---|---|---|---|---|---|---|---|---|
| Tumors /Deaths | Tumor Incidence (%) | Tumors /Survivals | Tumor Incidence (%) | Tumors | Tumor Incidence (%) | |||
| CEF-mock | CEF-mock | 22 | 0/0 | 0 | 0/22 | 0 | 0/22 | 0 |
| CEF-mock | SDCW01 | 26 | 11/23 | 47.8 | 3/3 | 100.0 a | 14/26 | 53.9 a |
| CVI988 | SDCW01 | 26 | 0/12 | 0 b | 2/14 | 14.3 b | 2/26 | 7.7 b |
| HVT | SDCW01 | 26 | 0/3 | 0 | 3/23 | 13.0 b | 3/26 | 11.5 b |
| CVI988+HVT | SDCW01 | 24 | 0/10 | 0 b | 0/14 | 0 b | 0/24 | 0 b |
| 814 | SDCW01 | 25 | 0/12 | 0 b | 0/13 | 0 b | 0/25 | 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-L.; Teng, M.; Zheng, L.-P.; Zhu, F.-X.; Ma, S.-X.; Li, L.-Y.; Zhang, Z.-H.; Chai, S.-J.; Yao, Y.; Luo, J. Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines. Viruses 2023, 15, 1434. https://doi.org/10.3390/v15071434
Liu J-L, Teng M, Zheng L-P, Zhu F-X, Ma S-X, Li L-Y, Zhang Z-H, Chai S-J, Yao Y, Luo J. Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines. Viruses. 2023; 15(7):1434. https://doi.org/10.3390/v15071434
Chicago/Turabian StyleLiu, Jin-Ling, Man Teng, Lu-Ping Zheng, Feng-Xia Zhu, Shu-Xue Ma, Lin-Yan Li, Zhi-Hui Zhang, Shu-Jun Chai, Yongxiu Yao, and Jun Luo. 2023. "Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines" Viruses 15, no. 7: 1434. https://doi.org/10.3390/v15071434
APA StyleLiu, J.-L., Teng, M., Zheng, L.-P., Zhu, F.-X., Ma, S.-X., Li, L.-Y., Zhang, Z.-H., Chai, S.-J., Yao, Y., & Luo, J. (2023). Emerging Hypervirulent Marek’s Disease Virus Variants Significantly Overcome Protection Conferred by Commercial Vaccines. Viruses, 15(7), 1434. https://doi.org/10.3390/v15071434







