Development of Dot-ELISA and Colloidal Gold Immunochromatographic Strip for Rapid and Super-Sensitive Detection of Plum Pox Virus in Apricot Trees
Abstract
1. Introduction
2. Materials and Methods
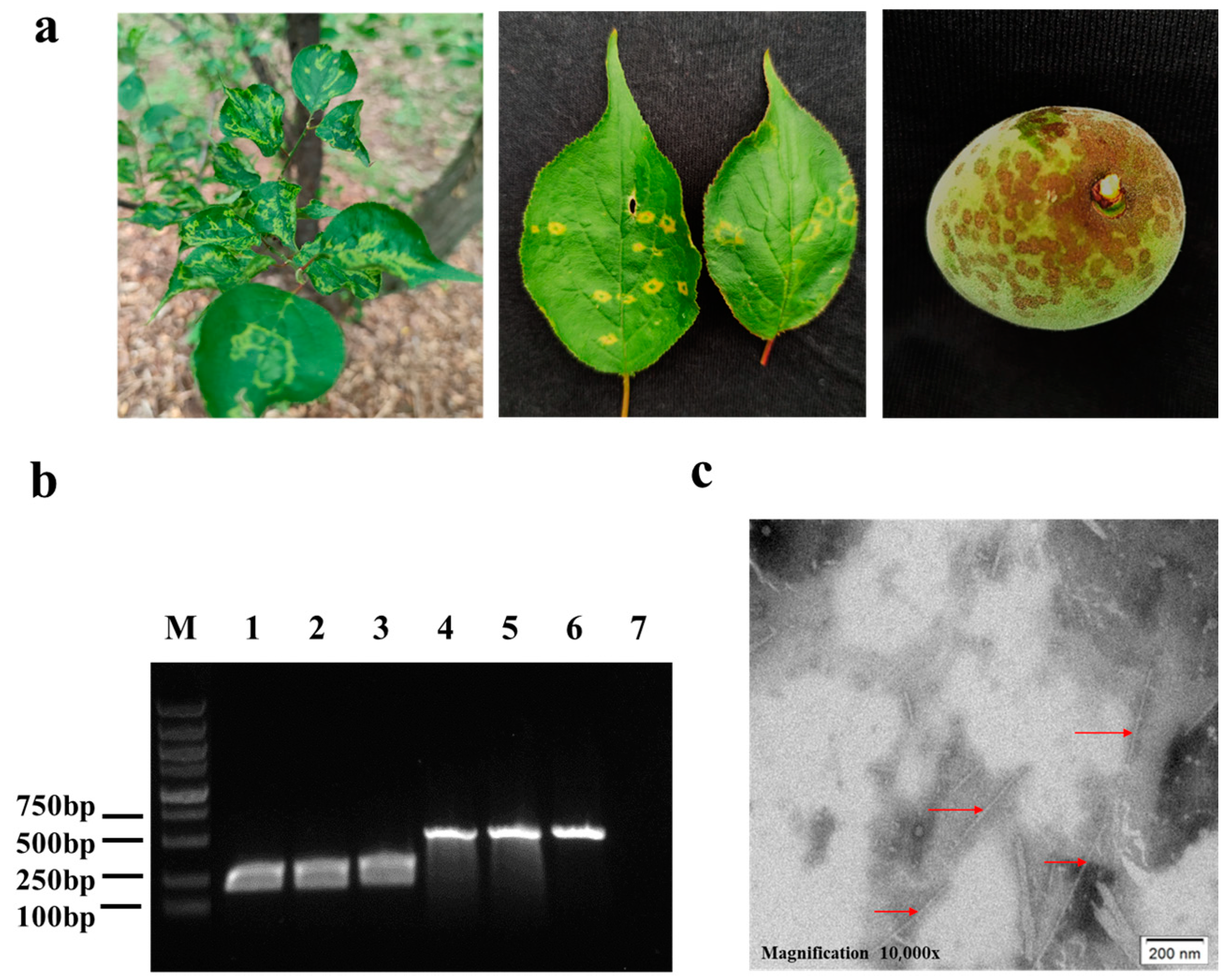
2.1. Virus Sources, Virion Purification
2.2. Generation and Characterization of Murine Monoclonal Antibodies
2.3. Dot-ELISA
2.4. Preparation of Colloidal Gold Nanoparticle-Conjugated Antibody
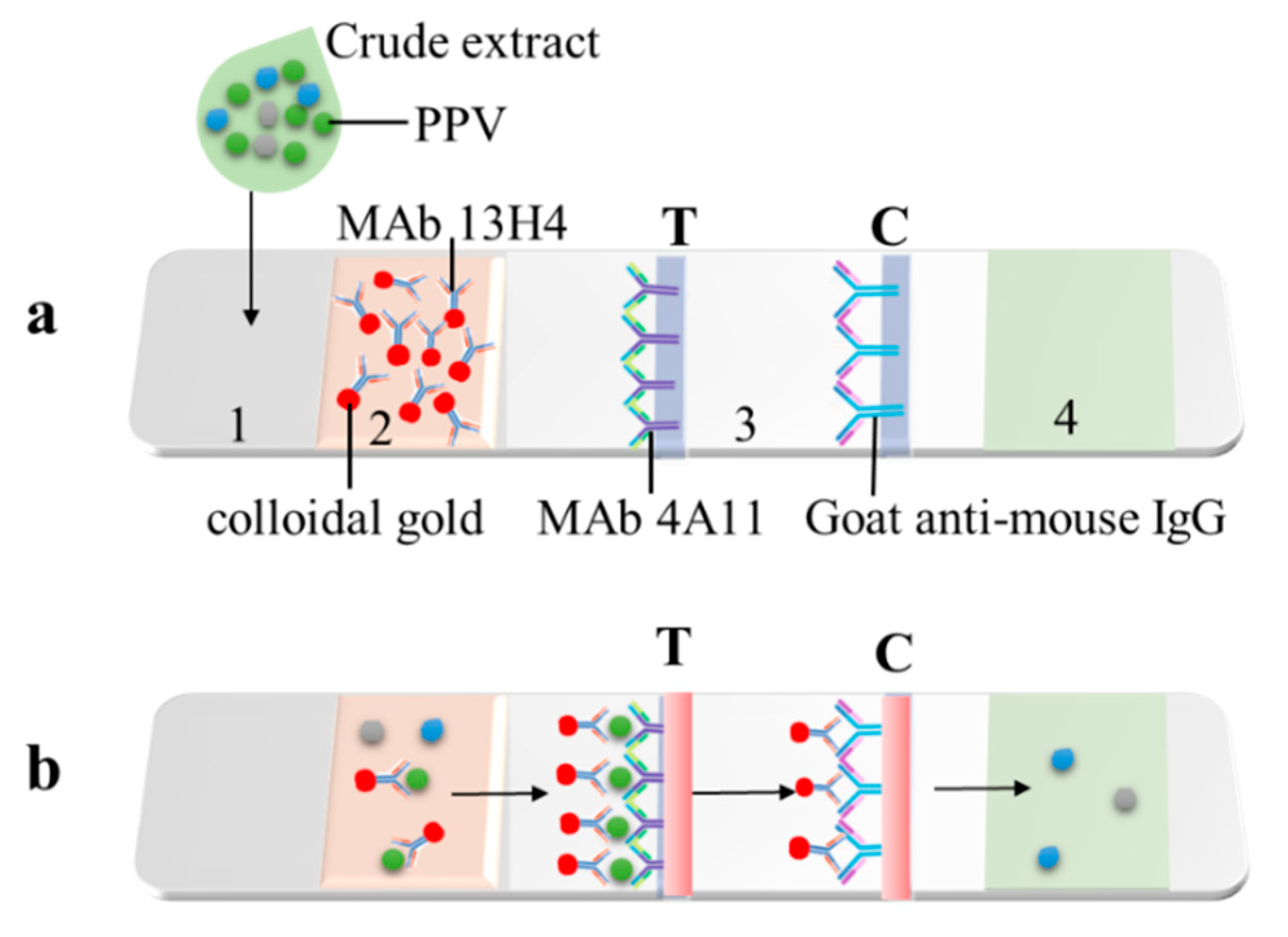
2.5. Development of a Colloidal Gold Immunochromatographic Strip (CGICS)
2.6. Test Procedure of the CGICS
2.7. Detection of PPV Infection through RT-PCR
3. Results
3.1. Preparations of PPV Virions and PPV MAbs
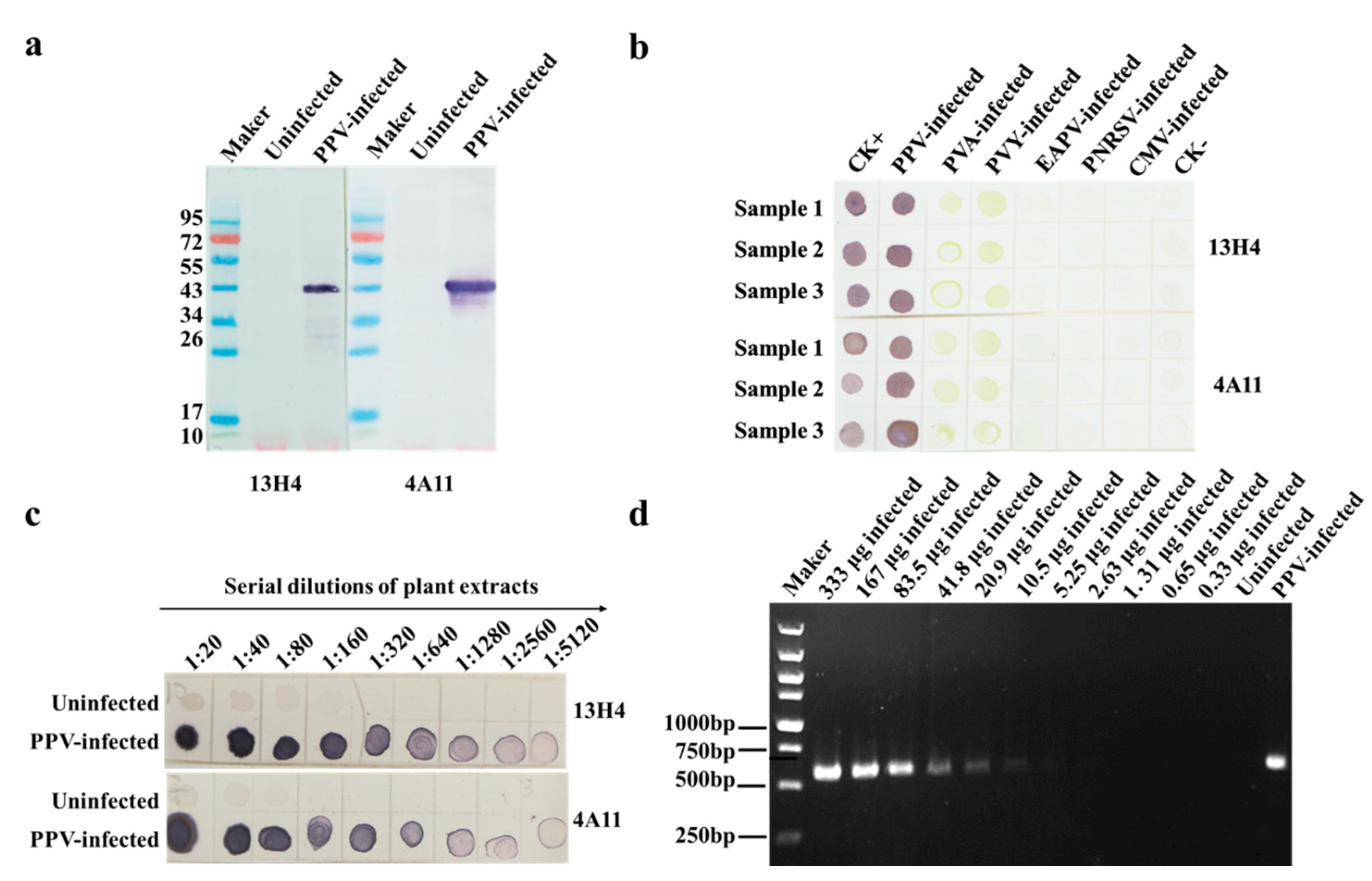
3.2. Characterization of MAbs 13H4 and 4A11, and Detection of PPV Using Dot-ELISA
3.3. Development of the CGICS for PPV Detection
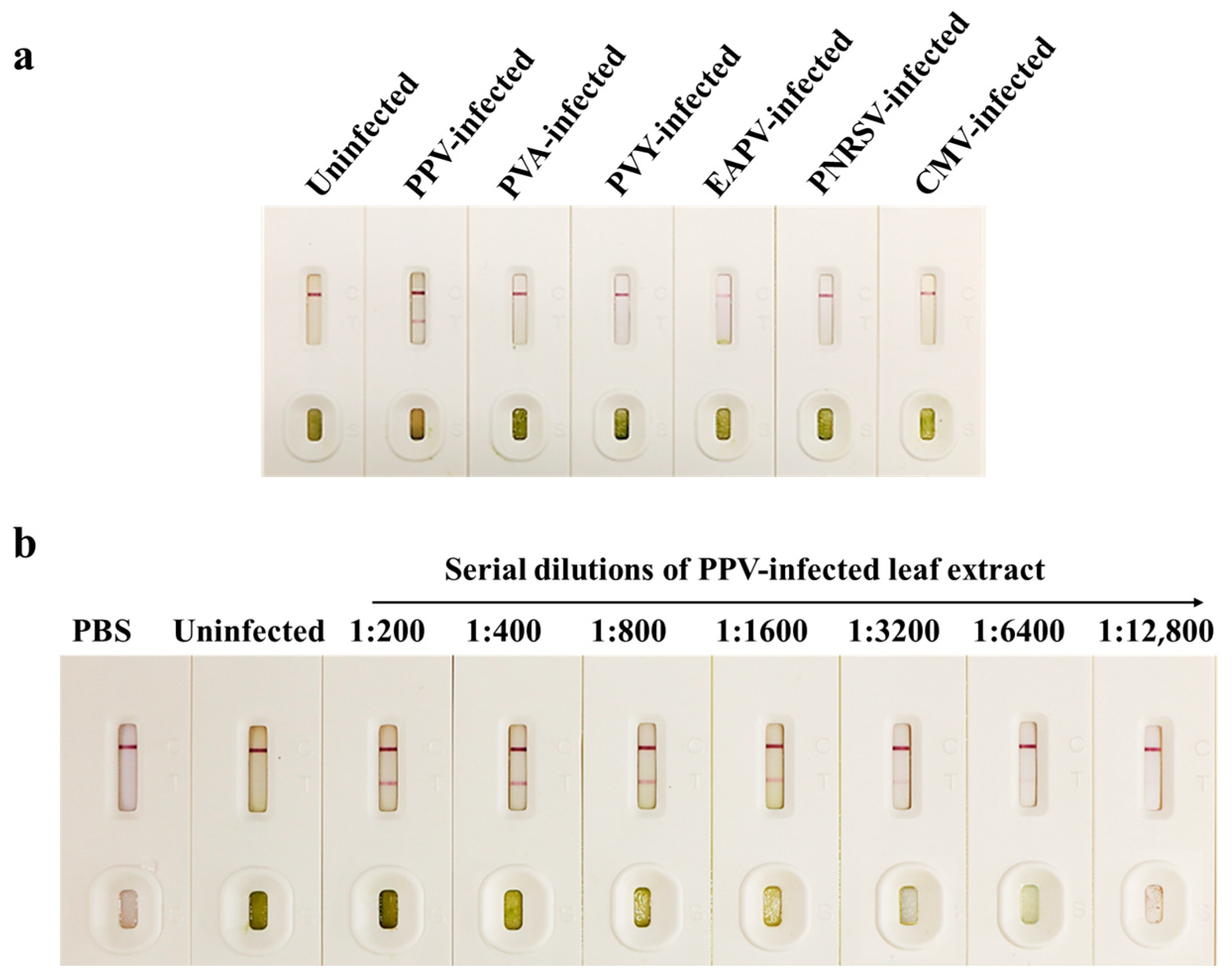
3.4. Specificity and Sensitivity of the CGICS for PPV Detection
3.5. Detection of PPV Infection in Field-Collected Japanese and Common Apricot Tree Leaves
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholthof, K.B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Babula, P.; Adam, V.; Krska, B.; Kizek, R. Sharka: The Past, The Present and The Future. Viruses 2012, 4, 2853–2901. [Google Scholar] [CrossRef] [PubMed]
- Llacer, G.; Cambra, M. Hosts and symptoms of Plum pox virus: Fruiting Prunus species. Bull. OEPP 2006, 36, 219–221. [Google Scholar] [CrossRef]
- Atanasoff, D. Plum pox. A new virus disease. In Yearbook University of Sofia; University of Sofia: Sofia, Bulgaria, 1932; Volume 11, pp. 49–69. [Google Scholar]
- CABI. Plum Pox Virus (Sharka) Invasive Species Compendium. 2022. Available online: https://www.cabi.org/isc/datasheet/42203#tosummaryOfInvasiveness (accessed on 20 October 2022).
- Zhou, J.; Xing, F.; Wang, H.; Li, S. Occurrence, Distribution, and Genomic Characteristics of Plum Pox Virus Isolates from Common Apricot (Prunus armeniaca) and Japanese Apricot (Prunus mume) in China. Plant Dis. 2021, 105, 3474–3480. [Google Scholar] [CrossRef]
- Frederick, G.; Vern, D.; Andrew, S.; William, S.; Douglas, L.; Laurene, L. Plum pox in north america: Identification of aphid vectors and a potential role for fruit in virus spread. Phytopathology 2004, 94, 868–874. [Google Scholar] [CrossRef]
- Manachini, B.; Casati, P.; Cinanni, L.; Bianco, P. Role of Myzus persicae (Hemiptera: Aphididae) and its secondary hosts in plum pox virus propagation. J. Econ. Entomol. 2007, 100, 1047–1052. [Google Scholar] [CrossRef]
- Cambra, M.; Vidal, E. Sharka, a vector-borne disease caused by Plum pox virus: Vector species, transmission mechanism, epidemiology and mitigation strategies to reduce its natural spread. In Proceedings of the 3rd International Symposium on Plum Pox Virus, Antalya, Turkey, 9–13 May 2016; pp. 57–67. [Google Scholar]
- Damsteegt, V.D.; Scorza, R.; Stone, A.L.; Schneider, W.L.; Webb, K.; Demuth, M.; Gildow, F.E. Prunus host range of Plum pox virus (PPV) in the United States by aphid and graft inoculation. Plant Dis. 2007, 91, 18–23. [Google Scholar] [CrossRef]
- Rubio, M.; Dicenta, F.; Masse, M.; Duval, H. Susceptibility of Prunus rootstocks against Marcus and Dideron isolates of Plum pox virus by graft-inoculation. Ann. Appl. Biol. 2013, 162, 214–220. [Google Scholar] [CrossRef]
- Laín, S.; Riechmann, J.; García, J.A. The complete nucleotide sequence of plum pox potyvirus RNA. Virus Res. 1989, 13, 157–172. [Google Scholar] [CrossRef]
- Hajizadeh, M.; Gibbs, A.J.; Amirnia, F.; Glasa, M. The global phylogeny of Plum pox virus is emerging. J. Gen. Virol. 2019, 100, 1457–1468. [Google Scholar] [CrossRef]
- Maejima, K.; Himeno, M.; Netsu, O.; Ishikawa, K.; Yoshida, T.; Fujita, N.; Hashimoto, M.; Komatsu, K.; Yamaji, Y.; Namba, S. Development of an on-site plum pox virus detection kit based on immunochromatography. J. Gen. Plant Pathol. 2014, 80, 176–183. [Google Scholar] [CrossRef]
- Kensaku, M.; Misako, H.; Ken, K.; Yusuke, T.; Masayoshi, H.; Shuichiro, T.; Yasuyuki, Y.; Kenro, O.; Shigetou, N. Molecular epidemiology of Plum pox virus in Japan. Phytopathology 2011, 101, 567–574. [Google Scholar] [CrossRef]
- Mori, T.; Warner, C.; Ohno, S.; Mori, K.; Tobimatsu, T.; Sera, T. Genome sequence analysis of new plum pox virus isolates from Japan. BMC Res. Notes 2021, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Hoshi, H.; Hashimoto, M.; Himeno, M.; Kawanishi, T.; Komatsu, K.; Yamaji, Y.; Hamamoto, H.; Namba, S. First report of plum pox virus infecting Japanese apricot (Prunus mume Sieb. et Zucc.) in Japan. J. Gen. Plant Pathol. 2010, 76, 229–231. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.S.; Kwak, H.R.; Kim, J.E.; Seo, J.K.; Hong, S.J.; Lee, G.J.; Kim, J.H.; Choi, M.K.; Kim, B.R.; et al. Occurrence and Eradication of Plum Pox Virus on Ornamentals in Korea, 2016–2017. Res. Plant Dis. 2019, 25, 8–15. [Google Scholar] [CrossRef]
- Navratil, M.; Safarova, D.; Karesova, R.; Petrzik, K. First incidence of Plum pox virus on apricot trees in China. Plant Dis. 2005, 89, 338. [Google Scholar] [CrossRef]
- Marais, A.; Faure, C.; Svanella Dumas, L.; Candresse, T. First Report of Cherry virus A in Prunus mume in China. Plant Dis. 2008, 92, 1589. [Google Scholar] [CrossRef]
- Capote, N.; Bertolini, E.; Olmos, A.; Vidal, E.; Martinez, M.C.; Cambra, M. Direct sample preparation methods for the detection of Plum pox virus by resal-time RT-PCR. Int. Microbiol. 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Sertkaya, G. Detection of some viruses of stone fruits in purple leaf plum (Prunus cerasifera) as ornamental plant in the eastern Mediterranean Region of Turkey. In Proceedings of the 20th International Symposium on Virus and Virus-like Diseases of Temperate Fruit Crops, Antalya, Turkey, 22–26 May 2006; pp. 97–99. [Google Scholar]
- Zagrai, L.; Zagrai, I.; Ferencz, B.; Gaboreanu, I.; Kovacs, K.; Petricele, I.; Popescu, O.; Pamfil, D.; Capote, N. Serological and molecular typing of Plum pox virus isolates in the North of Romania. J. Plant Pathol. 2008, 90, S41–S46. [Google Scholar]
- Elibuyuk, I.O. Detection of Plum pox virus in ornamental Prunus cerasifera. Phytoparasitica 2006, 34, 347–352. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kiss, T.; Peňázová, E.; Salava, J.; Nečas, T. Comparison of four techniques for plum pox virus detecton. J. Plant Dis. Prot. 2016, 123, 311–315. [Google Scholar] [CrossRef]
- Kajic, V.; Cerni, S.; Krajacic, M.; Mikec, I.; Skoric, D. Molecular typing of Plum pox virus isolates in Croatia. J. Plant Pathol. 2008, 90, S9–S13. [Google Scholar]
- Zagrai, I.; Zagrai, L.; Preda, S.; Kelemen, B.; Petricele, I.; Popescu, O.; Pamfil, D.; Isac, M. Genetic diversity of Plum pox virus Isolates in Muntenia, Romania. Rom. Biotechnol. Lett. 2010, 15, 5303–5309. [Google Scholar]
- Jeong, H.W.; Lee, H.J.; Cho, I.S.; Ju, H.J.; Jeong, R.D. Rapid detection of plum pox virus by reverse transcription recombinase polymerase amplification. J. Plant Dis. Prot. 2021, 128, 881–885. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Hong, J.; Wang, X.; Zhou, C.; Zhou, X.; Wu, J. Monoclonal antibody-based serological methods for detecting Citrus tristeza virus in citrus groves. Virol. Sin. 2016, 31, 324–330. [Google Scholar] [CrossRef]
- Huang, D.Q.; Chen, R.; Wang, Y.Q.; Hong, J.; Zhou, X.P.; Wu, J.X. Development of a colloidal gold-based immunochromatographic strip for rapid detection of Rice stripe virus. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2019, 20, 343–354. [Google Scholar] [CrossRef]
- Song, G.; Wu, J.Y.; Xie, Y.; Liu, Y.; Qian, Y.J.; Zhou, X.P.; Wu, J.X. Monoclonal antibody-based serological assays for detection of Potato virus S in potato plants. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2017, 18, 1075–1082. [Google Scholar] [CrossRef]
- Li, X.; Guo, L.; Guo, M.; Qi, D.; Zhou, X.; Li, F.; Wu, J. Three highly sensitive monoclonal antibody-based serological assays for the detection of tomato mottle mosaic virus. Phytopathol. Res. 2021, 3, 23. [Google Scholar] [CrossRef]
- Wu, J.X.; Wang, Q.; Liu, H.; Qian, Y.J.; Xie, Y.; Zhou, X.P. Monoclonal antibody-based serological methods for maize chlorotic mottle virus detection in China. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2013, 14, 555–562. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Guo, Y.L.; He, W.Q.; Wang, Y.Q.; Qian, Y.J.; Zhou, X.P.; Wu, J.X. Monoclonal antibody-based serological detection of potato virus M in potato plants and tubers. J. Integr. Agric. 2020, 19, 1283–1291. [Google Scholar] [CrossRef]
- Greice, J.; Pereira, C.-J.J.; Marquez, P.A.C.A.; Akio, T.E.; Samira, B.-S.; Mendes, B.L.A.; Lino, d.S.G.R. Development of a nanogold slot blot inhibition assay for the detection of antibodies against bovine herpesvirus type 1. Arch. Virol. 2018, 163, 1549–1557. [Google Scholar] [CrossRef]
- Zhang, G.P.; Wang, X.N.; Yang, J.F.; Yang, Y.Y.; Xing, G.X.; Li, Q.M.; Zhao, D.; Chai, S.J.; Guo, J.Q. Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist Clenbuterol residues. J. Immunol. Methods 2006, 312, 27–33. [Google Scholar] [CrossRef]
- Zhang, S.; Ravelonandro, M.; Chambers, M.; Briard, P.; Masson, M.; Amato, M.; Vrient, A. Rapid Diagnostic Detection of Plum Pox Virus by Isothermal AmplifyRP (R) and by ImmunoStrip (R). In Proceedings of the 2nd International Symposium on Plum Pox Virus, Olomouc, Czech Republic, 3–6 September 2013; pp. 167–172. [Google Scholar]
- He, W.Q.; Wu, J.Y.; Ren, Y.Y.; Zhou, X.P.; Zhang, S.B.; Qian, Y.J.; Li, F.F.; Wu, J.X. Highly sensitive serological approaches forPepino mosaic virusdetection. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2020, 21, 811–822. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, Y.; Zhou, X.P.; Qian, Y.J. Three sensitive and reliable serological assays for detection of potato virus A in potato plants. J. Integr. Agric. 2021, 20, 2966–2975. [Google Scholar] [CrossRef]
- Byzova, N.A.; Safenkova, I.V.; Chirkov, S.N.; Avdienko, V.G.; Guseva, A.N.; Mitrofanova, I.V.; Zherdev, A.V.; Dzantiev, B.B.; Atabekov, J.G. Interaction of Plum Pox Virus with Specific Colloidal Gold-Labeled Antibodies and Development of Immunochromatographic Assay of the Virus. Biochemistry 2010, 75, 1393–1403. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Qi, D.; Dong, J.; Dong, S.; Yang, X.; Qian, Y.; Zhou, X.; Wu, J. Development of Dot-ELISA and Colloidal Gold Immunochromatographic Strip for Rapid and Super-Sensitive Detection of Plum Pox Virus in Apricot Trees. Viruses 2023, 15, 169. https://doi.org/10.3390/v15010169
Guo M, Qi D, Dong J, Dong S, Yang X, Qian Y, Zhou X, Wu J. Development of Dot-ELISA and Colloidal Gold Immunochromatographic Strip for Rapid and Super-Sensitive Detection of Plum Pox Virus in Apricot Trees. Viruses. 2023; 15(1):169. https://doi.org/10.3390/v15010169
Chicago/Turabian StyleGuo, Mengmeng, Duo Qi, Jinxi Dong, Saiyu Dong, Xiuling Yang, Yajuan Qian, Xueping Zhou, and Jianxiang Wu. 2023. "Development of Dot-ELISA and Colloidal Gold Immunochromatographic Strip for Rapid and Super-Sensitive Detection of Plum Pox Virus in Apricot Trees" Viruses 15, no. 1: 169. https://doi.org/10.3390/v15010169
APA StyleGuo, M., Qi, D., Dong, J., Dong, S., Yang, X., Qian, Y., Zhou, X., & Wu, J. (2023). Development of Dot-ELISA and Colloidal Gold Immunochromatographic Strip for Rapid and Super-Sensitive Detection of Plum Pox Virus in Apricot Trees. Viruses, 15(1), 169. https://doi.org/10.3390/v15010169





