Co-Surveillance of Rotaviruses in Humans and Domestic Animals in Central Uganda Reveals Circulation of Wide Genotype Diversity in the Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Participants
2.3. Data and Sample Collection
2.4. Laboratory Investigations
2.5. Data Analysis
2.6. Phylogenetic Analysis
3. Results
3.1. Animal Host Characteristics
3.2. Risk Factors for Rotavirus Infection in Animals
3.3. Human Host Characteristics
4. Rotavirus Prevalence and Genotypes in Animals
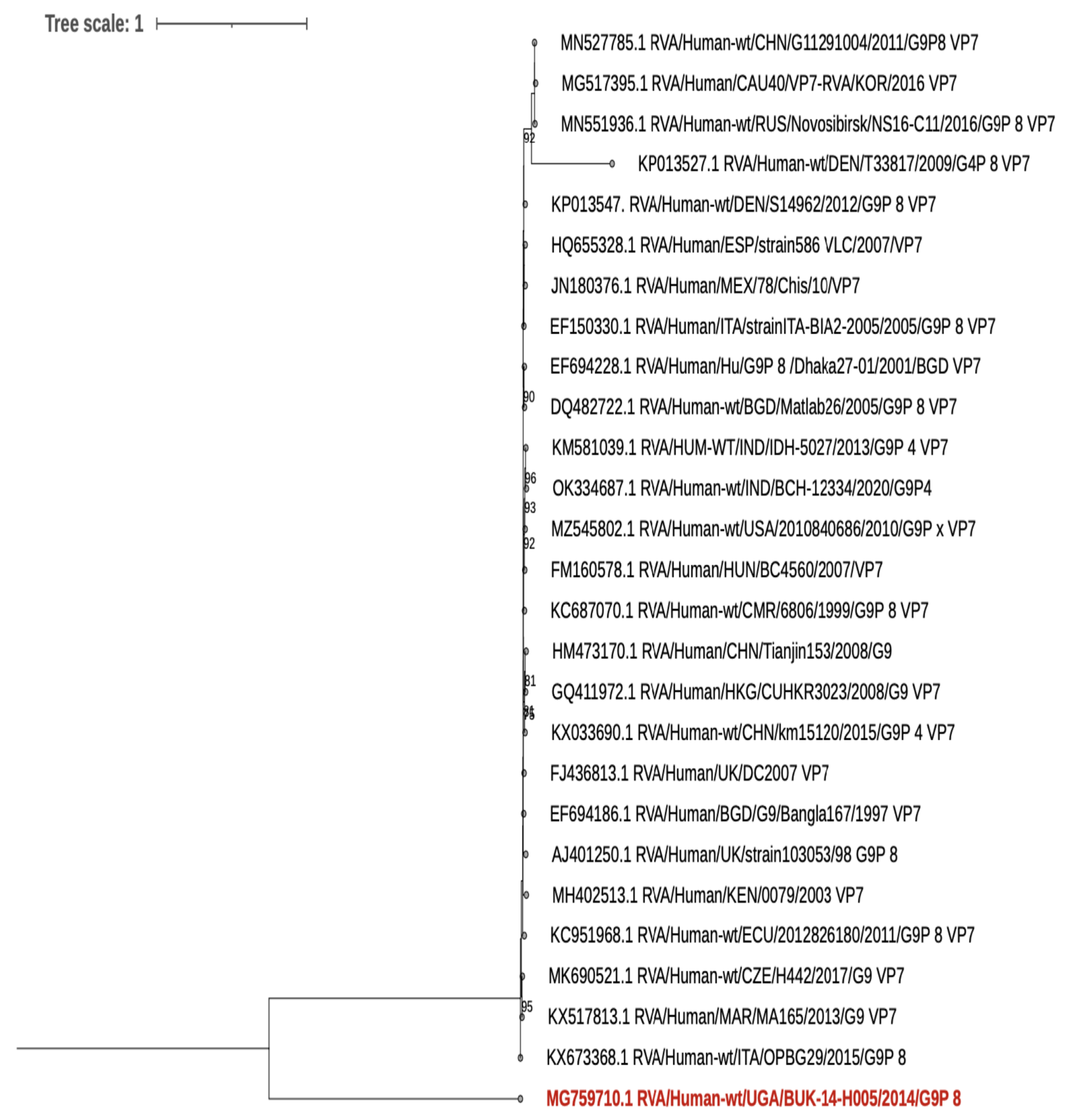
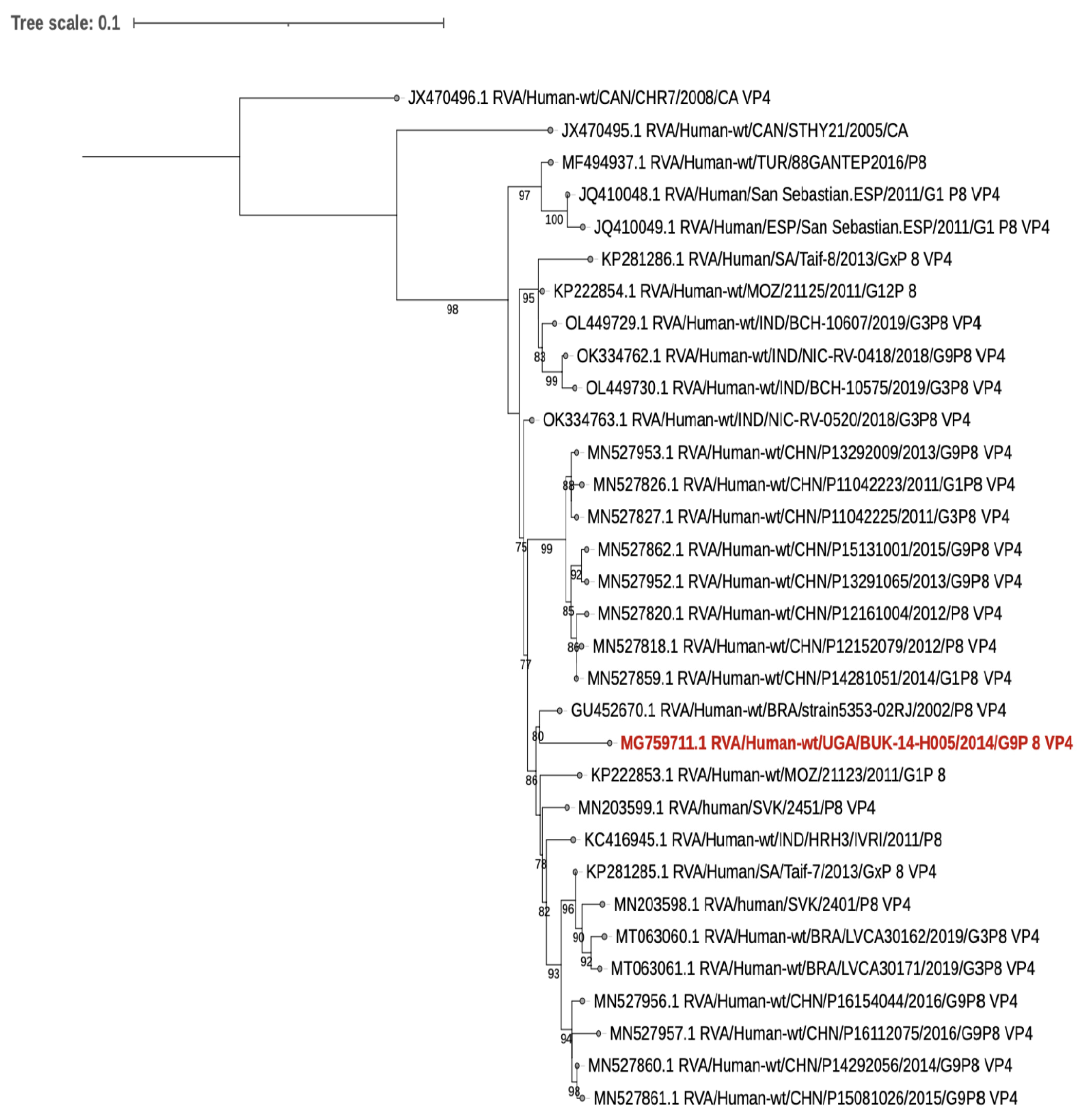
5. Human Rotavirus Sequence Results
6. Discussion
7. Study Limitations
8. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, A.L.; Platts-Mills, J.A.; Nakamura, T.; Operario, D.J.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Rey-Benito, G.; De Oliveira, L.H.; Ortiz, C.; et al. Aetiology and incidence of diarrhoea requiring hospitalisation in children under 5 years of age in 28 low-income and middle-income countries: Findings from the Global Pediatric Diarrhea Surveillance network. BMJ Glob. Health 2022, 7, e009548. [Google Scholar] [CrossRef] [PubMed]
- Bwogi, J.; Malamba, S.; Kigozi, B.; Namuwulya, P.; Tushabe, P.; Kiguli, S.; Byarugaba, D.K.; Desselberger, U.; Iturriza-Gomara, M.; Karamagi, C. The epidemiology of rotavirus disease in under-five-year-old children hospitalized with acute diarrhea in central Uganda, 2012–2013. Arch. Virol. 2016, 161, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin. Infect. Dis. 2016, 62 (Suppl. S2), S96–S105. [Google Scholar] [CrossRef] [PubMed]
- Marlow, R.; Finn, A.; Trotter, C. Quality of life impacts from rotavirus gastroenteritis on children and their families in the UK. Vaccine 2015, 33, 5212–5216. [Google Scholar] [CrossRef]
- Estes, M.K.; Greenberg, H.B. (Eds.) Rotaviruses and Their Replication, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1347–1401. [Google Scholar]
- Svensmark, B.; Nielsen, K.; Dalsgaard, K.; Willeberg, P. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. III. Rotavirus infection. Acta Vet. Scand. 1989, 30, 63–70. [Google Scholar] [CrossRef]
- Katsuda, K.; Kohmoto, M.; Kawashima, K.; Tsunemitsu, H. Frequency of enteropathogen detection in suckling and weaned pigs with diarrhea in Japan. J. Vet. Diagn. Investig. 2006, 18, 350–354. [Google Scholar] [CrossRef]
- Midgley, S.E.; Banyai, K.; Buesa, J.; Halaihel, N.; Hjulsager, C.K.; Jakab, F.; Kaplon, J.; Larsen, L.E.; Monini, M.; Poljsak-Prijatelj, M.; et al. Diversity and zoonotic potential of rotaviruses in swine and cattle across Europe. Vet. Microbiol. 2012, 156, 238–245. [Google Scholar] [CrossRef]
- Seid, U.; Dawo, F.; Tesfaye, A.; Ahmednur, M. Isolation and characterization of coronavirus and rotavirus associated with calves in central part of Oromia, Ethiopia. Vet. Med. Int. 2020, 2020, 8869970. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Liu, P.; Zhu, F. The performance of licensed rotavirus vaccines and the development of a new generation of rotavirus vaccines: A review. Hum. Vaccines Immunother. 2021, 17, 880–896. [Google Scholar] [CrossRef]
- Fritzen, J.T.; Oliveira, M.V.; Lorenzetti, E.; Miyabe, F.M.; Viziack, M.P.; Rodrigues, C.A.; Ayres, H.; Alfieri, A.F.; Alfieri, A.A. Longitudinal surveillance of rotavirus A genotypes circulating in a high milk yield dairy cattle herd after the introduction of a rotavirus vaccine. Vet. Microbiol. 2019, 230, 260–264. [Google Scholar] [CrossRef]
- Arista, S.; Giammanco, G.M.; De Grazia, S.; Ramirez, S.; Lo Biundo, C.; Colomba, C.; Cascio, A.; Martella, V. Heterogeneity and temporal dynamics of evolution of G1 human rotaviruses in a settled population. J. Virol. 2006, 80, 10724–10733. [Google Scholar] [CrossRef]
- Velasquez, D.E.; Parashar, U.D.; Jiang, B. Strain diversity plays no major role in the varying efficacy of rotavirus vaccines: An overview. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 28, 561–571. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Khan, J.; Aziz, A. Effect of rotavirus genetic diversity on vaccine impact. Rev. Med. Virol. 2022, 32, e2259. [Google Scholar] [CrossRef]
- Banyai, K.; Laszlo, B.; Duque, J.; Steele, A.D.; Nelson, E.A.; Gentsch, J.R.; Parashar, U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: Insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012, 30 (Suppl. S1), A122–A130. [Google Scholar] [CrossRef]
- Martella, V.; Banyai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar] [CrossRef]
- Kobayashi, N.; Ishino, M.; Wang, Y.; Chawla-Sarkar, M.; Krishnan, T.; Naik, T. Diversity of G-type and P-type of human and animal rotaviruses and its genetic background. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 847–858. [Google Scholar]
- Papp, H.; László, B.; Jakab, F.; Ganesh, B.; De Grazia, S.; Matthijnssens, J.; Ciarlet, M.; Martella, V.; Bányai, K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013, 165, 190–199. [Google Scholar] [CrossRef]
- Ghosh, S.; Kobayashi, N. Exotic rotaviruses in animals and rotaviruses in exotic animals. Virusdisease 2014, 25, 158–172. [Google Scholar] [CrossRef]
- German, A.; Iturriza-Gómara, M.; Dove, W.; Sandrasegaram, M.; Nakagomi, T.; Nakagomi, O.; Cunliffe, N.; Radford, A.; Morgan, K. Molecular epidemiology of rotavirus in cats in the United Kingdom. J. Clin. Microbiol. 2015, 53, 455–464. [Google Scholar] [CrossRef]
- Matthijnssens, J.; De Grazia, S.; Piessens, J.; Heylen, E.; Zeller, M.; Giammanco, G.M.; Bányai, K.; Buonavoglia, C.; Ciarlet, M.; Martella, V. Multiple reassortment and interspecies transmission events contribute to the diversity of feline, canine and feline/canine-like human group A rotavirus strains. Infect. Genet. Evol. 2011, 11, 1396–1406. [Google Scholar] [CrossRef]
- de Beer, M.; Steele, A.D. Characterization of the VP7 and VP4 genes of a South African Group A Caprine rotavirus. Genbank Record, 2002. [Google Scholar]
- Amimo, J.O.; Junga, J.O.; Ogara, W.O.; Vlasova, A.N.; Njahira, M.N.; Maina, S.; Okoth, E.A.; Bishop, R.P.; Saif, L.J.; Djikeng, A. Detection and genetic characterization of porcine group A rotaviruses in asymptomatic pigs in smallholder farms in East Africa: Predominance of P[8] genotype resembling human strains. Vet. Microbiol. 2015, 175, 195–210. [Google Scholar] [CrossRef] [PubMed]
- MAAIF; UBOS. The National Livestock Census Report 2008; UBOS: Kampala, Uganda, 2009. [Google Scholar]
- Kish, L. Survey Sampling; John Wesley & Sons. Inc: New York, NY, USA, 1965. [Google Scholar]
- Fodha, I.; Boumaiza, A.; Chouikha, A.; Dewar, J.; Armah, G.; Geyer, A.; Trabelsi, A.; Steele, A.D. Detection of group a rotavirus strains circulating in calves in Tunisia. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Geyer, A.; Sebata, T.; Peenze, I.; Steele, A.D. Group B and C porcine rotaviruses identified for the first time in South Africa. J. S. Afr. Vet. Assoc. 1996, 67, 115–116. [Google Scholar] [PubMed]
- Freeman, M.M.; Kerin, T.; Hull, J.; McCaustland, K.; Gentsch, J. Enhancement of detection and quantification of rotavirus in stool using a modified real-time RT-PCR assay. J. Med. Virol. 2008, 80, 1489–1496. [Google Scholar] [CrossRef]
- Amimo, J.O.; Otieno, T.; Okoth, E.; Onono, J.; Bett, B. Risk factors for rotavirus infection in pigs in Busia and Teso subcounties, Western Kenya. Trop. Anim. Health Prod. 2017, 49, 105–112. [Google Scholar] [CrossRef]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- World Health Organization. Manual of Rotavirus Detection and Characterization Methods; World Health Organization: Geneva, Switzerland, 2009.
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Iturriza-Gomara, M.; Green, J.; Brown, D.W.; Desselberger, U.; Gray, J.J. Diversity within the VP4 gene of rotavirus P[8] strains: Implications for reverse transcription-PCR genotyping. J. Clin. Microbiol. 2000, 38, 898–901. [Google Scholar] [CrossRef]
- Iturriza-Gomara, M.; Kang, G.; Gray, J. Rotavirus genotyping: Keeping up with an evolving population of human rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef]
- Aladin, F.; Nawaz, S.; Iturriza-Gomara, M.; Gray, J. Identification of G8 rotavirus strains determined as G12 by rotavirus genotyping PCR: Updating the current genotyping methods. J. Clin. Virol. 2010, 47, 340–344. [Google Scholar] [CrossRef]
- Dean, A.; Arner, T.; Sunki, G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.; Sullivan, K.; Brendel, K.; Gao, Z. Epi Info™, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2011; Volume 1. [Google Scholar]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Dione, M.; Masembe, C.; Akol, J.; Amia, W.; Kungu, J.; Lee, H.S.; Wieland, B. The importance of on-farm biosecurity: Sero-prevalence and risk factors of bacterial and viral pathogens in smallholder pig systems in Uganda. Acta Trop. 2018, 187, 214–221. [Google Scholar] [CrossRef]
- Khafagi, M.; Mahmoud, M.; Habashi, A. Prevalence of rotavirus infections in small ruminants. Glob. Vet. 2010, 4, 539–543. [Google Scholar]
- Ali, Y. Rotavirus infection in Humanand Domestic Animals in Sudan. J. Sci. Technol. 2011, 12, 58–63. [Google Scholar]
- Alkan, F.; Gulyaz, V.; Ozkan Timurkan, M.; Iyisan, S.; Ozdemir, S.; Turan, N.; Buonavoglia, C.; Martella, V. A large outbreak of enteritis in goat flocks in Marmara, Turkey, by G8P [1] group A rotaviruses. Arch. Virol. 2012, 157, 1183–1187. [Google Scholar] [CrossRef]
- Boene, S.S.; João, E.D.; Strydom, A.; Munlela, B.; Chissaque, A.; Bauhofer, A.F.L.; Nabetse, E.; Latifo, D.; Cala, A.; Mapaco, L. Prevalence and genome characterization of porcine rotavirus A in southern Mozambique. Infect. Genet. Evol. 2021, 87, 104637. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rahman, M.S.; Watson, O.J.; Islam, A.; Rahman, S.; Hasan, R.; Kafi, M.A.H.; Osmani, M.G.; Epstein, J.H.; Daszak, P. Epidemiology and genotypes of group A rotaviruses in cattle and goats of Bangladesh, 2009–2010. Infect. Genet. Evol. 2020, 79, 104170. [Google Scholar] [CrossRef]
- Mwenda, J.M.; Tate, J.E.; Parashar, U.D.; Mihigo, R.; Agócs, M.; Serhan, F.; Nshimirimana, D. African rotavirus surveillance network: A brief overview. Pediatr. Infect. Dis. J. 2014, 33, S6–S8. [Google Scholar] [CrossRef] [PubMed]
- Agutu, M.-T.; Ongus, J.; Kombich, J.; Kamenwa, R.; Nyangao, J.; Kagira, J.; Ogutu, A.A.; Bitek, A. Prevalence and genetic diversity of rotavirus infection in children with acute gastroenteritis in a hospital setting, Nairobi Kenya in post vaccination era: A cross-sectional study. Pan Afr. Med. J. 2017, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mwenda, J.M.; Ntoto, K.M.; Abebe, A.; Enweronu-Laryea, C.; Amina, I.; Mchomvu, J.; Kisakye, A.; Mpabalwani, E.M.; Pazvakavambwa, I.; Armah, G.E. Burden and epidemiology of rotavirus diarrhea in selected African countries: Preliminary results from the African Rotavirus Surveillance Network. J. Infect. Dis. 2010, 202, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Odiit, A.; Mulindwa, A.; Nalumansi, E.; Mphahlele, M.J.; Seheri, L.M.; Mwenda, J.M.; Kisakye, A. Rotavirus prevalence and genotypes among children younger than 5 years with acute diarrhea at Mulago National Referral Hospital, Kampala, Uganda. Pediatr. Infect. Dis. J. 2014, 33, S41–S44. [Google Scholar] [CrossRef]
- Bwogi, J.; Jere, K.C.; Karamagi, C.; Byarugaba, D.K.; Namuwulya, P.; Baliraine, F.N.; Desselberger, U.; Iturriza-Gomara, M. Whole genome analysis of selected human and animal rotaviruses identified in Uganda from 2012 to 2014 reveals complex genome reassortment events between human, bovine, caprine and porcine strains. PLoS ONE 2017, 12, e0178855. [Google Scholar] [CrossRef]
| Variable | Rotavirus Negative | Rotavirus Positive (%) | Total N = 1137 |
|---|---|---|---|
| Sex | |||
| Male | 423 | 21 (5.0) | 444 |
| Female | 669 | 24 (3.6) | 693 |
| Age (months) * | |||
| 0–2 | 218 | 12 (5.5) | 230 |
| 2.1–6 | 338 | 16 (4.7) | 354 |
| 6.1–12 | 205 | 5 (2.4) | 210 |
| 12.1–18 | 99 | 2 (2.0) | 101 |
| 18.1–24 | 76 | 2 (2.6) | 78 |
| ≥24.1 | 133 | 7 (5.3) | 140 |
| Animal type | |||
| Pig | 423 | 15 (3.5) | 438 |
| Goat | 409 | 9 (2.2) | 418 |
| Cattle | 260 | 21 (8.1) | 281 |
| Sub-county | |||
| Bukakata | 174 | 0 (0.0) | 174 |
| Buwunga | 88 | 14 (15.9) | 102 |
| Kabonera | 178 | 10 (5.6) | 188 |
| Kyanamukaka | 195 | 3 (1.5) | 198 |
| Kyesiiga | 233 | 6 (2.6) | 239 |
| Mukungwe | 231 | 12 (5.2) | 243 |
| Animal Suckling * | |||
| No | 821 | 32 (3.9) | 853 |
| Yes | 265 | 11 (4.2) | 276 |
| Diarrhoea in past 2 weeks * | |||
| No | 973 | 38 (3.9) | 1011 |
| Yes | 110 | 7 (6.4) | 117 |
| Univariate Analysis | Multi-Variable Analysis | |||||
|---|---|---|---|---|---|---|
| Animal Characteristic | Unadjusted Odds Ratio | 95% CI | p-Value | Adjusted Odds Ratio | 95% CI | p-Value |
| Sex of Animal | ||||||
| Male | 1.00 | |||||
| Female | 0.76 | 0.38–1.51 | 0.428 | - | - | - |
| Age of Animal in months | ||||||
| 0.0–2.0 | 1.00 | 1.00 | ||||
| 2.1–6.0 | 0.39 | 0.15–1.04 | 0.059 | 0.29 | 0.10–0.81 | 0.018 |
| 6.1–12.0 | 0.21 | 0.06–0.74 | 0.016 | 0.17 | 0.04–0.62 | 0.008 |
| 12.1–18.0 | 0.14 | 0.02–0.83 | 0.030 | 0.08 | 0.01–0.62 | 0.016 |
| 18.1–24.0 | 0.26 | 0.05–1.51 | 0.134 | 0.20 | 0.03–1.37 | 0.102 |
| >24.0 | 0.37 | 0.10–1.30 | 0.119 | 0.22 | 0.05–1.09 | 0.064 |
| Animal Type | ||||||
| Pigs | 1.00 | 1.00 | ||||
| Goats | 0.57 | 0.21–1.53 | 0.266 | 0.91 | 0.29–2.80 | 0.863 |
| Cows | 1.05 | 0.41–2.72 | 0.915 | 1.39 | 0.40–4.83 | 0.606 |
| Animal suckling | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.90 | 0.38–2.15 | 0.816 | 0.47 | 0.14–1.53 | 0.208 |
| Diarrhoea in past 2 weeks | ||||||
| No | 1.00 | |||||
| Yes | 1.51 | 0.54–4.25 | 0.430 | - | - | - |
| P-Genotype | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P[1] | P[6] | P[7] | P[8] | P[9] | P[10] | P[11] | P[5], P[10] | P[6], P[11] | P[6], P[7] | P[7], P[9] | P[8], P[10] | P[9], P[11] | P[4], P[5], P[11] | No P Type identified | Total | ||
| G3 | 1 | 1 | |||||||||||||||
| G8 | 1 | 3 | 5 | ||||||||||||||
| G10 | 1 | 1 | 2 | ||||||||||||||
| G-Genotype | G11 | 1 | 1 | 1 | 3 | ||||||||||||
| G12 | 1 | 1 | 2 | ||||||||||||||
| G9,G11 | 1 | 1 | |||||||||||||||
| G10,G12 | 1 | 2 | 1 | 1 | 4 | 9 | |||||||||||
| G11,G12 | 1 | 2 | 3 | ||||||||||||||
| G8,G9, G10,G12 | 1 | 1 | 2 | ||||||||||||||
| G9,G10, G11,G12 | 1 | 1 | 2 | ||||||||||||||
| No G-Type identified | 1 | 1 | 1 | 1 | 1 | 1 | 5 | 11 | |||||||||
| Total | 1 | 4 | 1 | 3 | 3 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 16 | 41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bwogi, J.; Karamagi, C.; Byarugaba, D.K.; Tushabe, P.; Kiguli, S.; Namuwulya, P.; Malamba, S.S.; Jere, K.C.; Desselberger, U.; Iturriza-Gomara, M. Co-Surveillance of Rotaviruses in Humans and Domestic Animals in Central Uganda Reveals Circulation of Wide Genotype Diversity in the Animals. Viruses 2023, 15, 738. https://doi.org/10.3390/v15030738
Bwogi J, Karamagi C, Byarugaba DK, Tushabe P, Kiguli S, Namuwulya P, Malamba SS, Jere KC, Desselberger U, Iturriza-Gomara M. Co-Surveillance of Rotaviruses in Humans and Domestic Animals in Central Uganda Reveals Circulation of Wide Genotype Diversity in the Animals. Viruses. 2023; 15(3):738. https://doi.org/10.3390/v15030738
Chicago/Turabian StyleBwogi, Josephine, Charles Karamagi, Denis Karuhize Byarugaba, Phionah Tushabe, Sarah Kiguli, Prossy Namuwulya, Samuel S. Malamba, Khuzwayo C. Jere, Ulrich Desselberger, and Miren Iturriza-Gomara. 2023. "Co-Surveillance of Rotaviruses in Humans and Domestic Animals in Central Uganda Reveals Circulation of Wide Genotype Diversity in the Animals" Viruses 15, no. 3: 738. https://doi.org/10.3390/v15030738
APA StyleBwogi, J., Karamagi, C., Byarugaba, D. K., Tushabe, P., Kiguli, S., Namuwulya, P., Malamba, S. S., Jere, K. C., Desselberger, U., & Iturriza-Gomara, M. (2023). Co-Surveillance of Rotaviruses in Humans and Domestic Animals in Central Uganda Reveals Circulation of Wide Genotype Diversity in the Animals. Viruses, 15(3), 738. https://doi.org/10.3390/v15030738








