Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Rotavirus Detection and Electropherotyping
2.3. Viral RNA Extraction and G/P Genotyping
2.4. Nucleotide Sequencing of the G8 RVA Segments
2.5. Phylogenetic Analyzes
2.6. Antigenic and Structural Analysis of VP7 Gene Segment
2.7. Ethical Approval
3. Results
3.1. RVA Detection and Genotyping
3.2. Bovine-like G8P[8] Strains with the DS-1-like Backbone
3.3. G8P[4] DS-1-like Strains
3.4. G8P[6] DS-1-like Strains
3.5. Modeling of the VP7 Gene
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benedicto-Matambo, P.; Bines, J.E.; Malamba-Banda, C.; Shawa, I.T.; Barnes, K.; Kamng’ona, A.W.; Hungerford, D.; Jambo, K.C.; Iturriza-Gomara, M.; Cunliffe, N.A.; et al. Leveraging Beneficial Off-Target Effects of Live-Attenuated Rotavirus Vaccines. Vaccines 2022, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo-Calvo, R.; Muñoz, C.; Buesa, J.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R. The Rotavirus Vaccine Landscape, an Update. Pathogens 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Costa, F.A.; de Assis, R.M.S.; Fialho, A.M.; Araújo, I.T.; Silva, M.F.; Gómez, M.M.; Andrade, J.S.; Rose, T.L.; Fumian, T.M.; Volotão, E.M.; et al. The evolving epidemiology of rotavirus A infection in Brazil a decade after the introduction of universal vaccination with Rotarix®. BMC Pediatr. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Laird, A.R.; Bielfelt, B.; Griffin, D.D.; Banyai, K.; Ramachandran, M.; Jain, V.; Cunliffe, N.A.; Nakagomi, O.; Kirkwood, C.D.; et al. Serotype diversity and reassortment between human and animal rotavirus strains: Implications for rotavirus vaccine programs. J. Infect. Dis. 2005, 192, 146–159. [Google Scholar] [CrossRef]
- Luchs, A.; Timenetsky, M.C. Group A rotavirus gastroenteritis: Post-vaccine era, genotypes and zoonotic transmission. Einstein 2016, 14, 278–287. [Google Scholar] [CrossRef]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Ide, T.; Haga, K.; Katayama, K.; Kato, T.; Ouchi, Y.; Kurahashi, H.; Tsuji, T.; et al. Whole Genomic Analysis of an Unusual Human G6P[14] Rotavirus Strain Isolated from a Child with Diarrhea in Thailand: Evidence for Bovine-To-Human Interspecies Transmission and Reassortment Events. PLoS ONE 2015, 10, e0139381. [Google Scholar] [CrossRef]
- Komoto, S.; Tacharoenmuang, R.; Guntapong, R.; Ide, T.; Sinchai, P.; Upachai, S.; Fukuda, S.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; et al. Identification and characterization of a human G9P[23] rotavirus strain from a child with diarrhoea in Thailand: Evidence for porcine-to-human interspecies transmission. J. Gen. Virol. 2017, 98, 532–538. [Google Scholar] [CrossRef]
- Mladenova, Z.; Papp, H.; Lengyel, G.; Kisfali, P.; Steyer, A.; Steyer, A.F.; Esona, M.D.; Iturriza-Gómara, M.; Bányai, K. Detection of rare reassortant G5P[6] rotavirus, Bulgaria. Infect. Genet. Evol. 2012, 12, 1676–1684. [Google Scholar] [CrossRef]
- Fukuda, S.; Tacharoenmuang, R.; Guntapong, R.; Upachai, S.; Singchai, P.; Ide, T.; Hatazawa, R.; Sutthiwarakom, K.; Kongjorn, S.; Onvimala, N.; et al. Full genome characterization of novel DS-1-like G9P[8] rotavirus strains that have emerged in Thailand. PLoS ONE 2020, 15, e0231099. [Google Scholar] [CrossRef] [PubMed]
- Jere, K.C.; Chaguza, C.; Bar-Zeev, N.; Lowe, J.; Peno, C.; Kumwenda, B.; Nakagomi, O.; Tate, J.E.; Parashar, U.D.; Heyderman, R.S.; et al. Emergence of Double- and Triple-Gene Reassortant G1P[8] Rotaviruses Possessing a DS-1-Like Backbone after Rotavirus Vaccine Introduction in Malawi. J. Virol. 2018, 92, e01246. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.C.C.; Boen, L.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.C. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.C.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.C. First Detection of DS-1-like G1P[8] Double-gene Reassortant Rotavirus Strains on The American Continent, Brazil, 2013. Sci. Rep. 2019, 9, 2210. [Google Scholar] [CrossRef] [PubMed]
- Moutelíková, R.; Sauer, P.; Heroldová, M.D.; Holá, V.; Prodělalová, J. Emergence of Rare Bovine-Human Reassortant DS-1-Like Rotavirus A Strains with G8P[8] Genotype in Human Patients in the Czech Republic. Viruses 2019, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Cowley, D.; Donato, C.M.; Roczo-Farkas, S.; Kirkwood, C.D. Emergence of a novel equine-like G3P[8] inter-genogroup reassortant rotavirus strain associated with gastroenteritis in Australian children. J. Gen. Virol. 2016, 97, 403–410. [Google Scholar] [CrossRef]
- Hoa-Tran, T.N.; Nakagomi, T.; Vu, H.M.; Do, L.P.; Gauchan, P.; Agbemabiese, C.A.; Nguyen, T.T.; Nakagomi, O.; Thanh, N.T. Abrupt emergence and predominance in Vietnam of rotavirus A strains possessing a bovine-like G8 on a DS-1-like background. Arch. Virol. 2016, 161, 479–482. [Google Scholar] [CrossRef]
- Papp, H.; László, B.; Jakab, F.; Ganesh, B.; De Grazia, S.; Matthijnssens, J.; Ciarlet, M.; Martella, V.; Bányai, K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013, 165, 190–199. [Google Scholar] [CrossRef]
- Matsuno, S.; Hasegawa, A.; Mukoyama, A.; Inouye, S. A candidate for a new serotype of human rotavirus. J. Virol. 1985, 54, 623–624. [Google Scholar] [CrossRef]
- Santos, N.; Hoshino, Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005, 15, 29–56. [Google Scholar] [CrossRef]
- Silva-Sales, M.; Leal, E.; Milagres, F.A.P.; Brustulin, R.; Morais, V.D.S.; Marcatti, R.; Araújo, E.L.L.; Witkin, S.S.; Deng, X.; Sabino, E.C.; et al. Genomic constellation of human Rotavirus A strains identified in Northern Brazil: A 6-year follow-up (2010–2016). Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e98. [Google Scholar] [CrossRef]
- Esona, M.D.; Mijatovic-Rustempasic, S.; Conrardy, C.; Tong, S.; Kuzmin, I.V.; Agwanda, B.; Breiman, R.F.; Banyai, K.; Niezgoda, M.; Rupprecht, C.E.; et al. Reassortant group A rotavirus from straw-colored fruit bat (Eidolon helvum). Emerg. Infect. Dis. 2010, 16, 1844–1852. [Google Scholar] [CrossRef]
- Heylen, E.; Zeller, M.; Ciarlet, M.; Lawrence, J.; Steele, D.; Van Ranst, M.; Matthijnssens, J. Comparative analysis of pentavalent rotavirus vaccine strains and G8 rotaviruses identified during vaccine trial in Africa. Sci. Rep. 2015, 5, 14658. [Google Scholar] [CrossRef] [PubMed]
- Dulgheroff, A.C.; Silva, G.A.; Naveca, F.G.; Oliveira, A.G.; Domingues, A.L. Diversity of group A rotavirus genes detected in the Triângulo Mineiro region, Minas Gerais, Brazil. Braz. J. Microbiol. 2016, 47, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.M.; Volotão, E.M.; de Mendonça, M.C.; Tort, L.F.; da Silva, M.F.; Leite, J.P. Detection of uncommon rotavirus A strains P[8]G8 and P[4]G8 in the city of Rio de Janeiro, 2002. J. Med. Virol. 2010, 82, 1272–1276. [Google Scholar] [CrossRef]
- Montenegro, F.M.; Correia, J.B.; Falbo, A.R.; Dove, W.; Nakagomi, T.; Nakagomi, O.; Cuevas, L.E.; Cunliffe, N.A.; Hart, C.A. Anticipating rotavirus vaccines in Brazil: Detection and molecular characterization of emerging rotavirus serotypes G8 and G9 among children with diarrhoea in Recife, Brazil. J. Med. Virol. 2007, 79, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Lima, R.C.; Pereira, C.F.; Gouvea, V. Detection of rotavirus types G8 and G10 among Brazilian children with diarrhea. J. Clin. Microbiol. 1998, 36, 2727–2729. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Heylen, E.; Zeller, M.; Rahman, M.; Lemey, P.; Van Ranst, M. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol. Biol. Evol. 2010, 27, 2431–2436. [Google Scholar] [CrossRef]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Gregório, D.S.; de Souza, K.A.; Vieira, H.R.; Fernandes, A.M.; Carmona, R.C.; Timenetsky, M.C. Detection of the emerging rotavirus G12P[8] genotype at high frequency in brazil in 2014: Successive replacement of predominant strains after vaccine introduction. Acta Trop. 2016, 156, 87–94. [Google Scholar] [CrossRef]
- Herring, A.J.; Inglis, N.F.; Ojeh, C.K.; Snodgrass, D.R.; Menzies, J.D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 1982, 16, 473–477. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–14373. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Timenetsky, M.C. G8P[6] rotaviruses isolated from Amerindian children in Mato Grosso do Sul, Brazil, during 2009: Close relationship of the G and P genes with those of bovine and bat strains. J. Gen. Virol. 2014, 95, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Varghese, V.; Ghosh, S.; Das, S.; Bhattacharya, S.K.; Krishnan, T.; Karmakar, P.; Kobayashi, N.; Naik, T.N. Characterization of VP1, VP2 and VP3 gene segments of a human rotavirus closely related to porcine strains. Virus Genes 2006, 32, 241–247. [Google Scholar] [CrossRef]
- Ramani, S.; Iturriza-Gomara, M.; Jana, A.K.; Kuruvilla, K.A.; Gray, J.J.; Brown, D.W.; Kang, G. Whole genome characterization of reassortant G10P[11] strain (N155) from a neonate with symptomatic rotavirus infection: Identification of genes of human and animal rotavirus origin. J. Clin. Virol. 2009, 45, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Pang, B.B.; Ghosh, S.; Zhou, X.; Shintani, T.; Urushibara, N.; Song, Y.W.; He, M.Y.; Liu, M.Q.; Tang, W.F.; et al. Molecular epidemiology and genetic evolution of the whole genome of G3P[8] human rotavirus in Wuhan, China, from 2000 through 2013. PLoS ONE 2014, 9, e88850. [Google Scholar] [CrossRef] [PubMed]
- Magagula, N.B.; Esona, M.D.; Nyaga, M.M.; Stucker, K.M.; Halpin, R.A.; Stockwell, T.B.; Seheri, M.L.; Steele, A.D.; Wentworth, D.E.; Mphahlele, M.J. Whole genome analyses of G1P[8] rotavirus strains from vaccinated and non-vaccinated South African children presenting with diarrhea. J. Med. Virol. 2015, 87, 79–101. [Google Scholar] [CrossRef]
- Mijatovic-Rustempasic, S.; Bányai, K.; Esona, M.D.; Foytich, K.; Bowen, M.D.; Gentsch, J.R. Genome sequence based molecular epidemiology of unusual US Rotavirus A G9 strains isolated from Omaha, USA between 1997 and 2000. Infect. Genet. Evol. 2011, 11, 522–527. [Google Scholar] [CrossRef]
- He, B.; Yang, F.; Yang, W.; Zhang, Y.; Feng, Y.; Zhou, J.; Xie, J.; Feng, Y.; Bao, X.; Guo, H.; et al. Characterization of a novel G3P[3] rotavirus isolated from a lesser horseshoe bat: A distant relative of feline/canine rotaviruses. J. Virol. 2013, 87, 12357–12366. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Agbemabiese, C.A.; Nakagomi, T.; Damanka, S.A.; Dennis, F.E.; Lartey, B.L.; Armah, G.E.; Nakagomi, O. Sub-genotype phylogeny of the non-G, non-P genes of genotype 2 Rotavirus A strains. PLoS ONE 2019, 14, e0217422. [Google Scholar] [CrossRef]
- Gupta, S.; Gauhar, M.; Bubber, P.; Ray, P. Phylogenetic analysis of VP7 and VP4 genes of the most predominant human group A rotavirus G12 identified in children with acute gastroenteritis in Himachal Pradesh, India during 2013–2016. J. Med. Virol. 2021, 93, 6200–6209. [Google Scholar] [CrossRef]
- Doan, Y.H.; Nakagomi, T.; Agbemabiese, C.A.; Nakagomi, O. Changes in the distribution of lineage constellations of G2P[4] Rotavirus A strains detected in Japan over 32 years (1980–2011). Infect. Genet. Evol. 2015, 34, 423–433. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using Modeller. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Carmona, R.C.; Timenetsky, M.C. Rotavirus genotypes circulating in Brazil, 2007-2012: Implications for the vaccine program. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Kapikian, A.Z. Rotaviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Philadelphia, P.A., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1917–1974. [Google Scholar]
- Kondo, K.; Tsugawa, T.; Ono, M.; Ohara, T.; Fujibayashi, S.; Tahara, Y.; Kubo, N.; Nakata, S.; Higashidate, Y.; Fujii, Y.; et al. Clinical and Molecular Characteristics of Human Rotavirus G8P[8] Outbreak Strain, Japan, 2014. Emerg. Infect. Dis. 2017, 23, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Tacharoenmuang, R.; Komoto, S.; Guntapong, R.; Ide, T.; Sinchai, P.; Upachai, S.; Yoshikawa, T.; Tharmaphornpilas, P.; Sangkitporn, S.; Taniguchi, K. Full Genome Characterization of Novel DS-1-Like G8P[8] Rotavirus Strains that Have Emerged in Thailand: Reassortment of Bovine and Human Rotavirus Gene Segments in Emerging DS-1-Like Intergenogroup Reassortant Strains. PLoS ONE 2016, 11, e0165826. [Google Scholar] [CrossRef] [PubMed]
- Degiuseppe, J.I.; Stupka, J.A. Argentinean Rotavirus Surveillance Network. Emergence of unusual rotavirus G9P[4] and G8P[8] strains during post vaccination surveillance in Argentina, 2017–2018. Infect. Genet. Evol. 2021, 93, 104940. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Petersen, L.; Patzer, L.; Liebert, U.G. Molecular characteristics of German G8P[4] rotavirus strain GER1H-09 suggest that a genotyping and subclassification update is required for G8. J. Clin. Microbiol. 2009, 47, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Poljsak-Prijatelj, M.; Bufon, T.L.; Marcun-Varda, N.; Marin, J. Rotavirus genotypes in Slovenia: Unexpected detection of G8P[8] and G12P[8] genotypes. J. Med. Virol. 2007, 79, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Esona, M.D.; Steele, D.; Kerin, T.; Armah, G.; Peenze, I.; Geyer, A.; Page, N.; Nyangao, J.; Agbaya, V.A.; Trabelsi, A. Determination of the G and P types of previously nontypeable rotavirus strains from the African Rotavirus Network, 1996–2004: Identification of unusual G types. J. Infect. Dis. 2010, 202, S49–S54. [Google Scholar] [CrossRef]
- Ianiro, G.; Delogu, R.; Bonomo, P.; Castiglia, P.; Ruggeri, F.M.; Fiore, L. Molecular characterization of human G8P[4] rotavirus strains in Italy: Proposal of a more complete subclassification of the G8 genotype in three major lineages. Infect. Genet. Evol. 2014, 21, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Rahman, M.; Yang, X.; Delbeke, T.; Arijs, I.; Kabue, J.P.; Muyembe, J.J.; Van Ranst, M. G8 rotavirus strains isolated in the Democratic Republic of Congo belong to the DS-1-like genogroup. J. Clin. Microbiol. 2006, 44, 1801–1809. [Google Scholar] [CrossRef]
- Heylen, E.; Batoko Likele, B.; Zeller, M.; Stevens, S.; De Coster, S.; Conceição-Neto, N.; Van Geet, C.; Jacobs, J.; Ngbonda, D.; Van Ranst, M.; et al. Rotavirus surveillance in Kisangani, the Democratic Republic of the Congo, reveals a high number of unusual genotypes and gene segments of animal origin in non-vaccinated symptomatic children. PLoS ONE 2014, 9, e100953. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, J.T.T.; Oliveira, M.V.; Lorenzetti, E.; Alfieri, A.F.; Alfieri, A.A. Genotype constellation of a rotavirus A field strain with an uncommon G8P[11] genotype combination in a rotavirus-vaccinated dairy cattle herd. Arch. Virol. 2020, 165, 1855–1861. [Google Scholar] [CrossRef]
- Medici, M.C.; Tummolo, F.; Martella, V.; Arcangeletti, M.C.; De Conto, F.; Chezzi, C.; Magrì, A.; Fehér, E.; Marton, S.; Calderaro, A.; et al. Whole genome sequencing reveals genetic heterogeneity of G3P[8] rotaviruses circulating in Italy. Infect. Genet. Evol. 2016, 40, 253–261. [Google Scholar] [CrossRef]
- Vrdoljak, M.; Guzvinec, M.; Trkulja, V.; Butic, I.; Ivic, I.; Krzelj, V.; Tonkic, M.; Jungvirth, M.H.; Pal, M.P.; Tesovic, G. Distribution of rotavirus genotypes in three Croatian regions among children ≤5 years of age (2012–2014). Int. J. Infect. Dis. 2019, 89, 3–9. [Google Scholar] [CrossRef]
- Pongsuwannna, Y.; Guntapong, R.; Tacharoenmuang, R.; Prapanpoj, M.; Kameoka, M.; Taniguchi, K. A long-term survey on the distribution of the human rotavirus G type in Thailand. J. Med. Virol. 2010, 82, 157–163. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Gondwe, J.S.; Broadhead, R.L.; Molyneux, M.E.; Woods, P.A.; Bresee, J.S.; Glass, R.I.; Gentsch, J.R.; Hart, C.A. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: Predominance of novel P[6]G8 strains. J. Med. Virol. 1999, 57, 308–312. [Google Scholar] [CrossRef]
- Cunliffe, N.A.; Gentsch, J.R.; Kirkwood, C.D.; Gondwe, J.S.; Dove, W.; Nakagomi, O.; Nakagomi, T.; Hoshino, Y.; Bresee, J.S.; Glass, R.I. Rotavirus G and P types in children with acute diarrhea in Blantyre, Malawi, from 1997 to 1998: Predominance of novel P[6]G8 strains. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology 2000, 274, 309–320. [Google Scholar] [CrossRef]
- Esona, M.D.; Geyer, A.; Page, N.; Trabelsi, A.; Fodha, I.; Aminu, M.; Agbaya, V.A.; Tsion, B.; Kerin, T.K.; Armah, G.E.; et al. Genomic characterization of human rotavirus G8 strains from the African rotavirus network: Relationship to animal rotaviruses. J. Med. Virol. 2009, 81, 937–951. [Google Scholar] [CrossRef]
- Istrate, C.; Sharma, S.; Nordgren, J.; Castro, S.V.E.; Lopes, Â.; Piedade, J.; Zaky, A.; Lima, A.; Neves, E.; Veiga, J.; et al. High rate of detection of G8P[6] rotavirus in children with acute gastroenteritis in São Tomé and Príncipe. Arch. Virol. 2015, 160, 423–428. [Google Scholar] [CrossRef]
- Mchaile, D.N.; Philemon, R.N.; Kabika, S.; Albogast, E.; Morijo, K.J.; Kifaro, E.; Mmbaga, B.T. Prevalence and genotypes of Rotavirus among children under 5 years presenting with diarrhoea in Moshi, Tanzania: A hospital based cross sectional study. BMC Res. Notes 2017, 10, 542. [Google Scholar] [CrossRef]
- Lorestani, N.; Moradi, A.; Teimoori, A.; Masodi, M.; Khanizadeh, S.; Hassanpour, M.; Javid, N.; Ardebili, A.; Tabarraei, A.; Nikoo, H.R. Molecular and serologic characterization of rotavirus from children with acute gastroenteritis in northern Iran, Gorgan. BMC Gastroenterol. 2019, 19, 100. [Google Scholar] [CrossRef]
- Yodmeeklin, A.; Khamrin, P.; Kumthip, K.; Malasao, R.; Ukarapol, N.; Ushijima, H.; Maneekarn, N. Increasing predominance of G8P[8] species A rotaviruses in children admitted to hospital with acute gastroenteritis in Thailand, 2010–2013. Arch. Virol. 2018, 163, 2165–2178. [Google Scholar] [CrossRef]
- Carvalho-Costa, F.A.; Volotão, E.M.; de Assis, R.M.; Fialho, A.M.; de Andrade, J.S.; Rocha, L.N.; Tort, L.F.; da Silva, M.F.; Gómez, M.M.; de Souza, P.M.; et al. Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005–2009. Pediatr. Infect. Dis. J. 2011, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.L.; Raguindin, P.F.; Silva, M.W.T. Prospects for rotavirus vaccine introduction in the Philippines: Bridging the available evidence into immunization policy. Hum. Vaccin. Immunother. 2019, 15, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Ciarlet, M.; Pratelli, A.; Arista, S.; Terio, V.; Elia, G.; Cavalli, A.; Gentile, M.; Decaro, N.; Greco, G.; et al. Molecular analysis of the VP7, VP4, VP6, NSP4, and NSP5/6 genes of a buffalo rotavirus strain: Identification of the rare P[3] rhesus rotavirus-like VP4 gene allele. J. Clin. Microbiol. 2003, 41, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Matsumoto, Y. Bovine rotavirus G and P types and sequence analysis of the VP7 gene of two G8 bovine rotaviruses from Japan. Vet. Microbiol. 2002, 84, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ciarlet, M.; Hoshino, Y.; Liprandi, F. Single point mutations may affect the serotype reactivity of serotype G11 porcine rotavirus strains: A widening spectrum? J. Virol. 1997, 71, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Graham, D.Y.; Mason, B.B. Proteolytic enhancement of rotavirus infectivity: Molecular mechanisms. J. Virol. 1981, 39, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Gorziglia, M.; Green, K.; Nishikawa, K.; Taniguchi, K.; Jones, R.; Kapikian, A.Z.; Chanock, R.M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J. Virol. 1988, 62, 2978–2984. [Google Scholar] [CrossRef]
- Guntapong, R.; Tacharoenmuang, R.; Singchai, P.; Upachai, S.; Sutthiwarakom, K.; Komoto, S.; Tsuji, T.; Tharmaphornpilas, P.; Yoshikawa, T.; Sangkitporn, S.; et al. Predominant prevalence of human rotaviruses with the G1P[8] and G8P[8] genotypes with a short RNA profile in 2013 and 2014 in Sukhothai and Phetchaboon provinces, Thailand. J. Med. Virol. 2017, 89, 615–620. [Google Scholar] [CrossRef]
- Degiuseppe, J.I.; Torres, C.; Mbayed, V.A.; Stupka, J.A. Phylogeography of Rotavirus G8P[8] Detected in Argentina: Evidence of Transpacific Dissemination. Viruses 2022, 14, 2223. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, L.N.; Wang, S.M.; Zhou, H.L.; Qiu, C.; Jiang, B.; Qiu, T.Y.; Chen, S.L.; von Seidlein, L.; Wang, X.Y. Genetic characterization of two G8P[8] rotavirus strains isolated in Guangzhou, China, in 2020/21: Evidence of genome reassortment. BMC Infect Dis. 2022, 22, 579. [Google Scholar] [CrossRef]
- Phan, T.; Kobayashi, M.; Nagasawa, K.; Hatazawa, R.; Pham, N.T.K.; Miyashita, H.; Komoto, S.; Tajima, T.; Baba, T.; Okitsu, S.; et al. Whole genome sequencing and evolutionary analysis of G8P[8] rotaviruses emerging in Japan. Virusdisease 2022, 33, 215–218. [Google Scholar] [CrossRef]
- Chan-it, W.; Chanta, C.; Ushijima, H. Predominance of novel DS-1-like G8P[8] rotavirus reassortant strains in children hospitalized with acute gastroenteritis in Thailand. Authorea. Preprint. 2023. Available online: https://www.authorea.com/doi/full/10.22541/au.167451365.57771803/v1 (accessed on 13 February 2023).
- Donato, C.M.; Cowley, D.; Donker, N.C.; Bogdanovic-Sakran, N.; Snelling, T.L.; Kirkwood, C.D. Characterization of G2P[4] rotavirus strains causing outbreaks of gastroenteritis in the Northern Territory, Australia, in 1999, 2004 and 2009. Infect. Genet. Evol. 2014, 28, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Agbemabiese, C.A.; Nakagomi, T.; Doan, Y.H.; Nakagomi, O. Whole genomic constellation of the first human G8 rotavirus strain detected in Japan. Infect. Genet. Evol. 2015, 35, 184–193. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mullick, S.; Deb, A.K.; Panda, S.; Chawla-Sarkar, M. First report of human rotavirus G8P[4] gastroenteritis in India: Evidence of ruminants-to-human zoonotic transmission. J. Med. Virol. 2013, 85, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.A.; Payne, D.C.; Teel, E.N.; Mijatovic-Rustempasic, S.; Bowen, M.D.; Wikswo, M.; Gentsch, J.R.; Parashar, U.D. First reports of human rotavirus G8P[4] gastroenteritis in the United States. J. Clin. Microbiol. 2012, 50, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Doan, Y.H.; Dove, W.; Ngwira, B.; Iturriza-Gómara, M.; Nakagomi, O.; Cunliffe, N.A. G8 rotaviruses with conserved genotype constellations detected in Malawi over 10 years (1997–2007) display frequent gene reassortment among strains co-circulating in humans. J. Gen. Virol. 2013, 94, 1273–1295. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Bányai, K.; Ciarlet, M.; Iturriza-Gómara, M.; Lorusso, E.; De Grazia, S.; Arista, S.; Decaro, N.; Elia, G.; Cavalli, A.; et al. Relationships among porcine and human P[6] rotaviruses: Evidence that the different human P[6] lineages have originated from multiple interspecies transmission events. Virology 2006, 344, 509–519. [Google Scholar] [CrossRef]



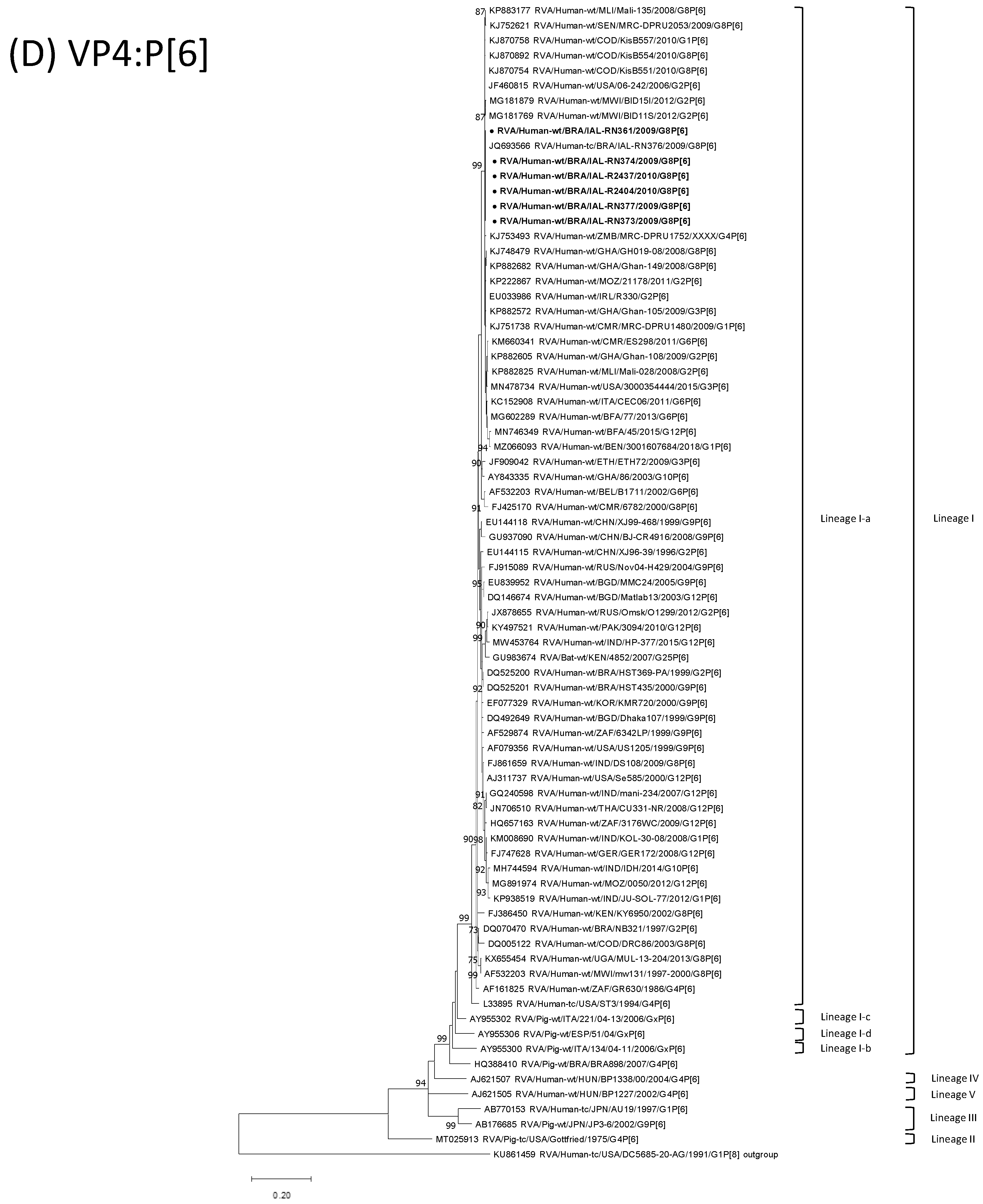
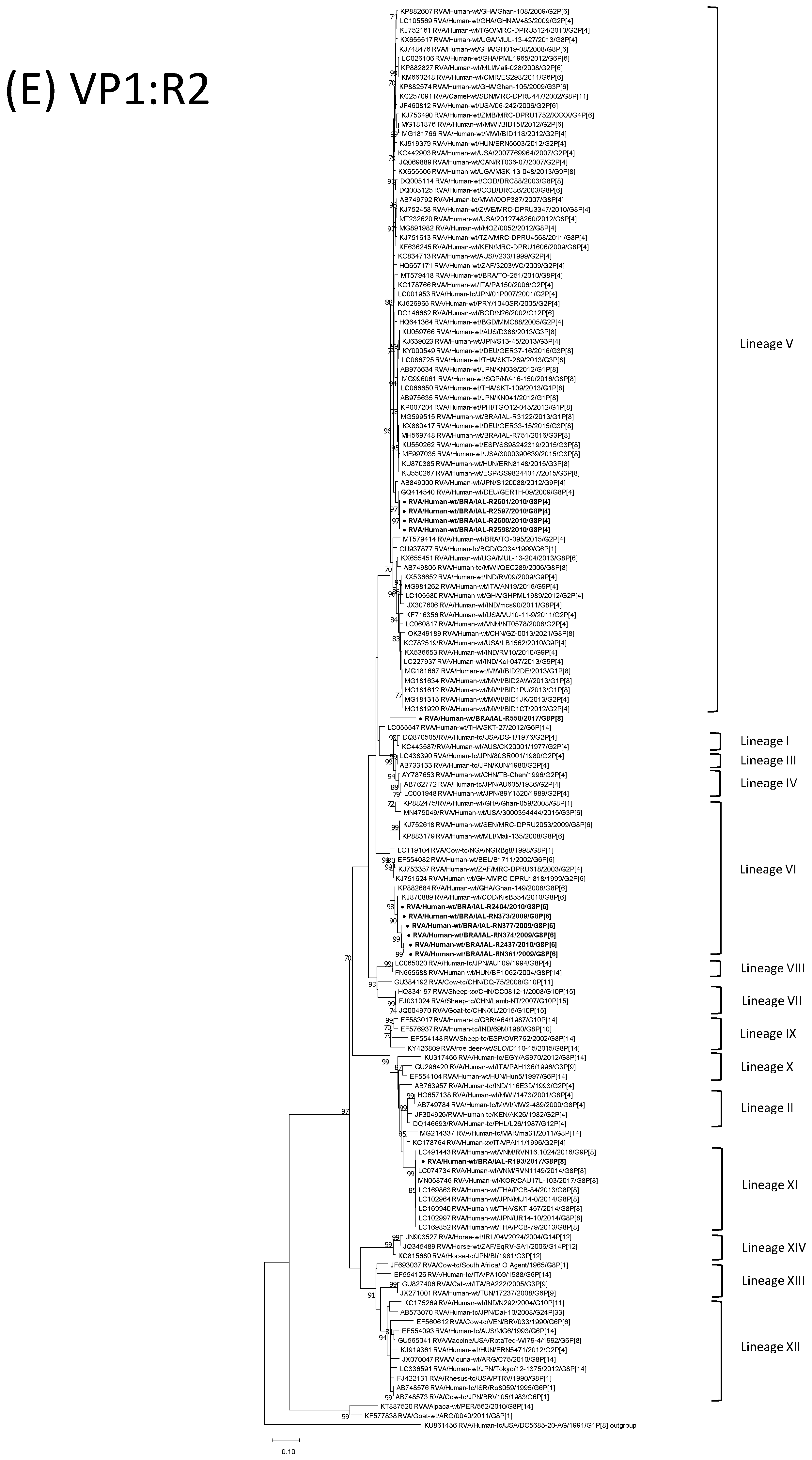
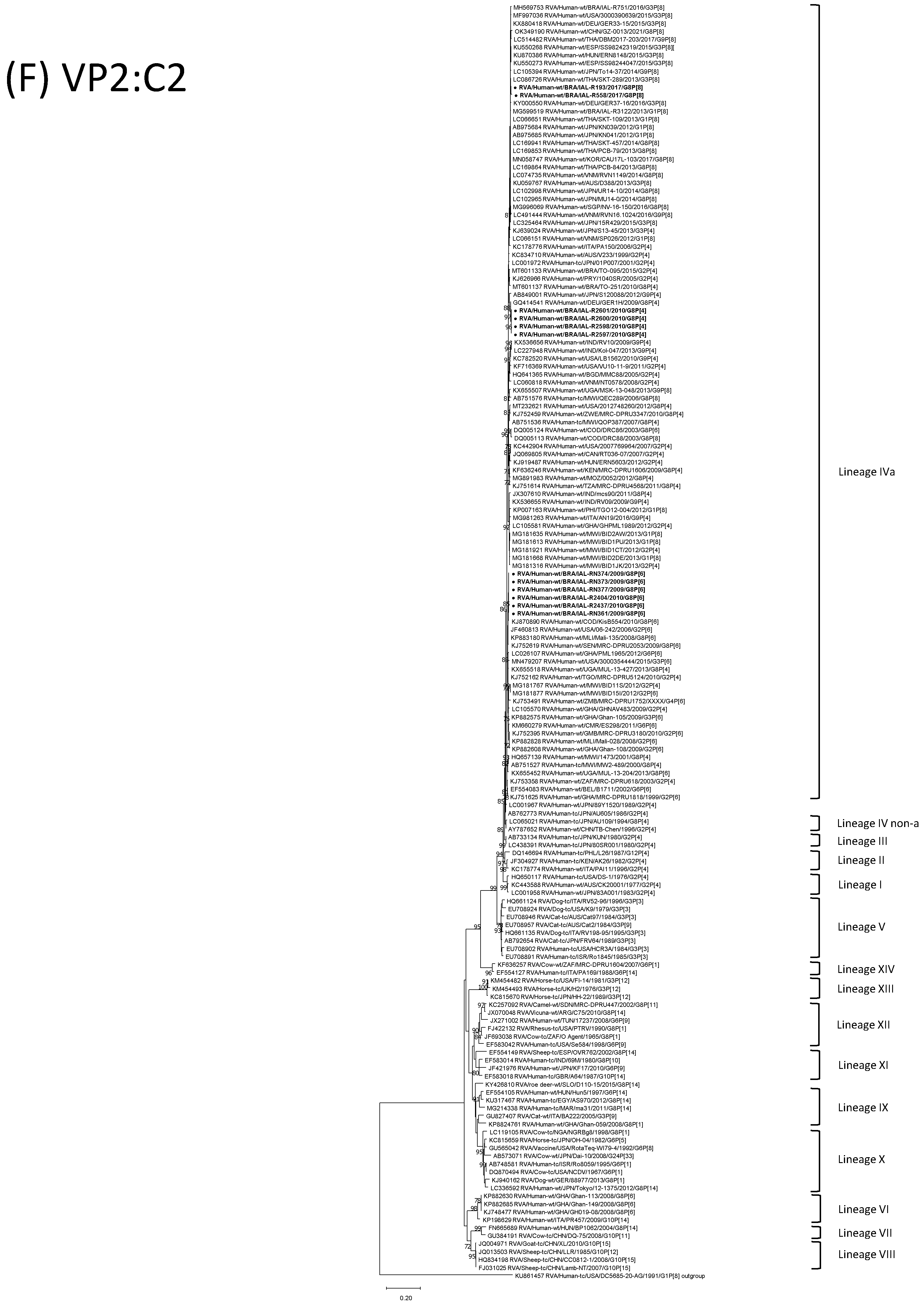

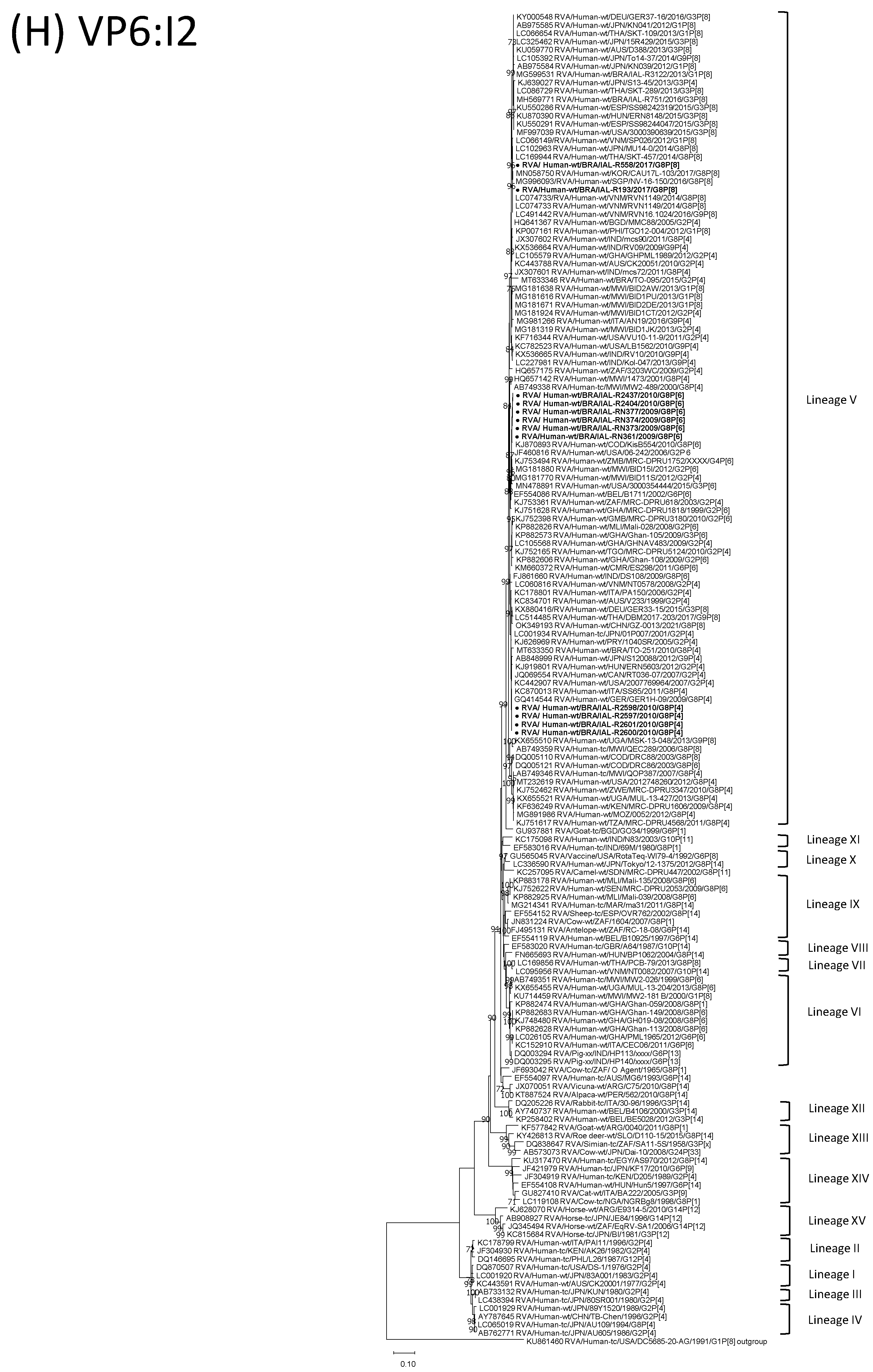

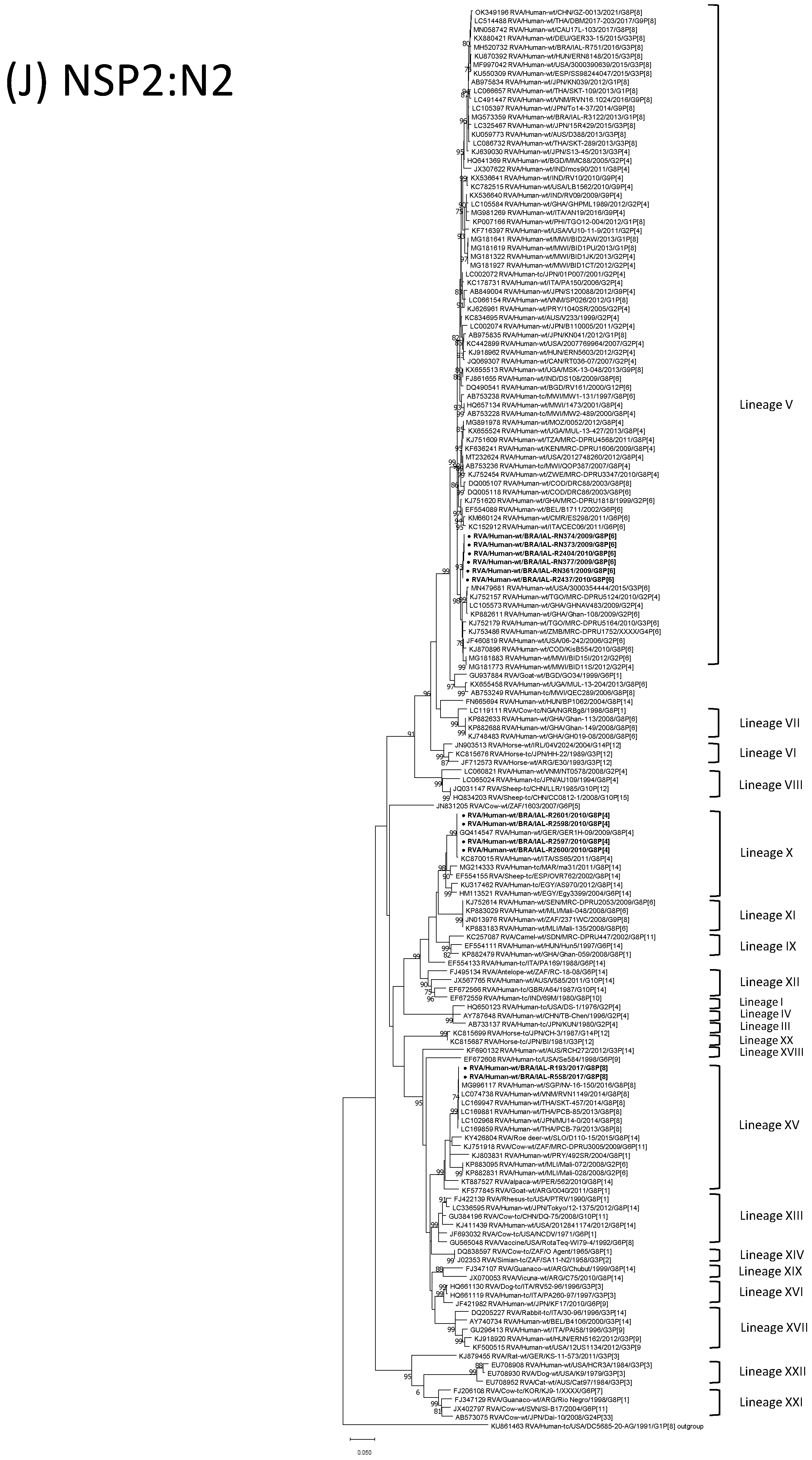
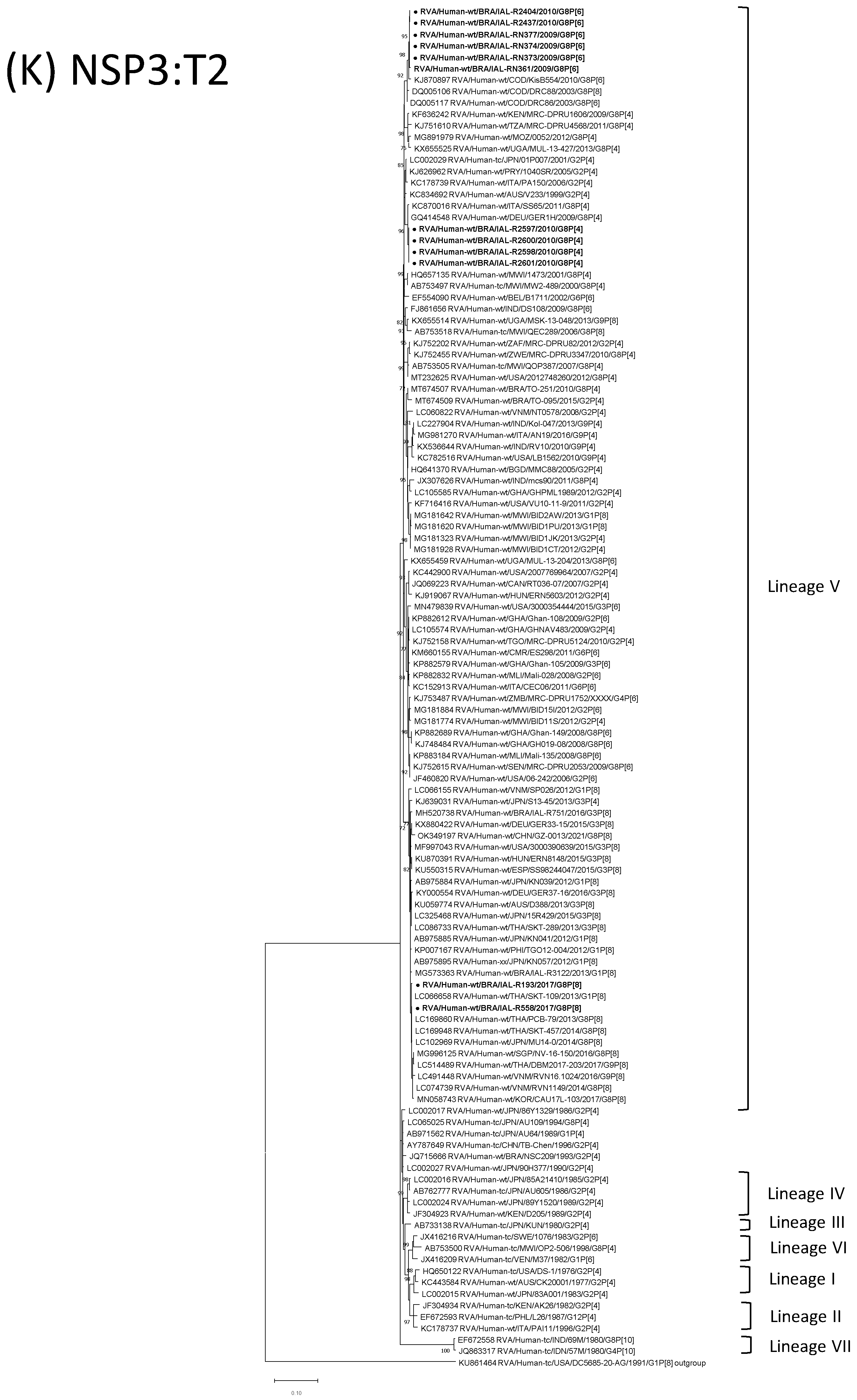
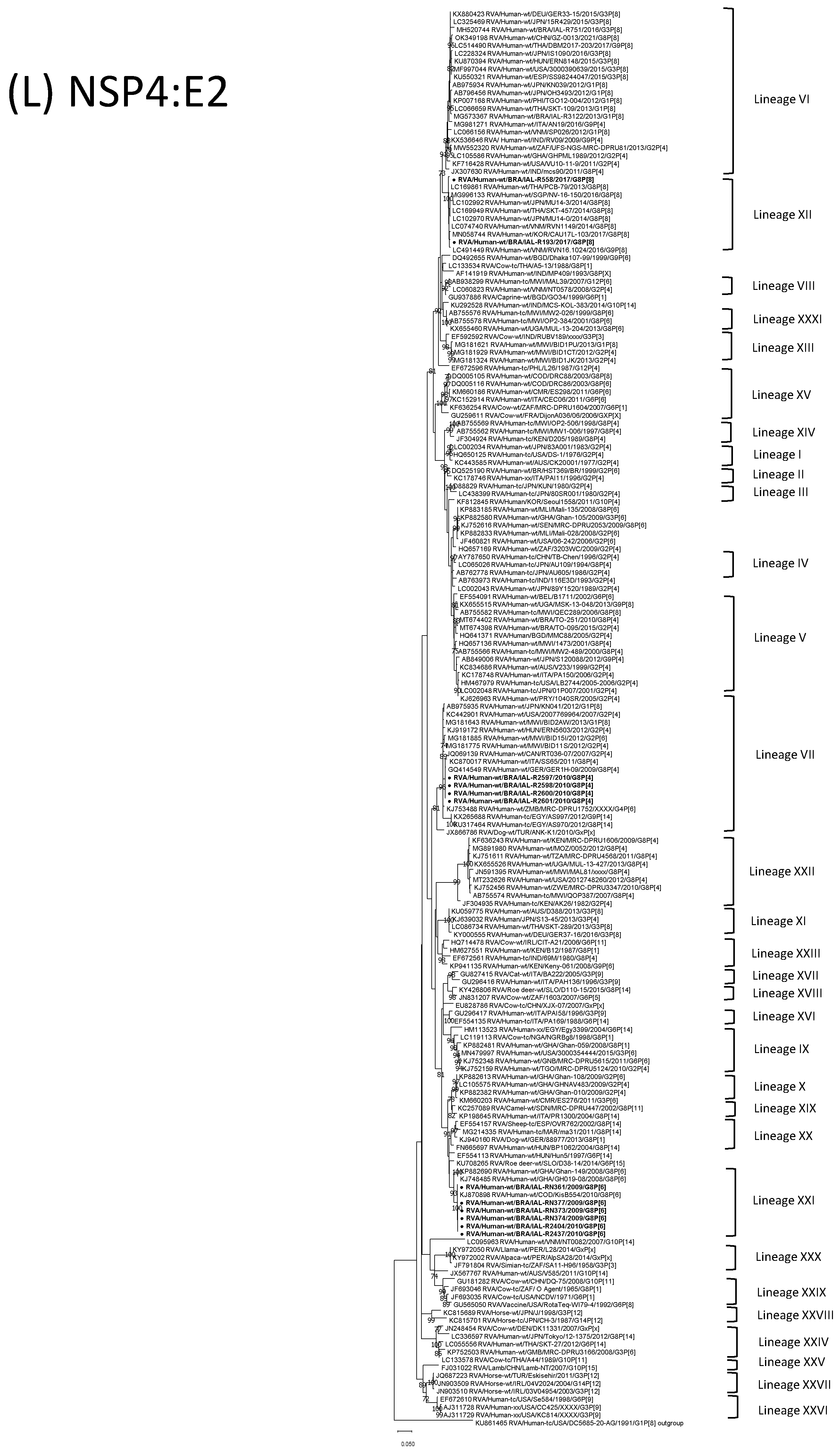
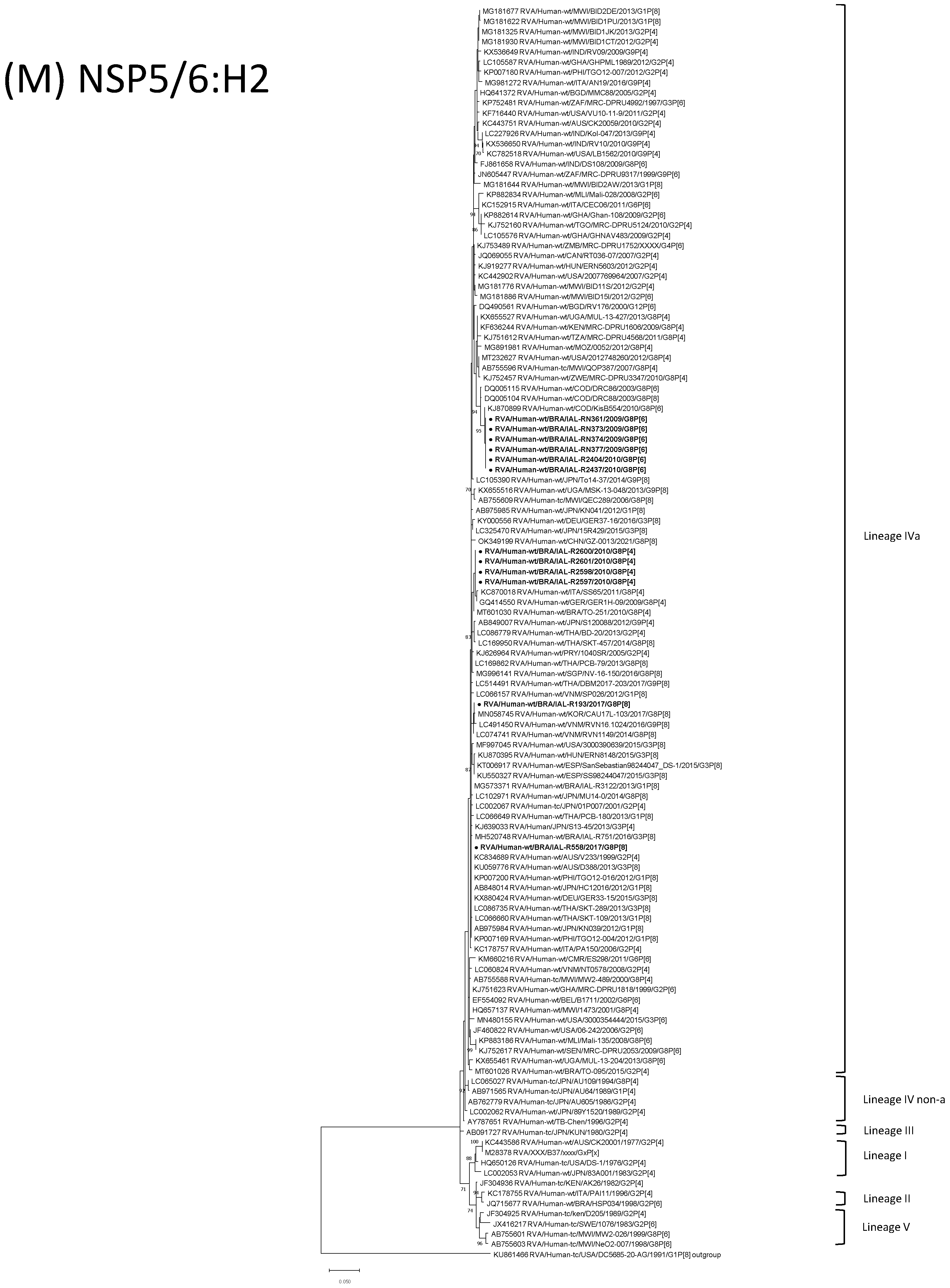
| Strain | Age | Gender | City | State | Profile | VP7 | VP4 | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G8 | P[8] | P[4] | P[6] | I2 | R2 | C2 | M2 | A2 | N2 | T2 | E2 | H2 | ||||||
| RVA/Human-wt/BRA/IAL-R193/2017/G8P[8] a | 7 months | M | Goiânia | GO | Short | IV | III | V | XI | IVa | V | IVa | XV | V | XII | IVa | ||
| RVA/Human-wt/BRA/IAL-R558/2017/G8P[8] a | 4 months | M | São Paulo | SP | Short | IV | III | V | Distinct | IVa | V | IVa | XV | V | XII | IVa | ||
| RVA/Human-wt/BRA/IAL-R2601/2010/G8P[4] a | 1 year | F | Brasília | DF | Short | I | IVa | V | V | IVa | V | II | X | V | VII | IVa | ||
| RVA/Human-wt/BRA/IAL-R2600/2010/G8P[4] a | 5 years | F | Brasília | DF | Short | I | IVa | V | V | IVa | V | II | X | V | VII | IVa | ||
| RVA/Human-wt/BRA/IAL-R2598/2010/G8P[4] a | 5 months | F | Brasília | DF | Short | I | IVa | V | V | IVa | V | II | X | V | VII | IVa | ||
| RVA/Human-wt/BRA/IAL-R2597/2010/G8P[4] a | 5 years | M | Brasília | DF | Short | I | IVa | V | V | IVa | V | II | X | V | VII | IVa | ||
| RVA/Human-wt/BRA/IAL-R2437/2010/G8P[6] a | 9 months | M | São Paulo | SP | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| RVA/Human-wt/BRA/IAL-R2404/2010/G8P[6] a | 1 year | M | São Paulo | SP | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| RVA/Human-wt/BRA/IAL-RN377/2009/G8P[6] a | 3 months | F | Dourados | MS | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| RVA/Human-wt/BRA/IAL-RN374/2009/G8P[6] a | 5 months | M | Dourados | MS | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| RVA/Human-wt/BRA/IAL-RN373/2009/G8P[6] a | 2 months | F | Dourados | MS | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| RVA/Human-wt/BRA/IAL-RN361/2009/G8P[6] a | 1 year | M | Dourados | MS | Short | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | ||
| Bovine-like G8P[8] DS-1 like | ||||||||||||||||||
| RVA/Human-wt/JPN/MU14-0/2014/G8P[8] | IV | III | V | XI | IVa | V | IVa | XV | V | XII | IVa | |||||||
| RVA/Human-wt/THA/SKT-457/2014/G8P[8] | IV | III | V | XI | IVa | V | IVa | XV | V | XII | IVa | |||||||
| RVA/Human-wt/THA/PCB-79/2013/G8P[8] | IV | III | V | XI | IVa | V | VII | XV | V | XII | IVa | |||||||
| RVA/Human-wt/VNM/RVN1149/2014/G8P[8] | IV | III | V | XI | IVa | V | IVa | XV | V | XII | IVa | |||||||
| RVA/Human-wt/KOR/CAU17L-103/2017/G8P[8] | IV | III | V | XI | IVa | V | IVa | V | V | XII | IVa | |||||||
| RVA/Human-wt/SGP/NV-16-150/2016/G8P[8] | IV | III | V | V | IVa | V | IVa | XV | V | XII | IVa | |||||||
| RVA/Human-wt/CHN/GZ-0013/2021/G8P[8] | IV | III | V | V | IVa | V | IVa | V | V | VI | IVa | |||||||
| Equine-like G3P[4] | ||||||||||||||||||
| RVA/Human-wt/JPN/S13-45/2013/G3P[4] | IVa | V | V | IVa | V | IVa | V | V | XI | IVa | ||||||||
| Equine-like G3P[8] reassortant | ||||||||||||||||||
| RVA/Human-wt/AUS/D388/2013/G3P[8] | III | V | V | IVa | V | IVa | V | V | XI | IVa | ||||||||
| RVA/Human-wt/BRA/IAL-R751/2016/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/DEU/GER33-15/2015/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/DEU/GER37-16/2016/G3P[8] | III | V | V | IVa | V | IVa | V | V | XI | IVa | ||||||||
| RVA/Human-wt/HUN/ERN8148/2015/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/JPN/15R429/2015/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/ESP/SS98244047/2015/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/THA/SKT-289/2013/G3P[8] | III | V | V | IVa | V | IVa | V | V | XI | IVa | ||||||||
| RVA/Human-wt/USA/3000390639/2015/G3P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| G1P[8] reassortant | ||||||||||||||||||
| RVA/Human-wt/JPN/KN039/2012/G1P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/JPN/KN041/2012/G1P[8] | III | V | V | IVa | V | II | V | V | VII | IVa | ||||||||
| RVA/Human-wt/MWI/BID2AW/2013/G1P[8] | III | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| RVA/Human-wt/MWI/BID1PU/2013/G1P[8] | III | V | V | IVa | V | IVa | V | V | XIII | IVa | ||||||||
| RVA/Human-wt/PHI/TGO12-004/2012/G1P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/THA/SKT-109/2013/G1P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/VNM/SP026/2012/G1P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/BRA/IAL-R3122/2013/G1P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| G9P[8] reassortant | ||||||||||||||||||
| RVA/Human-wt/VNM/RVN16.1024/2016/G9P[8] | III | V | XI | IVa | V | IVa | V | V | XII | IVa | ||||||||
| RVA/Human-wt/THA/DBM2017-203/2017/G9P[8] | III | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/JPN/To14-37/2014/G9P[8] | III | V | XI | IVa | V | IVa | V | T1 | E1 | IVa | ||||||||
| G8P[4] | ||||||||||||||||||
| RVA/Human-wt/MWI/1473/2001/G8P[4] | V | II | V | II | IVa | V | IVa | V | V | V | IVa | |||||||
| RVA/Human-tc/JPN/AU109/1994/G8P[4] | VI | IV non-a | IV | VIII | IV non-a | IV | IV non-a | VIII | Distinct | IV | IV non-a | |||||||
| RVA/Human-tc/MWI/MW2-489/2000/G8P[4] | V | II | V | II | IVa | V | IVa | V | V | V | IVa | |||||||
| RVA/Human-tc/MWI/QOP387/2007/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXII | IVa | |||||||
| RVA/Human-wt/BRA/TO-251/2010/G8P[4] | I | IVa | V | V | IVa | V | IVa | V | V | V | IVa | |||||||
| RVA/Human-wt/UGA/MUL-13-427/2013/G8P4 | V | II | V | V | IVa | V | IVa | V | V | XXIII | IVa | |||||||
| RVA/Human-wt/USA/2012748260/2012/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXII | IVa | |||||||
| RVA/Human-wt/ZWE/MRC-DPRU3347/2010/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXII | IVa | |||||||
| RVA/Human-wt/MOZ/0052/2012/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXII | IVa | |||||||
| RVA/Human-wt/DEU/GER1H-09/2009/G8P[4] | I | IVa | V | V | IVa | V | II | X | V | VII | IVa | |||||||
| RVA/Human-wt/TZA/MRC-DPRU4568/2011/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXII | IVa | |||||||
| RVA/Human-wt/KEN/MRC-DPRU1606/2009/G8P[4] | V | II | V | V | IVa | V | IVa | V | V | XXIII | IVa | |||||||
| RVA/Human-wt/ITA/SS65/2011/G8[4] | I | IVa | V | Rx | Cx | Mx | II | X | V | VII | IVa | |||||||
| RVA/Human-wt/IND/mcs90/2011/G8P[4] | II | IVa | V | V | IVa | VII | IVa | V | V | VI | H3 | |||||||
| G8P[6] | ||||||||||||||||||
| RVA/Human-wt/GHA/Ghan-149/2008/G8P[6] | V | I-a | VI | VI | VI | XIII | IVa | VII | V | XXI | H3 | |||||||
| RVA/Human-wt/MLI/Mali-135/2008/G8P[6] | V | I-a | IX | VI | IVa | VI | IVa | XI | V | Distinct | IVa | |||||||
| RVA/Human-wt/ COD/KisB554/2010/G8P[6] | V | I-a | V | VI | IVa | V | IVa | V | V | XXI | IVa | |||||||
| RVA/Human-wt/COD/DRC86/2003/G8P[6] | V | I-a | V | V | IVa | V | IVa | V | V | XV | IVa | |||||||
| RVA/Human-wt/UGA/MUL-13-204/2013/G8P[6] | V | I-a | VI | V | IVa | V | IVa | V | V | XXXI | IVa | |||||||
| RVA/Human-wt/SEN/MRC-DPRU2053/2009/G8P[6] | V | I-a | IX | VI | IVa | VI | IVa | XI | V | Distinct | IVa | |||||||
| RVA/Human-wt/IND/DS108/2009/G8P[6] | IV | I-a | V | Rx | Cx | Mx | IVa | V | V | E9 | IVa | |||||||
| RVA/Human-wt/GHA/GH019-08/2008/G8P[6] | V | I-a | VI | V | VI | XIII | IVa | VII | V | XXI | H3 | |||||||
| G8P[8] DS-1 like | ||||||||||||||||||
| RVA/Human-wt/COD/DRC88/2003/G8P[8] | V | III | V | V | IVa | V | IVa | V | V | XV | IVa | |||||||
| RVA/Human-tc/MWI/QEC289/2006/G8P[8] | V | III | V | V | IVa | V | IVa | V | V | V | IVa | |||||||
| G4P[6] | ||||||||||||||||||
| RVA/Human-wt/ZMB/MRC-DPRU1752/XXXX/G4P[6] | I-a | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| G8P[11] | ||||||||||||||||||
| RVA/Camel-wt/SDN/MRC-DPRU447/2002/G8P[11] | I | X | V | XII | XII | Ax | IX | T6 | XIX | H3 | ||||||||
| Vaccines strains | ||||||||||||||||||
| RVA/Vaccine/USA/Rotateq-WI79-4/1992/G6P[8] | II | X | XII | X | X | A3 | XIII | T6 | XXIX | H3 | ||||||||
| G8P[14] | ||||||||||||||||||
| RVA/Human-wt/JPN/TOKYO/12-1375/2012/G8P[14] | II | X | XII | X | X | A3 | XIII | T6 | XXIV | H3 | ||||||||
| RVA/Human-wt/HUN/BP1062/2004/G8P[14] | VI | VIII | VIII | VII | VI | A11 | Distinct | T6 | XX | H3 | ||||||||
| RVA/Vicuna-wt/ARG/C75/2010/G8P[14] | II | Distinct | XII | XII | XV | Ax | XIX | T6 | E3 | Hx | ||||||||
| RVA/Sheep-tc/ESP/OVR762/2002/G8P[14] | II | IX | IX | XI | VII | A11 | X | T6 | XX | H3 | ||||||||
| RVA/Human-tc/EGY/AS970/2012/G8P[14] | VI | XIV | X | IX | Distinct | A11 | X | T6 | VII | H3 | ||||||||
| RVA/Human-tc/MAR/ma31/2011/G8P[14] | II | IX | Distinct | IX | VIII | A11 | X | T6 | XX | H3 | ||||||||
| RVA/Alpaca-wt/PER/562/2010/G8P[14] | II | Distinct | Distinct | - | VIII | Ax | XV | T6 | E3 | H3 | ||||||||
| RVA/Roe deer-wt/SLO/D110-15/2015/G8P[14] | II | XIII | IX | IX | X | A3 | XV | T6 | XVIII | H3 | ||||||||
| G8P[1] | ||||||||||||||||||
| RVA/Human-wt/GHA/Ghan-059/2008/G8P[1] | V | VI | VI | IX | VI | A11 | IX | T6 | IX | H3 | ||||||||
| RVA/Goat-wt/ARG/0040/2011/G8P[1] | II | XIII | Distinct | - | XV | A3 | XV | T6 | E12 | H3 | ||||||||
| RVA/Cow-tc/NGA/NGRBg8/1998/G8P[1] | V | XIV | VI | X | VIII | A11 | VII | T6 | IX | H3 | ||||||||
| G2P[6] | ||||||||||||||||||
| RVA/Human-wt/MLI/Mali-028/2008/G2P[6] | I-a | V | V | IVa | V | IVa | XV | V | Distinct | IVa | ||||||||
| RVA/Human-wt/MWI/BID15I/2012/G2P[6] | I-a | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| RVA/Human-wt/GHA/Ghan-108/2009/G2P[6] | I-a | V | V | IVa | V | IVa | V | V | X | IVa | ||||||||
| RVA/Human-wt/USA/06-242/2006/G2P[6] | I-a | V | V | IVa | V | IVa | V | V | Distinct | IVa | ||||||||
| G3P[6] | ||||||||||||||||||
| RVA/Human-wt/USA/3000354444/2015/G3P[6] | I-a | V | VI | IVa | V | IVa | V | V | IX | IVa | ||||||||
| RVA/Human-wt/GHA/Ghan-105/2009/G3P[6] | I-a | V | V | IVa | V | IVa | N1 | V | Distinct | H3 | ||||||||
| G6P[6] | ||||||||||||||||||
| RVA/Human-wt/BEL/B1711/2002/G6P[6] | I-a | V | VI | IVa | XI | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/CMR/ES298/2011/G6P[6] | I-a | V | V | IVa | V | IVa | V | V | XV | IVa | ||||||||
| RVA/Human-wt/ITA/CEC06/2011/G6P[6] | I-a | VI | Rx | Cx | Mx | IVa | V | V | XV | IVa | ||||||||
| G9P[4] | ||||||||||||||||||
| RVA/Human-wt/IND/RV09/2009/G9P[4] | IVa | V | V | IVa | V | IVa | V | T1 | VI | IVa | ||||||||
| RVA/Human-wt/IND/RV10/2010/G9P[4] | IVa | V | V | IVa | VII | IVa | V | V | E6 | IVa | ||||||||
| RVA/Human-wt/IND/kol-047/2013/G9P[4] | IVa | V | V | IVa | VII | IVa | N1 | V | E6 | IVa | ||||||||
| RVA/Human-wt/ITA/AN19/2016/G9P[4] | IVa | V | V | IVa | VII | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/JPN/S120088/2012/G9P[4] | IVa | V | V | IVa | V | IVa | V | T1 | V | IVa | ||||||||
| RVA/Human-wt/USA/LB1562/2010/G9P[4] | IVa | V | V | IVa | VII | IVa | V | V | E6 | IVa | ||||||||
| G2P[4] | ||||||||||||||||||
| RVA/Human-wt/JPN/01P007/2001/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/USA/VU10-11-9/2011/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/USA/2007769964/2007/G2P[4] | IVa | V | V | IVa | V | II | V | V | VII | IVa | ||||||||
| RVA/Human-wt/MLW/BID1JK/2013/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | XIII | IVa | ||||||||
| RVA/Human-wt/VNM/NT0578/2008/G2P[4] | IVa | V | V | IVa | V | IVa | VIII | V | VIII | IVa | ||||||||
| RVA/Human-wt/TGO/MRC-DPRU5124/2010/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | IX | IVa | ||||||||
| RVA/Human-wt/GHA/GHNAV483/2009/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | X | IVa | ||||||||
| RVA/Human-wt/MWI/BID1CT/2012/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | XIII | IVa | ||||||||
| RVA/Human-wt/GHA/GHPML1989/2012/G2P[4] | IVa | V | V | IVa | VII | IVa | V | V | VI | IVa | ||||||||
| RVA/Human-wt/CHN/TB-Chen/1996/G2P[4] | IV non-a | IV | IV | IV non-a | IV | IV non-a | IV | Distinct | IV | IV non-a | ||||||||
| RVA/Human-wt/JPN/KUN/1980/G2P[4] | III | III | III | III | III | III | III | III | III | III | ||||||||
| RVA/Human-wt/ITA/PAI11/1996/G2P[4] | II | II | II | II | II | II | II | II | II | II | ||||||||
| RVA/Human-wt/USA/DS-1/1976/G2P[4] | I | I | I | I | I | I | I | I | I | I | ||||||||
| RVA/Human-wt/BRA/TO-095/2015/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | V | IVa | ||||||||
| RVA/Human-tc/JPN/AU605/1986/G2P[4] | IV non-a | IV | IV | IV non-a | IV | IV non-a | N1 | IV | IV | IV non-a | ||||||||
| RVA/Human-wt/JPN/89Y1520/1989/G2P[4] | IV non-a | IV | IV | IV non-a | IV | IV non-a | N1 | IV | IV | IVa | ||||||||
| RVA/Human-wt/BGD/MMC88/2005/G2P[4] | IVa | V | V | IVa | VII | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/PRY/1040SR/2005/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/ITA/PA150/2006/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/HUN/ERN5603/2012/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| RVA/Human-wt/AUS/V233/1999/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | V | IVa | ||||||||
| RVA/Human-wt/CAN/RT036-07/2007/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| RVA/Human-wt/MWI/BID11S/2012/G2P[4] | IVa | V | V | IVa | V | IVa | V | V | VII | IVa | ||||||||
| Protein | GQ225781 Bovine Chain_A_A | GU984760 Bovine Chain_A_A | KF305321 Bovine Chain_A_A | KX212865 Bovine Chain_A_A | LC119109 Bovine Chain_A_A | MF940609 Bovine Chain_A_A | MT633156 Human Chain_A_A | MN989610 Human Chain_A_A | LC102961 Human Chain_A_A | IAL-R193 Human Chain_A_A | IAL-R558 Human Chain_A_A | IAL-R2597 Human Chain_A_A | IAL-R2598 Human Chain_A_A | IAL-R2601 Human Chain_A_A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GQ225781 | None | 0.8403 | 0.71 | 1.1921 | 0.6988 | 0.7066 | 2.2261 | 1.5824 | 1.6236 | 1.2486 | 1.2736 | 1.322 | 1.2609 | 1.2433 |
| GU984760 | 0.8403 | None | 0.7676 | 1.0677 | 0.756 | 0.7377 | 2.3079 | 1.5151 | 1.6768 | 1.1326 | 1.0816 | 1.2795 | 1.0754 | 1.0572 |
| KF305321 | 0.71 | 0.7676 | None | 1.1332 | 0.7233 | 0.7164 | 2.2884 | 1.5478 | 1.6369 | 1.2352 | 1.2762 | 1.3485 | 1.2323 | 1.2301 |
| KX212865 | 1.1921 | 1.0677 | 1.1332 | None | 1.1732 | 1.1352 | 1.9266 | 1.2737 | 1.1325 | 0.999 | 0.9146 | 1.0105 | 0.9257 | 0.931 |
| LC119109 | 0.6988 | 0.756 | 0.7233 | 1.1732 | None | 0.6704 | 2.2777 | 1.6554 | 1.7588 | 1.1886 | 1.2276 | 1.4169 | 1.2077 | 1.1962 |
| MF940609 | 0.7066 | 0.7377 | 0.7164 | 1.1352 | 0.6704 | None | 2.2617 | 1.5988 | 1.725 | 1.1507 | 1.2141 | 1.3857 | 1.1806 | 1.1678 |
| MT633156 | 2.2261 | 2.3079 | 2.2884 | 1.9266 | 2.2777 | 2.2617 | None | 2.4741 | 2.8254 | 2.0582 | 2.0242 | 2.4111 | 2.0232 | 2.0604 |
| MN989610 | 1.5824 | 1.5151 | 1.5478 | 1.2737 | 1.6554 | 1.5988 | 2.4741 | None | 2.2828 | 1.2089 | 1.2356 | 1.6631 | 1.1921 | 1.2006 |
| LC102961 | 1.6236 | 1.6768 | 1.6369 | 1.1325 | 1.7588 | 1.725 | 2.8254 | 2.2828 | None | 0.947 | 1.0429 | 2.4073 | 1.0142 | 1.021 |
| IAL-R193 | 1.2486 | 1.1326 | 1.2352 | 0.999 | 1.1886 | 1.1507 | 2.0582 | 1.2089 | 0.947 | None | 0.5722 | 0.9145 | 0.5865 | 0.5743 |
| IAL-R558 | 1.2736 | 1.0816 | 1.2762 | 0.9146 | 1.2276 | 1.2141 | 2.0242 | 1.2356 | 1.0429 | 0.5722 | None | 0.9316 | 0.4324 | 0.4272 |
| IAL-R2597 | 1.322 | 1.2795 | 1.3485 | 1.0105 | 1.4169 | 1.3857 | 2.4111 | 1.6631 | 2.4073 | 0.9145 | 0.9316 | None | 0.8516 | 0.8224 |
| IAL-R2598 | 1.2609 | 1.0754 | 1.2323 | 0.9257 | 1.2077 | 1.1806 | 2.0232 | 1.1921 | 1.0142 | 0.5865 | 0.4324 | 0.8516 | None | 0.2972 |
| IAL-R2601 | 1.2433 | 1.0572 | 1.2301 | 0.931 | 1.1962 | 1.1678 | 2.0604 | 1.2006 | 1.021 | 0.5743 | 0.4272 | 0.4324 | 0.2972 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, R.S.; França, Y.; Viana, E.; de Azevedo, L.S.; Guiducci, R.; de Lima Neto, D.F.; da Costa, A.C.; Luchs, A. Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone. Viruses 2023, 15, 664. https://doi.org/10.3390/v15030664
Medeiros RS, França Y, Viana E, de Azevedo LS, Guiducci R, de Lima Neto DF, da Costa AC, Luchs A. Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone. Viruses. 2023; 15(3):664. https://doi.org/10.3390/v15030664
Chicago/Turabian StyleMedeiros, Roberta Salzone, Yasmin França, Ellen Viana, Lais Sampaio de Azevedo, Raquel Guiducci, Daniel Ferreira de Lima Neto, Antonio Charlys da Costa, and Adriana Luchs. 2023. "Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone" Viruses 15, no. 3: 664. https://doi.org/10.3390/v15030664
APA StyleMedeiros, R. S., França, Y., Viana, E., de Azevedo, L. S., Guiducci, R., de Lima Neto, D. F., da Costa, A. C., & Luchs, A. (2023). Genomic Constellation of Human Rotavirus G8 Strains in Brazil over a 13-Year Period: Detection of the Novel Bovine-like G8P[8] Strains with the DS-1-like Backbone. Viruses, 15(3), 664. https://doi.org/10.3390/v15030664







