SARS-CoV-2 Surveillance in Belgian Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Concentration, Extraction, and Quantification
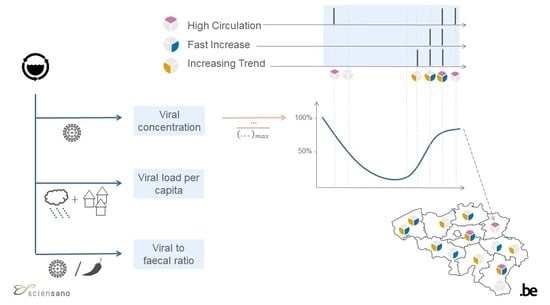
2.2. Concentrations in Wastewater and Indicators
2.3. COVID-19 Cases in the Covered Areas
2.4. Data Analysis
3. Results
3.1. Concentration in Wastewater and Daily New Cases
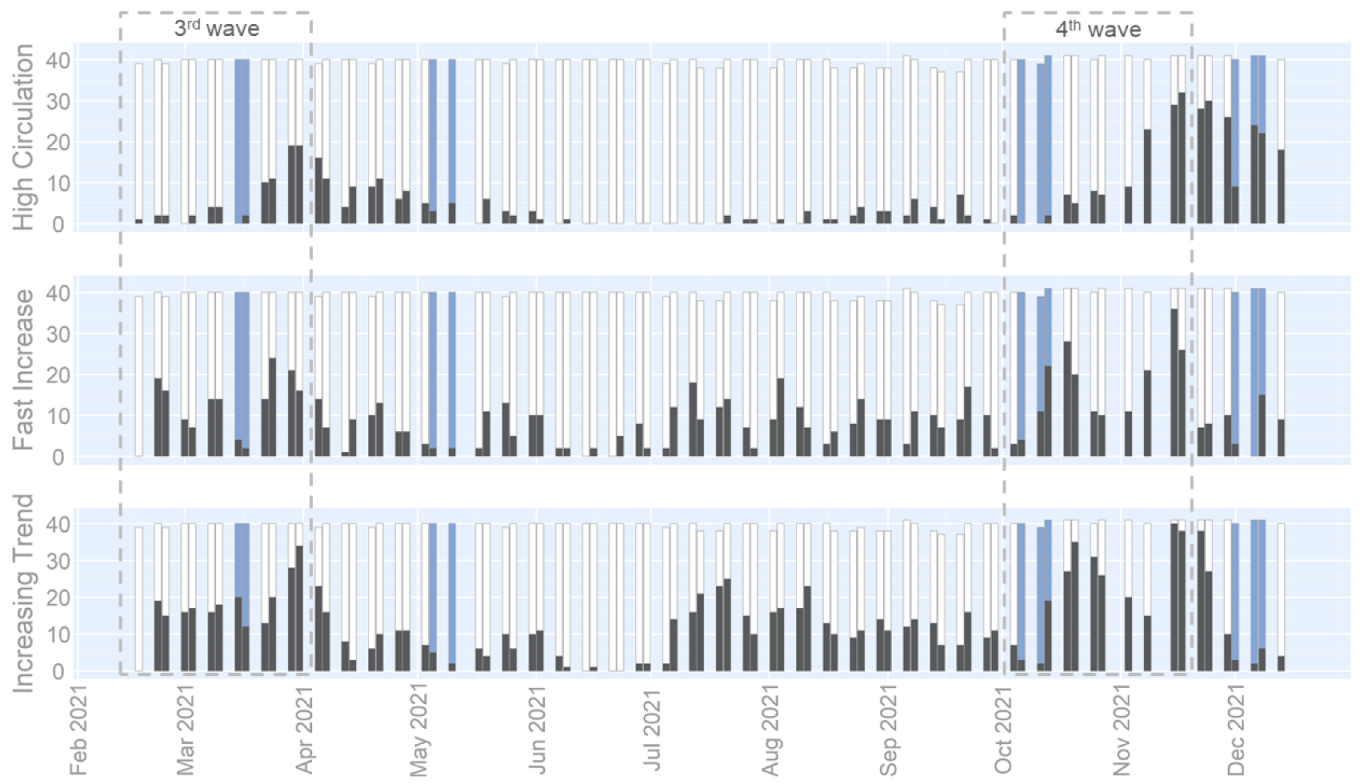
3.2. Wastewater Indicators
4. Discussion
4.1. Concentration in Wastewater and Daily New Cases
4.2. Wastewater Indicators
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitale, D.; Morales Suárez-Varela, M.; Picó, Y. Wastewater-based epidemiology, a tool to bridge biomarkers of exposure, contaminants, and human health. Curr. Opin. Environ. Sci. Health 2021, 20, 100229. [Google Scholar] [CrossRef]
- COVID-19 Wastewater-Base Epidemiology Collaborative: Publication Map. Available online: https://www.covid19wbec.org/publication-map (accessed on 19 January 2022).
- Hart, O.E.; Halden, R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020, 730, 138875. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, P.M.; Graber, T.E.; Mercier, E.; Montpetit, D.; Alexandrov, I.; Neault, N.; Baig, A.T.; Mayne, J.; Zhang, X.; Alain, T.; et al. Catching a resurgence: Increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci. Total Environ. 2021, 770, 145319. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xiao, A.; Zhang, J.; Moniz, K.; Endo, N.; Armas, F.; Bonneau, R.; Brown, M.A.; Bushman, M.; Chai, P.R.; et al. SARS-CoV-2 Titers in Wastewater Foreshadow Dynamics and Clinical Presentation of New COVID-19 Cases. medRxiv 2020. [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Ronquillo, N.; Belda-Ferre, P.; Alvarado, D.; Javidi, T.; Longhurst, C.A.; Knight, R. High-Throughput Wastewater SARS-CoV-2 Detection Enables Forecasting of Community Infection Dynamics in San Diego County. mSystems 2021, 6, e00045-21. [Google Scholar] [CrossRef]
- Gerrity, D.; Papp, K.; Stoker, M.; Sims, A.; Frehner, W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res. X 2021, 10, 100086. [Google Scholar] [CrossRef]
- D’Aoust, P.M.; Mercier, E.; Montpetit, D.; Jia, J.-J.; Alexandrov, I.; Neault, N.; Baig, A.T.; Mayne, J.; Zhang, X.; Alain, T.; et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021, 188, 116560. [Google Scholar] [CrossRef]
- Lazuka, A.; Arnal, C.; Soyeux, E.; Sampson, M.; Lepeuple, A.-S.; Deleuze, Y.; Pouradier Duteil, S.; Lacroix, S. COVID-19 wastewater based epidemiology: Long-term monitoring of 10 WWTP in France reveals the importance of the sampling context. Water Sci. Technol. 2021, 84, 1997–2013. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Kumar, M.; Islam, M.T.; Haque, R.; Chakraborty, S.; Ahmad, A.; Niazi, N.K.; Cetecioglu, Z.; Nilsson, D.; Ijumulana, J.; et al. Prevalence of SARS-CoV-2 in Communities Through Wastewater Surveillance—A Potential Approach for Estimation of Disease Burden. Curr. Pollut. Rep. 2021, 7, 160–166. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Shi, J.; Luby, S.P.; Jiang, G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021, 415, 129039. [Google Scholar] [CrossRef] [PubMed]
- Bertels, X.; Demeyer, P.; Van den Bogaert, S.; Boogaerts, T.; van Nuijs, A.L.; Delputte, P.; Lahousse, L. Factors influencing SARS-CoV-2 RNA concentrations in wastewater up to the sampling stage: A systematic review. Sci. Total Environ. 2022, 820, 153290. [Google Scholar] [CrossRef] [PubMed]
- Westhaus, S.; Weber, F.-A.; Schiwy, S.; Linnemann, V.; Brinkmann, M.; Widera, M.; Greve, C.; Janke, A.; Hollert, H.; Wintgens, T.; et al. Detection of SARS-CoV-2 in raw and treated wastewater in Germany—Suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021, 751, 141750. [Google Scholar] [CrossRef] [PubMed]
- Boogaerts, T.; Ahmed, F.; Choi, P.M.; Tscharke, B.; O’Brien, J.; De Loof, H.; Gao, J.; Thai, P.; Thomas, K.; Mueller, J.F.; et al. Current and future perspectives for wastewater-based epidemiology as a monitoring tool for pharmaceutical use. Sci. Total Environ. 2021, 789, 148047. [Google Scholar] [CrossRef]
- Scott, L.C.; Aubee, A.; Babahaji, L.; Vigil, K.; Tims, S.; Aw, T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021, 200, 111374. [Google Scholar] [CrossRef]
- Wilder, M.L.; Middleton, F.; Larsen, D.A.; Du, Q.; Fenty, A.; Zeng, T.; Insaf, T.; Kilaru, P.; Collins, M.; Kmush, B.; et al. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Res. X 2021, 11, 100100. [Google Scholar] [CrossRef]
- Róka, E.; Khayer, B.; Kis, Z.; Kovács, L.B.; Schuler, E.; Magyar, N.; Málnási, T.; Oravecz, O.; Pályi, B.; Pándics, T.; et al. Ahead of the second wave: Early warning for COVID-19 by wastewater surveillance in Hungary. Sci. Total Environ. 2021, 786, 147398. [Google Scholar] [CrossRef]
- Kantor, R.S.; Nelson, K.L.; Greenwald, H.D.; Kennedy, L.C. Challenges in Measuring the Recovery of SARS-CoV-2 from Wastewater. Environ. Sci. Technol. 2021, 55, 3514–3519. [Google Scholar] [CrossRef]
- Saguti, F.; Magnil, E.; Enache, L.; Churqui, M.P.; Johansson, A.; Lumley, D.; Davidsson, F.; Dotevall, L.; Mattsson, A.; Trybala, E.; et al. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021, 189, 116620. [Google Scholar] [CrossRef]
- WHO Public Health Surveillance for COVID-19: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-SurveillanceGuidance-2022.1 (accessed on 23 June 2022).
- European Commission. Commission Recommendation (EU) 2021/472 of 17 March 2021 on a Common Approach to Establish a Systematic Surveillance of SARS-CoV-2 and Its Variants in Wastewaters in the EU; European Commission: Brussels, Belgium, 2021; p. 8. [Google Scholar]
- COVIDPoops19. Available online: https://ucmerced.maps.arcgis.com/apps/dashboards/c778145ea5bb4daeb58d31afee389082 (accessed on 23 June 2022).
- Boogaerts, T.; Jacobs, L.; De Roeck, N.; Van den Bogaert, S.; Aertgeerts, B.; Lahousse, L.; van Nuijs, A.L.N.; Delputte, P. An alternative approach for bioanalytical assay optimization for wastewater-based epidemiology of SARS-CoV-2. Sci. Total Environ. 2021, 789, 148043. [Google Scholar] [CrossRef]
- Coupeau, D.; Burton, N.; Lejeune, N.; Loret, S.; Petit, A.; Pejakovic, S.; Poulain, F.; Bonil, L.; Trozzi, G.; Wiggers, L.; et al. SARS-CoV-2 Detection for Diagnosis Purposes in the Setting of a Molecular Biology Research Lab. Methods Protoc. 2020, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- CDC 2019-Novel Coronavirus (2019-NCoV) Real-Time RT-PCR Primers and Probes. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (accessed on 5 July 2022).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Breitbart, M.; Lee, W.H.; Run, J.-Q.; Wei, C.L.; Soh, S.W.L.; Hibberd, M.; Liu, E.T.; Rohwer, F.; Ruan, Y. RNA Viral Community in Human Feces: Prevalence of Plant Pathogenic Viruses. PLoS Biol. 2005, 4, e3. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Merkes, C.; Klymus, K.; Allison, M.; Goldberg, C.; Helbing, C.; Hunter, M.; Jackson, C.; Lance, R.; Mangan, A.; Monroe, E.; et al. Generic QPCR Limit of Detection (LOD)/Limit of Quantification (LOQ) Calculator; U.S. Geological Survey: Baltimore, MD, USA, 2019.
- Kitajima, M.; Sassi, H.P.; Torrey, J.R. Pepper mild mottle virus as a water quality indicator. npj Clean Water 2018, 1, 19. [Google Scholar] [CrossRef]
- Public Dashboard of the Belgian Wastewater-Based Epidemilogy. Available online: https://epistat.wiv-isp.be/covid/covid-19.html (accessed on 2 January 2022).
- Belgian Public Health Website. Available online: https://covid-19.sciensano.be/fr/covid-19-situation-epidemiologique (accessed on 2 January 2022).
- Meurisse, M.; Lajot, A.; Dupont, Y.; Lesenfants, M.; Klamer, S.; Rebolledo, J.; Lernout, T.; Leroy, M.; Capron, A.; Van Bussel, J.; et al. One year of laboratory-based COVID-19 surveillance system in Belgium: Main indicators and performance of the laboratories (March 2020–21). Arch. Public Health 2021, 79, 188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklöv, J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021, 28, taab124. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef]
- Rapport 26: Is er motivationeel draagvlak voor verstrengingen? De Motivatiebarometer. Available online: https://motivationbarometer.com/portfolio-item/rapport-26-is-er-motivationeel-draagvlak-voor-verstrengingen/ (accessed on 7 June 2022).
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef]

| Gene | Primer/Probe | Final cc. | Sequence (5′-3′) | Ref. |
|---|---|---|---|---|
| N1 | nCoV_N1-F | 500 nM | GACCCCAAAATCAGCGAAAT | [26] |
| nCoV_N1-R | 500 nM | TCTGGTTACTGCCAGTTGAATCTG | [26] | |
| nCoV_N1-P | 125 nM | ACCCCGCATTACGTTTGGTGGACC | [26] | |
| N2 | nCoV_N2-F | 500 nM | TTACAAACATTGGCCGCAAA | [26] |
| nCoV_N2-R | 500 nM | GCGCGACATTCCGAAGAA | [26] | |
| nCoV_N2-P | 125 nM | ACAATTTGCCCCCAGCGCTTCAG | [26] | |
| E | E_Sarbeco-F | 400 nM | ACAGGTACGTTAATAGTTAATAGCGT | [27] |
| E_Sarbeco-R | 400 nM | ATATTGCAGCAGTACGCACACA | [27] | |
| E_Sarbeco-P | 200 nM | ACACTAGCCATCCTTACTGCGCTTCG | [27] | |
| PMMoV | PMMV-rev-F | 400 nM | GAGTGGTTTGACCTTAACGTTTGA | [28] |
| PMMV-R | 400 nM | TTGTCGGTTGCAATGCAAGT | [28] | |
| PMMV-P | 200 nM | CCTACCGAAGCAAATG | [28] |
| Daily New Cases Correlation Against | Entire Period (N = 4984) | 2nd Wave (N = 507) | 3rd Wave (N = 558) | 4th Wave (N = 492) |
|---|---|---|---|---|
| Viral concentration (SARS-CoV-2 gene copies/mL) | 0.54 1 | 0.66 1 | 0.59 | 0.57 |
| Viral load per capita (SARS-CoV-2 gene copies/day/100 k inhab.) | n.d. 2 | n.d. 2 | 0.52 | 0.52 |
| Viral to faecal ratio (SRAS-CoV-2 gene copies/PMMoV gene copies) | n.d. 2 | n.d. 2 | 0.55 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssens, R.; Hanoteaux, S.; Maloux, H.; Klamer, S.; Laisnez, V.; Verhaegen, B.; Linard, C.; Lahousse, L.; Delputte, P.; Terwagne, M.; et al. SARS-CoV-2 Surveillance in Belgian Wastewaters. Viruses 2022, 14, 1950. https://doi.org/10.3390/v14091950
Janssens R, Hanoteaux S, Maloux H, Klamer S, Laisnez V, Verhaegen B, Linard C, Lahousse L, Delputte P, Terwagne M, et al. SARS-CoV-2 Surveillance in Belgian Wastewaters. Viruses. 2022; 14(9):1950. https://doi.org/10.3390/v14091950
Chicago/Turabian StyleJanssens, Raphael, Sven Hanoteaux, Hadrien Maloux, Sofieke Klamer, Valeska Laisnez, Bavo Verhaegen, Catherine Linard, Lies Lahousse, Peter Delputte, Matthieu Terwagne, and et al. 2022. "SARS-CoV-2 Surveillance in Belgian Wastewaters" Viruses 14, no. 9: 1950. https://doi.org/10.3390/v14091950
APA StyleJanssens, R., Hanoteaux, S., Maloux, H., Klamer, S., Laisnez, V., Verhaegen, B., Linard, C., Lahousse, L., Delputte, P., Terwagne, M., Marescaux, J., Pype, R., Didy, C., Dierick, K., Van Hoorde, K., & Lesenfants, M. (2022). SARS-CoV-2 Surveillance in Belgian Wastewaters. Viruses, 14(9), 1950. https://doi.org/10.3390/v14091950