FAdV-4 Promotes Expression of Multiple Cytokines and Inhibits the Proliferation of aHEV in LMH Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Infected Chickens and Their Sample Collection
2.3. Detection of FAdV-I Nucleic Acid in Liver Samples of Infected Poultry
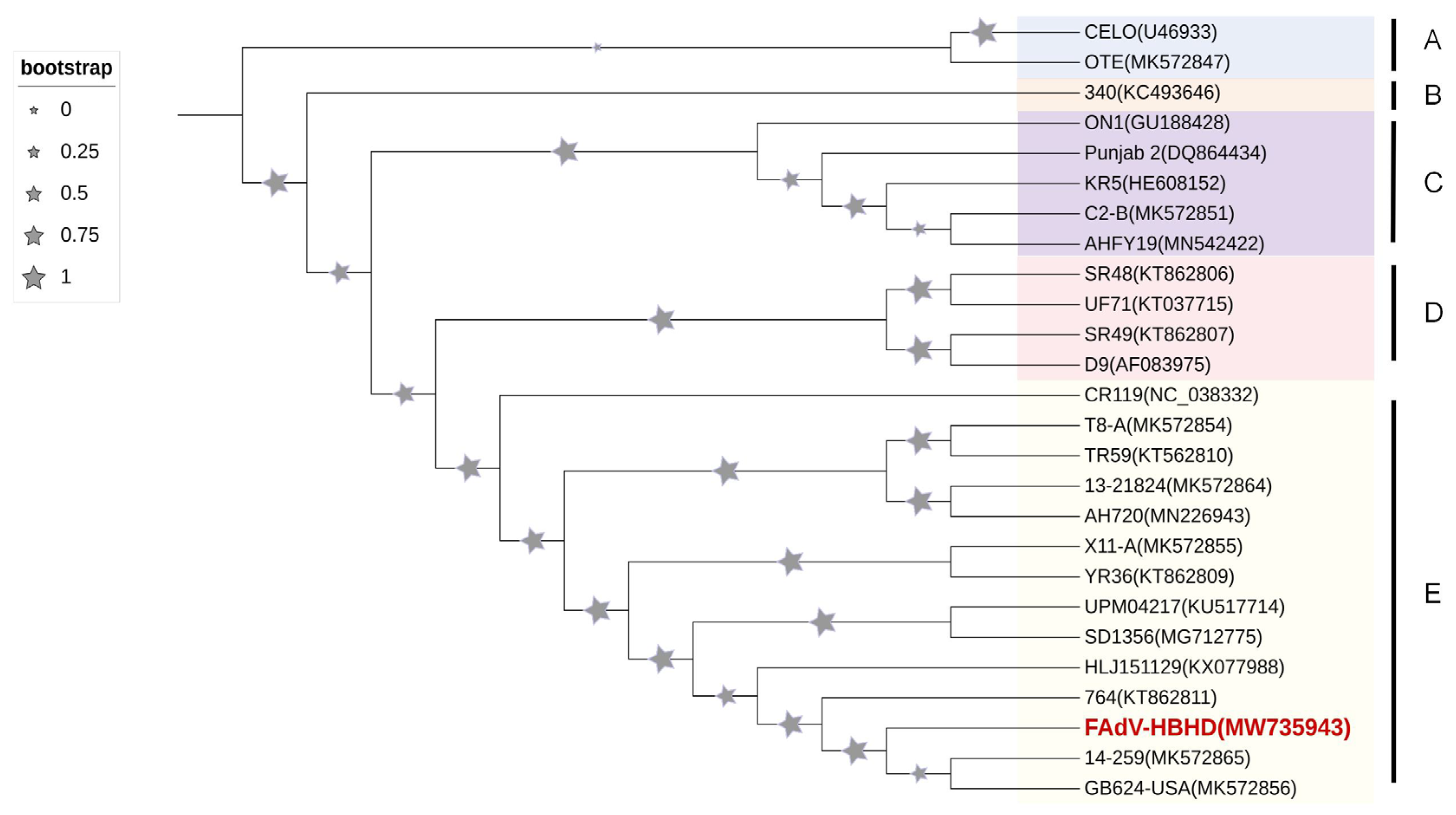
2.4. aHEV Detection and Sequence Analysis in Liver Samples of Diseased Chickens
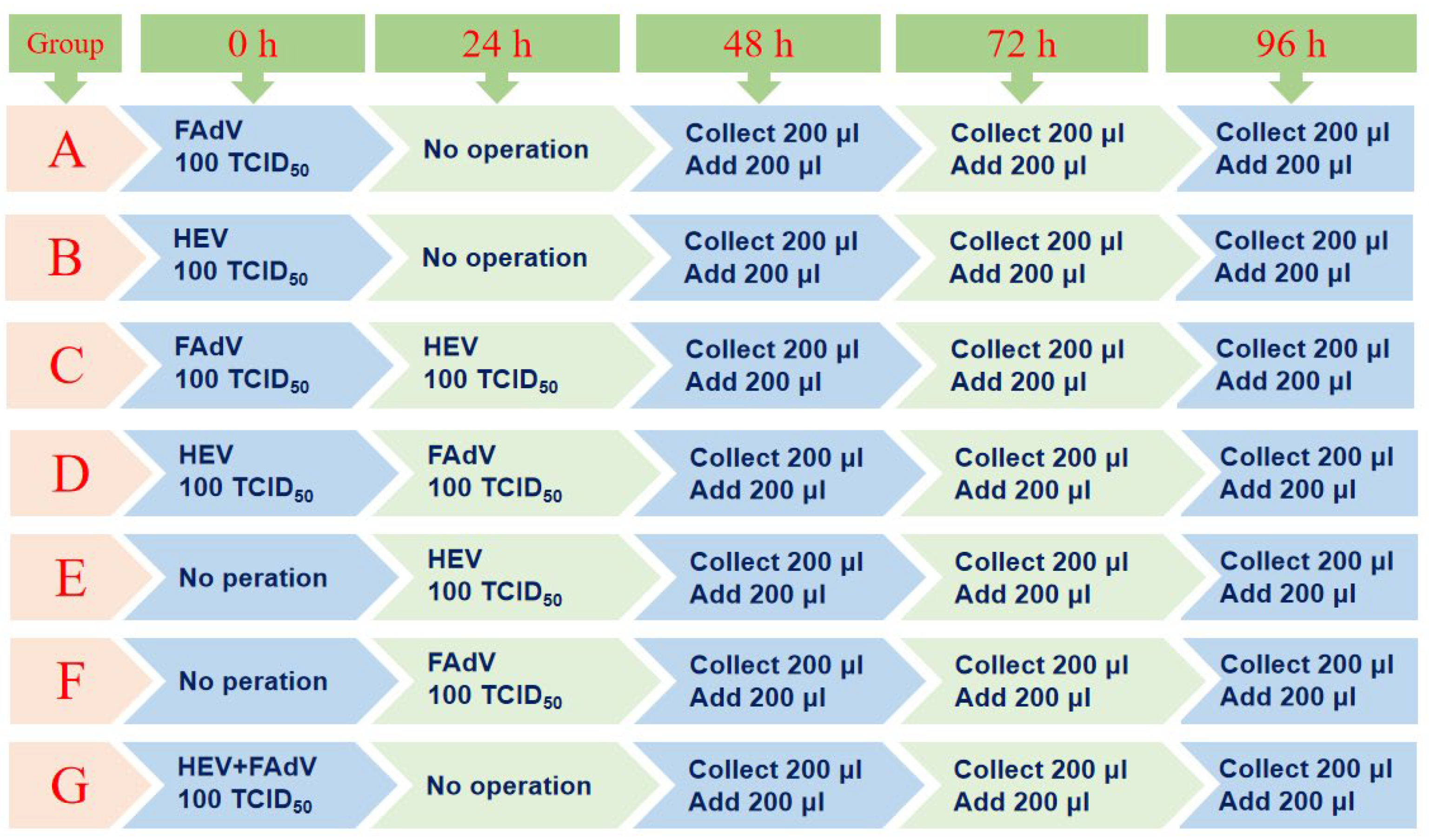
2.5. Detailed Experimental Protocol for Infection of aHEV and FAdV-I in LMH Cells
2.6. Confirmation of aHEV and FAdV-I Co-Infection in LMH Cells
2.7. The Effect of aHEV and FAdV-I Co-Infection on Cytokine Expression Levels in LMH Cells
2.8. Statistical Analysis
3. Results
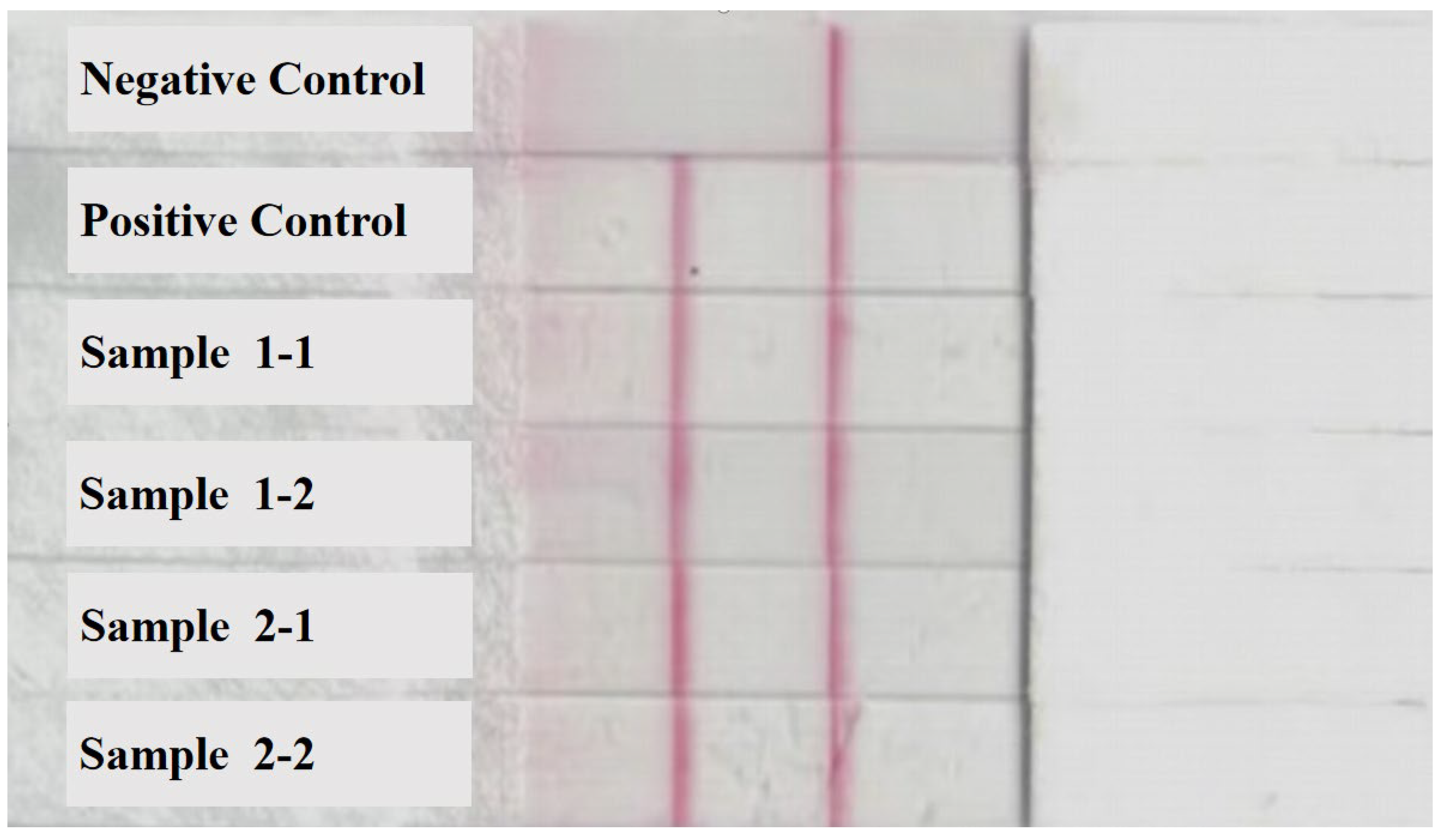
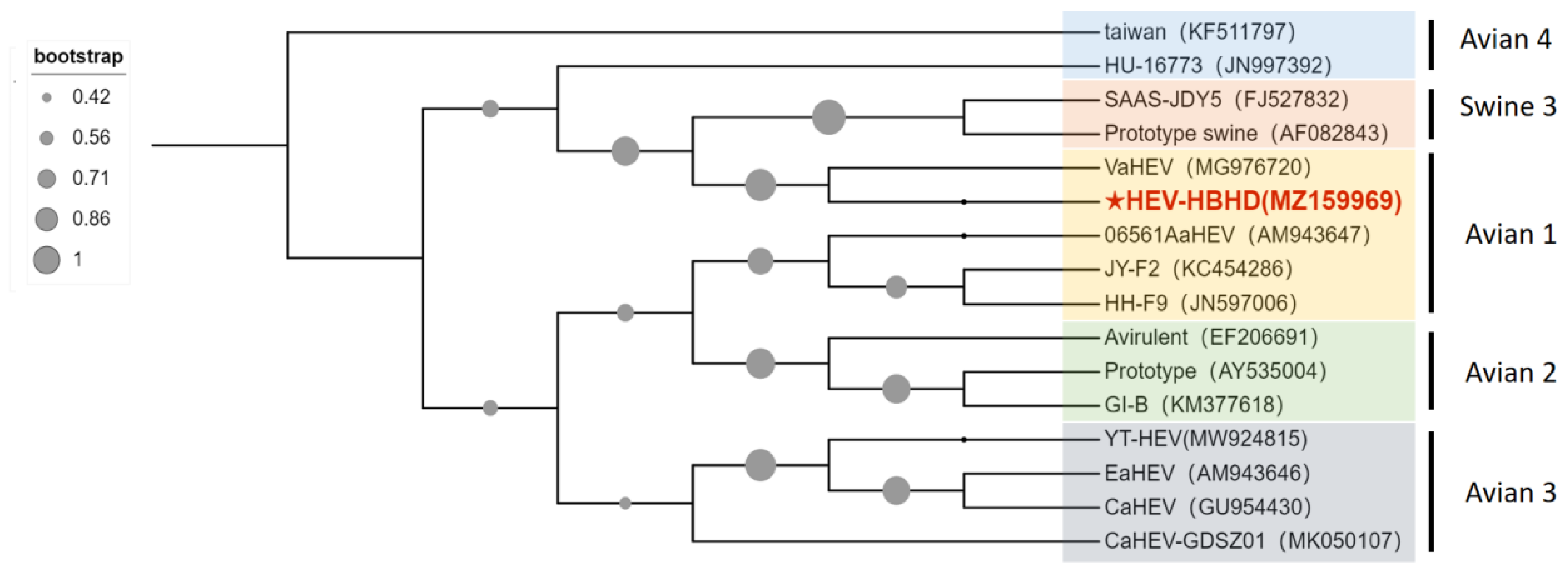
3.1. Identification of aHEV and FAdV-I Co-Infection in Liver Samples of Hy-Line Brown Dead Chickens
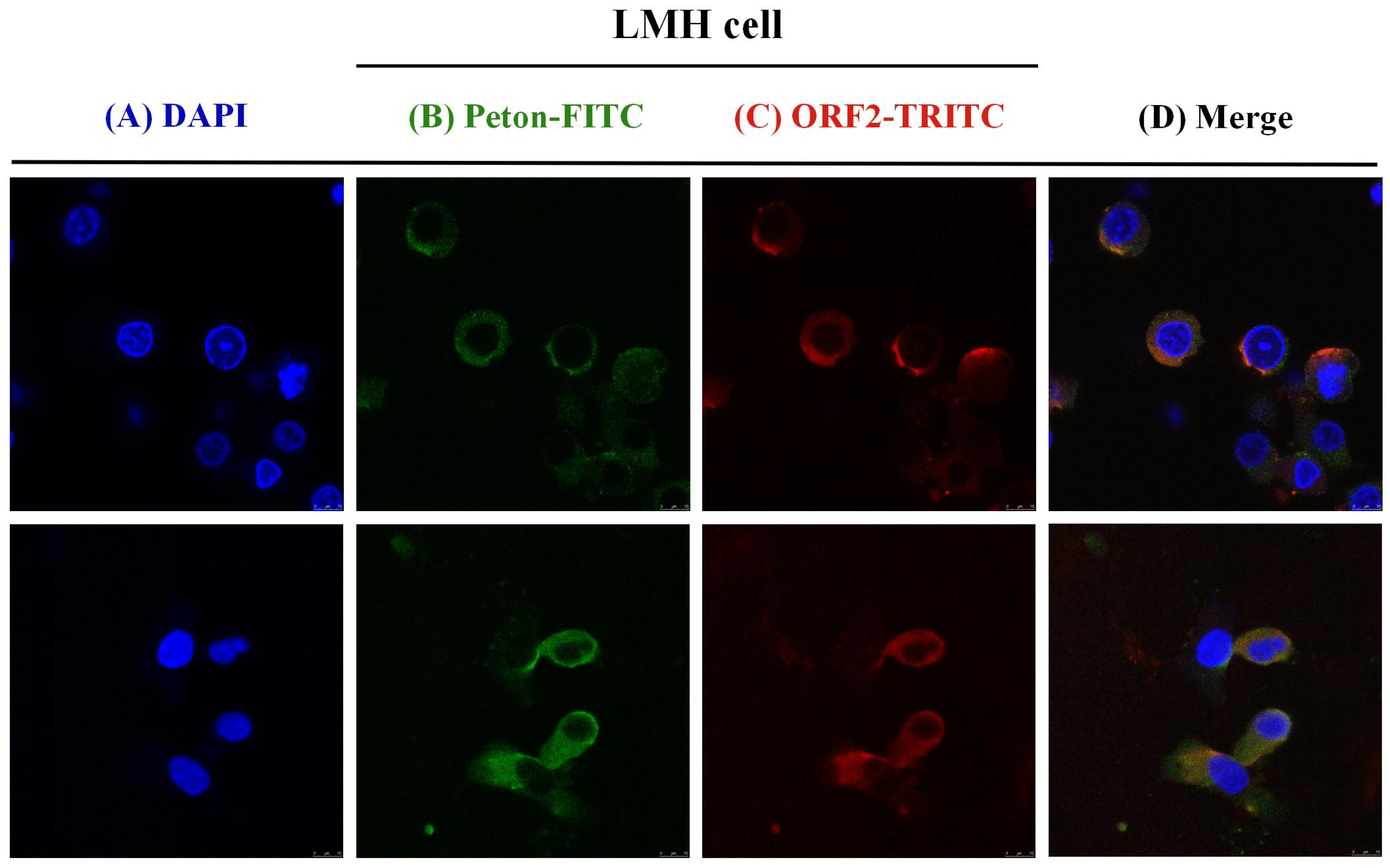
3.2. Co-Localization of aHEV and FAdV-I Infection in LMH Cells
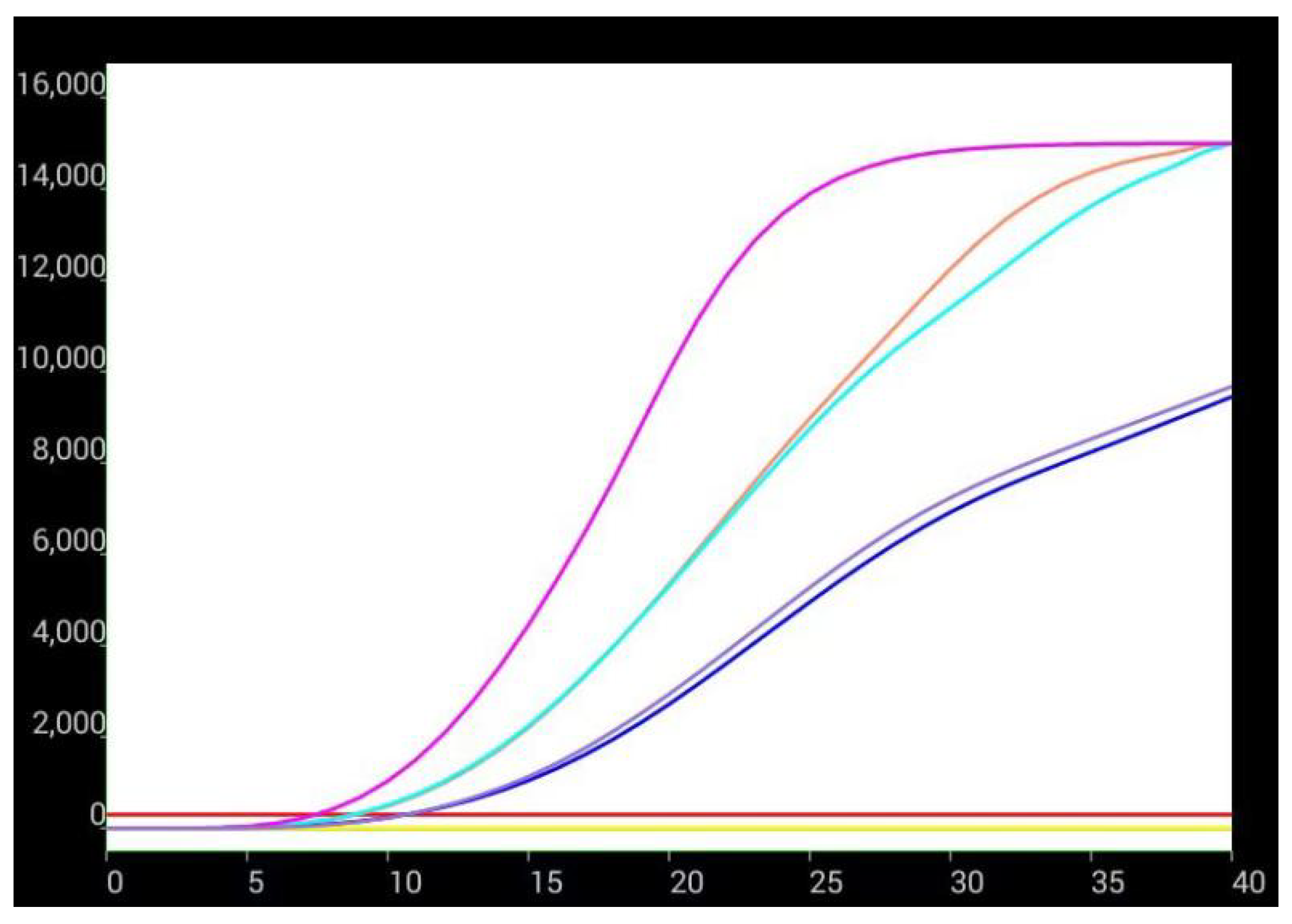
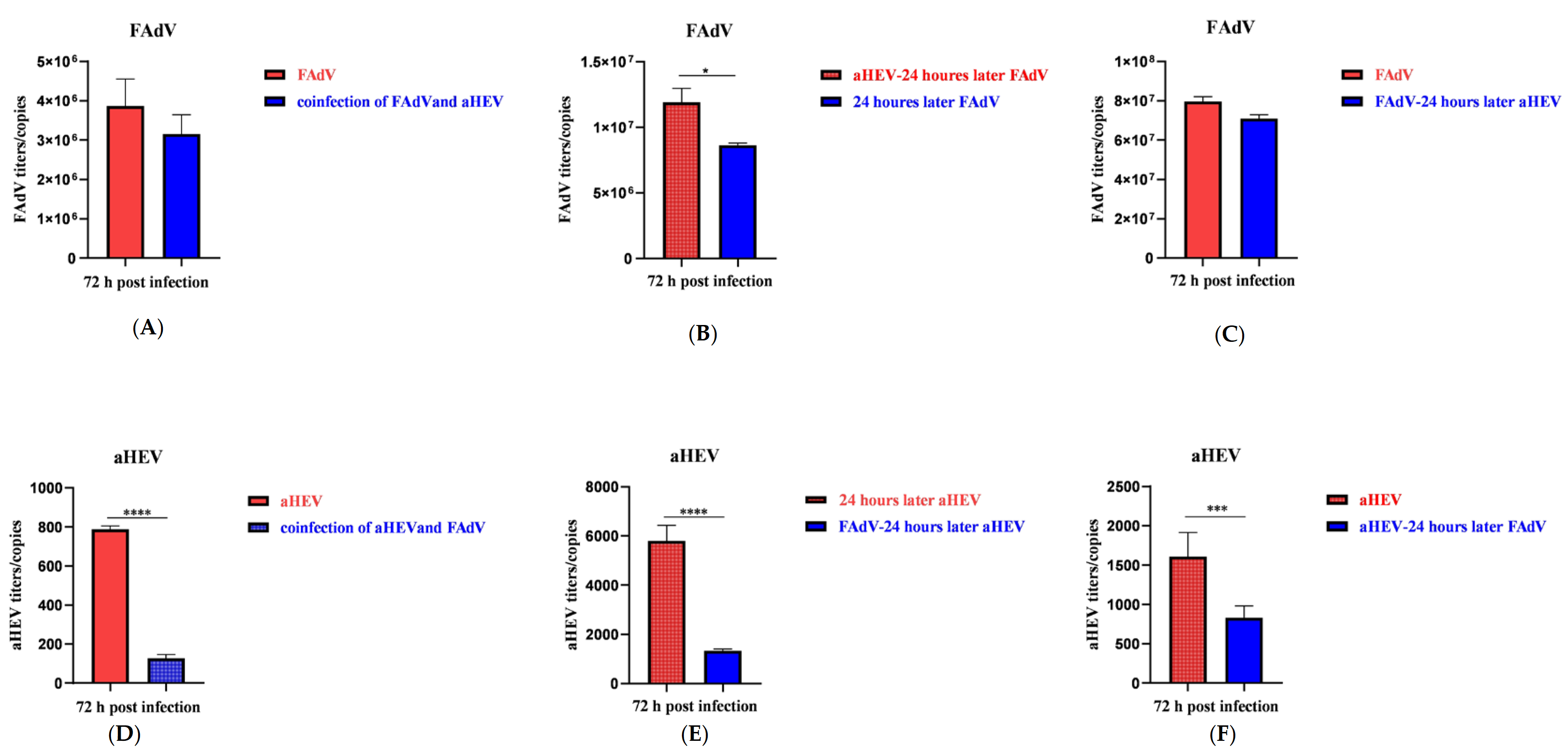
3.3. Effects of aHEV and FAdV-I on Each Other’s Replication upon Co-Infection in LMH Cells
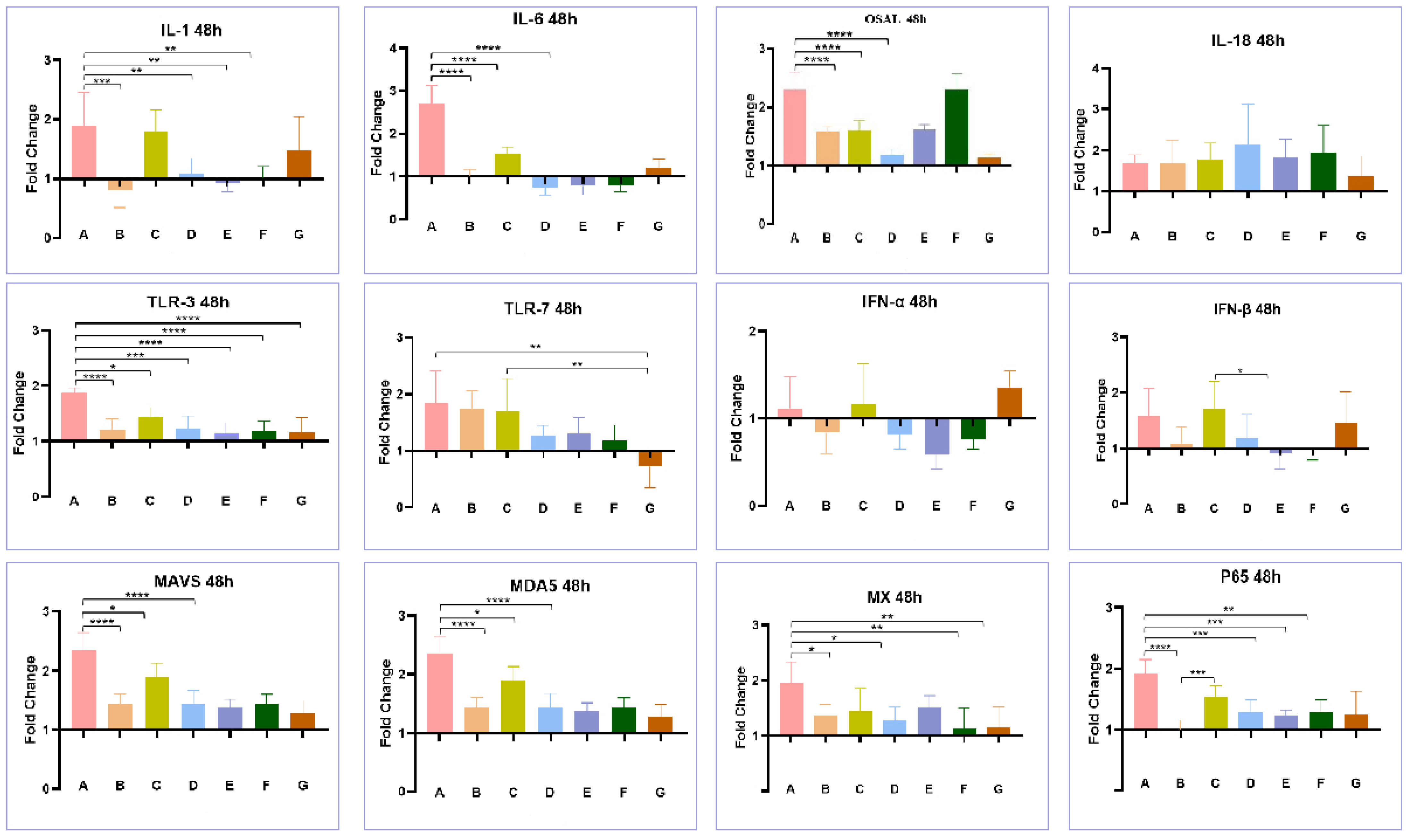
3.4. Effect of Co-Infection of HEV and FAdV-4 on Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, S.J.; Riddell, C. British Columbia. “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. Can. Vet J. 1991, 32, 500–501. [Google Scholar] [PubMed]
- Tablante, N.L.; Vaillancourt, J.P.; Julian, R.J. Necrotic, haemorrhagic, hepatomegalic hepatitis associated with vasculitis and amyloidosis in commercial laying hens. Avian Pathol. 1994, 23, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Haqshenas, G.; Shivaprasad, H.L.; Guenette, D.K.; Woolcock, P.R.; Larsen, C.T.; Pierson, F.W.; Elvinger, F.; Toth, T.E.; Meng, X.J. Heterogeneity and seroprevalence of a newly identified avian hepatitis e virus from chickens in the United States. J. Clin. Microbiol. 2002, 40, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Peralta, B.; Biarnes, M.; Ordonez, G.; Porta, R.; Martin, M.; Mateu, E.; Pina, S.; Meng, X.J. Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Vet. Microbiol. 2009, 137, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bilic, I.; Jaskulska, B.; Basic, A.; Morrow, C.J.; Hess, M. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J. Gen. Virol. 2009, 90, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, E.M.; Dong, S.W.; Qiu, H.K.; Zhang, L.; Hu, S.B.; Zhao, F.F.; Jiang, S.J.; Sun, Y.N. Analysis of avian hepatitis E virus from chickens, China. Emerg. Infect. Dis. 2010, 16, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Banyai, K.; Toth, A.G.; Ivanics, E.; Glavits, R.; Szentpali-Gavaller, K.; Dan, A. Putative novel genotype of avian hepatitis E virus, Hungary, 2010. Emerg. Infect. Dis. 2012, 18, 1365–1368. [Google Scholar] [CrossRef]
- Lim, T.H.; Kim, B.Y.; Kim, M.S.; Jang, J.H.; Lee, D.H.; Kwon, Y.K.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea. Poult. Sci. 2012, 91, 1113–1117. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, W.F.; Yuan, S.; Guo, J.Y.; Li, Z.L.; Chi, S.H.; Huang, W.J.; Li, X.W.; Huang, S.J.; Shao, J.W. Meta-transcriptomic analysis reveals a new subtype of genotype 3 avian hepatitis E virus in chicken flocks with high mortality in Guangdong, China. BMC Vet. Res. 2019, 15, 131. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Y.; Cui, Z.; Chang, S.; Zhao, P. Genetic diversity of avian hepatitis E virus in China, 2018–2019. Transbound. Emerg. Dis. 2020, 67, 2403–2407. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Q.; Zhang, J.; Li, X.; Zhao, J.; Fan, W.; Ji, P.; Wang, K.; Zhou, E.M.; Zhao, Q. Co-infection with avian hepatitis E virus and avian leukosis virus subgroup J as the cause of an outbreak of hepatitis and liver hemorrhagic syndromes in a brown layer chicken flock in China. Poult. Sci. 2020, 99, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Du, T.; Liu, B.; Syed, S.F.; Chen, Y.; Li, H.; Wang, X.; Zhang, G.; Zhou, E.M.; Zhao, Q. Seroprevalence of avian hepatitis E virus and avian leucosis virus subgroup J in chicken flocks with hepatitis syndrome, China. BMC Vet. Res. 2016, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Sun, S. Natural Infection with Avian Hepatitis E Virus and Marek’s Disease Virus in Brown Layer Chickens in China. Avian Dis. 2016, 60, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, Y.; Li, Y.; Cui, Z.; Chang, S.; Zhao, P. Epidemiological investigation of the novel genotype avian hepatitis E virus and co-infected immunosuppressive viruses in farms with hepatic rupture haemorrhage syndrome, recently emerged in China. Transbound. Emerg. Dis. 2019, 66, 776–784. [Google Scholar] [CrossRef]
- Meng, F.; Dong, G.; Zhang, Y.; Tian, S.; Cui, Z.; Chang, S.; Zhao, P. Co-infection of fowl adenovirus with different immunosuppressive viruses in a chicken flock. Poult. Sci. 2018, 97, 1699–1705. [Google Scholar] [CrossRef]
- Yu, G.; Lin, Y.; Dou, Y.; Tang, Y.; Diao, Y. Prevalence of Fowl Adenovirus Serotype 4 and Co-Infection by Immunosuppressive Viruses in Fowl with Hydropericardium Hepatitis Syndrome in Shandong Province, China. Viruses 2019, 11, 517. [Google Scholar] [CrossRef]
- Hou, L.; Su, Q.; Zhang, Y.; Liu, D.; Mao, Y.; Zhao, P. Development of a PCR-based dot blot assay for the detection of fowl adenovirus. Poult. Sci. 2022, 101, 101540. [Google Scholar] [CrossRef]
- Sun, Z.F.; Larsen, C.T.; Dunlop, A.; Huang, F.F.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J. Gen. Virol. 2004, 85, 693–700. [Google Scholar] [CrossRef]
- Yan, H.; Sun, W.; Wang, J.; Gao, Q.; Zhang, Y.; Wang, Y.; Chang, S.; Zhao, P. Combined inhibitory effect of interferon and antiserum on avian hepatitis E virus. Poult. Sci. 2023, 102, 102591. [Google Scholar] [CrossRef]
- Troxler, S.; Marek, A.; Prokofieva, I.; Bilic, I.; Hess, M. TaqMan real-time reverse transcription-PCR assay for universal detection and quantification of avian hepatitis E virus from clinical samples in the presence of a heterologous internal control RNA. J. Clin. Microbiol. 2011, 49, 1339–1346. [Google Scholar] [CrossRef]
- Heidari, M.; Wang, D.; Delekta, P.; Sun, S. Marek’s disease virus immunosuppression alters host cellular responses and immune gene expression in the skin of infected chickens. Vet. Immunol. Immunopathol. 2016, 180, 21–28. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, Z.; Dai, J.; Cao, Y.; Hou, J.; Zheng, Y.; Wei, T.; Mo, M.; Wei, P. Responses of the Toll-like receptor and melanoma differentiation-associated protein 5 signaling pathways to avian infectious bronchitis virus infection in chicks. Virol. Sin. 2016, 31, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, M.; Zhang, B.; Chen, Y.; Sun, Y.; Du, T.; Nan, Y.; Zhou, E.M.; Zhao, Q. Avian hepatitis E virus infection of duck, goose, and rabbit in northwest China. Emerg. Microbes Infect. 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; Zhao, L.; Zhang, M.; Ren, X.; Zhang, Y.; Zhang, B.; Fan, M.; Zhao, Q.; Zhou, E.M. Identification and pathogenicity of a novel genotype avian hepatitis E virus from silkie fowl (gallus gallus). Vet. Microbiol. 2020, 245, 108688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, F.; Tan, M.; Zeng, Y.; Yang, Q.; Tan, J.; Huang, J.; Huang, Y.; Kang, Z. Research Note: A putative novel subtype of the avian hepatitis E virus of genotype 3, Jiangxi province, China. Poult. Sci. 2020, 99, 6657–6663. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Zhao, J. The pros and cons of cytokines for fowl adenovirus serotype 4 infection. Arch. Virol. 2022, 167, 281–292. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Z.; Huang, K.; Lin, X.; Chen, H.; Jin, M. Insights into leghorn male hepatocellular cells response to fowl adenovirus serotype 4 infection by transcriptome analysis. Vet. Microbiol. 2018, 214, 65–74. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Pathogenesis of Hypervirulent Fowl Adenovirus Serotype 4: The Contributions of Viral and Host Factors. Viruses 2019, 11, 741. [Google Scholar] [CrossRef]

| Gene | Direction | Sequences (5′–3′) |
|---|---|---|
| FAdV | Forward | ATGGCKCAGATGGCYAAGG |
| Reverse | ATGGCKCAGATGGCYAAGG | |
| aHEV | Forward | AATGTGCTGCGGGGTGTCAA |
| Reverse | CATCTGGTACCGTGCGAGTA | |
| p65 | Forward | CCACAACACAATGCGCTCTG |
| Reverse | AACTCAGCGGCGTCGATG | |
| Mx | Forward | CAGCTCCAGAATGCATCAGA |
| Reverse | GGCAATTCCAGGAAGATCAA | |
| TLR3 | Forward | GCAACACTTCATTGAATAGCCTTGAT |
| Reverse | GCCAAACAGATTTCCAATTGCATGT | |
| TLR7 | Forward | AAGTCCCGGTATGTTCAGCT |
| Reverse | GGACAGGGTATTGTTCATAGC | |
| MDA5 | Forward | CAGCCAGTTGCCCTCGCCTCA |
| Reverse | AACAGCTCCCTTGCACCGTCT | |
| MAVS | Forward | CCTGACTCAAACAAGGGAAG |
| Reverse | AATCAGAGCGATGCCAACAG | |
| IL-1 | Forward | ATGACCAAACTGCTGCGGAG |
| Reverse | AGGTGACGGGCTCAAAAACC | |
| IL-6 | Forward | GACGAGGAGAAATGCCTGACG |
| Reverse | CGAGTCTGGGATGACCACTTC | |
| OASL | Forward | GAGATGGAGGTCCTGGTGAA |
| Reverse | CCAGCTCCTTGGTCTCGTAG | |
| IL-18 | Forward | GAGGTGAAATCTGGCAGTGG |
| Reverse | GAATGTCTTTGGGAACTTCTCC | |
| IFN-α | Forward | TACGGCATCCTGCTGCTCAC |
| Reverse | AGAGAAGGTGGCATCCTGGG | |
| IFN-β | Forward | GCCCACACACTCCAAAACACTG |
| Reverse | TGATGCTGAGGTGAGCGTTG | |
| β-Actin | Forward | CCCACCTGAGCGCAAGTACT |
| Reverse | AAGCATTTGCGGTGGACAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Wang, W.; Chi, Z.; Zhang, Y.; Zou, Z.; Zhao, P. FAdV-4 Promotes Expression of Multiple Cytokines and Inhibits the Proliferation of aHEV in LMH Cells. Viruses 2023, 15, 2072. https://doi.org/10.3390/v15102072
Hou L, Wang W, Chi Z, Zhang Y, Zou Z, Zhao P. FAdV-4 Promotes Expression of Multiple Cytokines and Inhibits the Proliferation of aHEV in LMH Cells. Viruses. 2023; 15(10):2072. https://doi.org/10.3390/v15102072
Chicago/Turabian StyleHou, Lidan, Wei Wang, Zengna Chi, Yawen Zhang, Zhong Zou, and Peng Zhao. 2023. "FAdV-4 Promotes Expression of Multiple Cytokines and Inhibits the Proliferation of aHEV in LMH Cells" Viruses 15, no. 10: 2072. https://doi.org/10.3390/v15102072
APA StyleHou, L., Wang, W., Chi, Z., Zhang, Y., Zou, Z., & Zhao, P. (2023). FAdV-4 Promotes Expression of Multiple Cytokines and Inhibits the Proliferation of aHEV in LMH Cells. Viruses, 15(10), 2072. https://doi.org/10.3390/v15102072




