Follow-Up of a Cohort of Patients with Post-Acute COVID-19 Syndrome in a Belgian Family Practice
Abstract
1. Introduction
PACS occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms and that last for at least 2 months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others, and generally have an impact on everyday functioning. Symptoms may be new onset following initial recovery from an acute COVID-19 episode, or persist from the initial illness. Symptoms may also fluctuate or relapse over time [8].
2. Methods
2.1. Clinical Data Collection
2.2. Data Collection from Narrative Medicine and Qualitative Approach
2.3. Laboratory
2.4. Nuclear Imaging
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Clinical Data
3.2. Clinical PACS Evolution on Two Years
- Grade 1, mild PACS; 16 patients (9 female, 7 male):The duration is 3 to 8 months, the impairment is mainly respiratory with fatigue, sternal pain, exhaustion with effort, no cognitive nor mnesic disorders. Other aspecific symptoms (skin redness, paresthesia, anosmia, dysgeusia, vertigo) may be present but disappear with time. These patients generally had a low DUSOI severity index and a low functional impairment score at the beginnig of the care and return to normal life after several months, sometimes with sequelae such as recurrent chest pain or taste or smell disorders.
- Grade 2, severe PACS; 15 patients (12 female, 3 male):The duration is 6 to 18 months, with extreme fatigue, effort exhaustion, anomia, cognitive disorder, mnesic disorder. Other symptoms of the digestive, cardiac or autonomic system could be present. Nevertheless, all the symptoms diminish after 12 to 18 months and the resumption of activities is possible with sometimes sequelae (procedural memory disorder, fatigue on exertion, sudden deep breathing). A relapse is possible. These patients most often had a high severity index (DUSOI 3 or 4) and poor functional status (COOP > 20).
- Grade 3, very severe PACS; 21 patients (17 female, 4 male):After 12 to 27 months (maximum at the time of writing) patients are not able to resume their activity or only part-time at the most. Exhaustion is constant, efforts impossible, cognitive revalidation useless, hypersomnia, weight gain due to inactivity, persistent severe memory disorders and of course a considerable anxiety about the future and the feeling of having contracted an unknown and incurable disease. The repercussions on the family life are very severe. The oldest patient (F, 79) is hospitalized with a hypothetical diagnosis of Alzheimer’s disease. At the beginning of the care, these patients could not be distinguished from the others and no predictive elements were found that would allow us to make a prognosis of their evolution.
Concerning Vaccines
3.3. PACS Clinical Picture
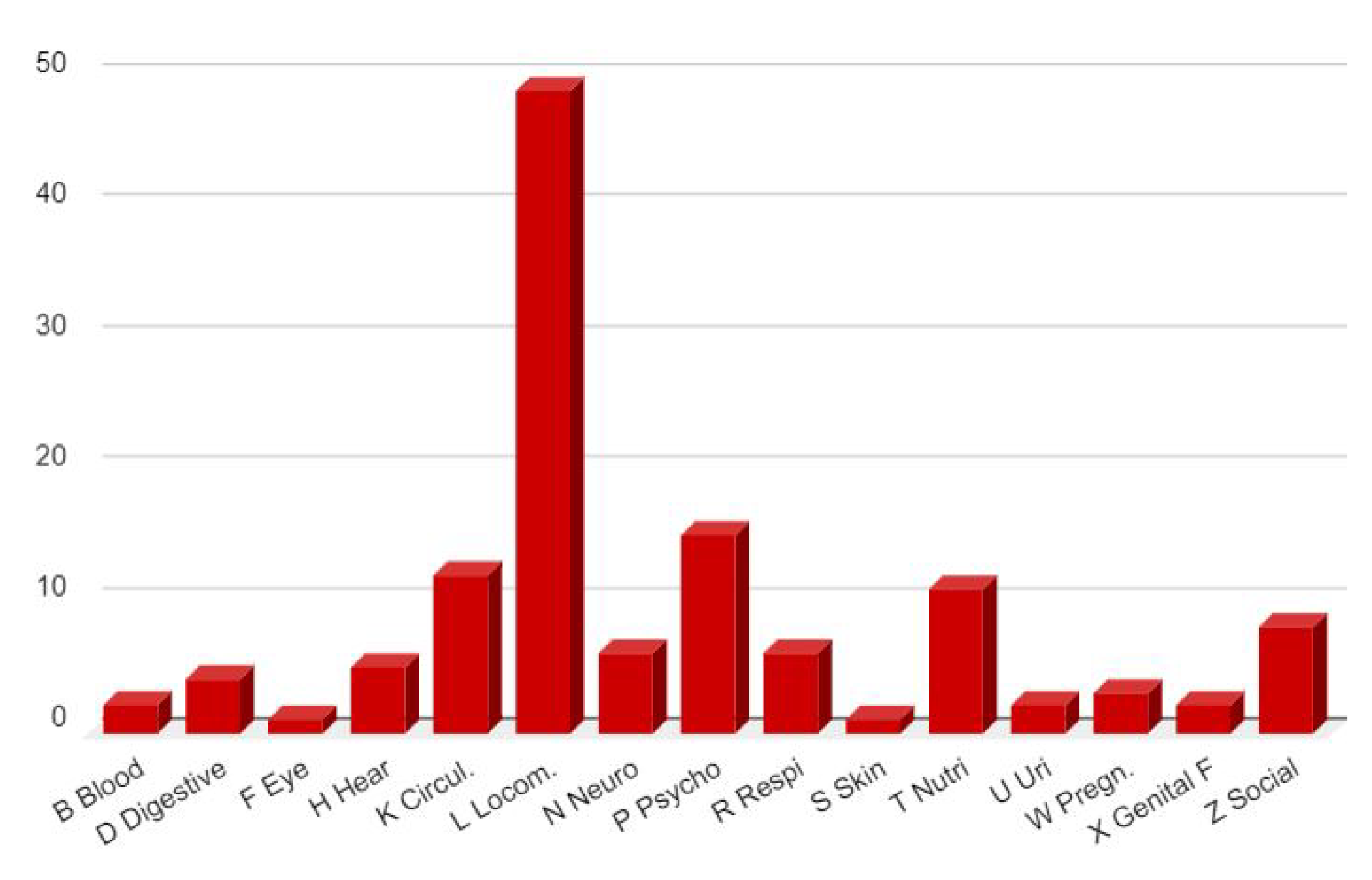
3.4. Nuclear Imaging Interest
- Clinical symptoms suggesting a brain disorder in the context of the COVID-19 pandemic
- A degree of severity of 3 or 4 on the DUSOI/WONCA
- A functional status of more than 20 points on the COOP/WONCA
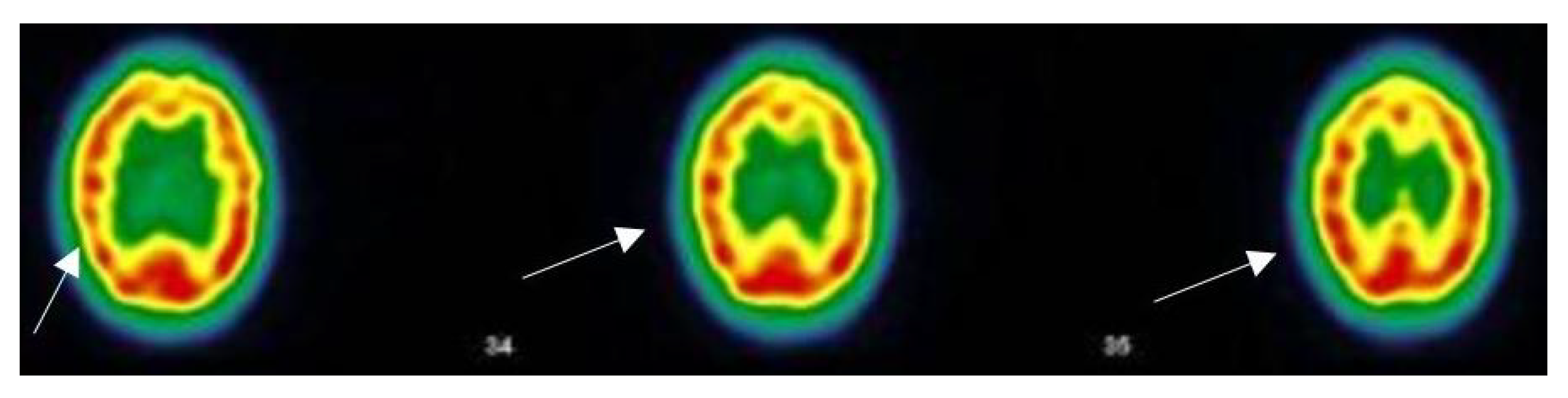
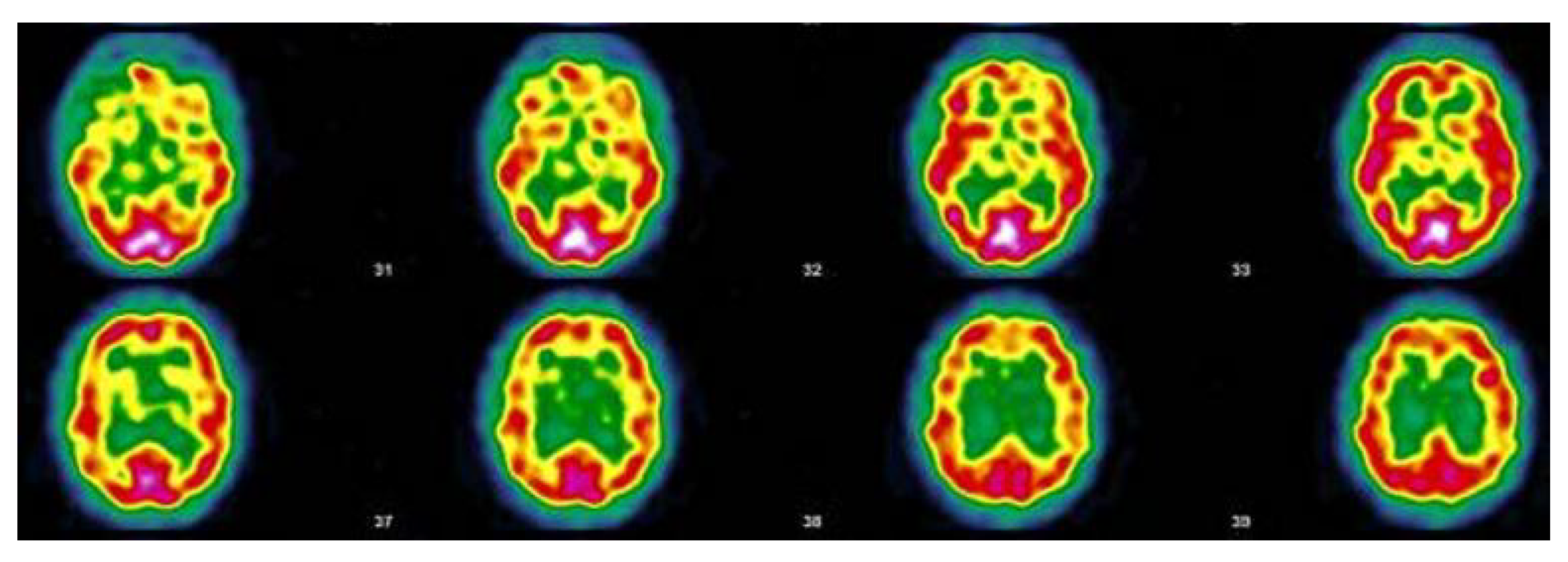
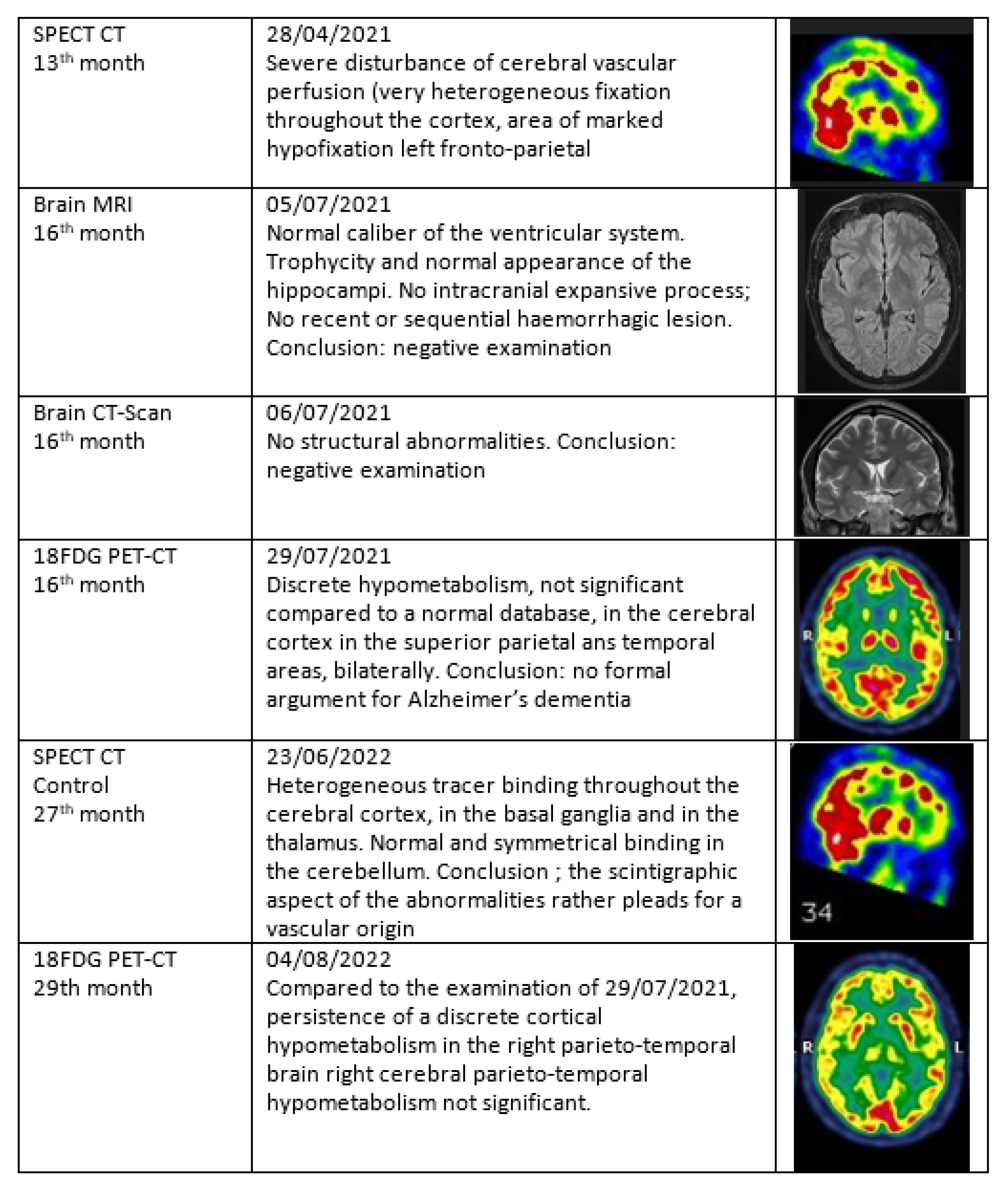
3.5. Narrative and SPECT-CT Images of Some Exemplary Cases
3.5.1. Patient MGA010
3.5.2. Patient MGA017
3.5.3. Patient MGA005
3.5.4. Patient MGA058
4. Discussion
4.1. Clinical Approach
4.2. Global Indicators of Severity and Functional Status
4.3. Limitations
4.4. Hypo-Perfusion and Hypercoagulation Looks Central to PACS Pathophysiology
4.5. Impact of Imaging Diagnosis on Patients’ Experiences
4.6. Severe Reactions to Vaccines in Many Patients
4.7. An Empathetic Therapeutic Approach
4.8. Further Studies
5. Conclusions
- PACS syndrome has a high prevalence in primary care for those who want to see it.
- Clinical skills and narrative medicine are essential to identify and understand patients’ experiences. This needs time, open mindedness and empathy.
- Cerebral hypo-perfusion demonstrated by SPECT-CT seems to correlate with the clinical symptoms in a cohort of PACS patients. This needs further studies.
- Uncertainty about the primary acute infection is a problem. The participation of 48 patients to the European Consortium for Genetic and Immunological Studies on COVID-19 will probably provide some answers and further questions.
- The impact of PACS is substantial, with many social and economic implications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COOP/WONCA | Charts for Primary Care Practice; Cooperative/WONCA |
| DUSOI/WONCA | Duke Severity of Illness Score/WONCA |
| EBM | Evidence Based Medicine |
| ECD Tc-99m | 99mTc-ethyl cysteinate dimer |
| EBM | Evidence Based Medicine |
| ECD Tc-99m | 9mTc-ethyl cysteinate dimer |
| EMR | Electronic Medical Record |
| GP | general practitioner |
| ICPC-2 | International Classification of Primary Care, second version |
| ISPPC | Public Health Community of the Charleroi region |
| NMR | Nuclear Magnetic Resonance |
| MCA | Mast Cell Activation |
| MUS | Medically Unexplained Symptoms |
| 18FDG PET-CT | Positron emission tomography with 18 fluoro-D-glucose integrated with computed tomography |
| PACS | Post-Acute COVID-19 Syndrome |
| PCR | Polymerase Chain Reaction |
| SPECT-CT | Single Photon Emission Tomography-Computed Tomography |
| WHO | World Health Organization |
| WONCA | World Organization of Family Doctors |
Appendix A
- Au : longCovidAT
- Ch : LongCovidCH
- Fr : apresj20.fr
- UK : longCovidsos.org
- UK : longCovid.org
- UK : longCovidwork.co.uk
- UK : https://www.facebook.com/LongCovidPage
- UK : longCovid.scot
- UK : LongCovidWales
- UK : LongCovidPhysio
- EU : LongCOVIDEurope
- Nl : coronaplein.nu
- De : longCoviddeutschland.org
- Ca: Covidlonghaulcanada.com
- USA : Survivorcorps.com
- USA : Body Politic
- USA : Long COVID Alliance
- USA : Long COVID Kids
References
- Blomberg, B.; Mohn, K.G.I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Baraff, A.; Fox, A.; Shahoumian, T.; Hickok, A.; O’Hare, A.M.; Bohnert, A.S.B.; Boyko, E.J.; Maciejewski, M.L.; Bowling, C.B.; et al. Rates and Factors Associated with Documentation of Diagnostic Codes for Long COVID in the National Veterans Affairs Health Care System. JAMA Netw. Open 2022, 5, e2224359. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Abou Shaar, B.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: What do we know about “long covid”? BMJ 2020, 370. [Google Scholar] [CrossRef]
- Burgers, J. “Long covid”: The Dutch response. BMJ 2020, 370. [Google Scholar] [CrossRef]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.E.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv 2021. [Google Scholar] [CrossRef]
- WHO. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 7 July 2022).
- Ayoubkhani, D.; Munro, M. Prevalence of Ongoing Symptoms following Coronavirus (COVID-19) Infection in the UK. 7 July 2022. Available online: https://tinyurl.com/long-covid-uk (accessed on 7 July 2022).
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021, 12, 1494. [Google Scholar] [CrossRef]
- Brodin, P.; Casari, G.; Townsend, L.; O’Farrelly, C.; Tancevski, I.; Löffler-Ragg, J.; Mogensen, T.H.; Casanova, J.L. Studying severe long COVID to understand post-infectious disorders beyond COVID-19. Nat. Med. 2022, 28, 879–882. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.; Olde Hartman, T.C.; Rosmalen, J.G.; Initiative, L.C.R. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211009385. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370. [Google Scholar] [CrossRef]
- Becker, J.H.; Lin, J.J.; Doernberg, M.; Stone, K.; Navis, A.; Festa, J.R.; Wisnivesky, J.P. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw. Open 2021, 4, e2130645. [Google Scholar] [CrossRef]
- Malik, P.; Patel, K.; Pinto, C.; Jaiswal, R.; Tirupathi, R.; Pillai, S.; Patel, U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—A systematic review and meta-analysis. J. Med. Virol. 2021. [Google Scholar] [CrossRef]
- Yelin, D.; Wirtheim, E.; Vetter, P.; Kalil, A.C.; Bruchfeld, J.; Runold, M.; Guaraldi, G.; Mussini, C.; Gudiol, C.; Pujol, M.; et al. Long-term consequences of COVID-19: Research needs. Lancet Infect. Dis. 2020, 20, 1115–1117. [Google Scholar] [CrossRef]
- Kas, A.; Soret, M.; Pyatigoskaya, N.; Habert, M.O.; Hesters, A.; Le Guennec, L.; Paccoud, O.; Bombois, S.; Delorme, C. The cerebral network of COVID-19-related encephalopathy: A longitudinal voxel-based 18F-FDG-PET study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1–15. [Google Scholar] [CrossRef]
- Barizien, N.; Le Guen, M.; Russel, S.; Touche, P.; Huang, F.; Vallée, A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef]
- van Kessel, S.A.; Olde Hartman, T.C.; Lucassen, P.L.; van Jaarsveld, C.H. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022. [Google Scholar] [CrossRef]
- Godeau, D.; Petit, A.; Richard, I.; Roquelaure, Y.; Descatha, A. Return-to-work, disabilities and occupational health in the age of COVID-19. Scand. J. Work. Environ. Health 2021. [Google Scholar] [CrossRef]
- Messin, L.; Puyraveau, M.; Benabdallah, Y.; Lepiller, Q.; Gendrin, V.; Zayet, S.; Klopfenstein, T.; Toko, L.; Pierron, A.; Royer, P.Y. COVEVOL: Natural Evolution at 6 Months of COVID-19. Viruses 2021, 13, 2151. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Long COVID: Pathophysiology-Epidemiology and Patient Needs. KCE, Belgium. Available online: https://kce.fgov.be/sites/default/files/atoms/files/2020-04HSR_Long%20COVID_Scientifi (accessed on 7 July 2022).
- Ledford, H. Do vaccines protect against long COVID? What the data say. Nature 2021, 599, 546–548. [Google Scholar] [CrossRef]
- WHO-News. A Life Altered by Long COVID—Susan’s Experience. Available online: https://www.who.int/europe/news/item/09-08-2022-a-life-altered-by-long-covid—susan-s-experience (accessed on 7 July 2022).
- Aghaei, A.; Zhang, R.; Taylor, S.; Tam, C.C.; Yang, C.H.; Li, X.; Qiao, S. Impact of COVID-19 symptoms on social aspects of life among female long haulers: A qualitative study. Res. Sq. 2022, rs.3.rs, 1285284. [Google Scholar] [CrossRef]
- Van de Vyver, J.; Leite, A.C.; Alwan, N.A. Navigating the social identity of long covid. BMJ 2021, 375, n2933. [Google Scholar] [CrossRef]
- Runco, M.A.; Chand, Y. Problem Finding, Evaluative Thinking, and Creativity. In Problem Finding, Problem Solving, and Creativity; Book Section 2; Ablex Publishing: New York, NY, USA, 1974; pp. 40–47. [Google Scholar]
- Dirkzwager, A.J.; Verhaak, P.F. Patients with persistent medically unexplained symptoms in general practice: Characteristics and quality of care. BMC Fam. Pract. 2007, 8, 1–10. [Google Scholar] [CrossRef]
- Greenhalgh, T. Narrative based medicine in an evidence based world. BMJ 1999, 318, 323–325. [Google Scholar] [CrossRef]
- Wood, M. Naming the illness: The power of words. Fam. Med. 1991, 23, 534–538. [Google Scholar] [PubMed]
- Resnik, D.B. Glossary of Commonly Used Terms in Research Ethics. Available online: https://www.niehs.nih.gov/research/resources/bioethics/glossary/index.cfm (accessed on 24 August 2022).
- Bentzen, N. WONCA Dictionary of General/Family Practice. 2004. Available online: http://www.ph3c.org/PH3C/docs/27/000092/0000052.pdf (accessed on 1 September 2022).
- Jamoulle, M.; Kazeneza-Mugisha, G. Descriptive and Narrative Study of Long Covid Cases in General Practice and Diagnostic Value of Single Photon Emission Computed Tomography. medRxiv 2022. [Google Scholar] [CrossRef]
- Okkes, I.; Jamoulle, M.; Lamberts, H.; Bentzen, N. ICPC-2-E: The electronic version of ICPC-2. Differences from the printed version and the consequences. Fam. Pract. 2000, 17, 101–107. [Google Scholar] [CrossRef][Green Version]
- Llewelyn, H. Reasoning in Medicine and Science. 2013. Available online: https://blog.oup.com/2013/09/medical-diagnosis-reasoning-probable-elimination (accessed on 7 July 2022).
- Parkerson, G.R., Jr.; Broadhead, W.E.; Chiu-Kit, J. The Duke Severity of Illness Checklist (DUSOI) for measurement of severity and comorbidity. J. Clin. Epidemiol. 1993, 46, 379–393. [Google Scholar] [CrossRef]
- Parkerson, G., Jr. Description of the DUSOI-WONCA Severity of Illness Instrument. 1999. Available online: http://www.ph3c.org/4daction/w3_CatVisu/en/description-of-the-dusoi—-wonca–severity-of-illness-instrument.html?wDocID=157 (accessed on 7 July 2022).
- Jamoulle, M.; Roland, M.; Elkinne, J.; Parkerson, G. Le DUSOI/WONCA un indice de gravité pour la médecine générale. 2000. Available online: https://orbi.uliege.be/handle/2268/228936 (accessed on 7 July 2022).
- van Weel, C.; König Zahn, C.; Touw Otten, F.; van Duijn, N.; Meyboom de Jong, B. Measuring Functional Health Status with the COOP/WONCA Charts. A Manual. 1995. Available online: http://www.ph3c.org/PH3C/docs/27/000150/0000103.pdf (accessed on 7 July 2022).
- Casanova, J.L.; Su, H.C.; Abel, L.; Aiuti, A.; Almuhsen, S.; Arias, A.A.; Bastard, P.; Biggs, C.; Bogunovic, D.; Boisson, B.; et al. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell 2020, 181, 1194–1199. [Google Scholar] [CrossRef]
- Guedj, E.; Million, M.; Dudouet, P.; Tissot-Dupont, H.; Bregeon, F.; Cammilleri, S.; Raoult, D. 18 F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: Substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 592–595. [Google Scholar] [CrossRef]
- Guedj, E.; Lazarini, F.; Morbelli, S.; Ceccaldi, M.; Hautefort, C.; Kas, A.; Radulesco, T.; Salmon-Ceron, D.; Eldin, C. Long COVID and the brain network of Proust’s madeleine: Targeting the olfactory pathway. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Rodríguez-Alfonso, B.; Solís, S.R.; Silva-Hernández, L.; Pascual, I.P.; Ibáñez, S.A.; Antón, C.S. 18F-FDG-PET/CT in SARS-CoV-2 infection and its sequelae. Rev. Esp. Med. Nucl. Imagen Mol. 2021. [Google Scholar] [CrossRef]
- Hugon, J. Long-covid: Cognitive deficits (brain fog) and brain lesions in non-hospitalized patients. Presse Med. 2021. [Google Scholar] [CrossRef]
- Verger, A.; Kas, A.; Dudouet, P.; Goehringer, F.; Salmon, D.; Guedj, E. Visual Interpretation of Brain Hypometabolisms Related to Neurological Long COVID: A French Multicentric Experience. Eur. J. Nucl. Med. Mol. Imaging 2022, 32. [Google Scholar] [CrossRef]
- Donnemiller, E.; Heilmann, J.; Wenning, G.K.; Berger, W.; Decristoforo, C.; Moncayo, R.; Poewe, W.; Ransmayr, G. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-beta -CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur. J. Nucl. Med. 2021, 24, 320–325. [Google Scholar] [CrossRef]
- Shepstone, B.J. Cerebral scintigraphy–the phoenix rises again. Postgrad. Med. J. 1988, 64, 4–17. [Google Scholar] [CrossRef][Green Version]
- Catafau, A.M. Brain SPECT in clinical practice. Part I: Perfusion. J. Nucl. Med. 2001, 42, 259–271. [Google Scholar]
- Koyama, M.; Kawashima, R.; Ito, H.; Ono, S.; Sato, K.; Goto, R.; Kinomura, S.; Yoshioka, S.; Sato, T.; Fukuda, H. SPECT imaging of normal subjects with technetium-99m-HMPAO and technetium-99m-ECD. J. Nucl. Med. 1997, 38, 587–592. [Google Scholar]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020, 27, 582–603. [Google Scholar] [CrossRef]
- Conklin, J.; Frosch, M.P.; Mukerji, S.S.; Rapalino, O.; Maher, M.D.; Schaefer, P.W.; Lev, M.H.; Gonzalez, R.; Das, S.; Champion, S.N.; et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J. Neurol. Sci. 2021, 421, 117308. [Google Scholar] [CrossRef]
- Kremer, S.; Lersy, F.; Anheim, M.; Merdji, H.; Schenck, M.; Oesterlé, H.; Bolognini, F.; Messie, J.; Khalil, A.; Gaudemer, A.; et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology 2020, 95, e1868–e1882. [Google Scholar] [CrossRef]
- Gamal, S.M.T.; Azab, A.O.M.; El Refaei, S.M.; Houseni, M. The role of 18-FDG PET/CT assessment of functional brain metabolism in cancer patients after chemotherapy. Egypt. J. Radiol. Nucl. Med. 2021, 52, 1–7. [Google Scholar] [CrossRef]
- Davison, C.M.; O’Brien, J.T. A comparison of FDG-PET and blood flow SPECT in the diagnosis of neurodegenerative dementias: A systematic review. Int. J. Geriatr. Psychiatry 2014, 29, 551–561. [Google Scholar] [CrossRef]
- Blazhenets, G.; Schröter, N.; Bormann, T.; Thurow, J.; Wagner, D.; Frings, L.; Weiller, C.; Meyer, P.T.; Dressing, A.; Hosp, J.A. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Lennon, O.C.; Carey, A.; Creed, A.; Durcan, S.; Blake, C. Reliability and validity of COOP/WONCA functional health status charts for stroke patients in primary care. J. Stroke Cerebrovasc. Dis. 2011, 20, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, R. Design, validation and implementation of the post-acute (long) COVID-19 quality of life (PAC-19QoL) instrument. Health Qual. Life Outcomes 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. Br. Med. J. 1996, 312. [Google Scholar] [CrossRef]
- Unger, J.P.; Morales, I.; De Paepe, P. Medical heuristics and action-research: Professionalism versus science. BMC Health Serv. Res. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Pols, J. Knowing patients: Turning patient knowledge into science. Sci. Technol. Hum. Values 2014, 39, 73–97. [Google Scholar] [CrossRef]
- McWhinney, I. Problem-solving & decision-making in family practice. Can. Fam. Physician 1979, 25, 1473. [Google Scholar]
- Malterud, K. The art and science of clinical knowledge: Evidence beyond measures and numbers. Lancet 2001, 358, 397–400. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Radionuclide Basics: Technetium-99. 2021. Available online: https://www.epa.gov/radiation/radionuclide-basics-technetium-99 (accessed on 7 July 2022).
- Vucina, J. Technetium-99m production for use in nuclear medicine. Med. Pregl. 2000, 53, 631:4. [Google Scholar]
- Haines, A.; Scheelbeek, P.; Abbasi, K. Challenges for health in the Anthropocene epoch. BMJ 2019, 364, 1460. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Travieso, A.; Sagir, A.; Martínez-Gómez, E.; Carrascosa-Granada, A.; Toya, T.; Núñez-Gil, I.J.; Estrada, V.; Lerman, A.; Escaned, J. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int. J. Cardiol. 2021, 345, 153–155. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front. Med. 2021, 8, 754667. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 2020, 21, 8234. [Google Scholar] [CrossRef]
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 spike protein S1 induces fibrin (ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 2021, 41, BSR20210611. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 1–18. [Google Scholar] [CrossRef]
- Jones, O.Y.; Yeralan, S. Is Long COVID a State of Systemic Pericyte Disarray? J. Clin. Med. 2022, 11, 572. [Google Scholar] [CrossRef]
- Rutkai, I.; Mayer, M.G.; Hellmers, L.M.; Ning, B.; Huang, Z.; Monjure, C.J.; Coyne, C.; Silvestri, R.; Golden, N.; Hensley, K.; et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the nervous system. Cell 2020, 183, 16–27. [Google Scholar] [CrossRef]
- McMahon, D.E.; Gallman, A.E.; Hruza, G.J.; Rosenbach, M.; Lipoff, J.B.; Desai, S.R.; French, L.E.; Lim, H.; Cyster, J.G.; Fox, L.P.; et al. Long COVID in the skin: A registry analysis of COVID-19 dermatological duration. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Nirenberg, M.S.; Requena, L.; Santonja, C.; Smith, G.T.; McClain, S.A. Histopathology of Persistent Long COVID Toe: A Case Report. J. Cutan. Pathol. 2022, 49, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Bunker, C.B.; Ciurtin, C.; Porter, J.C.; Chambers, R.C.; Papdopoulou, C.; Garthwaite, H.; Hillman, T.; Heightman, M.; Howell, K.J.; et al. Chilblain-like acral lesions in long COVID-19: Management and implications for understanding microangiopathy. Lancet Infect. Dis. 2021, 7, 912. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent exertional intolerance after COVID-19: Insights from invasive cardiopulmonary exercise testing. Chest 2022, 161, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Vallejo Camazón, N.; Teisa, A.; Martínez Membrivea, M.J.; Llibre, C.; Bayés-Genís, A.; Mateu, L. Long COVID-19 and microvascular disease-related angina. Rev. EspañOla Cardiol. 2021. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent brainstem dysfunction in long-COVID: A hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast cell activation symptoms are prevalent in Long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Balagny, P.; Arnoult, F.; Mangin, L.; Bancal, C.; d’Ortho, M.P.; Frija-Masson, J. Hyperventilation: A possible explanation for long-lasting exercise intolerance in mild Covid-19 survivors? Front. Physiol. 2021, 11, 1856. [Google Scholar] [CrossRef]
- Geng, L.N.; Bonilla, H.F.; Shafer, R.W.; Miglis, M.G.; Yang, P.C. Case Report of Breakthrough Long COVID and the Use of Nirmatrelvir-Ritonavir. Researchsquare 2022. [Google Scholar] [CrossRef]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals With Long-COVID: Identification of Diagnostic Biomarkers. Front. Immunol. 2022, 13, 848886. [Google Scholar] [CrossRef]
- Ziporyn, T.D. Nameless Diseases; Rudger Universty Press: New Brunswick, NJ, USA, 1992. [Google Scholar]
- Arnold, D.T.; Milne, A.; Samms, E.; Stadon, L.; Maskell, N.A.; Hamilton, F.W. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: A case series. Ann. Intern. Med. 2021, 174, 1334–1336. [Google Scholar] [CrossRef]
- Wynberg, E.; Han, A.X.; Boyd, A.; van Willigen, H.D.; Verveen, A.; Lebbink, R.; van der Straten, K.; Kootstra, N.; van Gils, M.J.; Russell, C.; et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine 2022, 40, 4424–4431. [Google Scholar] [CrossRef]
- Leen, B.; Delaunois, I.; Carrigan, M.; McCarthy, S. Evidence Summary: What Is the Latest Evidence about the Existence of Long-COVID or Post-COVID and Its Persistence for COVID-19 Survivors? What Evidence Is Currently Available on the Management of Patients Who Have Post Viral Fatigue Syndrome Due to COVID-19? [v1.1]. 2021. Available online: https://doi.org/http://hdl.handle.net/10147/628919 (accessed on 1 September 2022).
- Moghimi, N.; Di Napoli, M.; Biller, J.; Siegler, J.E.; Shekhar, R.; McCullough, L.D.; Harkins, M.S.; Hong, E.; Alaouieh, D.A.; Mansueto, G.; et al. The Neurological Manifestations of Post-Acute Sequelae of SARS-CoV-2 infection. Curr. Neurol. Neurosci. Rep. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Swain, O.; Romano, S.K.; Miryala, R.; Tsai, J.; Parikh, V.; Umanah, G.K. SARS-CoV-2 Neuronal Invasion and Complications: Potential Mechanisms and Therapeutic Approaches. J. Neurosci. 2021, 41, 5338–5349. [Google Scholar] [CrossRef]
- Housman, H.A. Exploring Neuroplasticity in the Classroom: Teaching Cortical Reorganization in the Visual System with a Stroke Patient Study. 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/33880107/ (accessed on 7 July 2022).
- Kuut, T.; Müller, F.; Aldenkamp, A.; Assmann-Schuilwerve, E.; Braamse, A.; Geerlings, S.; Gibney, K.; Kanaan, R.; Nieuwkerk, P.; Olde Hartman, T.; et al. A randomised controlled trial testing the efficacy of Fit after COVID, a cognitive behavioural therapy targeting severe post-infectious fatigue following COVID-19 (ReCOVer): Study protocol. Trials 2021, 22, 867. [Google Scholar] [CrossRef]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5185. [Google Scholar] [CrossRef]
- Jamoulle, M. Trajet de Soins «Long Covid»: Comment ça marche?—[Long Covid Pathway, How It Works?]. 2022. Available online: https://orbi.uliege.be/handle/2268/293637 (accessed on 1 September 2022).
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 1–18. [Google Scholar] [CrossRef]
- Krzych, Ł.J.; Putowski, Z.; Czok, M.; Hofman, M. What Is the Role of Therapeutic Plasma Exchange as an Adjunctive Treatment in Severe COVID-19: A Systematic Review. Viruses 2021, 13, 1484. [Google Scholar] [CrossRef]
- Parry, C. Opening the Black Box: The Researchers Trying to Find Treatments for Long COVID—The Pharmaceutical Journal. 2022. Available online: https://pharmaceutical-journal.com/article/feature/opening-the-black-box-the-researchers-trying-to-find-treatments-for-long-covid (accessed on 1 September 2022).
- Szolnoky, G.; González-Ochoa, A.J. Sulodexide: A Benefit for Cardiovascular Sequelae of Long COVID Patients? Clin. Appl. Thromb. 2022, 28, 10760296221084300. [Google Scholar] [CrossRef]
- Robbins, T.; Gonevski, M.; Clark, C.; Baitule, S.; Sharma, K.; Magar, A.; Patel, K.; Sankar, S.; Kyrou, I.; Ali, A.; et al. Hyperbaric oxygen therapy for the treatment of long COVID: Early evaluation of a highly promising intervention. Clin. Med. 2021, 21, e629–e632. [Google Scholar] [CrossRef]
- Kander, T. Coagulation disorder in COVID-19. Lancet Haematol. 2020, 7, e630–e632. [Google Scholar] [CrossRef]
- Ahmed, H.A.S.; Merrell, E.; Ismail, M.; Joudeh, A.I.; Riley, J.B.; Shawkat, A.; Habeb, H.; Darling, E.; Goweda, R.A.; Shehata, M.H.; et al. Rationales and uncertainties for aspirin use in COVID-19: A narrative review. Fam. Med. Community Health 2021, 9, e000741. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Moremi, K.; Oladejo, S.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]


| Status | Recovered | Still Sick | |
|---|---|---|---|
| Mild | Severe | Very Severe | |
| Grade | 1 | 2 | 3 |
| Label | mild Long Covid | severe Long Covid | very severe Long Covid |
| Length | 3 à 8 months | 6 à 18 months | > 18 months |
| Number | 16 patients (9 f, 7 m) | 17 patients ( 13 f, 4 m) | 22 patients (18 f, 4 m) |
| Symptoms | No after-effects | Minor sequelae (e.g. difficult exertion or minor loss of working memory) | Major fatigue, exertional exhaustion, difficulty concentrating, emotional disturbances, paresthesia, persistent memory problems |
| Capacity | Normal course of life resumed | Unable to resume normal life | |
| Outcome | |||||||
|---|---|---|---|---|---|---|---|
| Recovered Mild and Severe | Still ill Very Severe | ||||||
| n | % | n | % | Test | p | ||
| Sex | Female | 22 | 55 | 18 | 45 | 1.528 # | 0.216 |
| Male | 11 | 73.3 | 4 | 26.7 | |||
| Number of COVID episodes | 1 | 28 | 63.6 | 16 | 36.4 | 3.918 * | 0.117 |
| 2 or 3 | 4 | 44.4 | 5 | 55.6 | |||
| # of vaccines | 0 | 6 | 85.7 | 1 | 14.3 | 4.292 * | 0.202 |
| 1 | 0 | 0 | 1 | 100 | |||
| 2 | 12 | 66.7 | 6 | 33.3 | |||
| 3 | 15 | 51.7 | 14 | 48.3 | |||
| Vaccine reaction | Yes | 18 | 50 | 18 | 50 | 4.238 * | 0.121 |
| No | 9 | 75 | 3 | 25 | |||
| Not vaccinated | 6 | 85.7 | 1 | 14.3 | |||
| Vaccine reaction type | Local | 4 | 66.7 | 2 | 33.3 | 0.800 * | 0.658 |
| Systemic | 14 | 46.7 | 16 | 53.3 | |||
| First SPECT | No | 19 | 86.4 | 3 | 13.6 | 10.617 # | 0.001 |
| Yes | 13 | 41.9 | 18 | 58.1 | |||
| First SPECT status | Yes | 13 | 43.3 | 17 | 56.7 | 0.034 * | 1.000 |
| No | 1 | 50.0 | 1 | 50.0 | |||
| Second SPECT | No | 28 | 73.7 | 10 | 26.3 | 12.766 # | <0.001 |
| Yes | 3 | 20.0 | 12 | 80.0 | |||
| Second SPECT status | Improved | 3 | 37.5 | 5 | 62.5 | 3.281 * | 0.123 |
| Worsened | 0 | 0 | 7 | 100 | |||
| PCR test in the first episode of COVID | No | 15 | 65.2 | 8 | 34.8 | 0.448 # | 0.503 |
| Yes | 18 | 56.3 | 14 | 43.8 | |||
| DUSOI | 2 | 6 | 100 | 0 | 0 | 13.847 * | 0.001 |
| 3 | 18 | 78.3 | 5 | 21.7 | |||
| 4 | 9 | 34.6 | 17 | 65.4 | |||
| Mean | SD | Mean | SD | ||||
| Age (years) | 42.9 | 15.6 | 42.0 | 12.9 | 0.222 ## | 0.825 | |
| COOP Total score | 20.8 | 3.7 | 23.4 | 2.3 | 2.758 ## | 0.008 | |
| Months after acute COVID | 13.3 | 8.9 | 18.3 | 5.9 | 2.347 ** | 0.019 | |
| The acute COVID-19 experience | Before I got sick with COVID-19, I was generally feeling fine because I was used to my condition. But when I got COVID-19, everything became more difficult …“ ”Before having COVID I was mentally in a pretty good place, a little depressed but not much. But with COVID, I was at my lowest point. A week to ten days later, I became extremely sick and more and more afraid. I could hardly see, I couldn’t even hold my phone. I felt like I had no oxygen left in my brain and I had to go to the window to breathe. I had awful headaches. I was thinking, ‘I’m going to die, I’m going to die,’ ‘Why me? Why did I get COVID?’. I wanted to die. Life seemed very dark to me, and I didn’t feel like living, I didn’t feel like eating. Even tea, which I usually like to drink, disgusted me. (…) For more than three weeks, I didn’t eat, I lost weight, I couldn’t sleep, I woke up at night, I cried and cried …I didn’t know how to do anything. |
| The PACS period | For nine months I didn’t laugh, I was always tired, I didn’t go out, I was always in a chair. I had to eat all the time, I gained 7 kgs. I would have hunger attacks, and when I didn’t eat, I would shake. In the morning, when I woke up I ate, at night I woke up and ate. Every morning I was waiting for the night to come and every night I waited for the morning. The days were endless because I was sick, I did nothing. I couldn’t stand the TV or the noise. Before COVID, I thought I was a beautiful woman but after when I looked at myself in the mirror, I said to myself ‘I am so old, as if I had aged ten years’. I forgot a lot, words, names, …I had to repeat to myself ‘I must not forget, I must not forget’. My brain was working backwards. I was angry for no reason. I was wondering ‘when will I die?’ |
| Acute Symptoms | Long-Lasting Symptoms | SPECT-CT Protocol |
|---|---|---|
| MGA001, F, 48 | ||
| 13 October 2020 Throat pain, rhinorrhea, bad aches, severe fatigue and headache, but no breathing difficulties, dysgeusia, anosmia. Stays at home, cured after 12 days. Home care only | 11 November 2020 Pain in both eyes, ocular pruritus, rapid ocular fatigue, noise intolerance, memory loss (forgets to pick up her daughter at school), concentration problems, remains isolated in her room, dyspnea at the slightest effort and at speech, almost continuous osteoarticular and muscular pains often with headaches, abnormal dreams, depressive feeling, fatigue, post-exertional malaise (PEM) | 27 July 2021 “On the images taken, left fronto-parietal, left frontal and left thalamic hypofixation is observed. No preservation of the sensory motor cortices. The fixation in front of the cerebellum is correct. Conclusion: Scintigraphic examination compatible with a cerebral pathology of the vascular type with clearer left fronto-parietal, left frontal and left thalamic vascular disorders”. |
| MGA013, F, 39 | ||
| 3 March 2021 Cough, aching, elevated temperature, headache, 20 days in bed, loss of taste, loss of smell, severe tinnitus, 20 days in total. Home care | 5 October 2021 Hearing loss in right ear, balance always disturbed, dizziness, loss of vision, quickly tired, severe weight gain, quickly out of breath, became depressed, pain in left hip every night, post-vaccinations headaches, memory loss, word retrieval deficit, repeats herself and doesn’t realize it, forgets which groceries she went to get, has trouble concentrating, disseminated myalgia, insomnia, loss of sense of direction | 17 November 2021 “Heterogeneous tracer distribution throughout the cortex, with more marked hypofixation in the bilateral predominantly left superior parietal, left parietal, bilateral medial temporal and bilateral predominantly right parieto-occipital areas. Diffuse subcortical periventricular hypofixation. The basal ganglia and cerebellum show preserved and symmetrical tracer uptake. Scintigraphic image suggestive of vascular damage in the broad sense.” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamoulle, M.; Kazeneza-Mugisha, G.; Zayane, A. Follow-Up of a Cohort of Patients with Post-Acute COVID-19 Syndrome in a Belgian Family Practice. Viruses 2022, 14, 2000. https://doi.org/10.3390/v14092000
Jamoulle M, Kazeneza-Mugisha G, Zayane A. Follow-Up of a Cohort of Patients with Post-Acute COVID-19 Syndrome in a Belgian Family Practice. Viruses. 2022; 14(9):2000. https://doi.org/10.3390/v14092000
Chicago/Turabian StyleJamoulle, Marc, Gisele Kazeneza-Mugisha, and Ayoub Zayane. 2022. "Follow-Up of a Cohort of Patients with Post-Acute COVID-19 Syndrome in a Belgian Family Practice" Viruses 14, no. 9: 2000. https://doi.org/10.3390/v14092000
APA StyleJamoulle, M., Kazeneza-Mugisha, G., & Zayane, A. (2022). Follow-Up of a Cohort of Patients with Post-Acute COVID-19 Syndrome in a Belgian Family Practice. Viruses, 14(9), 2000. https://doi.org/10.3390/v14092000





