Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Virus
2.2. Preparation of CSP
2.3. Cytotoxicity Assays
2.4. Determination of Antiviral Abilities In Vitro
2.5. Time-of-Addition Assay
2.6. Inactivation Assay
2.7. Inhibitory Action Assays
2.8. IFAT
2.9. RT-qPCR Analysis
2.10. Statistics Analysis
3. Results
3.1. Cytotoxicity of CSP In Vitro
3.2. Antiviral Activity of CSP on IHNV
3.2.1. Antiviral Effects of CSP on IHNV
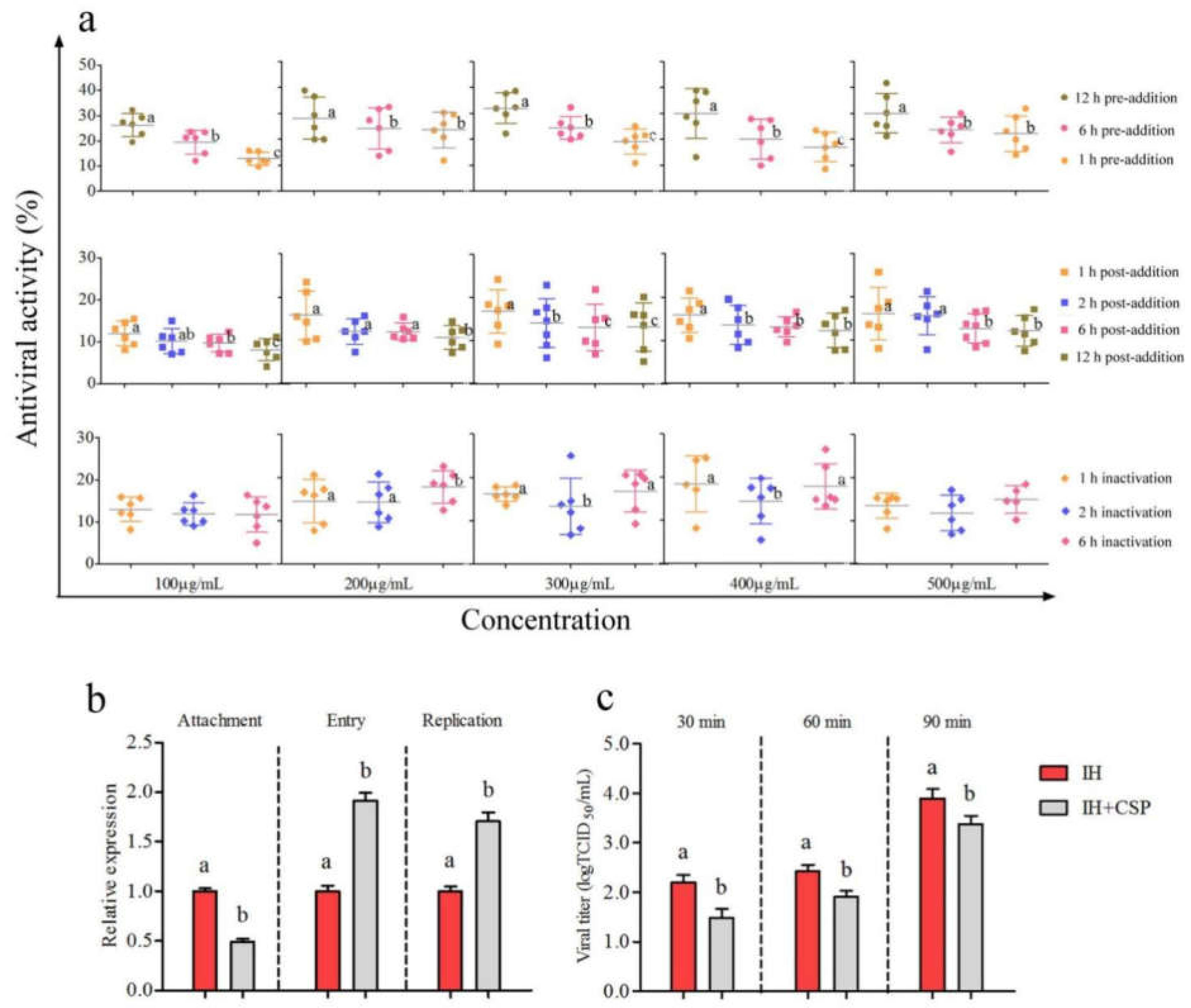
3.2.2. Determination of Time-Addition Mode of CSP to IHNV
3.2.3. Analysis of Inhibitory Action of CSP to IHNV
3.3. Antiviral Activity of CSP on IPNV
3.3.1. Antiviral Capabilities of CSP on IPNV
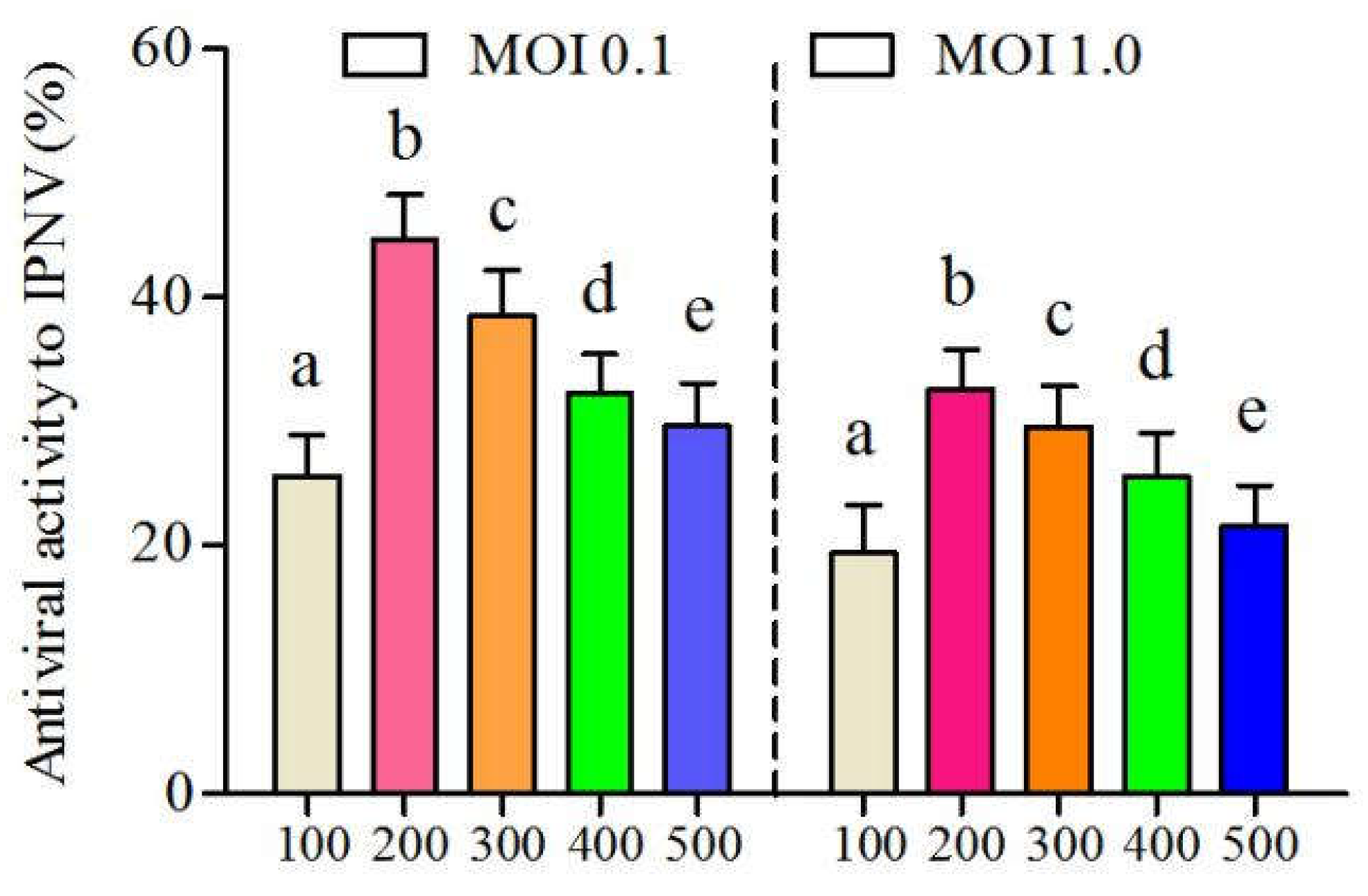
3.3.2. Determination of Time-Addition Mode of CSP on IPNV
3.3.3. Analysis of Inhibitory Action of CSP on IPNV
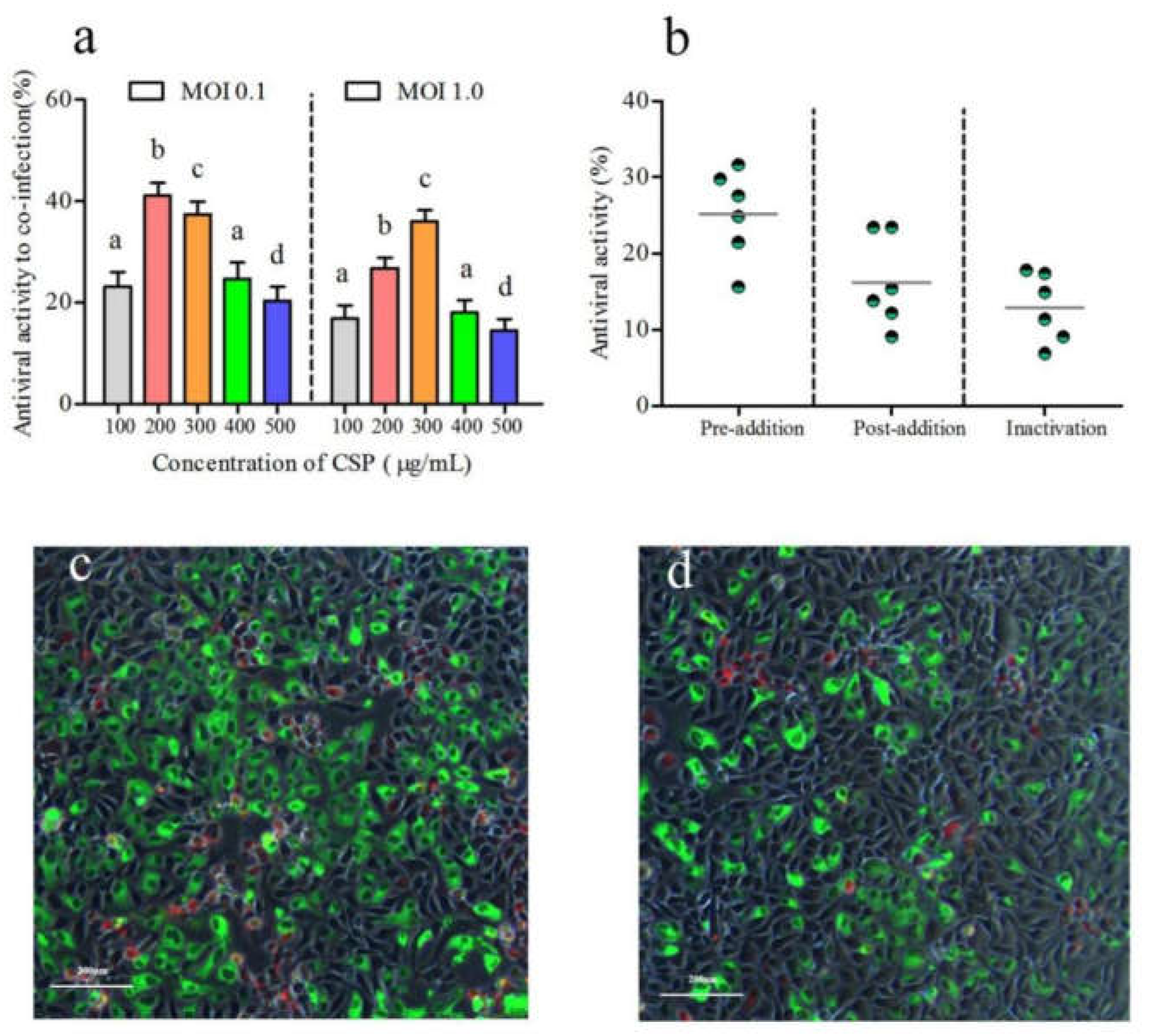
3.4. Antiviral Capability of CSP on Co-Infection of IHNV and IPNV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurath, G.; Garver, K.A.; Troyer, R.M.; Emmenegger, E.J.; Einer-Jensen, K.; Anderson, E.D. Phylogeography of infectious haematopoietic necrosis virus in North America. J. Gen. Virol. 2003, 84, 803–814. [Google Scholar] [CrossRef] [PubMed]
- OIE. World Organization for Animal Health, Aquatic Animal Health Code. Available online: http://www.oie.int/international-standardsetting/aquatic-code/access-online (accessed on 12 October 2018).
- Alonso, M.; Rodriguez Saint-Jean, S.; Perez-Prieto, S.I. Virulence of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus coinfection in rainbow trout (Oncorhynchus mykiss) and nucleotide sequence analysis of the IHNV glycoprotein gene. Arch. Virol. 2003, 148, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Rucker, R.R.; Whippie, W.J.; Parvin, J.R. A contagious disease of salmon possibly of virus origin, US Fish Wildl. Serv. Fish Bull. 1953, 54, 174–175. [Google Scholar]
- Dixon, P.; Paley, R.; Alegria-Moran, R.; Oidtmann, B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): A review. Vet. Res. 2016, 47, 63. [Google Scholar] [CrossRef]
- Enzmann, P.J.; Castric, J.; Bovo, G.; Thiery, R.; Fichtner, D.; Schütze, H.; Wahli, T. Evolution of infectious hematopoietic necrosis virus (IHNV), a fish rhabdovirus, in Europe over 20 years: Implications for control. Dis. Aquat. Org. 2010, 89, 9–15. [Google Scholar] [CrossRef]
- Wood, E.M.; Snieszko, S.F.; Yasutake, W.T. Infectious pancreatic necrosis in brook trout AMA Arch. Pathology 1995, 60, 26–28. [Google Scholar]
- Bootland, L.M.; Leong, J.C. Infectious hematopoietic necrosis virus. In Fish Diseases and Disorders; Woo, P.T.K., Bruno, D.W., Eds.; CAB International: New York, NY, USA, 1999; Volume 3, pp. 57–121. [Google Scholar]
- Santi, E.N. Infectious pancreatic necrosis virus. In Encyclopedia of Virology; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008; pp. 83–89. [Google Scholar]
- Dadar, M.; Memari, H.R.; Vakharia, V.N.; Peyghan, R.; Shapouri, M.S.A.; Mohammadian, T.; Hasanzadeh, R.; Ghasemi, M. Protective and immunogenic effects of Escherichia coli-expressed infectious pancreatic necrosis virus (IPNV) VP2-VP3 fusion protein in rainbow trout. Fish Shellfish. Immunol. 2015, 47, 390–396. [Google Scholar] [CrossRef]
- Rodriguez, S.; Alonso, M.; Perez-Prietol, S.I. Comparison of two birnavirus-rhabdovirus coinfections in fish cell lines. Dis. Aquat. Org. 2005, 67, 183–190. [Google Scholar] [CrossRef]
- Panzarin, V.; Holmes, E.C.; Abbadi, M.; Zamperin, G.; Quartesan, R.; Milani, A.; Schivo, A.; Bille, L.; Pozza, M.D.; Monne, I.; et al. Low evolutionary rate of infectious pancreatic necrosis virus (IPNV) in Italy is associated with reduced virulence in trout. Virus Evol. 2018, 2, 2. [Google Scholar] [CrossRef]
- LaPatra, S.E.; Lauda, K.A.; Woolley, M.J.; Armstrong, R. Detection of a naturally occurring co-infection of IHNV and IPNV in rainbow trout. Fish Health Sect. Am. Fish. Soc. 1993, 21, 9–10. [Google Scholar]
- Salonius, K.; Simard, N.; Harland, R.; Ulmer, J.B. The road to licensure of a DNA vaccine. Curr. Opin. Investig. Drugs 2007, 8, 635–641. [Google Scholar] [PubMed]
- Kouakou, K.; Schepetkin, I.A.; Yapi, A.; Kirpotina, L.N.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of polysaccharides isolated from Alchornea cordifolia. J. Ethnopharmacol. 2013, 146, 232–242. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef]
- Kim, M.; Yim, H.J.; Kim, S.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.; Lee, C. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef]
- Mendes, G.S.; Duarte, M.E.R.; Colodi, F.G.; Noseda, M.D.; Ferreira, L.G.; Berté, S.D.; Cavalcanti, J.F.; Santos, N.; Romanos, M.T.V. Structure and anti-metapneumovirus activity of sulfated galactans from the red seaweed Cryptonemia seminervis. Carbohyd. Polym. 2014, 101, 313–323. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chotigeat, W.; Tongsupa, S.; Supamataya, K.; Phongdara, A. Effect of fucoidan on disease resistance of black tiger shrimp. Aquaculture 2004, 233, 23–30. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish. Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef]
- Ji, F.; Zhao, J.; Liu, M.; Lu, T.; Liu, H.; Yin, J.; Xu, L. Complete genomic sequence of an infectious pancreatic necrosis virus isolated from rainbow trout (Oncorhynchus mykiss) in China. Virus Genes 2017, 53, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Ren, G.; Dong, Y.; Cao, Y.; Shao, Y.; Liu, H.; Yin, J.; Lu, T. Complete genome sequence and phylogenetic analysis of Sn1203 strain of infectious hematopoietic necrosis virus. Chin. J. Fish. 2020, 33, 1–9. [Google Scholar]
- Miller, G.L. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar]
- Reed, L.J.; Münch, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Ren, G.; Xu, L.; Zhao, J.; Shao, Y.; Chen, X.; Lu, T.; Zhang, Q. Supplementation of dietary crude lentinan improves the intestinal microbiota and immune barrier in rainbow trout (Oncorhynchus mykiss) infected by infectious hematopoietic necrosis virus. Front. Immunol. 2022, 13, 920065. [Google Scholar] [CrossRef]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Béress, A.; Wassermann, O.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Chen, J.; Hu, Y.L.; Wang, D.; Fan, Y.; Wang, J.; Abula, S.; Zhang, J.; Qin, T.; Chen, X.; et al. In vitro antiviral activity of sulfated Auricularia auricula polysaccharides. Carbohydr. Polym. 2012, 90, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Maguire, T.; Hileman, R.E.; Fromm, J.R.; Esko, J.D.; Linhardt, R.J.; Marks, R.M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J. Virol. 2005, 79, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Era, S.; Watanabe, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.P.J.; Suzuki, T. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hidari, K.I.P.J.; Abe, T.; Suzuki, T. Crabohydrate-related inhibitors of dengue virus entry. Viruses 2013, 5, 605–618. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Du, H.; Yang, J.; Ming, K.; Song, M.; Liu, J. Comparison of the anti-duck hepatitis a virus activities of phosphorylated and sulfated Astragalus polysaccharides. Exp. Biol. Med. 2016, 242, 344–353. [Google Scholar] [CrossRef]
- Ming, K.; Chen, Y.; Shi, J.; Yang, J.; Yao, F.; Du, H.; Zhang, W.; Bai, J.; Liu, J.; Wang, D.; et al. Effects of Chrysanthemum indicum polysaccharide and its phosphate on anti-duck hepatitis a virus and alleviating hepatic injury. J. Biol. Macromol. 2017, 102, 813–821. [Google Scholar] [CrossRef]
- Levican, J.; Miranda-Cardenas, C.; Soto-Rifo, R.; Aguayo, F.; Gaggero, A.; León, O. Infectious pancreatic necrosis virus enters CHSE-214 cells via macropinocytosis. Sci. Rep. 2017, 7, 3068. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, X.; Ma, J.; Zhang, M.; Wu, H. Structural elucidation of a novel pectin-polysaccharide from the petal of Saussurea laniceps and the mechanism of its anti-HBV activity. Carbohydr. Polym. 2019, 223, 115077. [Google Scholar] [CrossRef] [PubMed]
- Faccin-Galhardi, L.C.; Aimi Yamamoto, K.; Ray, S.; Ray, B.; Linhares, R.E.C.; Nozawa, C. The in vitro antiviral property of Aazadirachta indica polysaccharides for poliovirus. J. Ethnopharmacol. 2012, 142, 86–90. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.; Zhang, C.; Li, P.; Li, X.; Chen, X.; Qiu, S.; et al. Solomonseal polysaccharide and sulfated Codonopsis pilosula polysaccharide synergistically resist Newcastle disease virus. PLoS ONE 2015, 10, e0117916. [Google Scholar] [CrossRef]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Park, K.; Quan, F.; Song, J.; Wee, J.J.; Wang, B.; Cho, Y.; Compans, R.W.; et al. Protective effect of Ginseng polysaccharides on influenza viral infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Guo, Q.; Wang, M.; Li, Y.; Meng, Y.; Huang, L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, J.; Ren, G.; Dong, Y.; Lin, J.; Cao, Y.; Yin, J.; Liu, H.; Lu, T.; Zhang, Q. Co-infection of infectious hematopoietic necrosis virus (IHNV) and infectious pancreatic necrosis virus (IPNV) caused high mortality in farmed rainbow trout (Oncorhynchus mykiss) in China. Aquaculture 2019, 512, 734286. [Google Scholar] [CrossRef]
- Lin, Q.; Fu, X.; Li, N.; Wan, Q.; Chen, W.; Huang, Y.; Huang, Z.; Li, J.; Zhao, L.; Lin, L. Co-infections of infectious spleen and kidney necrosis virus and Siniperca chuatsi rhabdovirus in Chinese perch (Siniperca chuatsi). Microb. Pathog. 2017, 111, 422–430. [Google Scholar] [CrossRef]
- Long, A.; Garver, K.A.; Jones, S.R.M. Synergistic osmoregulatory dysfunction during salmon lice (Lepeophtheirus salmonis) and infectious hematopoietic necrosis virus co-infection in sockeye salmon (Oncorhynchus nerka) smolts. J. Fish Dis. 2019, 42, 869–882. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Xu, L.; Zhao, J.; Shao, Y.; Lin, Y.; Li, L.; Liu, Q.; Lu, T.; Zhang, Q. Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro. Viruses 2022, 14, 2080. https://doi.org/10.3390/v14092080
Ren G, Xu L, Zhao J, Shao Y, Lin Y, Li L, Liu Q, Lu T, Zhang Q. Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro. Viruses. 2022; 14(9):2080. https://doi.org/10.3390/v14092080
Chicago/Turabian StyleRen, Guangming, Liming Xu, Jingzhuang Zhao, Yizhi Shao, Yujie Lin, Linfang Li, Qi Liu, Tongyan Lu, and Qiya Zhang. 2022. "Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro" Viruses 14, no. 9: 2080. https://doi.org/10.3390/v14092080
APA StyleRen, G., Xu, L., Zhao, J., Shao, Y., Lin, Y., Li, L., Liu, Q., Lu, T., & Zhang, Q. (2022). Antiviral Activity of Crude Polysaccharide Derived from Seaweed against IHNV and IPNV In Vitro. Viruses, 14(9), 2080. https://doi.org/10.3390/v14092080





