Nipah Virus: An Overview of the Current Status of Diagnostics and Their Role in Preparedness in Endemic Countries
Abstract
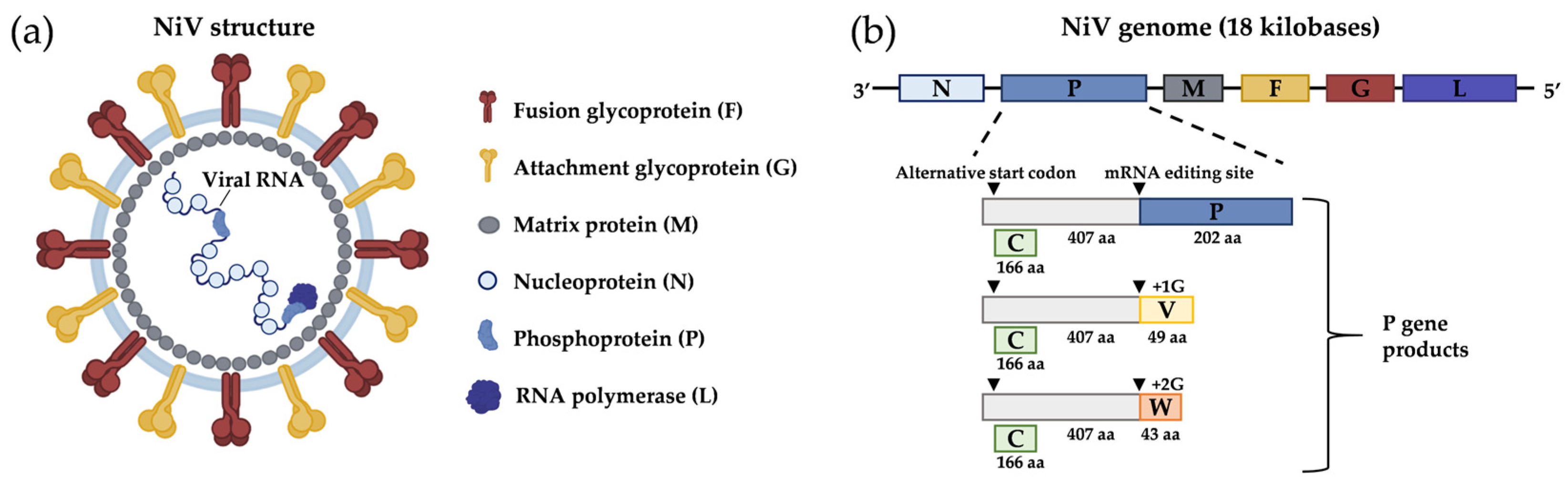
1. General Aspects
2. Epidemiology
2.1. Fruit Bats
2.2. Pigs
2.3. Other Hosts
3. Modes of Transmission
4. Symptoms and Pathogenesis in Humans
5. Diagnostics
5.1. Molecular Diagnostics
| Test | Test Type | Virus Detected | Target Gene | Biological Matrix | Sensivity | Specificity | Author |
|---|---|---|---|---|---|---|---|
| Home made | RT-PCR broad range | Paramyxoviruses | L gene of NiV | Paramyxovirus viral strains (henipavirus, Morbilliviruses, respiroviruses, and Rubulaviruses) | 10–100 copies/reaction | No cross reactivity with Influenza A and B viruses, Rhinoviruses, adenoviruses, coronaviruses 229E, and OC-43. Chlamidia pneumoniae, Haemophilus influenzae, Streptococcus pneumoniae, and Mycoplasma pneumoniae | Tong et al., 2008 [59] |
| Home made | Duplex RT-PCR | NiV | N gene of NiV | Urine, saliva, and blood | 0.37 pg/µL | ND | Chua et al., 2000 [6] |
| Home made | Real-time RT-PCR with fluorescent reporter dye detected at each PCR cycle | NiV | N gene of NiV M strain | Viral stock | Whole blood: 103 copies/mL | No cross-reactivity with measles virus was declared | Guillaume et al., 2004 [62] |
| Home made | Real-time RT-PCR with fluorescent reporter dye detected at each PCR cycle | NiV | N, M, P genes | In vitro transcribed RNA | 20 copies/reaction | Whole blood: 100% [95.9–100]. | Feldman et al., 2009 [63] |
| Home made | SYBR Green RT-PCR | NiV | N gene | Whole blood and urine | 20 copies/reaction | Whole blood: 100% for Nipah virus | Feldman et al., 2009 [63] |
| Home made | Transcription-loop-mediated isothermal amplification (RT-LAMP) | NIV | N gene of NiV | Whole blood sample, fecal sample, throat swab sample, and urine sample | 107 copies/ reaction | No cross reactivity with HeV, Newcastle disease virus, Japanese encephalitis virus, and Influenza A virus. | Ma et al., 2019 [68] |
| Home made | SYBR Green RT-PCR | NiV | N gene of NiV | Viral replication in vitro | 100 pfu/µL | ND | Chang et al., 2006 [64] |
| Home made | Real-time RT-PCR with fluorescent dye-labelled probes to detect PCR amplicons | NiV | Intergenic region separating the viral F and G protein coding regions | Serum, body fluid, and urine | LOD: NiV-B assay 1.63 × 104 genomes/mL; NiV-M 5.82 × 103 genomes/mL | No cross reactivity with HeV, RSV, EBOV, and LASV | Jensen et al., 2018 [65] |
| Home made | NGS | 35 epizootic and zoonotic viruses | Full genome | Whole blood, serum, plasma, and urine | 21 genomes/reaction | ND | Wylezich et al., 2021 [70] |
| EZ1 test (DOD) | Real-time TaqMan RT-PCR with fluorescent reporter dye detected at each PCR cycle | Several pathogens | N gene | Whole blood and plasma | Whole blood: 54 copies/well | 100%; no cross-reactivity with other Viral Haemorrhagic Fever | Onyango et al., 2017 [66] |
| Home made | TaqMan array CARD | 15 viruses, 8 bacteria and 3 protozoa | Blood samples 104 copies/mL | Liu et al., 2016 [67] | |||
| Home made | Reverse transcription recombinase-based isothermal amplification coupled with lateral flow detection | N gene | Viral stock produced in vitro | 103 copies/µL (analytical sensitivity) | No cross reactivity with Hendra virus | Pollak et al., 2023 [69] |
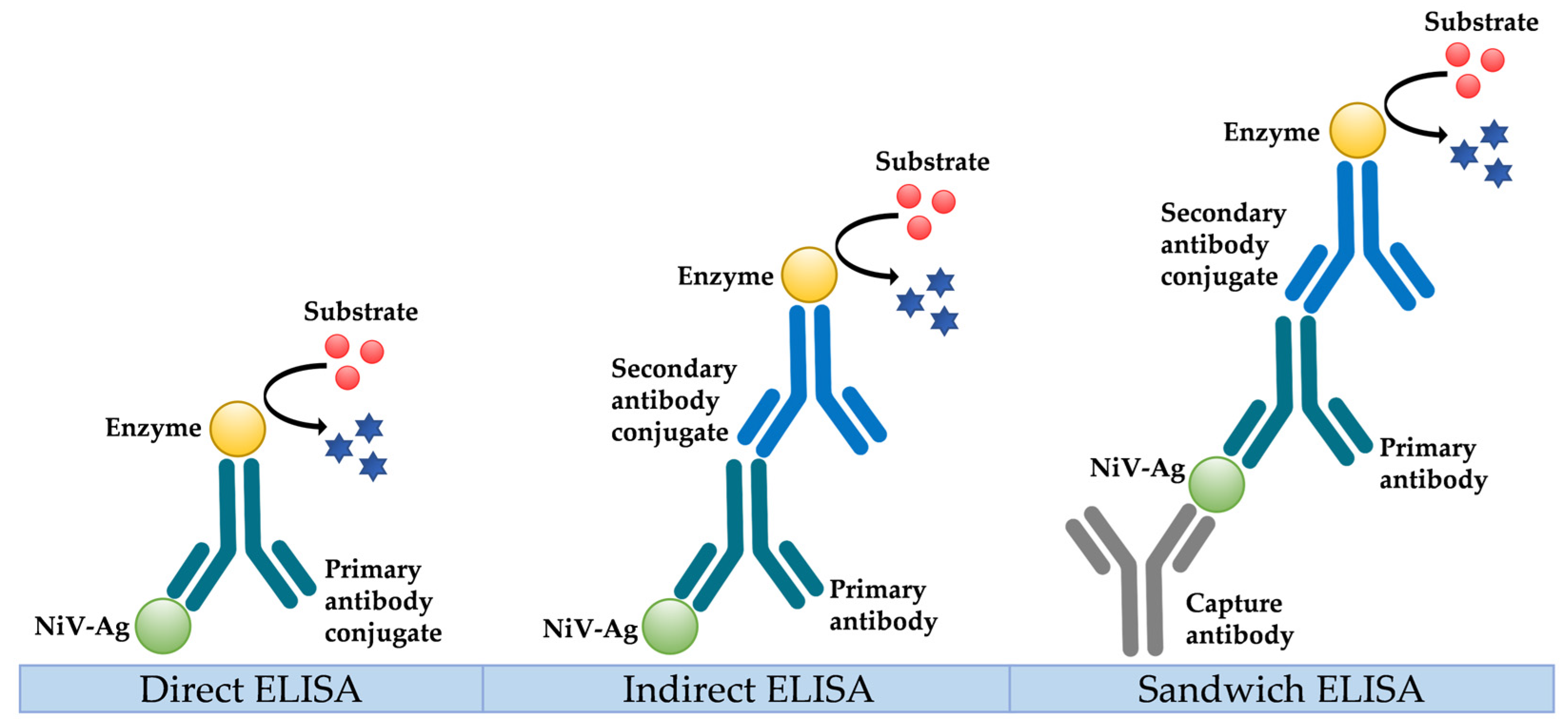
5.2. Serological Diagnosis
5.3. Neutralization Assays
5.4. Virus Isolation
6. One Health Concept for Preparedness Applied to Nipah Virus
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shoemaker, T.; Choi, M.J. Henipaviruses. In Centers for Disease Control and Prevention CDC Yellow Book 2020: Health Information for International Travel; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Bruno, L.; Nappo, M.A.; Ferrari, L.; Di Lecce, R.; Guarnieri, C.; Cantoni, A.M.; Corradi, A. Nipah Virus Disease: Epidemiological, Clinical, Diagnostic and Legislative Aspects of This Unpredictable Emerging Zoonosis. Animals 2022, 13, 159. [Google Scholar] [CrossRef]
- Kummer, S.; Kranz, D.-C. Henipaviruses—A Constant Threat to Livestock and Humans. PLoS Negl. Trop. Dis. 2022, 16, e0010157. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Kumar, R.; Yadav, J.P.; Kaushik, S. Emerging Trends of Nipah Virus: A Review. Rev. Med. Virol. 2019, 29, e2010. [Google Scholar] [CrossRef]
- Uchida, S.; Horie, R.; Sato, H.; Kai, C.; Yoneda, M. Possible Role of the Nipah Virus V Protein in the Regulation of the Interferon Beta Induction by Interacting with UBX Domain-Containing Protein1. Sci. Rep. 2018, 8, 7682. [Google Scholar] [CrossRef]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.-J.; et al. Nipah Virus: A Recently Emergent Deadly Paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef]
- Guillaume, V.; Aslan, H.; Ainouze, M.; Guerbois, M.; Fabian Wild, T.; Buckland, R.; Langedijk, J.P.M. Evidence of a Potential Receptor-Binding Site on the Nipah Virus G Protein (NiV-G): Identification of Globular Head Residues with a Role in Fusion Promotion and Their Localization on an NiV-G Structural Model. J. Virol. 2006, 80, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Bonaparte, M.I.; Dimitrov, A.S.; Bossart, K.N.; Crameri, G.; Mungall, B.A.; Bishop, K.A.; Choudhry, V.; Dimitrov, D.S.; Wang, L.-F.; Eaton, B.T.; et al. Ephrin-B2 Ligand Is a Functional Receptor for Hendra Virus and Nipah Virus. Proc. Natl. Acad. Sci. USA 2005, 102, 10652–10657. [Google Scholar] [CrossRef] [PubMed]
- Negrete, O.A.; Levroney, E.L.; Aguilar, H.C.; Bertolotti-Ciarlet, A.; Nazarian, R.; Tajyar, S.; Lee, B. EphrinB2 Is the Entry Receptor for Nipah Virus, an Emergent Deadly Paramyxovirus. Nature 2005, 436, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.C.; Matreyek, K.A.; Filone, C.M.; Hashimi, S.T.; Levroney, E.L.; Negrete, O.A.; Bertolotti-Ciarlet, A.; Choi, D.Y.; McHardy, I.; Fulcher, J.A.; et al. N-Glycans on Nipah Virus Fusion Protein Protect against Neutralization but Reduce Membrane Fusion and Viral Entry. J. Virol. 2006, 80, 4878–4889. [Google Scholar] [CrossRef]
- Patch, J.R.; Crameri, G.; Wang, L.-F.; Eaton, B.T.; Broder, C.C. Quantitative Analysis of Nipah Virus Proteins Released as Virus-like Particles Reveals Central Role for the Matrix Protein. Virol. J. 2007, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Leo, Y.S.; Zaki, S.R.; Auchus, A.P.; Lee, K.E.; Ling, A.E.; Chew, S.K.; Ang, B.; Rollin, P.E.; Umapathi, T.; et al. Outbreak of Nipah-Virus Infection among Abattoir Workers in Singapore. Lancet 1999, 354, 1253–1256. [Google Scholar] [CrossRef]
- Parashar, U.D.; Sunn, L.M.; Ong, F.; Mounts, A.W.; Arif, M.T.; Ksiazek, T.G.; Kamaluddin, M.A.; Mustafa, A.N.; Kaur, H.; Ding, L.M.; et al. Case-Control Study of Risk Factors for Human Infection with a New Zoonotic Paramyxovirus, Nipah Virus, during a 1998–1999 Outbreak of Severe Encephalitis in Malaysia. J. Infect. Dis. 2000, 181, 1755–1759. [Google Scholar] [CrossRef]
- Rahman, S.A.; Hassan, S.S.; Olival, K.J.; Mohamed, M.; Chang, L.-Y.; Hassan, L.; Saad, N.M.; Shohaimi, S.A.; Mamat, Z.C.; Naim, M.S.; et al. Characterization of Nipah Virus from Naturally Infected Pteropus vampyrus Bats, Malaysia. Emerg. Infect. Dis. 2010, 16, 1990–1993. [Google Scholar] [CrossRef]
- Anderson, D.E.; Islam, A.; Crameri, G.; Todd, S.; Islam, A.; Khan, S.U.; Foord, A.; Rahman, M.Z.; Mendenhall, I.H.; Luby, S.P.; et al. Isolation and Full-Genome Characterization of Nipah Viruses from Bats, Bangladesh. Emerg. Infect. Dis. 2019, 25, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Yob, J.M.; Field, H.; Rashdi, A.M.; Morrissy, C.; Van Der Heide, B.; Rota, P.; Bin Adzhar, A.; White, J.; Daniels, P.; Jamaluddin, A.; et al. Nipah Virus Infection in Bats (Order Chiroptera) in Peninsular Malaysia. Emerg. Infect. Dis. 2001, 7, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Towner, J.S.; Raut, C.G.; Shete, A.M.; Nichol, S.T.; Mourya, D.T.; Mishra, A.C. Detection of Nipah Virus RNA in Fruit Bat (Pteropus giganteus) from India. Am. J. Trop. Med. Hyg. 2012, 87, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; et al. Pteropid Bats Are Confirmed as the Reservoir Hosts of Henipaviruses: A Comprehensive Experimental Study of Virus Transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef]
- Chow, V.T.K.; Tambyah, P.A.; Yeo, W.M.; Phoon, M.C.; Howe, J. Diagnosis of Nipah Virus Encephalitis by Electron Microscopy of Cerebrospinal Fluid. J. Clin. Virol. 2000, 19, 143–147. [Google Scholar] [CrossRef]
- Ambat, A.S.; Zubair, S.M.; Prasad, N.; Pundir, P.; Rajwar, E.; Patil, D.S.; Mangad, P. Nipah Virus: A Review on Epidemiological Characteristics and Outbreaks to Inform Public Health Decision Making. J. Infect. Public Health 2019, 12, 634–639. [Google Scholar] [CrossRef]
- Plowright, R.K.; Becker, D.J.; Crowley, D.E.; Washburne, A.D.; Huang, T.; Nameer, P.O.; Gurley, E.S.; Han, B.A. Prioritizing Surveillance of Nipah Virus in India. PLoS Negl. Trop. Dis. 2019, 13, e0007393. [Google Scholar] [CrossRef]
- Yadav, P.D.; Shete, A.M.; Kumar, G.A.; Sarkale, P.; Sahay, R.R.; Radhakrishnan, C.; Lakra, R.; Pardeshi, P.; Gupta, N.; Gangakhedkar, R.R.; et al. Nipah Virus Sequences from Humans and Bats during Nipah Outbreak, Kerala, India, 2018. Emerg. Infect. Dis. 2019, 25, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Mhod Nor, M.N.; Gan, C.H.; Ong, B.L. Nipah Virus Infection of Pigs in Peninsular Malaysia. Rev. Sci. Tech. OIE 2000, 19, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B. Nipah Virus Outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Enserink, M. New Virus Fingered in Malaysian Epidemic. Science 1999, 284, 407–410. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Update: Outbreak of Nipah Virus—Malaysia and Singapore, 1999. MMWR Morb. Mortal. Wkly. Rep. 1999, 48, 335–337. [Google Scholar]
- Chowdhury, S.; Khan, S.U.; Crameri, G.; Epstein, J.H.; Broder, C.C.; Islam, A.; Peel, A.J.; Barr, J.; Daszak, P.; Wang, L.-F.; et al. Serological Evidence of Henipavirus Exposure in Cattle, Goats and Pigs in Bangladesh. PLoS Negl. Trop. Dis. 2014, 8, e3302. [Google Scholar] [CrossRef]
- Hegde, S.T.; Sazzad, H.M.S.; Hossain, M.J.; Alam, M.-U.; Kenah, E.; Daszak, P.; Rollin, P.; Rahman, M.; Luby, S.P.; Gurley, E.S. Investigating Rare Risk Factors for Nipah Virus in Bangladesh: 2001–2012. EcoHealth 2016, 13, 720–728. [Google Scholar] [CrossRef]
- Rahman, M.; Chakraborty, A. Nipah Virus Outbreaks in Bangladesh: A Deadly Infectious Disease. WHO South-East Asia J. Public Health 2012, 1, 208–212. [Google Scholar] [CrossRef]
- Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Ali Khan, S.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah Virus Dynamics in Bats and Implications for Spillover to Humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. [Google Scholar] [CrossRef]
- Ching, P.K.G.; De Los Reyes, V.C.; Sucaldito, M.N.; Tayag, E.; Columna-Vingno, A.B.; Malbas, F.F.; Bolo, G.C.; Sejvar, J.J.; Eagles, D.; Playford, G.; et al. Outbreak of Henipavirus Infection, Philippines, 2014. Emerg. Infect. Dis. 2015, 21, 328–331. [Google Scholar] [CrossRef]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.K.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal Encephalitis Due to Nipah Virus among Pig-Farmers in Malaysia. Lancet 1999, 354, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Gurley, E.S.; Spiropoulou, C.F.; De Wit, E. Twenty Years of Nipah Virus Research: Where Do We Go From Here? J. Infect. Dis. 2020, 221, S359–S362. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.A.; Bandyopadhyay, B.T.; Ramdasi, A.Y.; Jadi, R.; Patil, D.R.; Rahman, M.; Majumdar, M.; Banerjee, P.S.; Hati, A.K.; Goswami, R.P.; et al. Genomic Characterization of Nipah Virus, West Bengal, India. Emerg. Infect. Dis. 2011, 17, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.P.G.; Sugunan, A.P.; Yadav, P.; Kurup, K.K.; Aarathee, R.; Manickam, P.; Bhatnagar, T.; Radhakrishnan, C.; Thomas, B.; Kumar, A.; et al. Infections among Contacts of Patients with Nipah Virus, India. Emerg. Infect. Dis. 2019, 25, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Gurley, E.S.; Montgomery, J.M.; Bell, M.; Carroll, D.S.; Hsu, V.P.; Formenty, P.; Croisier, A.; Bertherat, E.; Faiz, M.A.; et al. Clinical Presentation of Nipah Virus Infection in Bangladesh. Clin. Infect. Dis. 2008, 46, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; McCall, B.; Smith, G.; Slinko, V.; Allen, G.; Smith, I.; Moore, F.; Taylor, C.; Kung, Y.-H.; Field, H. Human Hendra Virus Encephalitis Associated with Equine Outbreak, Australia, 2008. Emerg. Infect. Dis. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Arunkumar, G.; Chandni, R.; Mourya, D.T.; Singh, S.K.; Sadanandan, R.; Sudan, P.; Bhargava, B.; Nipah Investigators People and Health Study Group; Gangakhedkar, R.R.; Gupta, N.; et al. Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018. J. Infect. Dis. 2019, 219, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.J.; Tan, C.T.; Chew, N.K.; Tan, P.S.K.; Kamarulzaman, A.; Sarji, S.A.; Wong, K.T.; Abdullah, B.J.J.; Chua, K.B.; Lam, S.K. Clinical Features of Nipah Virus Encephalitis among Pig Farmers in Malaysia. N. Engl. J. Med. 2000, 342, 1229–1235. [Google Scholar] [CrossRef]
- Aditi; Shariff, M. Aditi; Shariff, M. Nipah Virus Infection: A Review. Epidemiol. Infect. 2019, 147, e95. [Google Scholar] [CrossRef]
- World Health Organization. Nipah Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed on 8 May 2023).
- Hauser, N.; Gushiken, A.C.; Narayanan, S.; Kottilil, S.; Chua, J.V. Evolution of Nipah Virus Infection: Past, Present, and Future Considerations. Trop. Med. Infect. Dis. 2021, 6, 24. [Google Scholar] [CrossRef]
- Ang, B.S.P.; Lim, T.C.C.; Wang, L. Nipah Virus Infection. J. Clin. Microbiol. 2018, 56, e01875-17. [Google Scholar] [CrossRef] [PubMed]
- Pallivalappil, B.; Ali, A.; Thulaseedharan, N.; Karadan, U.; Chellenton, J.; Dipu, K.; Anoop Kumar, A.; Sajeeth Kumar, K.; Rajagopal, T.; Suraj, K.; et al. Dissecting an Outbreak: A Clinico-Epidemiological Study of Nipah Virus Infection in Kerala, India, 2018. J. Glob. Infect. Dis. 2020, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Shieh, W.-J.; Kumar, S.; Norain, K.; Abdullah, W.; Guarner, J.; Goldsmith, C.S.; Chua, K.B.; Lam, S.K.; Tan, C.T.; et al. Nipah Virus Infection: Pathology and Pathogenesis of an Emerging Paramyxoviral Zoonosis. Am. J. Pathol. 2002, 161, 2153–2167. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Dhama, K.; Chakraborty, S.; Tiwari, R.; Natesan, S.; Khandia, R.; Munjal, A.; Vora, K.S.; Latheef, S.K.; Karthik, K.; et al. Nipah Virus: Epidemiology, Pathology, Immunobiology and Advances in Diagnosis, Vaccine Designing and Control Strategies—A Comprehensive Review. Vet. Q. 2019, 39, 26–55. [Google Scholar] [CrossRef]
- Siva, S.R.; Chong, H.T.; Tan, C.T. Ten Year Clinical and Serological Outcomes of Nipah Virus Infection. Neurol. Asia 2009, 14, 53–58. [Google Scholar]
- Sejvar, J.J.; Hossain, J.; Saha, S.K.; Gurley, E.S.; Banu, S.; Hamadani, J.D.; Faiz, M.A.; Siddiqui, F.M.; Mohammad, Q.D.; Mollah, A.H.; et al. Long-Term Neurological and Functional Outcome in Nipah Virus Infection. Ann. Neurol. 2007, 62, 235–242. [Google Scholar] [CrossRef]
- Thakur, N.; Bailey, D. Advances in Diagnostics, Vaccines and Therapeutics for Nipah Virus. Microbes Infect. 2019, 21, 278–286. [Google Scholar] [CrossRef]
- Tan, K.S.; Tan, C.T.; Goh, K.J. Epidemiological Aspects of Nipah Virus Infection. Neurol. J. Southeast Asia 1999, 4, 77–81. [Google Scholar]
- Chan, K.P.; Rollin, P.E.; Ksiazek, T.G.; Leo, Y.S.; Goh, K.T.; Paton, N.I.; Sng, E.H.; Ling, A.E. A Survey of Nipah Virus Infection among Various Risk Groups in Singapore. Epidemiol. Infect. 2002, 128, 93–98. [Google Scholar] [CrossRef]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah Virus Encephalitis Reemergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef]
- Banerjee, S.; Gupta, N.; Kodan, P.; Mittal, A.; Ray, Y.; Nischal, N.; Soneja, M.; Biswas, A.; Wig, N. Nipah Virus Disease: A Rare and Intractable Disease. Intractable Rare Dis. Res. 2019, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Robertson, T.; Ong, B.B.; Chong, J.W.; Yaiw, K.C.; Wang, L.F.; Ansford, A.J.; Tannenberg, A. Human Hendra Virus Infection Causes Acute and Relapsing Encephalitis. Neuropathol. Appl. Neurobiol. 2009, 35, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.T.; Kunjapan, S.R.; Thayaparan, T.; Geok Tong, J.M.; Petharunam, V.; Jusoh, M.R.; Tan, C.T. Nipah Encephalitis Outbreak in Malaysia, Clinical Features in Patients from Seremban. Can. J. Neurol. Sci. 2002, 29, 83–87. [Google Scholar] [CrossRef]
- Middleton, D.J.; Westbury, H.A.; Morrissy, C.J.; Van Der Heide, B.M.; Russell, G.M.; Braun, M.A.; Hyatt, A.D. Experimental Nipah Virus Infection in Pigs and Cats. J. Comp. Pathol. 2002, 126, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Nipah Virus (NiV). Available online: https://www.cdc.gov/vhf/nipah/index.html (accessed on 12 June 2023).
- Centers for Disease Control and Prevention. Nipah Virus (NiV): Diagnosis. Available online: https://www.cdc.gov/vhf/nipah/diagnosis/index.html (accessed on 10 June 2023).
- Tong, S.; Chern, S.-W.W.; Li, Y.; Pallansch, M.A.; Anderson, L.J. Sensitive and Broadly Reactive Reverse Transcription-PCR Assays To Detect Novel Paramyxoviruses. J. Clin. Microbiol. 2008, 46, 2652–2658. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Lumlertdacha, B.; Boongird, K.; Wanghongsa, S.; Chanhome, L.; Rollin, P.; Stockton, P.; Rupprecht, C.E.; Ksiazek, T.G.; Hemachudha, T. Bat Nipah Virus, Thailand. Emerg. Infect. Dis. 2005, 11, 1949–1951. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Phumesin, P.; Hemachudha, T. Drinking Bat Blood May Be Hazardous to Your Health. Clin. Infect. Dis. 2006, 43, 269. [Google Scholar] [CrossRef][Green Version]
- Guillaume, V.; Lefeuvre, A.; Faure, C.; Marianneau, P.; Buckland, R.; Lam, S.K.; Wild, T.F.; Deubel, V. Specific Detection of Nipah Virus Using Real-Time RT-PCR (TaqMan). J. Virol. Methods 2004, 120, 229–237. [Google Scholar] [CrossRef]
- Feldman, K.S.; Foord, A.; Heine, H.G.; Smith, I.L.; Boyd, V.; Marsh, G.A.; Wood, J.L.N.; Cunningham, A.A.; Wang, L.-F. Design and Evaluation of Consensus PCR Assays for Henipaviruses. J. Virol. Methods 2009, 161, 52–57. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Mohd Ali, A.; Hassan, S.S.; AbuBakar, S. Quantitative Estimation of Nipah Virus Replication Kinetics in Vitro. Virol. J. 2006, 3, 47. [Google Scholar] [CrossRef]
- Jensen, K.S.; Adams, R.; Bennett, R.S.; Bernbaum, J.; Jahrling, P.B.; Holbrook, M.R. Development of a Novel Real-Time Polymerase Chain Reaction Assay for the Quantitative Detection of Nipah Virus Replicative Viral RNA. PLoS ONE 2018, 13, e0199534. [Google Scholar] [CrossRef] [PubMed]
- Onyango, C.O.; Loparev, V.; Lidechi, S.; Bhullar, V.; Schmid, D.S.; Radford, K.; Lo, M.K.; Rota, P.; Johnson, B.W.; Munoz, J.; et al. Evaluation of a TaqMan Array Card for Detection of Central Nervous System Infections. J. Clin. Microbiol. 2017, 55, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ochieng, C.; Wiersma, S.; Ströher, U.; Towner, J.S.; Whitmer, S.; Nichol, S.T.; Moore, C.C.; Kersh, G.J.; Kato, C.; et al. Development of a TaqMan Array Card for Acute-Febrile-Illness Outbreak Investigation and Surveillance of Emerging Pathogens, Including Ebola Virus. J. Clin. Microbiol. 2016, 54, 49–58. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Guan, W.; Chen, Q.; Liu, D. Rapid and Specific Detection of All Known Nipah Virus Strains’ Sequences With Reverse Transcription-Loop-Mediated Isothermal Amplification. Front. Microbiol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Pollak, N.M.; Olsson, M.; Marsh, G.A.; Macdonald, J.; McMillan, D. Evaluation of Three Rapid Low-Resource Molecular Tests for Nipah Virus. Front. Microbiol. 2023, 13, 1101914. [Google Scholar] [CrossRef]
- Wylezich, C.; Calvelage, S.; Schlottau, K.; Ziegler, U.; Pohlmann, A.; Höper, D.; Beer, M. Next-Generation Diagnostics: Virus Capture Facilitates a Sensitive Viral Diagnosis for Epizootic and Zoonotic Pathogens Including SARS-CoV-2. Microbiome 2021, 9, 51. [Google Scholar] [CrossRef]
- Arunkumar, G.; Devadiga, S.; McElroy, A.K.; Prabhu, S.; Sheik, S.; Abdulmajeed, J.; Robin, S.; Sushama, A.; Jayaram, A.; Nittur, S.; et al. Adaptive Immune Responses in Humans During Nipah Virus Acute and Convalescent Phases of Infection. Clin. Infect. Dis. 2019, 69, 1752–1756. [Google Scholar] [CrossRef]
- Ramasundram, V.; Tan, C.T.; Chua, K.B.; Chong, H.T.; Goh, K.J.; Chew, N.K.; Tan, K.S.; Thayaparan, T.; Kunjapan, S.R.; Petharunam, V.; et al. Kinetics of IgM and IgG Seroconversion in Nipah Virus Infection. Neurol. J. Southeast Asia 2000, 5, 23–28. [Google Scholar]
- Shete, A.; Radhakrishnan, C.; Pardeshi, P.; Yadav, P.; Jain, R.; Sahay, R.; Sugunan, A. Antibody Response in Symptomatic & Asymptomatic Nipah Virus Cases from Kerala, India. Indian J. Med. Res. 2021, 154, 533–535. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Tosh, C.; Venkatesh, G.; Senthil Kumar, D. Nipah Virus Infection: Current Scenario. Indian J. Virol. 2013, 24, 398–408. [Google Scholar] [CrossRef]
- Abdullah, S.; Tan, C.T. Henipavirus Encephalitis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 663–670. ISBN 978-0-444-53488-0. [Google Scholar]
- Satterfield, B.A.; Dawes, B.E.; Milligan, G.N. Status of Vaccine Research and Development of Vaccines for Nipah Virus. Vaccine 2016, 34, 2971–2975. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.; Kumar, R.; Kumary, S.; Kajal, F.; David, J. Nipah Virus Epidemic in Southern India and Emphasizing “One Health” Approach to Ensure Global Health Security. J. Fam. Med. Prim. Care 2018, 7, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Daniels, P.; Ksiazek, T.; Eaton, B.T. Laboratory Diagnosis of Nipah and Hendra Virus Infections. Microbes Infect. 2001, 3, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Field, H.; Young, P.; Yob, J.M.; Mills, J.; Hall, L.; Mackenzie, J. The Natural History of Hendra and Nipah Viruses. Microbes Infect. 2001, 3, 307–314. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Tamin, A.; Ksiazek, T.G.; Rollin, P.E.; Anderson, L.J.; Bellini, W.J.; Rota, P.A. Molecular Characterization of Nipah Virus, a Newly Emergent Paramyxovirus. Virology 2000, 271, 334–349. [Google Scholar] [CrossRef]
- Yu, F.; Khairullah, N.S.; Inoue, S.; Balasubramaniam, V.; Berendam, S.J.; Teh, L.K.; Ibrahim, N.S.W.; Abdul Rahman, S.; Hassan, S.S.; Hasebe, F.; et al. Serodiagnosis Using Recombinant Nipah Virus Nucleocapsid Protein Expressed in Escherichia coli. J. Clin. Microbiol. 2006, 44, 3134–3138. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Venkatesh, G.; Tosh, C.; Patel, P.; Mashoria, A.; Gupta, V.; Gupta, S.; Senthilkumar, D. Development and Evaluation of Recombinant Nucleocapsid Protein Based Diagnostic ELISA for Detection of Nipah Virus Infection in Pigs. J. Immunoassay Immunochem. 2016, 37, 154–166. [Google Scholar] [CrossRef]
- Chiang, C.-F.; Lo, M.K.; Rota, P.A.; Spiropoulou, C.F.; Rollin, P.E. Use of Monoclonal Antibodies against Hendra and Nipah Viruses in an Antigen Capture ELISA. Virol. J. 2010, 7, 115. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Rollin, P.E.; Jahrling, P.B.; Johnson, E.; Dalgard, D.W.; Peters, C.J. Enzyme Immunosorbent Assay for Ebola Virus Antigens in Tissues of Infected Primates. J. Clin. Microbiol. 1992, 30, 947–950. [Google Scholar] [CrossRef]
- Saijo, M.; Georges-Courbot, M.-C.; Fukushi, S.; Mizutani, T.; Philippe, M.; Georges, A.-J.; Kurane, I.; Morikawa, S. Marburgvirus Nucleoprotein-Capture Enzyme-Linked Immunosorbent Assay Using Monoclonal Antibodies to Recombinant Nucleoprotein: Detection of Authentic Marburgvirus. Jpn. J. Infect. Dis. 2006, 59, 323–325. [Google Scholar]
- Warnes, A.; Fooks, A.R.; Stephenson, J.R. Design and Preparation of Recombinant Antigens as Diagnostic Reagents in Solid-Phase Immunosorbent Assays. Methods Mol. Med. 2004, 94, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Yu, M.; Morrissy, C.; Zhao, Y.-G.; Meehan, G.; Sun, Y.-X.; Wang, Q.-H.; Zhang, W.; Wang, L.-F.; Wang, Z.-L. A Comparative Indirect ELISA for the Detection of Henipavirus Antibodies Based on a Recombinant Nucleocapsid Protein Expressed in Escherichia Coli. J. Virol. Methods 2006, 136, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, Y.; Na, Y.N.; Tanimura, N.; Imada, T. A Solid-Phase Blocking ELISA for Detection of Antibodies to Nipah Virus. J. Virol. Methods 2004, 121, 259–261. [Google Scholar] [CrossRef]
- McNabb, L.; Barr, J.; Crameri, G.; Juzva, S.; Riddell, S.; Colling, A.; Boyd, V.; Broder, C.; Wang, L.-F.; Lunt, R. Henipavirus Microsphere Immuno-Assays for Detection of Antibodies against Hendra Virus. J. Virol. Methods 2014, 200, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Foord, A.J.; White, J.R.; Colling, A.; Heine, H.G. Microsphere Suspension Array Assays for Detection and Differentiation of Hendra and Nipah Viruses. BioMed Res. Int. 2013, 2013, 289295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Daniels, P. Diagnosis of Henipavirus Infection: Current Capabilities and Future Directions. In Henipavirus; Lee, B., Rota, P.A., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 359, pp. 179–196. ISBN 978-3-642-29818-9. [Google Scholar]
- Bae, S.E.; Kim, S.S.; Moon, S.T.; Cho, Y.D.; Lee, H.; Lee, J.-Y.; Shin, H.Y.; Lee, H.-J.; Kim, Y.B. Construction of the Safe Neutralizing Assay System Using Pseudotyped Nipah Virus and G Protein-Specific Monoclonal Antibody. Biochem. Biophys. Res. Commun. 2019, 513, 781–786. [Google Scholar] [CrossRef]
- Kaku, Y.; Noguchi, A.; Marsh, G.A.; McEachern, J.A.; Okutani, A.; Hotta, K.; Bazartseren, B.; Fukushi, S.; Broder, C.C.; Yamada, A.; et al. A Neutralization Test for Specific Detection of Nipah Virus Antibodies Using Pseudotyped Vesicular Stomatitis Virus Expressing Green Fluorescent Protein. J. Virol. Methods 2009, 160, 7–13. [Google Scholar] [CrossRef]
- Khetawat, D.; Broder, C.C. A Functional Henipavirus Envelope Glycoprotein Pseudotyped Lentivirus Assay System. Virol. J. 2010, 7, 312. [Google Scholar] [CrossRef]
- Kaku, Y.; Noguchi, A.; Marsh, G.A.; Barr, J.A.; Okutani, A.; Hotta, K.; Bazartseren, B.; Fukushi, S.; Broder, C.C.; Yamada, A.; et al. Second Generation of Pseudotype-Based Serum Neutralization Assay for Nipah Virus Antibodies: Sensitive and High-Throughput Analysis Utilizing Secreted Alkaline Phosphatase. J. Virol. Methods 2012, 179, 226–232. [Google Scholar] [CrossRef]
- Tamin, A.; Harcourt, B.H.; Lo, M.K.; Roth, J.A.; Wolf, M.C.; Lee, B.; Weingartl, H.; Audonnet, J.-C.; Bellini, W.J.; Rota, P.A. Development of a Neutralization Assay for Nipah Virus Using Pseudotype Particles. J. Virol. Methods 2009, 160, 1–6. [Google Scholar] [CrossRef]
- Hyatt, A.D.; Zaki, S.R.; Goldsmith, C.S.; Wise, T.G.; Hengstberger, S.G. Ultrastructure of Hendra Virus and Nipah Virus within Cultured Cells and Host Animals. Microbes Infect. 2001, 3, 297–306. [Google Scholar] [CrossRef]
- Chua, K.B.; Lam, S.K.; Tan, C.T.; Hooi, P.S.; Goh, K.J.; Chew, N.K.; Tan, K.S.; Kamarulzaman, A.; Wong, K.T. High Mortality in Nipah Encephalitis Is Associated with Presence of Virus in Cerebrospinal Fluid. Ann. Neurol. 2000, 48, 802–805. [Google Scholar] [CrossRef]
- World Health Organization. One Health. Available online: https://www.who.int/health-topics/one-health#tab=tab_1 (accessed on 10 July 2023).
- Latinne, A.; Nga, N.T.T.; Long, N.V.; Ngoc, P.T.B.; Thuy, H.B.; PREDICT Consortium; Long, N.V.; Long, P.T.; Phuong, N.T.; Quang, L.T.V.; et al. One Health Surveillance Highlights Circulation of Viruses with Zoonotic Potential in Bats, Pigs, and Humans in Viet Nam. Viruses 2023, 15, 790. [Google Scholar] [CrossRef]
- Charlier, J.; Barkema, H.W.; Becher, P.; De Benedictis, P.; Hansson, I.; Hennig-Pauka, I.; La Ragione, R.; Larsen, L.E.; Madoroba, E.; Maes, D.; et al. Disease Control Tools to Secure Animal and Public Health in a Densely Populated World. Lancet Planet. Health 2022, 6, e812–e824. [Google Scholar] [CrossRef] [PubMed]
- Morcatty, T.Q.; Pereyra, P.E.R.; Ardiansyah, A.; Imron, M.A.; Hedger, K.; Campera, M.; Nekaris, K.A.-I.; Nijman, V. Risk of Viral Infectious Diseases from Live Bats, Primates, Rodents and Carnivores for Sale in Indonesian Wildlife Markets. Viruses 2022, 14, 2756. [Google Scholar] [CrossRef]
- Singhai, M.; Jain, R.; Jain, S.; Bala, M.; Singh, S.; Goyal, R. Nipah Virus Disease: Recent Perspective and One Health Approach. Ann. Glob. Health 2021, 87, 102. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, P.; Feng, L.; Li, R.; Yang, J.; Zhang, R.; Li, J. External Quality Assessment of Molecular Testing of 9 Viral Encephalitis-Related Viruses in China. Virus Res. 2021, 306, 198598. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.T.; Fooks, A.R.; Hayman, D.T.S.; Horton, D.L.; Müller, T.; Plowright, R.; Peel, A.J.; Bowen, R.; Wood, J.L.N.; Mills, J.; et al. Deciphering Serology to Understand the Ecology of Infectious Diseases in Wildlife. EcoHealth 2013, 10, 298–313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbuglia, A.R.; Lapa, D.; Pauciullo, S.; Raoul, H.; Pannetier, D. Nipah Virus: An Overview of the Current Status of Diagnostics and Their Role in Preparedness in Endemic Countries. Viruses 2023, 15, 2062. https://doi.org/10.3390/v15102062
Garbuglia AR, Lapa D, Pauciullo S, Raoul H, Pannetier D. Nipah Virus: An Overview of the Current Status of Diagnostics and Their Role in Preparedness in Endemic Countries. Viruses. 2023; 15(10):2062. https://doi.org/10.3390/v15102062
Chicago/Turabian StyleGarbuglia, Anna Rosa, Daniele Lapa, Silvia Pauciullo, Hervé Raoul, and Delphine Pannetier. 2023. "Nipah Virus: An Overview of the Current Status of Diagnostics and Their Role in Preparedness in Endemic Countries" Viruses 15, no. 10: 2062. https://doi.org/10.3390/v15102062
APA StyleGarbuglia, A. R., Lapa, D., Pauciullo, S., Raoul, H., & Pannetier, D. (2023). Nipah Virus: An Overview of the Current Status of Diagnostics and Their Role in Preparedness in Endemic Countries. Viruses, 15(10), 2062. https://doi.org/10.3390/v15102062








